Hybrid Wetting Surface with Plasmonic Alloy Nanocomposites for Sensitive SERS Detection
Abstract
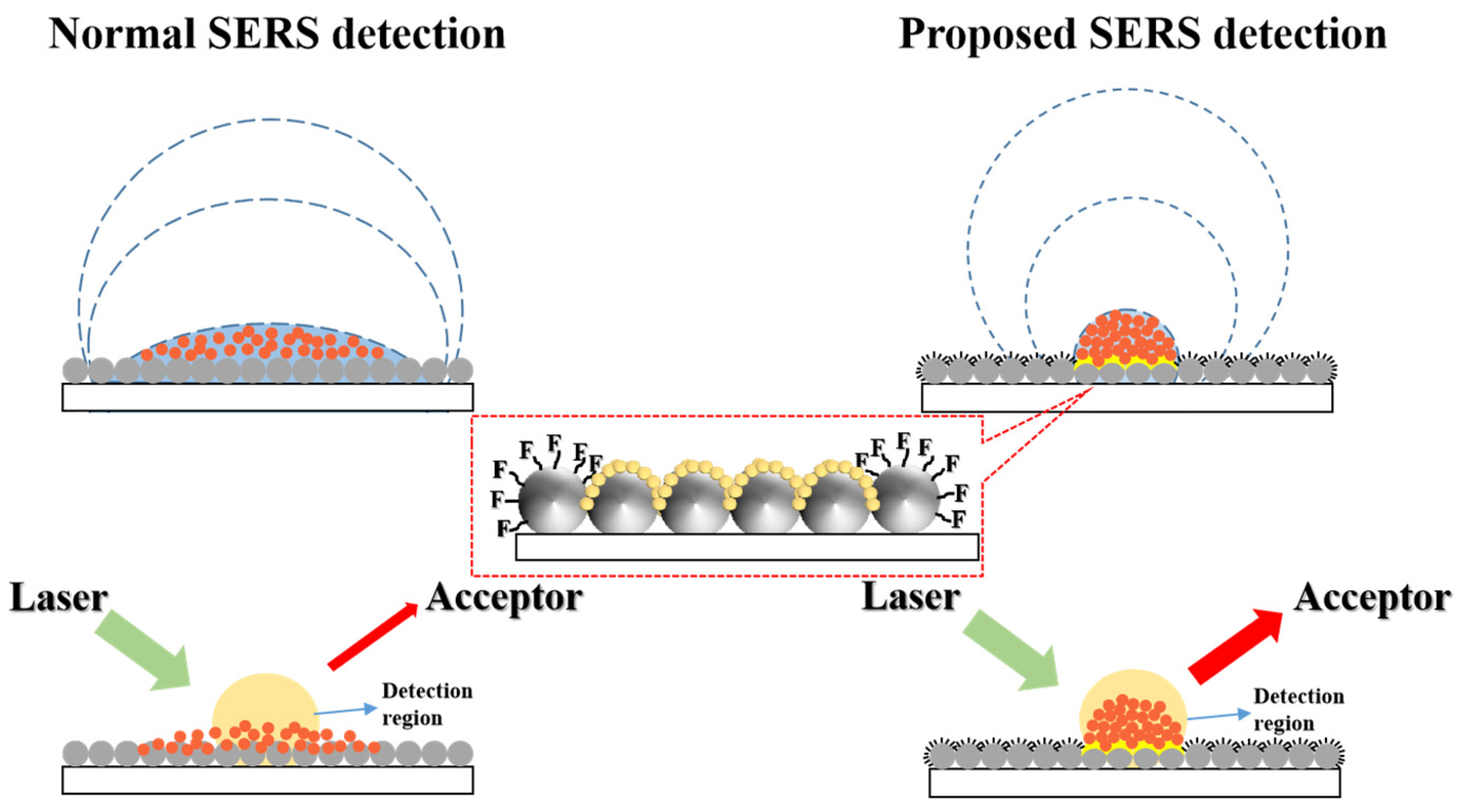
1. Introduction
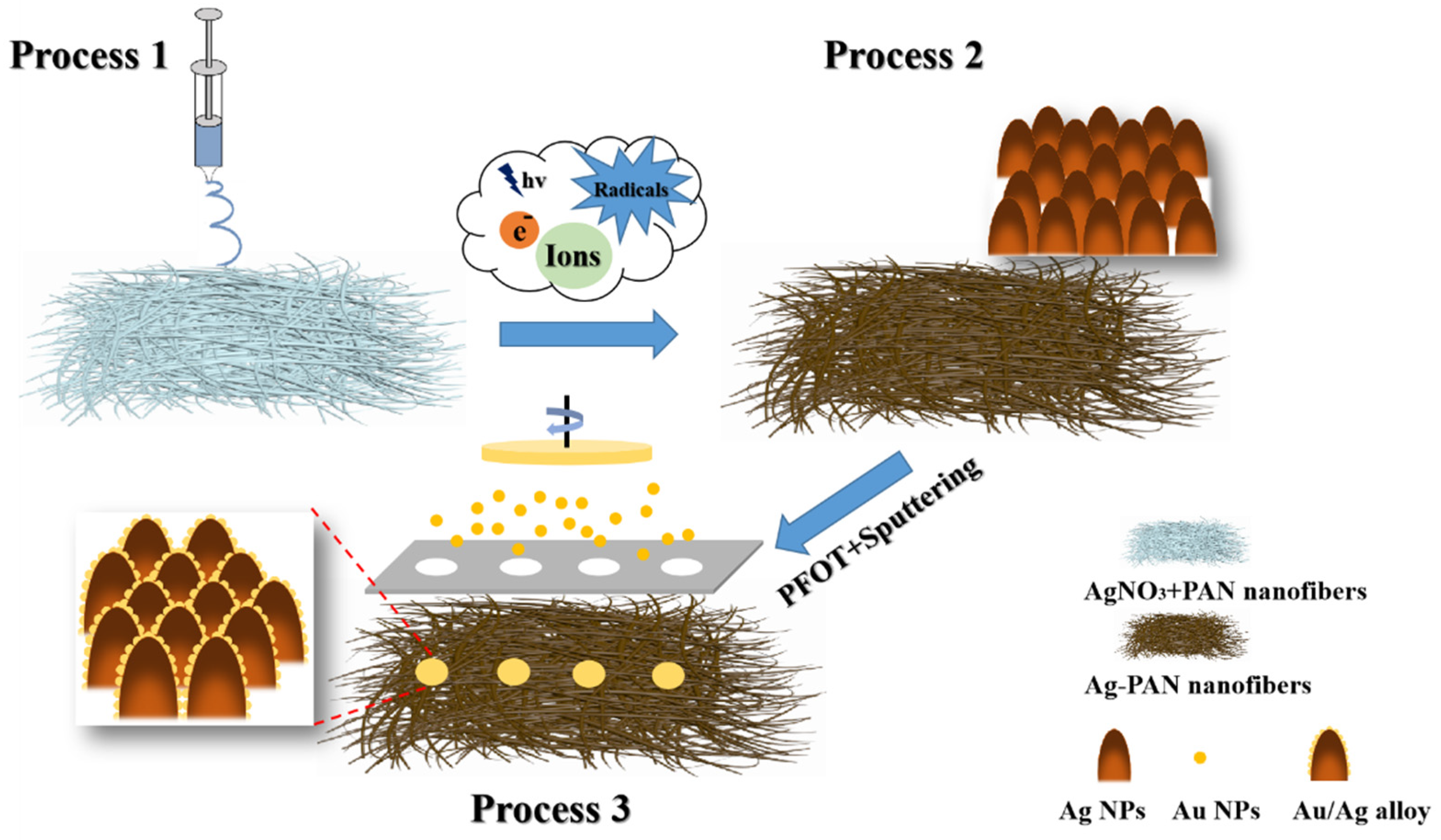
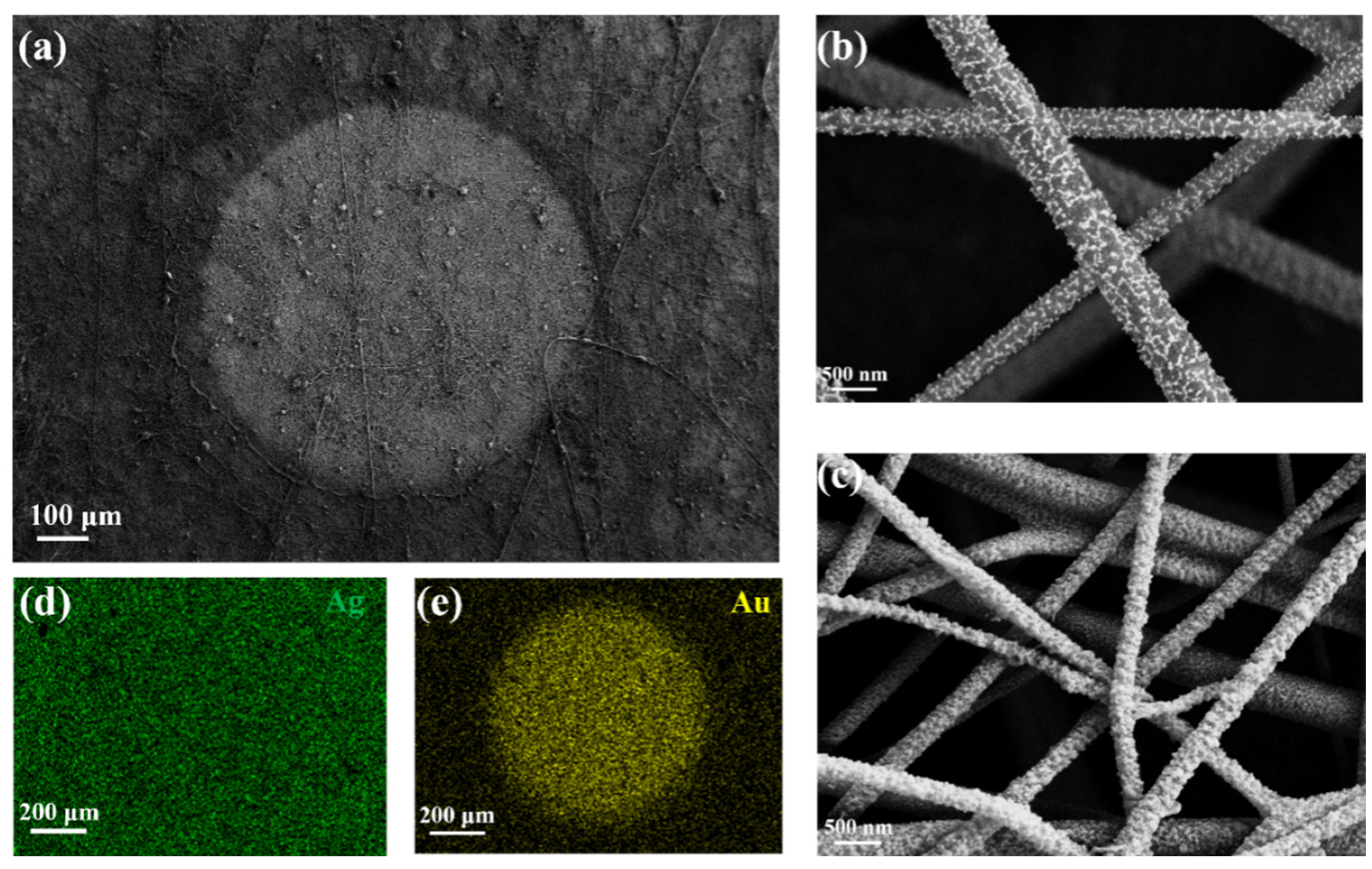
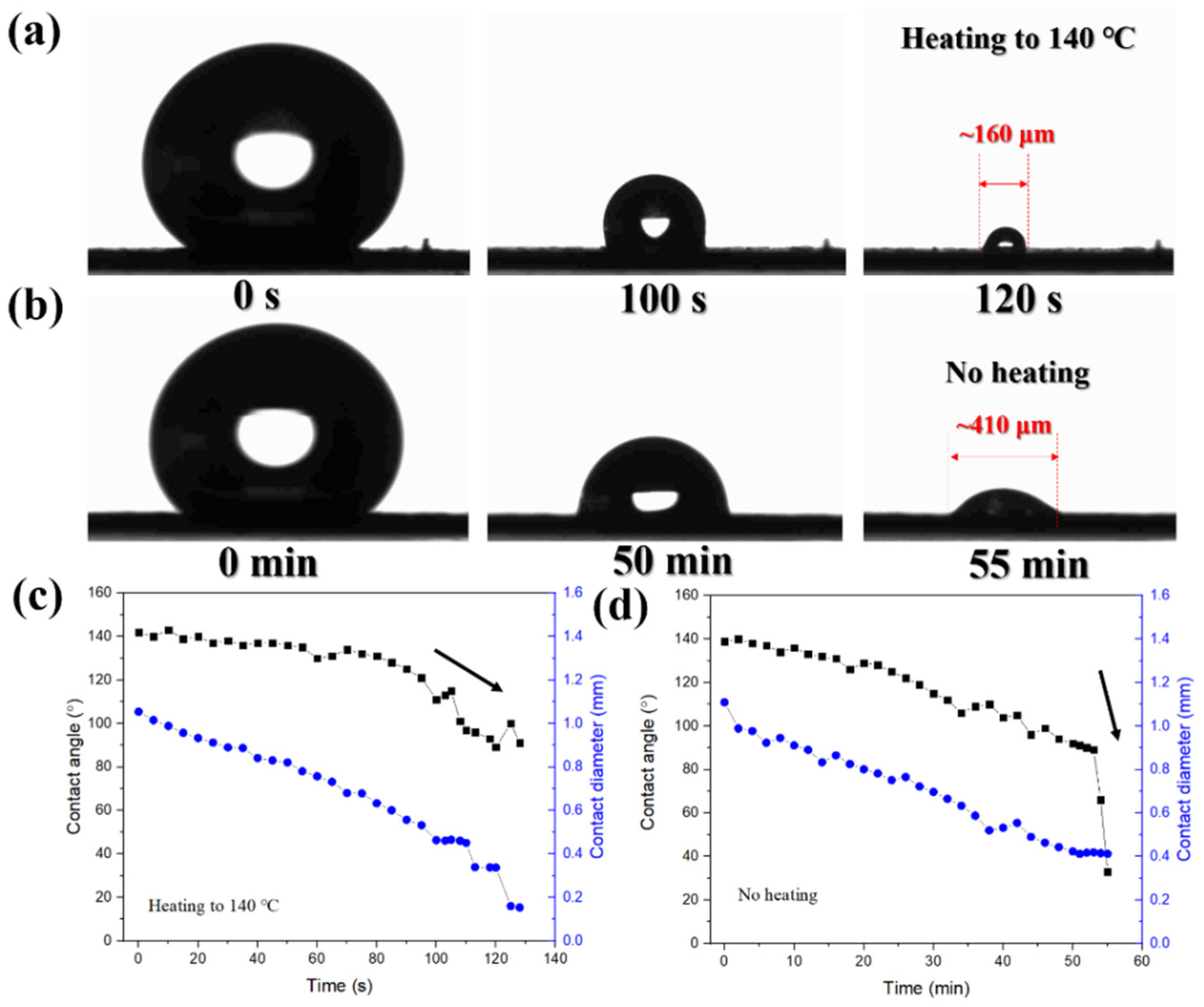
2. Results and Discussion
3. Experimental Section
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Zong, S.; Wu, L.; Zhu, D.; Cui, Y. SERS-Activated Platforms for Immunoassay: Probes, Encoding Methods, and Applications. Chem. Rev. 2017, 117, 7910–7963. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, R.A.; Vikesland, P.J. Surface-Enhanced Raman Spectroscopy (SERS) for Environmental Analyses. Environ. Sci. Technol. 2010, 44, 7749–7755. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.P.; Franca, A.S.; Irudayaraj, J. Surface-Enhanced Raman Spectroscopy Applied to Food Safety. Annu. Rev. Food Sci. Technol. 2013, 4, 369–380. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.-J.; Wei, T.; Ma, X.; Zheng, X.-S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Wang, H.; Yin, B.; Wong, S.H.D.; Zhang, A.P.; Tam, H.-Y. Ultraminiature optical fiber-tip directly-printed plasmonic biosensors for label-free biodetection. Biosens. Bioelectron. 2022, 218, 114761. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, B.; Hao, J.; Ma, L.; Huang, Y.; Shao, X.; Li, C.; Chu, Z.; Yi, C.; Wong, S.H.D.; et al. An AIEgen/graphene oxide nanocomposite (AIEgen@GO)-based two-stage “turn-on” nucleic acid biosensor for rapid detection of SARS-CoV-2 viral sequence. Aggregate 2022, 4, e195. [Google Scholar] [CrossRef]
- Bell, S.E.J.; Sirimuthu, N.M.S. Quantitative Surface-Enhanced Raman Spectroscopy. Chem. Soc. Rev. 2008, 37, 1012–1024. [Google Scholar] [CrossRef]
- Lin, E.-C.; Fang, J.; Park, S.-C.; Johnson, F.W.; Jacobs, H.O. Effective Localized Collection and Identification of Airborne Species Through Electrodynamic Precipitation and SERS-Based Detection. Nat. Commun. 2013, 4, 1636. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, H.; Chen, X.; Yang, J.; Shi, L.; Chen, T.; Bao, Z.; Liu, J.; Wu, Y. Highly Efficient Photoinduced Enhanced Raman Spectroscopy (PIERS) from Plasmonic Nanoparticles Decorated 3D Semiconductor Arrays for Ultrasensitive, Portable, and Recyclable Detection of Organic Pollutants. ACS Sens. 2019, 4, 1670–1681. [Google Scholar] [CrossRef]
- Huang, J.; Wen, Y.; Li, J.; Li, Y.; Gou, T.; Ma, Y.; Qu, Y.; Zhang, Z.; Ren, W.; Zhang, Z.; et al. Superhydrophobic-Superhydrophilic Hybrid Surface with Highly Ordered Tip-Capped Nanopore Arrays for Surface-Enhanced Raman Scattering Spectroscopy. ACS Appl. Mater. Interfaces 2020, 12, 37499–37505. [Google Scholar] [CrossRef]
- Li, H.Z.; Yang, Q.; Hou, J.; Li, Y.A.; Li, M.Z.; Song, Y.L. Bioinspired Micropatterned Superhydrophilic Au-Areoles for SurfaceEnhanced Raman Scattering (SERS) Trace Detection. Adv. Funct. Mater. 2018, 28, 1800448. [Google Scholar] [CrossRef]
- Song, W.; Psaltis, D.; Crozier, K.B. Superhydrophobic bull’s-eye for surfaceenhanced Raman scattering. Lab Chip 2014, 14, 3907–3911. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, S.; Xu, T.; Song, Y.; Zhang, X. Microdroplet-captured tapes for rapid sampling and SERS detection of food contaminants. Biosens. Bioelectron. 2020, 152, 112013. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ying, B.; Song, P.; Liu, X.A. Nanocellulose-Paper-Based SERS Multiwell Plate with High Sensitivity and High Signal Homogeneity. Adv. Mater. Interfaces 2019, 6, 1901346. [Google Scholar] [CrossRef]
- Fan, M.; Cheng, F.; Wang, C.; Gong, Z.; Tang, C.; Man, C.; Brolo, A.G. SERS Optrode as a “Fishing Rod” to Direct PreConcentrate Analytes from Superhydrophobic Surfaces. Chem. Commun. 2015, 51, 1965–1968. [Google Scholar] [CrossRef]
- Cheung, M.; Lee, W.W.Y.; McCracken, J.N.; Larmour, I.A.; Brennan, S.; Bell, S.E.J. Raman Analysis of Dilute Aqueous Samples by Localized Evaporation of Submicroliter Droplets on the Tips of Superhydrophobic Copper Wires. Anal. Chem. 2016, 88, 4541–4547. [Google Scholar] [CrossRef]
- Li, J.-F.; Zhang, Y.-J.; Ding, S.-Y.; Panneerselvam, R.; Tian, Z.-Q. Core-Shell Nanoparticle-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 5002. [Google Scholar] [CrossRef]
- Shan, F.; Zhang, X.-Y.; Fu, X.-C.; Zhang, L.-J.; Su, D.; Wang, S.-J.; Wu, J.-Y.; Zhang, T. Investigation of simultaneously existed Raman scattering enhancement and inhibiting fluorescence using surface modified gold nanostars as SERS probes. Sci. Rep. 2017, 7, 6813. [Google Scholar] [CrossRef]
- Su, D.; Zhang, X.-Y.; Chen, X.-Y.; Wang, S.-J.; Wan, Q.-D.; Zhang, T. Centrifugation-induced assembly of dense hotspots based SERS substrate for enhanced Raman scattering and quenched fluorescence. Nanotechnology 2022, 33, 235304. [Google Scholar] [CrossRef]
- Perassi, E.M.; Canali, L.R.; Coronado, E.A. Enhancement and confinement analysis of the electromagnetic fields inside Hot spots. J. Phys. Chem. C 2009, 113, 6315–6319. [Google Scholar] [CrossRef]
- Wang, S.-J.; Su, D.; Zhang, T. Research progress of surface plasmons mediated photothermal effects. Acta Phys. Sin. 2019, 68, 144401. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, Z.; Jia, L.; Song, P.; Mei, L.; Bai, L.; Liu, Y. Gold nanoparticle decorated electrospun nanofibers: A 3D reproducible and sensitive SERS substrate. Appl. Surf. Sci. 2017, 403, 29–34. [Google Scholar] [CrossRef]
- Park, M.; Hwang, C.S.H.; Jeong, K.-H. Nanoplasmonic Alloy of Au/Ag Nanocomposites on Paper Substrate for Biosensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 290–295. [Google Scholar] [CrossRef]
- Liu, K.; Bai, Y.; Zhang, L.; Yang, Z.; Fan, Q.; Zheng, H.; Yin, Y.; Gao, C. Porous Au-Ag Nanospheres with High-Density and Highly Accessible Hotspots for SERS Analysis. Nano Lett. 2016, 16, 3675–3681. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Ho, W.K.H.; Zhang, Q.; Li, C.; Huang, Y.; Yan, J.; Yang, H.; Hao, J.; Wong, S.H.D.; Yang, M. Magnetic-Responsive Surface-Enhanced Raman Scattering Platform with Tunable Hot Spot for Ultrasensitive Virus Nucleic Acid Detection. ACS Appl. Mater. Interfaces 2022, 14, 4714–4724. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, Q.; Xia, X.; Li, C.; Ho, W.K.H.; Yan, J.; Huang, Y.; Wu, H.; Wang, P.; Yi, C.; et al. A CRISPR-Cas12a integrated SERS nanoplatform with chimeric DNA/RNA hairpin guide for ultrasensitive nucleic acid detection. Theranostics 2022, 12, 5914–5930. [Google Scholar] [CrossRef]
- Yin, B.; Ho, W.K.H.; Xia, X.; Chan, C.K.W.; Zhang, Q.; Ng, Y.M.; Lam, C.Y.K.; Cheung, J.C.W.; Wang, J.; Yang, M.; et al. A Multilayered Mesoporous Gold Nanoarchitecture for Ultraeffective Near-Infrared Light-Controlled Chemo/Photothermal Therapy for Cancer Guided by SERS Imaging. Small 2023, 19, 2206762. [Google Scholar] [CrossRef]
- Kong, T.; Luo, G.; Zhao, Y.; Liu, Z. Bioinspired Superwettability Micro/Nanoarchitectures: Fabrications and Applications. Adv. Funct. Mater. 2019, 29, 1808012. [Google Scholar] [CrossRef]
- Hou, L.; Wang, N.; Wu, J.; Cui, Z.; Jiang, L.; Zhao, Y. Bioinspired Superwettability Electrospun Micro/Nanofibers and Their Applications. Adv. Funct. Mater. 2018, 28, 1801114. [Google Scholar] [CrossRef]
- Wang, S.-J.; Zhang, X.-Y.; Su, D.; Yan, X.; Zhou, H.-L.; Xue, X.-M.; Wang, Y.-F.; Zhang, T. Enhanced Photocatalytic Reactions via Plasmonic metal-Semiconductor Heterostructures Combing with Solid-Liquid-Gas Interfaces. Appl. Catal. B 2022, 306, 121102. [Google Scholar] [CrossRef]
- Luo, X.; Pan, R.; Cai, M.; Liu, W.; Chen, C.; Jiang, G.; Hu, X.; Zhang, H.; Zhong, M. Atto-Molar Raman detection on patterned superhydrophilic-superhydrophobic platform via localizable evaporation enrichment. Sens. Actuat. B-Chem. 2021, 326, 128826. [Google Scholar] [CrossRef]
- Jia, P.; Cao, B.; Wang, J.; Qu, J.; Liu, Y.; Pan, K. Self-assembly of various silver nanocrystals on PmPD/PAN nanofibers as a high-performance 3D SERS substrate. Analyst 2015, 140, 5707. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; Gentile, F.; Mecarini, F.; Das, G.; Moretti, M.; Candeloro, P.; Coluccio, M.L.; Cojoc, G.; Accardo, A.; Liberale, C.; et al. Breaking the diffusion limit with super-hydrophobic delivery of molecules to plasmonic nanofocusing SERS structures. Nat. Photo. 2011, 5, 683–688. [Google Scholar] [CrossRef]
- Xu, T.; Song, Y.; Gao, W.; Wu, T.; Xu, L.-P.; Zhang, X.; Wang, S. Superwettable Electrochemical Biosensor toward Detection of Cancer Biomarkers. ACS Sens. 2018, 3, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jiang, L.; Li, X.; Li, B.; Huang, J.; Sun, J.; Wang, Z.; Xu, Z.; Qu, L.; Lu, Y.; et al. Hybrid superhydrophilic–superhydrophobic micro/nanostructures fabricated by femtosecond laserinduced forward transfer for sub-femtomolar Raman detection. Microsyst. Nanoeng. 2018, 5, 48. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Su, D.; Zhou, H.; Jiang, X.; Zhang, X.; Zhang, T. Hybrid Wetting Surface with Plasmonic Alloy Nanocomposites for Sensitive SERS Detection. Molecules 2023, 28, 2190. https://doi.org/10.3390/molecules28052190
Wang S, Su D, Zhou H, Jiang X, Zhang X, Zhang T. Hybrid Wetting Surface with Plasmonic Alloy Nanocomposites for Sensitive SERS Detection. Molecules. 2023; 28(5):2190. https://doi.org/10.3390/molecules28052190
Chicago/Turabian StyleWang, Shanjiang, Dan Su, Huanli Zhou, Xiaohan Jiang, Xiaoyang Zhang, and Tong Zhang. 2023. "Hybrid Wetting Surface with Plasmonic Alloy Nanocomposites for Sensitive SERS Detection" Molecules 28, no. 5: 2190. https://doi.org/10.3390/molecules28052190
APA StyleWang, S., Su, D., Zhou, H., Jiang, X., Zhang, X., & Zhang, T. (2023). Hybrid Wetting Surface with Plasmonic Alloy Nanocomposites for Sensitive SERS Detection. Molecules, 28(5), 2190. https://doi.org/10.3390/molecules28052190





