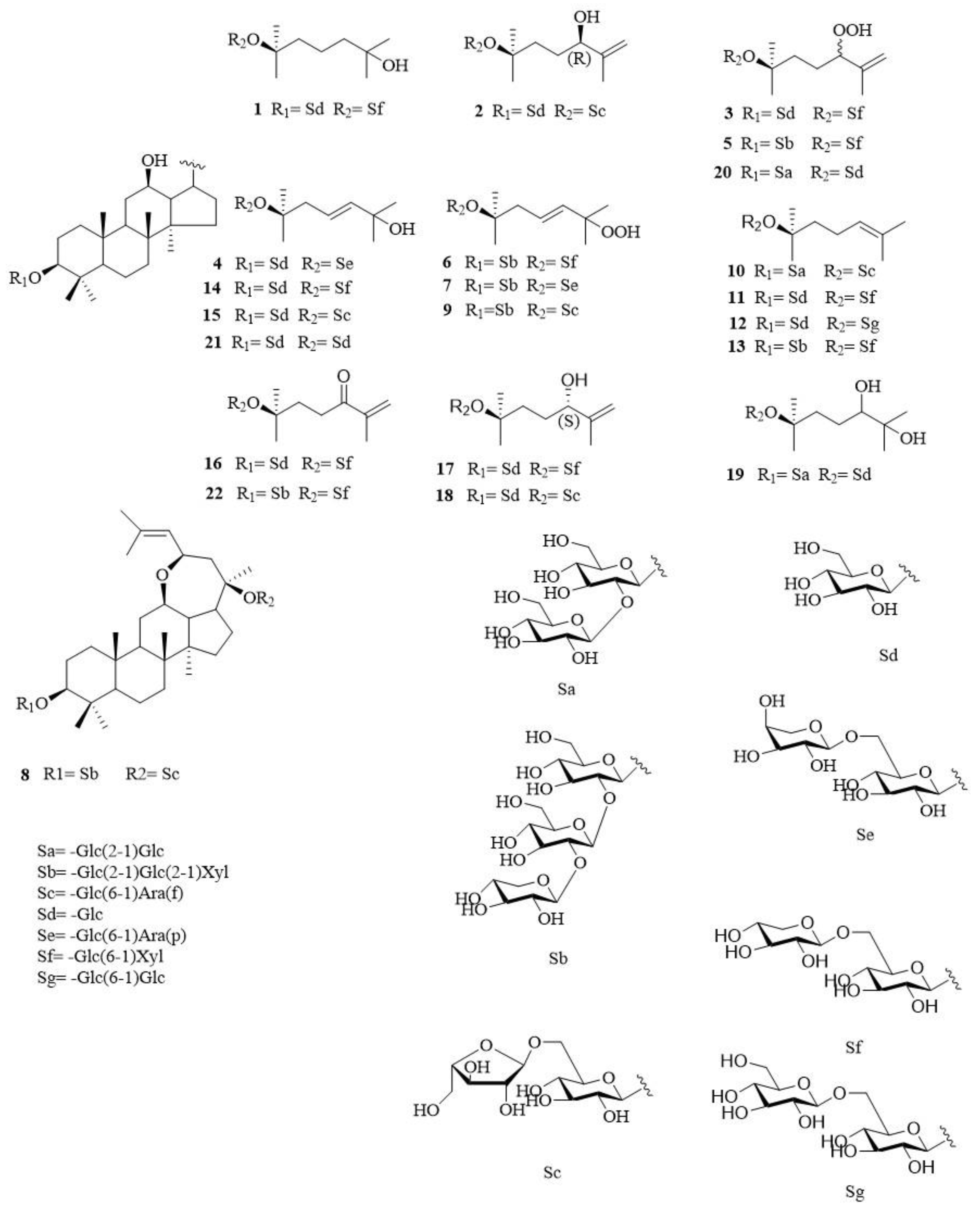
2.1. Structural Elucidation
Compound
1 (4.0 mg): a white amorphous powder. The molecular formula was assigned as C
47H
82O
18 by positive HR-ESI-MS spectrum at
m/z 957.5231 [M + Na]
+ (calcd. For. C
47H
82O
18Na: 957.5542).
+ 7.00 (c 0.19, MeOH). The IR spectrum illustrated the presence of hydroxyl (3416 cm
−1). In the
1H-NMR spectrum (shown in
Table 1), eight methyl groups
δH: 0.90 (3H, s), 0.81 (3H, s), 1.61 (3H, s), 1.59 (3H, s), 1.53 (3H, s), 1.30 (3H, s), 0.99 (3H, s), and 1.00 (3H, s), were perceived in the high field. In addition, four characteristic signals
δH: 3.43 (1H, dd,
J = 4.8, 10.8 Hz), 3.65 (1H, m), 1.34 (1H, m), and 1.46 (1H, m) were presented. The
13C-NMR spectrum indicated 47 carbon signals (shown in
Table 1), which included
δC as: 88.6, 18.2, 70.2 and 83.0. The above indicated compound
1 was a protopanaxadiol saponin substituted by sugars at C-3 and C-20. A series of carbon signals (
δC: 70.5, 39.8, 23.6, 27.5, 26.4, 25.2, 24.8) and hydrogen signals (
δH: 1.93, 2.45, 1.81, 2.05) showed that compound
1 is a saponin without a double bond at C-24/25, and
δC: 70.5 of which was protons bearing an oxygenated quaternary carbon at C-25. The HMBC correlations from H
2-23/H
3-26/H
3-27 to C-24, and H
3-26/H
3-27 to C-25 (shown in
Supplementary Materials), indicated that a hydroxyl was located at C-25. The
1H-NMR and
13C-NMR data of the side chain was similar to 20(
R)-Dammarane-3
β, 12
β, 20, 25-tetrol [
19], who displays no double carbon signal, and it showed a hydroxyl in this constituent (
δC: 70.5).
Three anomeric carbon resonances (
δC: 106.8 (Glc H-1′), 97.9 (Glc C-1″), 105.5 (Xyl C-1‴)) of sugars were observed in the
13C-NMR spectrum. Besides, three hydrogen signals (
δH: 4.97 (d,
J = 7.8 Hz, Glc H-1′), 5.12 (d,
J = 7.8 Hz, Glc H-1″), 4.97 (d,
J = 7.2 Hz, Xyl H-1‴)) of anomeric carbons were present from the
1H-NMR spectrum, and all were
β configurations. The HMBC spectrum showed the correlations from Glc H-1′ (
δH: 4.97) to C-3 (
δC: 88.6), Glc H-1″ (
δH: 5.12) to C-20 (
δC: 83.0), and Xyl H-1‴ (
δH: 4.97) to C-6″ (
δC: 69.4) (shown in
Figure 2), respectively, from which indicated Glc C-1′ was connected with C-3, and Glc C-6″ was connected with Xyl C-1‴, finally Glc C-1″ was connected with C-20. Multiple methods were applied to determine the configurations of sugars, such as hydrolysis, derivatization, and GC analysis. The
1H-NMR and
13C-NMR data of sugars was highly consistent with gypenoside IX [
20], and the structure of this compound was then determined and named as notoginsenoside SL
1.
Compound
2 (24.0 mg) was obtained as a white amorphous powder. Its molecular formula was determined as C
47H
80O
18, evidenced by positive HR-ESI-MS data
m/z 955.5245 [M + Na]
+ (calcd. For. C
47H
80O
18Na, 955.5232).
− 1.46 (c 0.20, MeOH). As shown in its IR spectrum, the signals of hydroxyl (3406 cm
−1) and double bond (1230 cm
−1) were significant. In the
1H-NMR spectrum (shown in
Table 2), seven methyl groups (
δH: 0.93, 0.79, 1.63, 1.91, 1.31, 0.96 and 0.94, each 3H, s) were revealed. Moreover, signals [
δH: 3.37 (1H, dd,
J = 4.8, 10.8 Hz), 3.12 (1H, m), 1.36 (1H, m), 1.47 (1H, m)] were presented in the
1H-NMR spectrum. In the
13C-NMR spectrum (shown in
Table 2), 47 carbon signals were revealed, including
δC: 88.6, 18.2, 70.1, and 83.2. Compound
2 was a protopanaxadiol saponin substituted by sugars at C-3 and C-20 indicating from above. A set of carbon signals (
δC: 150.0, 110.3, 22.3, 32.6, 76.6, 30.6, 17.8) and hydrogen signals (
δH: 4.95, 5.26, 2.54, 2.24, 4.47, 4.95, 5.26, 1.91) indicated a hydroxyl was connected with C-24, and this compound was a saponin with a changeable side chain. Furthermore, the HMBC correlations from H
3-27/H
2-26/H
1-24 to C-25, and H
2-22/H
2-26/H
3-27 to C-24 showed the existence of a double bond between C-25 and C-26, and a hydroxyl at C-24 (shown in
Supplementary Materials). The absolute configuration of the hydroxyl at C-24 was determined by its chemical shift of
13C-NMR. The chemical shift of a relatively low field corresponded to the
R configuration of C-24, and a relatively high field corresponded to the
S configuration of C-24. As shown in the
13C-NMR spectrum, a hydroxyl existed in the relatively low field (
δC: 76.6), and indicated the configuration of C-24 was
R. The
1H-NMR and
13C-NMR data of side chain was similar to majoroside F
1 [
21].
Three anomeric carbon signals (
δC: 106.8 (Glc C-1′), 97.9 (Glc C-1″), 109.8 (Ara(f) C-1‴) and hydrogen signals [
δH: 4.97 (d,
J = 7.8 Hz, Glc H-1′), 5.17 (d,
J = 7.7 Hz, Glc H-1″), 4.69 (br.s, Ara (f) H-1‴] were observed, and it was revealed that the configurations of two glucoses were
β, and that arabinose (f) was
α. In the HMBC spectrum, the correlations from Glc H-1′ (
δH: 4.97) to C-3 (
δC: 88.6), Glc H-1″ (
δH: 5.17) to C-20 (
δC: 83.2), and Ara (f) H-1‴ (
δH: 4.69) to C-6″ (
δC: 68.3), respectively, indicated that Glc C-1′ was connected with C-3, and Glc C-6″ was connected with Ara (f) C-1‴, and finally, Glc C-1″ was connected with C-20 (shown in
Figure 2). Three glycosides were
β-D-glucoses and
α-L-arabinose determined by hydrolysis, derivatization, and GC analysis. The
1H-NMR and
13C-NMR data of sugars was highly consistent with notoginsenoside Fe [
22]. Consequently, the structure of compound
2 was determined and named as notoginsenoside SL
2.
Compound
3 (40.5 mg): white amorphous powder. The molecular formula of 3 was deduced to be C
47H
80O
19 by positive HR-ESI-MS data at
m/
z 971.5255 [M + Na]
+ (calculated for C
47H
80O
19Na, 971.5242).
+ 7.89 (c 0.18, MeOH). The IR absorptions revealed the existence of hydroxyl (3421 cm
−1) and double bond (1261 cm
−1). In the
1H-NMR spectrum (shown in
Table 3), seven angular methyl groups (
δH: 0.97, 0.81, 1.63, 1.96, 1.32, 1.01 and 0.98, each 3H, s), and two hydrogen signals of oxymethine [
δH: 3.38 (1H, dd, J = 4.2 Hz, 11.6 Hz), 4.18 (1H, m)] were illustrated. The
13C-NMR spectrum indicated 47 carbon signals (shown in
Table 3), including three characteristic carbon signals (
δC: 88.6, 18.2, and 83.0). Compound
3 was a protopanaxadiol saponin substituted by sugars at C-3 and C-20 from above. The
1H-NMR signals [
δH: 1.97, 2.23 (m, H
2-23), 4.80 (m, H-24), 5.09, 5.27 (br.s, H
2-26)] and
13C-NMR signals (
δC: 145.9, 113.3, 22.2, 23.6, 89.8, 26.0, 17.3) indicated that C-24 of this compound was substituted, and its lateral chain was changed. Combining the
13C-NMR (
δC: 89.8) with the data of MS, it revealed that C-24 of this compound was replaced by hydroxyperoxy. Besides, the HMBC correlations from H
2-26/H
3-27 to C-25, and H
2-23/H
2-26/H
3-27 to C-24 verified that an alkene proton signal existed between C-25 and C-26 (shown in
Supplementary Materials). Furthermore, a hydroxyperoxy existed at C-24. As for the configuration of hydroxyperoxy at C-24, it was necessary to convert hydroxyperoxy into hydroxyl. The
1H-NMR and
13C-NMR data of side chain was similar to ginsenoside II [
23].
Anomeric carbon signals [
δC: 106.8 (Glc C-1′), 97.9 (Glc C-1′′), 105.5(Xyl C-1′″)] and hydrogen signals [
δH: 4.97 (d,
J = 7.6 Hz, Glc H-1′), 5.12 (br.s, Glc H-1″), 4.97 (d,
J = 7.2 Hz, Xyl H-1‴)] were observed and revealed the configurations of two glucoses were
β. In the HMBC spectrum, Glc H-1′ (
δH: 4.97) was correlated with C-3 (
δC: 88.6), Glc H-1″ (
δH: 5.12) was correlated with C-20 (
δC 83.0), and Xyl H-1‴ (
δH: 4.97) was correlated with C-6″ (
δC: 68.3) (shown in
Figure 2), respectively, from which it was indicated that Glc C-1′ was connected with C-3, and Glc C-6″ was connected with Xyl C-1‴, finally Glc C-1″ was connected with C-20. Three glycosides were
β-D-glucoses and
β-D-xylose, determined by hydrolysis, derivatization, and GC analysis. The
1H-NMR and
13C-NMR data of sugars was highly consistent with gypenoside IX [
20]. Consequently, the structure of compound
3 was determined and named as notoginsenoside SL
3.
Compound
4 (3.0 mg) was obtained as a white amorphous powder. Its molecular formula was determined as C
47H
80O
18, evidenced by positive HR-ESI-MS data (
m/
z 955.5247 [M + Na]
+, calculated for C
47H
80O
18Na, 955.5641).
+ 19.20 (c 0.15, MeOH). The IR absorptions revealed the existence of hydroxyl (3433 cm
−1) and double bond (1260 cm
−1). The
1H-NMR showed eight angular methyl groups (
δH: 0.87, 0.95, 1.48, 1.59, 1.53, 1.30, 0.99, and 1.02, each 3H, s) and four characteristic hydrogen signals [3.43 (1H, dd,
J = 4.8 Hz, 10.8 Hz), 3.65 (1H, m), 1.34, 1.47 (2H, m)] (shown in
Table 4). In its
13C-NMR spectrum, 47 carbon signals were indicated, including three characteristic carbon signals (
δC: 89.2, 18.5, 83.8) (shown in
Table 4). Compound
4 was a protopanaxadiol saponin substituted by sugars at C-3 and C-20 indicating from above. The
1H-NMR signals [
δH: 6.09 (d,
J = 15.5 Hz, H
1-24), 6.23 (ddd,
J = 5.8, 8.5, 15.5 Hz, H
1-23) and
13C-NMR signals (
δC: 122.7, 142.2, 70.0, 17.7, 17.8) indicated that C-25 of this compound was substituted, and its lateral chain was changed. Furthermore, its
13C-NMR indicated a quaternary carbon replaced by hydroxyl at C-25. The HMBC correlations from H
1-23/H
1-24/H
3-26/H
3-27 to C-25 and H
2-22/H
1-23/H
3-26/H
3-27 to C-24 indicated that an alkene proton signal existed between C-23 and C-24 (shown in
Supplementary Materials), and a hydroxyl at C-25. The
1H-NMR and
13C-NMR data of the side chain was similar to quinquenoside L3 [
24].
Anomeric carbon signals [
δC: 106.8 (Glc C-1′), 97.9 (Glc C-1″), 104.6 (Ara (p) C-1‴)] and hydrogen signals [
δH: 4.93 (d,
J = 7.8 Hz, Glc H-1′), 5.14 (d,
J = 7.8 Hz, Glc H-1″), 5.00 (d,
J = 6.0 Hz, Ara (p) C-1‴)] were observed, and revealed that the configurations of two glucoses were
β and the configuration of an arabinose was
α. Besides, the carbon signals of sugars [104.6 (Ara (p) C-1‴), 72.1 (Ara (p) C-2‴), 74.1 (Ara (p) C-3‴), 68.5 (Ara (p) C-4‴), and 65.6 (Ara (p) C-5‴)] revealed that this arabinose was a pyranose. In the HMBC spectrum, Glc H-1′ (
δH: 4.93) was correlated with C-3 (
δC: 89.2). Glc H-1″ (
δH: 5.14) was correlated with C-20 (
δC: 83.8), and Ara (p) H-1‴ (
δH: 5.00) was correlated with Glc C-6″ (
δC: 68.3) (shown in
Figure 2), respectively. It was indicated Glc C-1′ was connected with C-3, and Glc C-6″ was connected with Ara (p) C-1‴, and finally, Glc C-1″ was connected with C-20. The configurations of glycosides were
β-D-glucoses and
α-L- arabinose determined by similar methods above. The
1H-NMR and
13C- NMR data of sugars was highly consistent with ginsenoside Rd
2. Then, the structure of compound 4 was elucidated and named as notoginsenoside SL
4.
Compound
5 (57.1 mg) was obtained as a white amorphous powder. The molecular formula was deduced to be C
58H
98O
28 by positive HR-ESI-MS data at
m/
z 1265.6144 [M + Na]
+ (calculated for C
58H
98O
28Na, 1265.6142).
+ 10.67 (c 0.15, MeOH). The existence of hydroxyl (3417 cm
−1) and double bond (1258 cm
−1) was revealed from its IR spectrum. In its
1H-NMR spectrum (shown in
Table 5), seven angular methyl groups (
δH: 0.96, 0.80, 1.64, 1.96, 1.28, 1.11 and 0.95, each 3H, s), and four characteristic hydrogen signals [
δH: 3.30 (dd,
J = 3.9, 11.5 Hz, 1H), 1.34 (m, 1H), 1.47 (m, 1H), 4.18 (m, 1H)] were indicated. The
13C-NMR spectrum revealed 58 carbon signals (shown in
Table 5), including four characteristic carbon signals (
δC: 88.6, 18.2, 69.9, and 83.2). Compound
5 was a protopanaxadiol saponin substituted by sugars at C-3 and C-20 from above. The
1H-NMR signals [
δH: 1.97, 2.20 (m, H
2-23), 4.80 (t,
J = 6.7 Hz, H-24), 5.09, 5.28 (br.s, H
2-26)], and
13C-NMR signals (
δC: 146.1, 113.1, 32.7, 90.0, 26.4, 17.3) indicated that C-24 of this compound was substituted and a double bond was revealed between C-25 and C-26. Combining the
13C-NMR (
δC: 90.0) with the data of MS, it revealed that C-24 of this compound was replaced by hydroxyperoxy. Besides, the HMBC correlations from H
2-23/H
2-26/H
3-27 to C-24, and H
2-26/H
3-27 to C-25 verified that an alkene proton signal existed between C-25 and C-26 (shown in
Supplementary Materials), and a hydroxyperoxy existed at C-24. The
1H-NMR and
13C-NMR data of the side chain was similar to ginsenoside Ⅱ [
23].
Anomeric carbon signals [
δC: 104.6 (Glc C-1′), 103.0 (Glc C-1″), 106.2 (Xyl C-1‴), 97.8 (Glc H-1′′′′), 105.5 (Xyl C-1′′′′′)] and hydrogen signals [(
δH: 4.95 (d,
J = 7.7 Hz, Glc H-1′), 5.54 (d,
J = 6.8 Hz, Glc H-1″), 5.44 (d,
J = 6.8 Hz, Xyl H-1‴), 5.12 (br.s, Glc H-1′′′′), and 5.01 (d,
J = 7.4 Hz, Xyl H-1′′′′′)] were observed and revealed that the configurations of three glucoses were
β. In the HMBC spectrum, Glc H-1′ (
δH: 4.95) was correlated with C-3 (
δC 88.6), Glc H-1″ (
δH: 5.54) was correlated with Glc C-2′ (
δC: 82.3), Xyl H-1‴ (
δH: 5.44) was correlated with C-2″ (
δC: 84.2), Glc H-1′′′′ (
δH: 5.12) was correlated with C-20 (
δC: 83.2), and Xyl H-1′′′′′ (
δH 5.01) was correlated with Glc C-6′′′′ (
δC: 69.9) (shown in
Figure 2), respectively. From this it was indicated that Glc C-1′ was connected with C-3, Glc C-1″ was connected with Glc C-2′, Glc C-1‴was connected with Xyl C-2″, and Glc C-1′′′′ was connected with C-20, and finally Xyl C-1′′′′′ was connected with Glc C-6′′′′. The glycosides were
β-D-glucoses and
β-D-xyloses, which were determined by hydrolysis, derivatization, and GC analysis. The
1H-NMR and
13C-NMR data of sugars was highly consistent with notoginsenoside Fc [
25]. Then, the structure of compound
5 was determined and named as notoginsenoside SL
5.
Compound
6 (10.0 mg) was a white amorphous powder. The molecular formula of 6 was deduced to be C
58H
98O
28 by positive HR-ESI-MS data at
m/
z 1265.6149 [M + Na]
+ (calculated for C
58H
98O
28Na, 1265.6142).
+ 7.45 (c 0.15, MeOH). The IR absorptions revealed the existence of hydroxyl (3415 cm
−1) and double bond (1258 cm
−1). In the
1H-NMR spectrum (shown in
Table 6), eight angular methyl groups (
δH: 1.01, 0.83, 1.63, 1.62, 1.62, 1.29, 1.12, and 0.92, each 3H, s) were shown. Four characteristic hydrogen signals [
δH: 3.30 (dd-like, 1H), 1.38 (m, 1H), 1.54 (m, 1H), 4.07 (m, 1H)] were revealed. The
13C-NMR spectrum indicated 58 carbon signals, including four characteristic carbon signals (
δC: 88.6, 18.2, 70.2, and 83.0) (shown in
Table 6). Compound
6 was a protopanaxadiol saponin substituted by sugars at C-3 and C-20 from above. According to HSQC, H-23 (
δH: 6.20, m) was correlated with C-23 (
δC: 126.5), and H-24 (
δH: 6.15, br.s) was correlated with C-24 (
δC: 137.8), which indicated that a double bond existed between C-23 and C-24. Combining MS spectrum with carbon spectrum (
δC: 81.1), a hydroperoxyl was presented at C-25. Furthermore, the HMBC correlations from H
3-26/H
3-27/H
1-24/H
1-23 to C-25 and H
3-27/H
3-26/H
1-23/H
2-22 to C-24 verified that an alkene proton signal existed between C-23 and C-24, and a hydroxyperoxy existed at C-25 (shown in
Supplementary Materials). The
1H-NMR and
13C-NMR data of the side chain was similar to notoginsenoside E [
26].
Anomeric carbon signals [
δC: 104.6 (Glc C-1′), 102.9 (Glc C-1″), 106.2 (Xyl C-1‴), 98.0 (Glc C-1′′′′), 105.4 (Xyl C-1′′′′′)] and hydrogen signals [
δH: 4.96 (d,
J = 6.2 Hz, Glc H-1′), 5.54 (d-like, Glc H-1″), 5.45 (d,
J = 6.0 Hz, Xyl H-1‴), 5.20 (br.s, Glc H-1′′′′), 5.00 (d,
J = 6.8 Hz, Xyl H-1′′′′′)] were observed, and the configurations of five glycosyl signals were all
β. In the HMBC spectrum, the correlations from Glc H-1′ (
δH: 4.96) to C-3 (
δC: 88.6), Glc H-1″ (
δH: 5.54) to Glc C-2′ (
δC: 82.7), Xyl H-1‴ (
δH: 5.45) to Glc C-2″ (
δH: 84.3), Glc H-1′′′′ (
δH: 5.20) to C-20 (
δC: 83.0), and Xyl H-1′′′′ (
δH: 5.00) to Glc C-6′′′′ (
δC: 69.7) (shown in
Figure 2), respectively, from which indicated Glc C-1′ was connected with C-3, Glc C-1″ was connected with Glc C-2′, Glc C-1‴ was connected with Xyl C-2″, and Glc C-1′′′′ was connected with C-20, finally Xyl C-1′′′′′ was connected with Glc C-6′′′′. Five glycosides were determined as
β-D-glucoses and
β-D-xyloses by same methods above. The
1H-NMR and
13C-NMR data of sugars was highly consistent with notoginsenoside Fc [
27]. Finally, the structure of compound
6 was elucidated and named as notoginsenoside SL
6.
Compound
7 (24.6 mg) was obtained as a white amorphous powder. The molecular formula of 7 was deduced to be C
58H
98O
28 by positive HR-ESI-MS data at
m/
z 1265.6146 [M + Na]
+ (calculated for C
58H
98O
28Na, 1265.6142).
+ 4.40 (c 0.18, MeOH). The IR absorptions revealed the existence of hydroxyl (3425 cm
−1) and double bond (1257 cm
−1). In its 1H-NMR spectrum (shown in
Table 7), there were eight angular methyl groups (
δH: 0.79, 0.93, 1.63, 1.61, 1.61, 1.27, 1.14, and 0.94, each 3H, s). Four characteristic hydrogen signals [
δH: 3.30 (dd, J = 4.2, 11.4 Hz, 1H), 1.37 (m, 1H), 1.55 (m, 1H), 4.13 (m, 1H)] were revealed. The
13C-NMR spectrum indicated 58 carbon signals, including four characteristic carbon signals (
δC: 88.6, 18.2, 70.5 and 83.3) (shown in
Table 7). Compound
7 was a protopanaxadiol saponin substituted by sugars at C-3 and C-20 from above. According to the hydrogen spectrum [
δH: 5.20 (m, H-23), 6.15 (br.s, H-24)] and carbon spectrum [
δC: 126.2 (C-23), 137.8 (C-24)], a double bond existed between C-23 and C-24. In combining MS spectrum with carbon spectrum (
δC: 81.1), a hydroxyperoxy was presented at C-25. Furthermore, the HMBC correlations from H
3-26/H
3-27/H
1-24/H
1-23 to C-25, and H
3-27/H
3-26/H
1-23/H
2-22 to C-24 verified that an alkene proton signal existed between C-23 and C-24 (shown in
Supplementary Materials), and a hydroxyperoxy existed at C-25. The
1H-NMR and
13C-NMR data of side chain was similar to notoginsenoside E [
26].
Anomeric carbon signals (
δC: 104.6 (Glc C-1
′), 102.9 (Glc C-1″), 106.2 (Xyl C-1‴), 98.0 (Glc C-1′′′′), 104.1 (Ara (p) C-1′′′′′) and hydrogen signals [
δH: 4.95 (d,
J = 6.2 Hz, Glc H-1′), 5.52 (d,
J = 7.8 Hz, Glc H-1″), 5.43 (d,
J = 6.6 Hz, Xyl H-1‴), 5.20 (br.s, Glc H-1′′′′), 5.00 (d,
J = 6.8 Hz, Ara (p) H-1′′′′′)] were observed, what were the signals of five glycosyl and the configurations of glucoses were
β and arabinose was
α. In the HMBC spectrum, Glc H-1′ (
δH: 4.95) was correlated with C-3 (
δC: 88.6). Glc H-1″ (
δH: 5.52 ) was correlated with Glc C-2′ (
δC: 82.7), Xyl H-1‴ (
δH: 5.43) was correlated with Glc C-2″ (
δH: 84.2), Glc H-1′′′′ (
δH: 5.20) was correlated with C-20 (
δC: 83.3), and Ara (p) H-1′′′′′ (
δH: 5.00) was correlated with Glc C-6′′′′ (
δC: 68.7) (shown in
Figure 2), respectively, from which indicated Glc C-1′ was connected with C-3, Glc C-1″ was connected with Glc C-2′, Glc C-1‴ was connected with Xyl C-2″, and Glc C-1′′′′ was connected with C-20, and finally, Ara (p) C-1′′′′′ was connected with Glc C-6′′′′. Five glycosides were
β-D-glucoses,
β-D-xylose and
α-L-arabinose, which was determined by same methods above. The
1H-NMR and
13C-NMR data of sugars was highly consistent with notoginsenoside Fz [
4]. Accordingly, the structure of compound
7 was determined and named as notoginsenoside SL
7.
Compound
8 (6.9 mg) was obtained as a white amorphous powder. The molecular formula of 8 was deduced to be C
58H
96O
26 by positive HR-ESI-MS data at
m/
z 1232.4306 [M + Na]
+ (calculated for C58H96O26Na, 1232.3532).
− 7.20 (
c 0.32, MeOH). The IR absorptions revealed the existence of hydroxyl (3412 cm
−1) and double bond (1265 cm
−1). In its 1H-NMR spectrum (shown in
Table 8), eight angular methyl groups (
δH: 0.88, 0.92, 1.61, 1.65, 1.94, 1.27, 1.10, 0.79, each 3H, s) were shown, including two characteristic methyl groups linked to a sp2 bond (
δH: 1.65 and 1.94). The 1H-NMR of 8 showed only an olefinic proton at
δH 6.02 (d, J = 7.6 Hz, 1H). On the basis of four characteristic carbon signals (
δC: 89.1, 18.5, 79.1 and 83.6), compound 8 was a protopanaxadiol saponin substituted by sugars at C-3 and C-20 from above. Furthermore, the HMBC correlations from H
2-22/H
1-23/H
3-26/H
3-27 to C-24 and H
1-25/H
3-26/H
3-27 to C-25 verified that an alkene proton signal existed between C-24 and C-25 (shown in
Supplementary Materials). In addition, the HMBC correlations from H
1-12/H
2-22/H
1-24 to C-23 and the positive HR-ESI-MS data showed a molecule oxygen between C-12 and C-23. The
1H-NMR and
13C-NMR data of the side chain was similar to quinquefoloside-Lb [
28], whose
1H-NMR and
13C-NMR data was assigned by comparing it with that in the literature.
Anomeric carbon signals (
δC: 105.3 (Glc C-1′), 105.6 (Glc C-1″), 106.2 (Xyl C-1‴), 98.4 (Glc C-1′′′′), 110.3 (Ara (f) C-1′′′′′) and hydrogen signals [
δH: 4.96 (d,
J = 8.3 Hz, Glc H-1′), 5.40 (d,
J = 6.9 Hz, Glc H-1″), 5.44 (d,
J = 6.6 Hz, Xyl H-1‴), 5.18 (br.s, Glc H-1′′′′), 5.68 (d,
J = 6.8 Hz, Ara (f) H -1′′′′′) ] were observed, which revealed five glycosyl signals existed and the configurations of glucoses were
β and arabinose was
α. In the HMBC spectrum, Glc H-1′ (
δH: 4.96) was correlated with C-3 (
δC: 89.1). Glc H-1″ (
δH: 5.40) was correlated with Glc C-2′ (
δC: 83.2), Xyl H-1‴ (
δH: 5.44) was correlated with Glc C-2″ (
δH: 75.2), Glc H-1′′′′ (
δH: 5.18) was correlated with C-20 (
δC: 83.6), and Ara (f) H-1′′′′′ (
δH: 5.68) was correlated with Glc C-6′′′′ (
δC: 67.2) (shown in
Figure 2), respectively, from which it was indicated that Glc C-1′ was connected with C-3, Glc C-1″ was connected with Glc C-2′, Glc C-1‴ was connected with Xyl C-2″, and Glc C-1′′′′ was connected with C-20, finally Ara (f) C-1′′′′′ was connected with Glc C-6′′′′. Five glycosides were
β-D-glucoses,
β-D-xylose and
α-L- arabinose determining by same methods above. The
1H-NMR and
13C-NMR data of sugars was highly consistent with notoginsenoside NL-A
3 [
29]. Consequently, the structure of compound
8 was elucidated and named as notoginsenoside SL
8.