Preparation of Porous Ti/RuO2-IrO2@Pt, Ti/RuO2-TiO2@Pt and Ti/Y2O3-RuO2-TiO2@Pt Anodes for Efficient Electrocatalytic Decomposition of Tetracycline
Abstract
1. Introduction
2. Results and Discussion
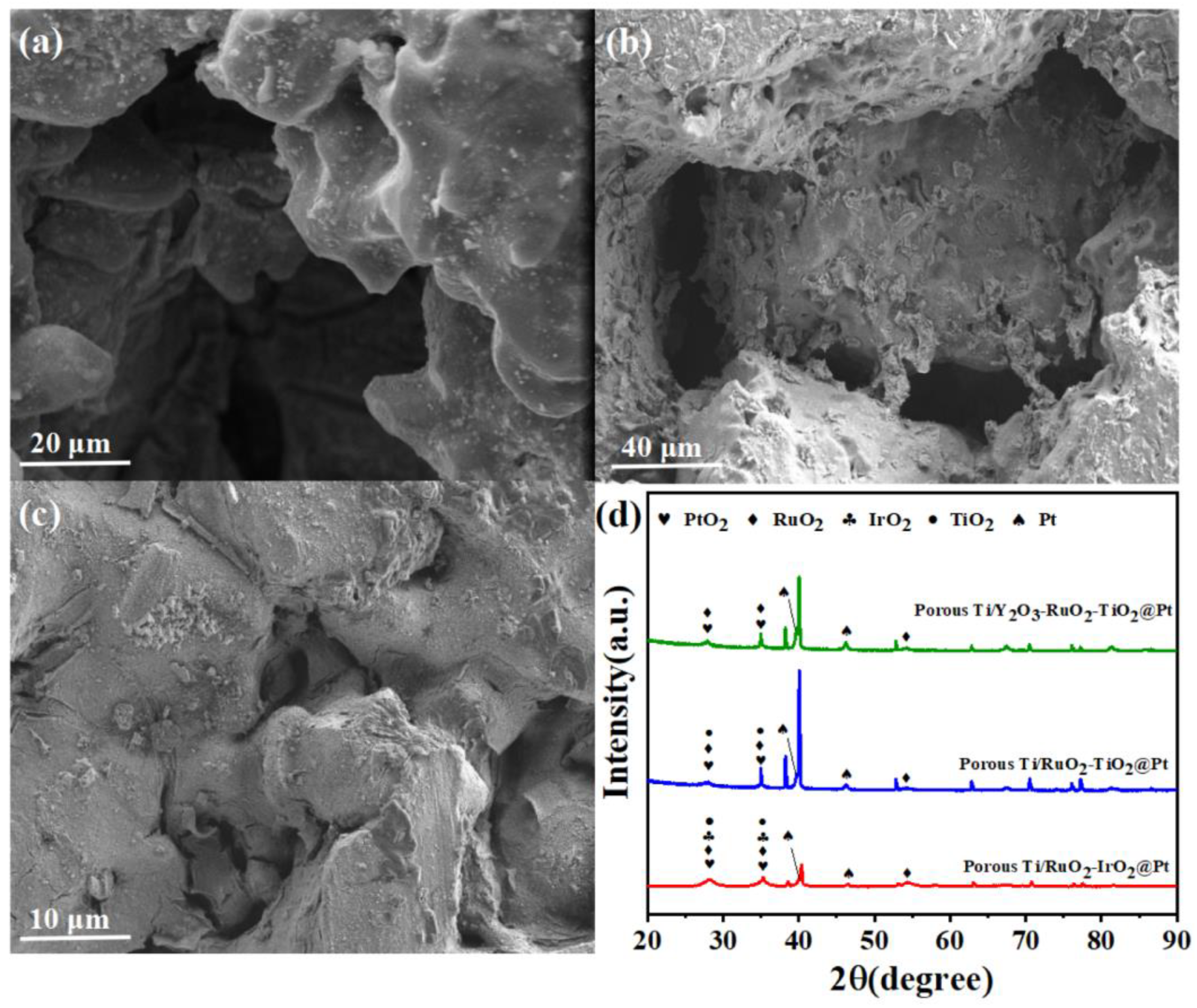
2.1. Morphological and Structural Characterization
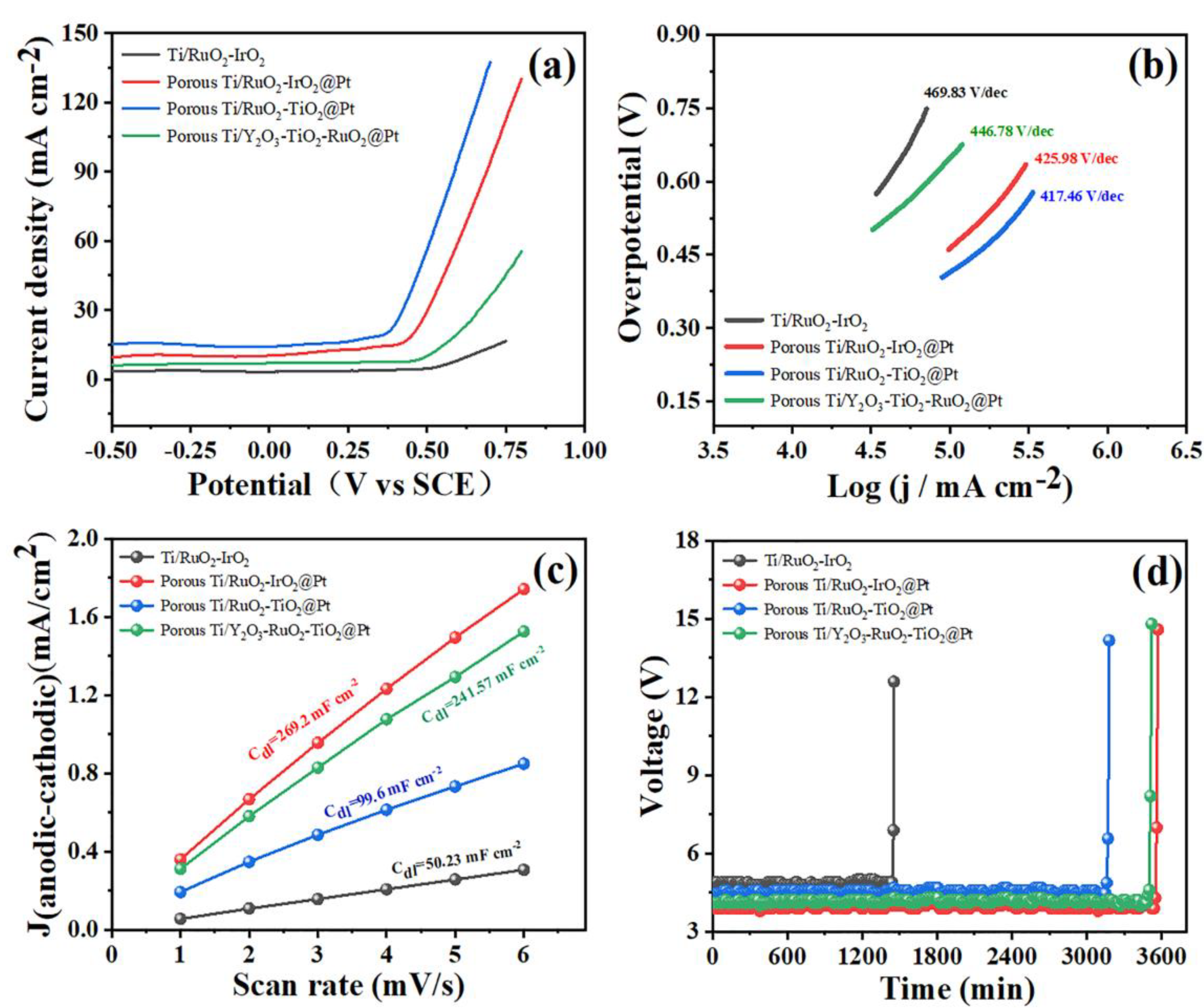
2.2. Electrochemical Performance Analysis
2.3. Electrocatalytic Degradation of Tetracycline
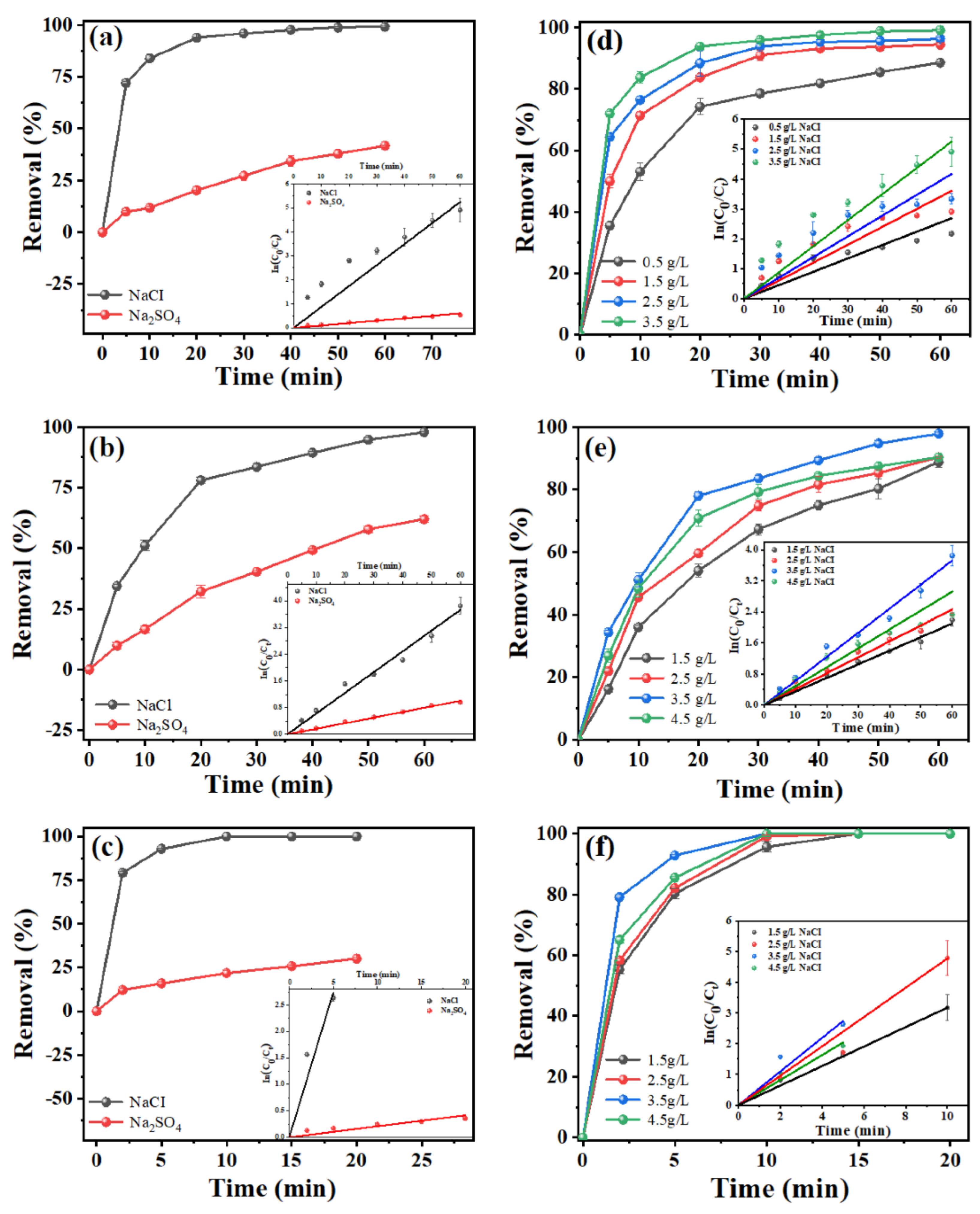
2.3.1. Effect of Electrolyte Type and Concentration
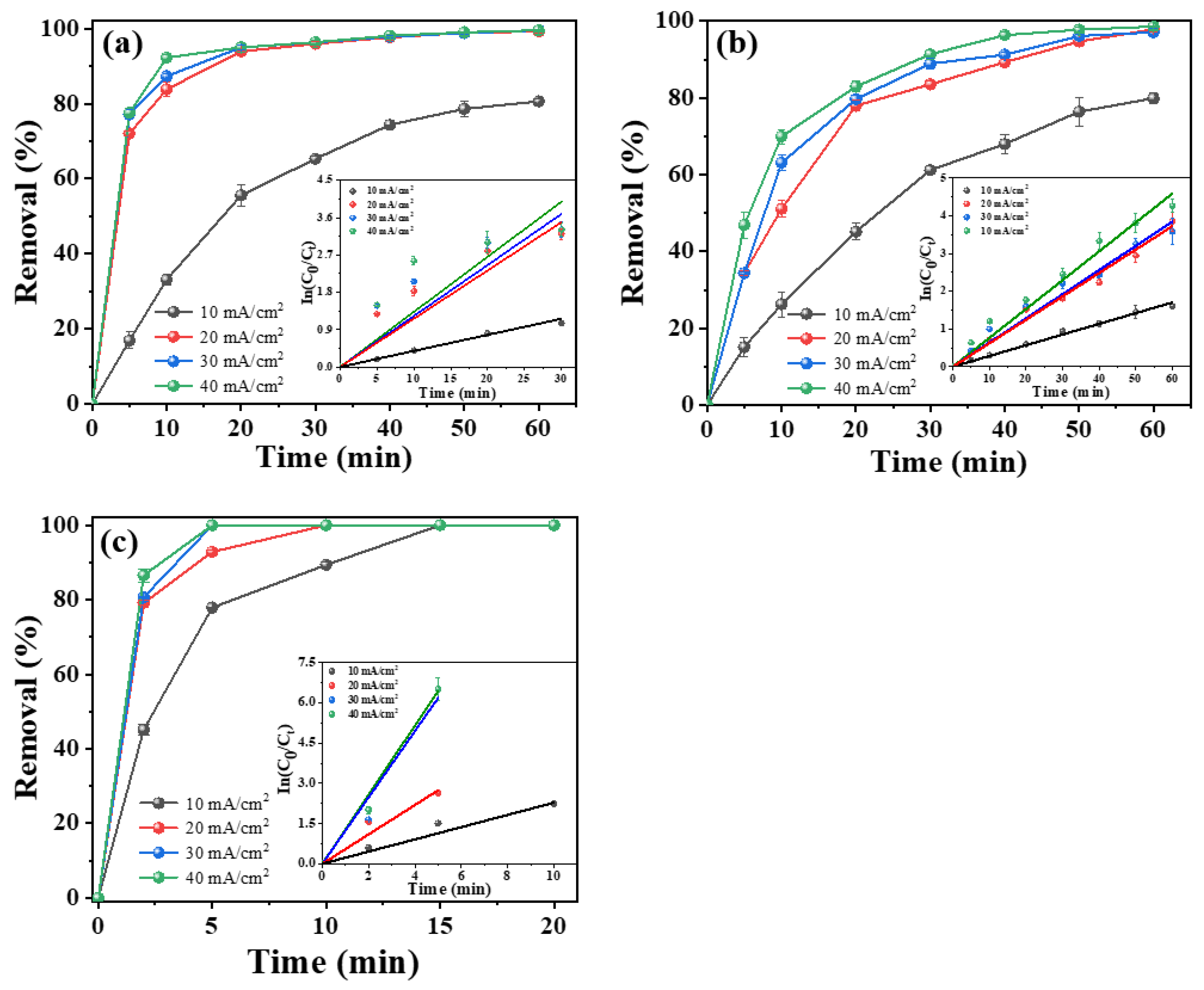
2.3.2. Effect of Current Density
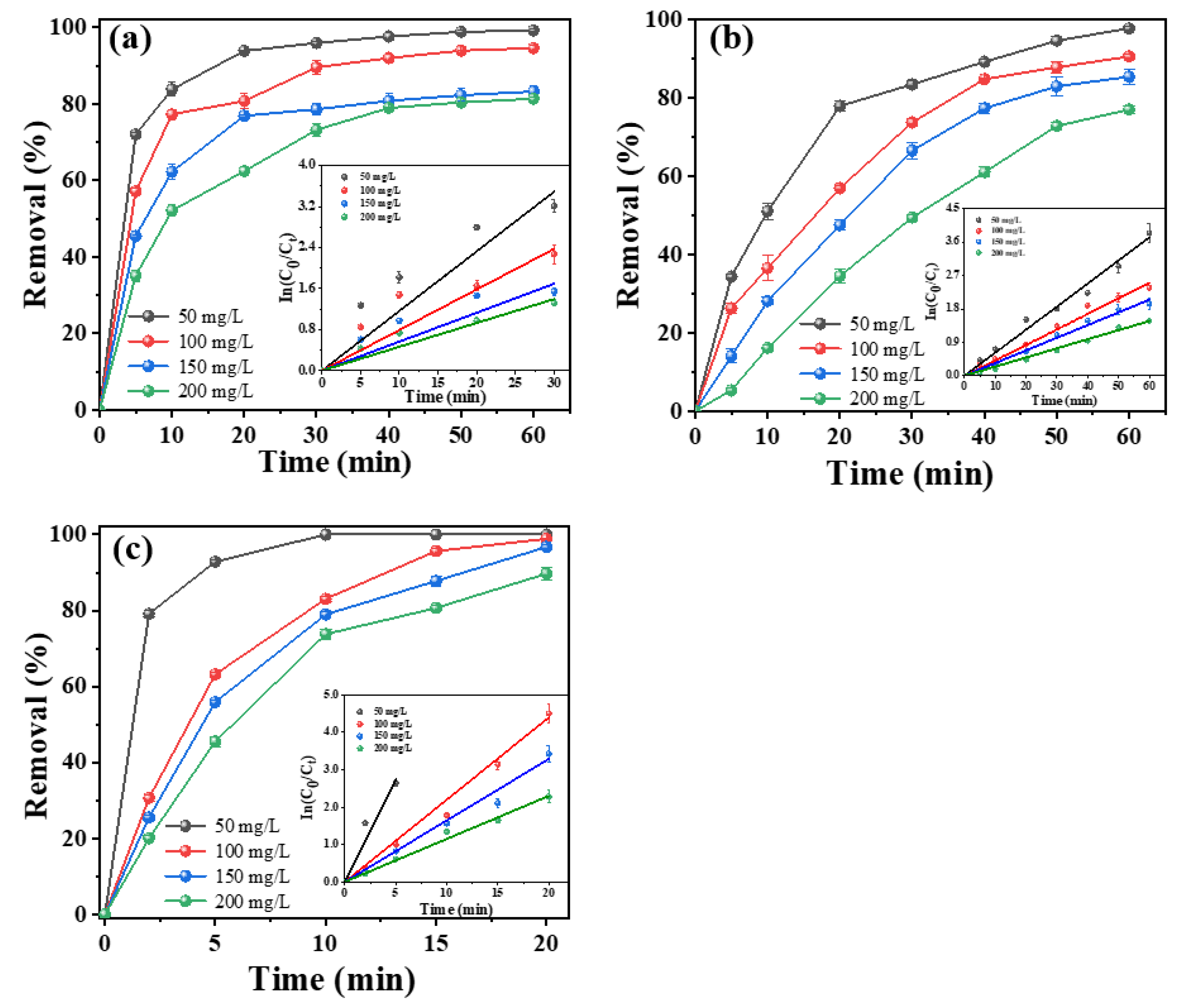
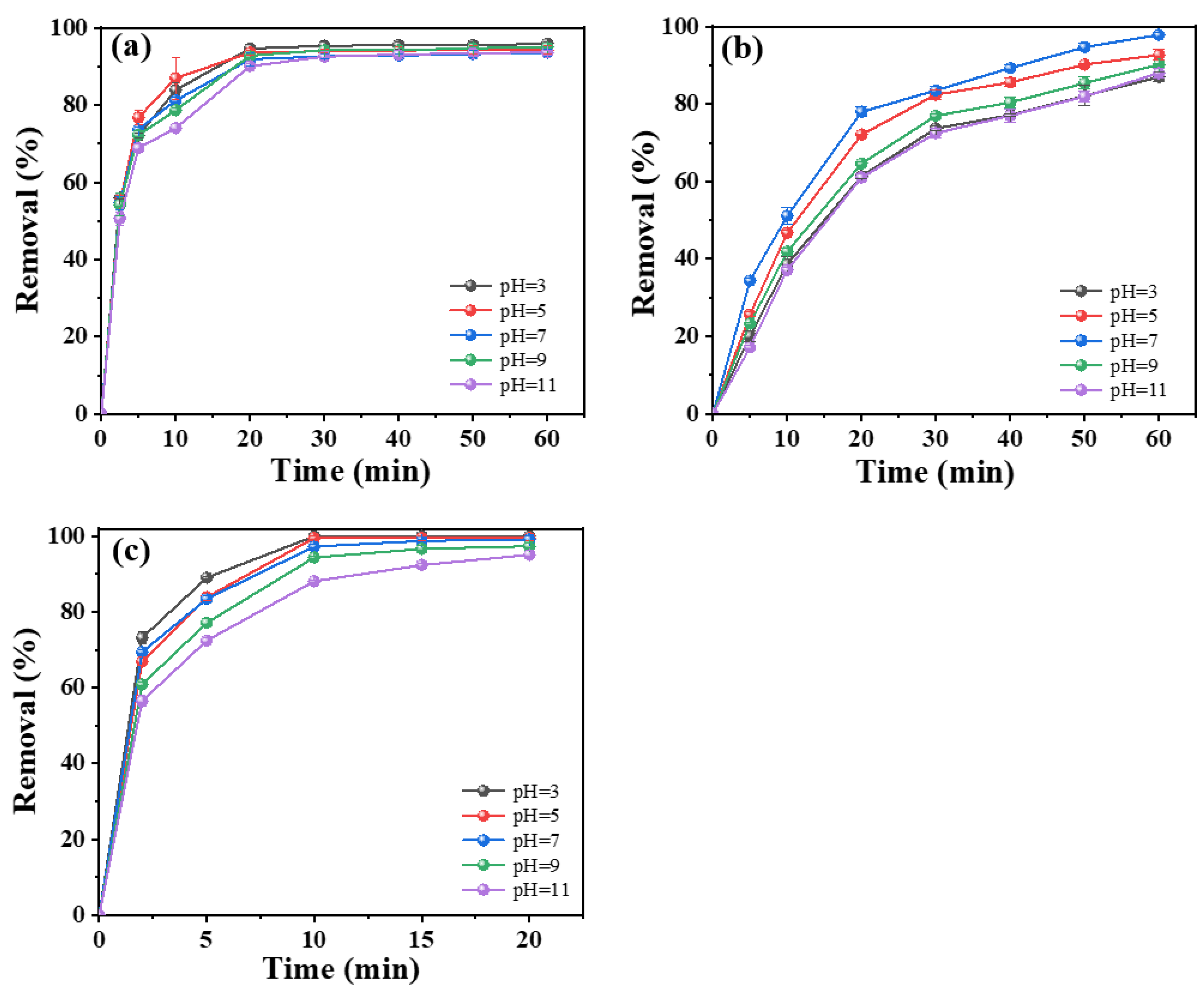
2.3.3. Effect of Initial Tetracycline Concentration and pH
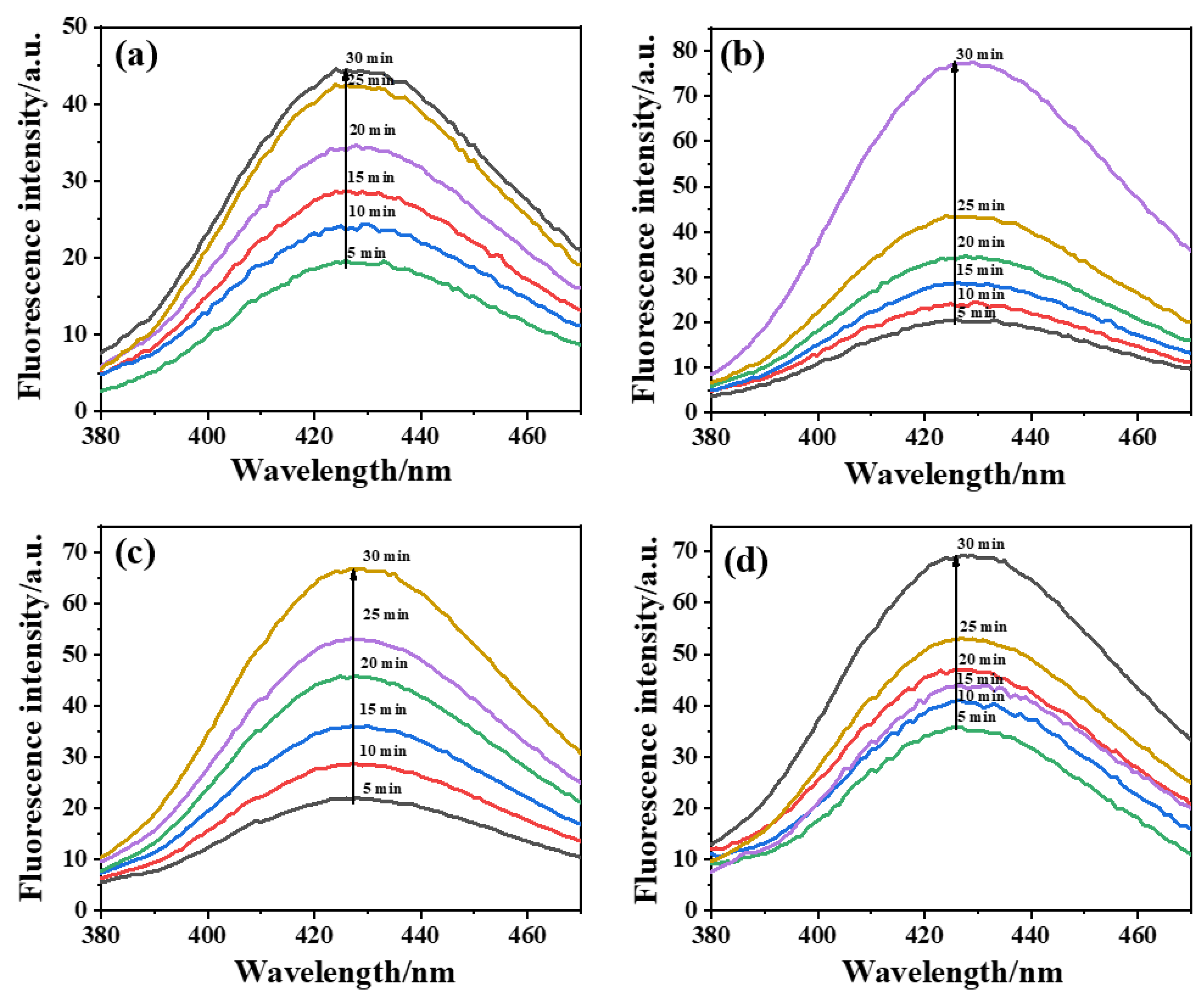
2.4. Analysis of •OH Production
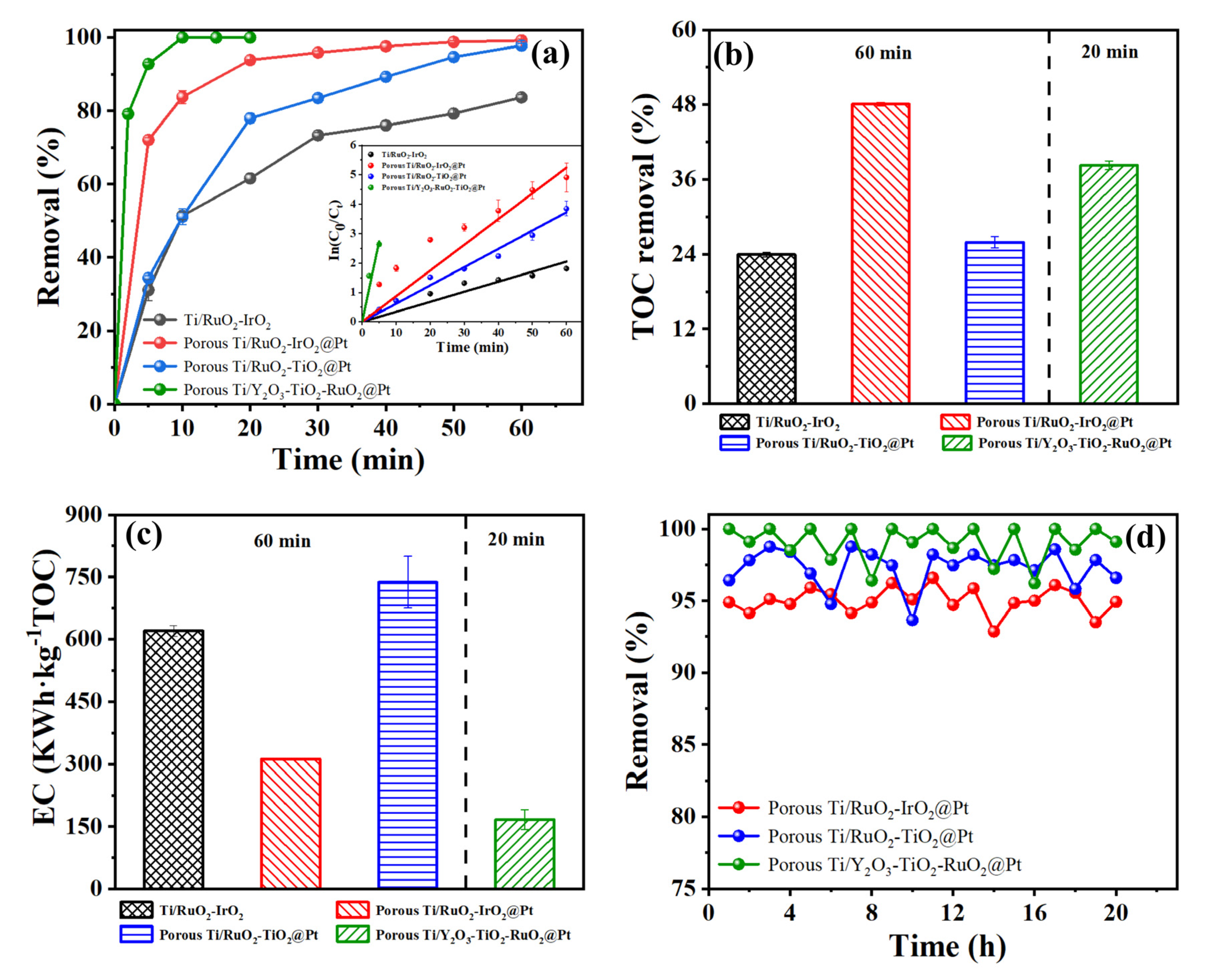
2.5. Evaluation of Different Anodes in Tetracycline Degradation
3. Experimental
3.1. Chemical Reagents and Materials
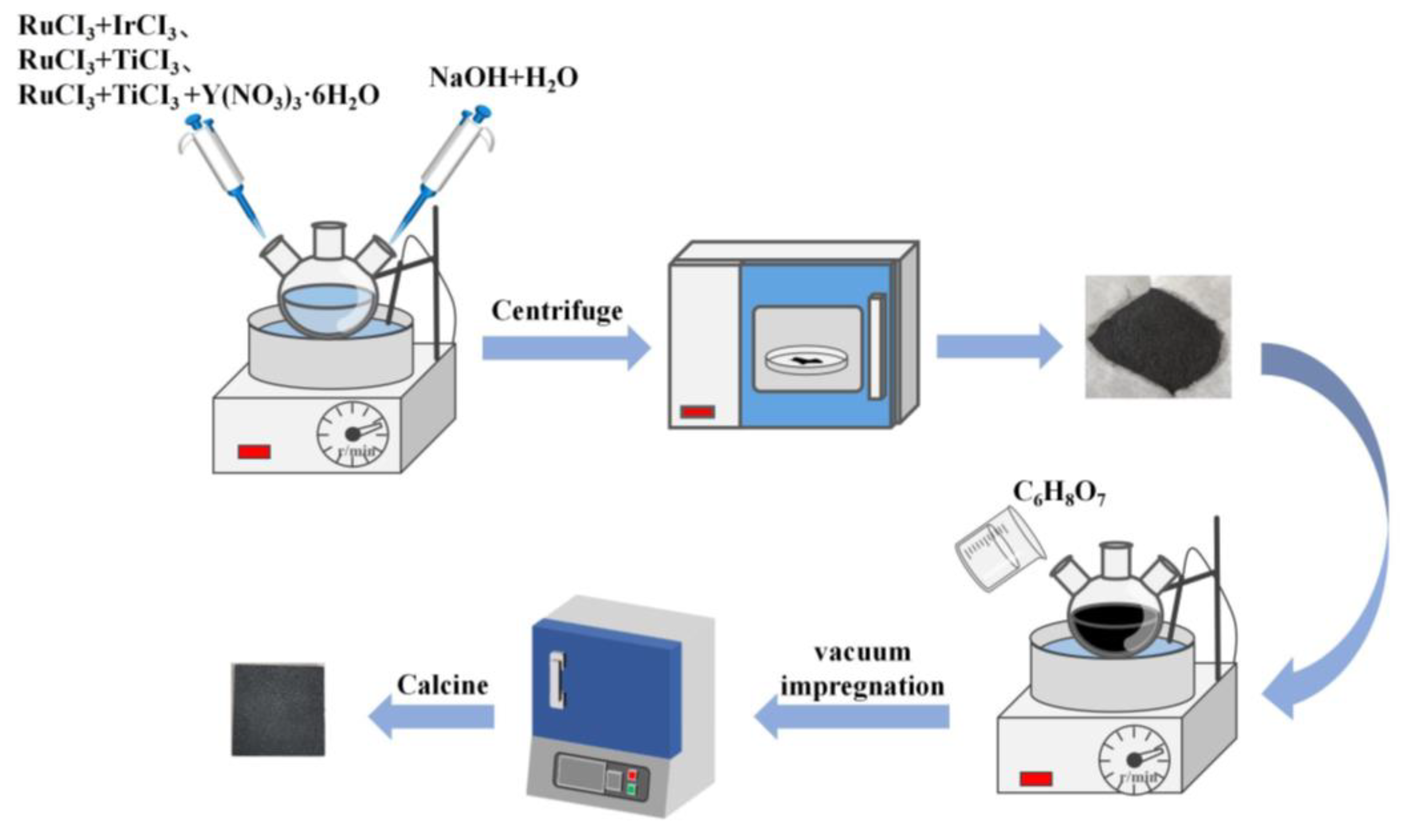
3.2. Electrode Preparation
3.3. Electrode Characterization and Electrochemical Testing
3.4. Tetracycline Electrocatalytic Degradation Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, H.; Chen, T.; Chen, D.; Zou, X.; Li, M.; Huang, F.; Sun, F.; Wang, C.; Shu, D.; Liu, H. Sulfurized oolitic hematite as a heterogeneous Fenton-like catalyst for tetracycline antibiotic degradation. Appl. Catal. B Environ. 2020, 260, 118203. [Google Scholar] [CrossRef]
- Zhang, B.; He, X.; Yu, C.; Liu, G.; Ma, D.; Cui, C.; Yan, Q.; Zhang, Y.; Zhang, G.; Ma, J.; et al. Degradation of tetracycline hydrochloride by ultrafine TiO2 nanoparticles modified g-C3N4 heterojunction photocatalyst: Influencing factors, products and mechanism insight. Chin. Chem. Lett. 2022, 33, 1337–1342. [Google Scholar] [CrossRef]
- Wu, Z.; Tong, Z.; Xie, Y.; Sun, H.; Gong, X.; Qin, P.; Liang, Y.; Yuan, X.; Zou, D.; Jiang, L. Efficient degradation of tetracycline by persulfate activation with Fe, Co and O co−doped g−C3N4: Performance, mechanism and toxicity. Chem. Eng. J. 2022, 434, 134732. [Google Scholar] [CrossRef]
- Cai, A.; Deng, J.; Xu, M.; Zhu, T.; Zhou, S.; Li, J.; Wang, G.; Li, X. Degradation of tetracycline by UV activated monochloramine process: Kinetics, degradation pathway, DBPs formation and toxicity assessment. Chem. Eng. J. 2020, 395, 125090. [Google Scholar] [CrossRef]
- Wang, Y.; Rao, L.; Wang, P.; Shi, Z.; Zhang, L. Photocatalytic activity of N-TiO2/O-doped N vacancy g-C3N4 and the intermediates toxicity evaluation under tetracycline hydrochloride and Cr(VI) coexistence environment. Appl. Catal. B Environ. 2020, 262, 118308. [Google Scholar] [CrossRef]
- Shen, Q.; Wei, L.; Bibi, R.; Wang, K.; Hao, D.; Zhou, J.; Li, N. Boosting photocatalytic degradation of tetracycline under visible light over hierarchical carbon nitride microrods with carbon vacancies. J. Hazard. Mater. 2021, 413, 125376. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, Y.; Zhang, Y.; Feng, J.; Li, Y.; Lan, J.; Cheng, X. Fabrication of NiCoP decorated TiO2/polypyrrole nanocomposites for the effective photocatalytic degradation of tetracycline. Chin. Chem. Lett. 2022, 33, 2741–2746. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Wang, P.; Wang, Z.; Zuo, C.; Chen, W.; Ao, T. Facile fabrication of N-doped hierarchical porous carbons derived from soft-templated ZIF-8 for enhanced adsorptive removal of tetracycline hydrochloride from water. J. Hazard. Mater. 2022, 423, 127103. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Yoo, S.; Choi, Y.K.; Park, S.; Kan, E. Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar. Bioresour. Technol. 2018, 259, 24–31. [Google Scholar] [CrossRef]
- Xin, S.; Huo, S.; Xin, Y.; Gao, M.; Wang, Y.; Liu, W.; Zhang, C.; Ma, X. Heterogeneous photo-electro-Fenton degradation of tetracycline through nitrogen/oxygen self-doped porous biochar supported CuFeO2 multifunctional cathode catalyst under visible light. Appl. Catal. B Environ. 2022, 312, 121442. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, K.; Hu, X.; Xue, Y.; Zhang, L.; Sun, Y. Ball-milled biochar incorporated polydopamine thin-film composite (PDA/TFC) membrane for high-flux separation of tetracyclic antibiotics from wastewater. Sep. Purif. Technol. 2021, 272, 118957. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Z.; Zhang, J.; Shan, D.; Wu, Y.; Bai, L.; Wang, B. Treatment of industrial dye wastewater and pharmaceutical residue wastewater by advanced oxidation processes and its combination with nanocatalysts: A review. J. Water Process Eng. 2021, 42, 102122. [Google Scholar] [CrossRef]
- Xie, W.; Shi, Y.; Wang, Y.; Zheng, Y.; Liu, H.; Hu, Q.; Wei, S.; Gu, H.; Guo, Z. Electrospun iron/cobalt alloy nanoparticles on carbon nanofibers towards exhaustive electrocatalytic degradation of tetracycline in wastewater. Chem. Eng. J. 2021, 405, 126585. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; van Hullebusch, E.D.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hubner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Sires, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. Int. 2014, 21, 8336–8367. [Google Scholar] [CrossRef]
- Ammar, H.B.; Brahim, M.B.; Abdelhédi, R.; Samet, Y. Green electrochemical process for metronidazole degradation at BDD anode in aqueous solutions via direct and indirect oxidation. Sep. Purif. Technol. 2016, 157, 9–16. [Google Scholar] [CrossRef]
- Kaur, R.; Kushwaha, J.P.; Singh, N. Electro-oxidation of Ofloxacin antibiotic by dimensionally stable Ti/RuO2 anode: Evaluation and mechanistic approach. Chemosphere 2018, 193, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Jiao, L.; Yu, N.; Guo, F.; Chen, X. Comparison of electrocatalytic characterization of Ti/Sb-SnO2 and Ti/F-PbO2 electrodes. J. Solid State Electrochem. 2015, 20, 353–359. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, C.; Zhang, M.; Zhang, B.-T.; Yu, Y.-G. The electrochemical degradation of ciprofloxacin using a SnO2-Sb/Ti anode: Influencing factors, reaction pathways and energy demand. Chem. Eng. J. 2016, 296, 79–89. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, S.; Yin, L.; Dai, Y. Electrocatalytic degradation of pesticide micropollutants in water by high energy pulse magnetron sputtered Pt/Ti anode. Chin. Chem. Lett. 2022, 33, 5196–5199. [Google Scholar] [CrossRef]
- Yang, W.; Tan, J.; Chen, Y.; Li, Z.; Liu, F.; Long, H.; Wei, Q.; Liu, L.; Ma, L.; Zhou, K.; et al. Relationship between substrate type and BDD electrode structure, performance and antibiotic tetracycline mineralization. J. Alloys Compd. 2022, 890, 161760. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Han, W.; Sun, X.; Li, J.; Wang, L. Preparation of porous TiO2-NTs/m-SnO2-Sb electrode for electrochemical degradation of benzoic acid. RSC Adv. 2016, 6, 19848–19856. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Gao, Q.; Jiang, B.; Li, C.; Zhao, Q. Synthesis of low-cost Ti4O7 membrane electrode for electrooxidation of tetracycline under flow-through conditions: Performance, kinetics and mechanism. Process Saf. Environ. Prot. 2022, 159, 931–943. [Google Scholar] [CrossRef]
- Asim, S.; Zhu, Y.; Batool, A.; Hailili, R.; Luo, J.; Wang, Y.; Wang, C. Electrochemical treatment of 2, 4-dichlorophenol using a nanostructured 3D-porous Ti/Sb-SnO2-Gr anode: Reaction kinetics, mechanism, and continuous operation. Chemosphere 2017, 185, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Asim, S.; Zhu, Y.; Rana, M.; Yin, J.; Shah, M.W.; Li, Y.; Wang, C. Nanostructured 3D-porous graphene hydrogel based Ti/Sb-SnO2-Gr electrode with enhanced electrocatalytic activity. Chemosphere 2017, 169, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.; Liu, Y.; Song, H.; Chen, D.; Zhu, W.; Liu, R.; Yang, H.; Li, A.; Zhang, S. Trace Ti3+- and N-codoped TiO2 nanotube array anode for significantly enhanced electrocatalytic degradation of tetracycline and metronidazole. Chem. Eng. J. 2021, 405, 126982. [Google Scholar] [CrossRef]
- Chen, M.; Pan, S.; Zhang, C.; Wang, C.; Zhang, W.; Chen, Z.; Zhao, X.; Zhao, Y. Electrochemical oxidation of reverse osmosis concentrates using enhanced TiO2-NTA/SnO2-Sb anodes with/without PbO2 layer. Chem. Eng. J. 2020, 399, 125756. [Google Scholar] [CrossRef]
- Lin, H.; Xiao, R.; Xie, R.; Yang, L.; Tang, C.; Wang, R.; Chen, J.; Lv, S.; Huang, Q. Defect Engineering on a Ti4O7 Electrode by Ce3+ Doping for the Efficient Electrooxidation of Perfluorooctanesulfonate. Environ. Sci. Technol. 2021, 55, 2597–2607. [Google Scholar] [CrossRef]
- You, S.; Liu, B.; Gao, Y.; Wang, Y.; Tang, C.Y.; Huang, Y.; Ren, N. Monolithic Porous Magnéli-phase Ti4O7 for Electro-oxidation Treatment of Industrial Wastewater. Electrochim. Acta 2016, 214, 326–335. [Google Scholar] [CrossRef]
- Xu, K.; Peng, J.; Chen, P.; Gu, W.; Luo, Y.; Yu, P. Preparation and Characterization of Porous Ti/SnO2–Sb2O3/PbO2 Electrodes for the Removal of Chloride Ions in Water. Processes 2019, 7, 762. [Google Scholar] [CrossRef]
- Yang, D.; Tian, Z.; Song, J.; Lu, T.; Qiu, G.; Kang, J.; Zhou, H.; Mao, H.; Xiao, J. Influences of sintering temperature on pore morphology, porosity, and mechanical behavior of porous Ti. Mater. Res. Express 2021, 8, 106519. [Google Scholar] [CrossRef]
- Shen, J.; Chen, D.; Zhao, W.; Zhang, W.W.; Zhou, H. Study on the Preparation and Characterizations of an Improved Porous Ti/TiO2/CdS-CNT/C3N4 Photoelectrode and Photoelectric Catalytic Degradation of Methylene Blue. ChemistrySelect 2018, 3, 3363–3373. [Google Scholar] [CrossRef]
- Moradi, F.; Dehghanian, C. Addition of IrO2 to RuO2+TiO2 coated anodes and its effect on electrochemical performance of anodes in acid media. Prog. Nat. Sci. Mater. Int. 2014, 24, 134–141. [Google Scholar] [CrossRef]
- Okur, M.C.; Akyol, A.; Nayir, T.Y.; Kara, S.; Ozturk, D.; Civas, A. Performance of Ti/RuO2-IrO2 electrodes and comparison with BDD electrodes in the treatment of textile wastewater by electro-oxidation process. Chem. Eng. Res. Des. 2022, 183, 398–410. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, H.; Chen, G.; Zhang, Y. Study of IrxRu1−xO2 oxides as anodic electrocatalysts for solid polymer electrolyte water electrolysis. Electrochim. Acta 2009, 54, 6250–6256. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, T.; Cheng, C.; Pan, F.; Zhu, Y.; Ma, H.; Niu, J. Ultralong-lifetime Ti/RuO2-IrO2@Pt anodes with a strong metal-support interaction for efficient electrochemical mineralization of perfluorooctanoic acid. Nanoscale 2022, 14, 3579–3588. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Pang, D.; Song, H.; Zhang, S. Simultaneous electrochemical degradation of tetracycline and metronidazole through a high-efficiency and low-energy-consumption advanced oxidation process. Chemosphere 2022, 292, 133469. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Wang, C.; Meng, X.; Zhang, W.; Chen, Z.; Crittenden, J. Electrochemical degradation of methylisothiazolinone by using Ti/SnO2-Sb2O3/α, β-PbO2 electrode: Kinetics, energy efficiency, oxidation mechanism and degradation pathway. Chem. Eng. J. 2019, 374, 626–636. [Google Scholar] [CrossRef]
- Zhi, D.; Zhang, J.; Wang, J.; Luo, L.; Zhou, Y.; Zhou, Y. Electrochemical treatments of coking wastewater and coal gasification wastewater with Ti/Ti4O7 and Ti/RuO2-IrO2 anodes. J Environ. Manag. 2020, 265, 110571. [Google Scholar] [CrossRef]
- Sheng, S.; Ye, K.; Gao, Y.; Zhu, K.; Yan, J.; Wang, G.; Cao, D. Simultaneously boosting hydrogen production and ethanol upgrading using a highly-efficient hollow needle-like copper cobalt sulfide as a bifunctional electrocatalyst. J. Colloid. Interface Sci. 2021, 602, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, J.; Wang, S.; Huang, H.; He, Y.; Guo, Z. Preparation and electrochemical properties of a novel porous Ti/Sn-Sb-RuOx/β-PbO2/MnO2 anode for zinc electrowinning. RSC Adv. 2021, 11, 19136–19146. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chang, L.; Chen, B.; Li, J.; Huang, H.; Guo, Z.; He, Y. Effects of coating precursor states on performance of titanium-based metal oxide coating anode for Mn electrowinning. Electrochim. Acta 2021, 400, 139459. [Google Scholar] [CrossRef]
- Chen, J.; Xia, Y.; Dai, Q. Electrochemical degradation of chloramphenicol with a novel Al doped PbO2 electrode: Performance, kinetics and degradation mechanism. Electrochim. Acta 2015, 165, 277–287. [Google Scholar] [CrossRef]
- Li, D.; Tang, J.; Zhou, X.; Li, J.; Sun, X.; Shen, J.; Wang, L.; Han, W. Electrochemical degradation of pyridine by Ti/SnO2–Sb tubular porous electrode. Chemosphere 2016, 149, 49–56. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Tang, S.; Wang, Y.; Yu, H.; Niu, J. Insights into the electrochemical degradation of triclosan from human urine: Kinetics, mechanism and toxicity. Chemosphere 2021, 264, 128598. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Li, D.; Li, L.; Zhang, Y.; Chen, S.; Wu, Q.; Wang, P.; Zhang, C.; Sun, J. Insights into the electrooxidation of florfenicol by a highly active La-doped Ti4O7 anode. Sep. Purif. Technol. 2022, 291, 120904. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, K.; Xu, H.; Yan, W. Electrochemical oxidation of rhodamine B by PbO2/Sb-SnO2/TiO2 nanotube arrays electrode. Chin. J. Catal. 2019, 40, 917–927. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Xu, Z.; Niu, J.; Hou, L.-A. Electrochemical degradation of enrofloxacin by lead dioxide anode: Kinetics, mechanism and toxicity evaluation. Chem. Eng. J. 2017, 326, 911–920. [Google Scholar] [CrossRef]
- Chen, S.; He, P.; Wang, X.; Xiao, F.; Zhou, P.; He, Q.; Jia, L.; Dong, F.; Zhang, H.; Jia, B.; et al. Co/Sm-modified Ti/PbO2 anode for atrazine degradation: Effective electrocatalytic performance and degradation mechanism. Chemosphere 2021, 268, 128799. [Google Scholar] [CrossRef]
- Song, S.; Fan, J.; He, Z.; Zhan, L.; Liu, Z.; Chen, J.; Xu, X. Electrochemical degradation of azo dye C.I. Reactive Red 195 by anodic oxidation on Ti/SnO2–Sb/PbO2 electrodes. Electrochim. Acta 2010, 55, 3606–3613. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Yang, W.; Chen, X.; Li, W.; Luo, B.; Wang, K. Preparation of 3D PbO2 nanospheres@SnO2 nanowires/Ti Electrode and Its Application in Methyl Orange Degradation. Electrochim. Acta 2014, 146, 15–22. [Google Scholar] [CrossRef]
- Dai, Q.; Zhou, J.; Meng, X.; Feng, D.; Wu, C.; Chen, J. Electrochemical oxidation of cinnamic acid with Mo modified PbO2 electrode: Electrode characterization, kinetics and degradation pathway. Chem. Eng. J. 2016, 289, 239–246. [Google Scholar] [CrossRef]
- Zhao, W.; Xing, J.; Chen, D.; Bai, Z.; Xia, Y. Study on the performance of an improved Ti/SnO2–Sb2O3/PbO2 based on porous titanium substrate compared with planar titanium substrate. RSC Adv. 2015, 5, 26530–26539. [Google Scholar] [CrossRef]
- Ishibashi, K.-i.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
- Bai, C.; Yang, G.; Zhang, S.; Deng, S.; Zhang, Y.; Chen, C.; He, J.; Xu, M.; Long, L. A synergistic system of electrocatalytic-anode/α-MnO2/peroxymonosulfate for removing combined pollution of tetracycline and Cr(VI). Chem. Eng. J. 2021, 423, 130284. [Google Scholar] [CrossRef]
- Xin, S.; Ma, B.; Liu, G.; Ma, X.; Zhang, C.; Ma, X.; Gao, M.; Xin, Y. Enhanced heterogeneous photo-Fenton-like degradation of tetracycline over CuFeO2/biochar catalyst through accelerating electron transfer under visible light. J Environ. Manag. 2021, 285, 112093. [Google Scholar] [CrossRef]
- Wang, P.; Yuan, Q. Photocatalytic degradation of tetracyclines in liquid digestate: Optimization, kinetics and correlation studies. Chem. Eng. J. 2021, 410, 128327. [Google Scholar] [CrossRef]
- Bugueno-Carrasco, S.; Monteil, H.; Toledo-Neira, C.; Sandoval, M.A.; Thiam, A.; Salazar, R. Elimination of pharmaceutical pollutants by solar photoelectro-Fenton process in a pilot plant. Environ. Sci. Pollut. Res. Int. 2021, 28, 23753–23766. [Google Scholar] [CrossRef]
- Steter, J.R.; Brillas, E.; Sirés, I. Solar photoelectro-Fenton treatment of a mixture of parabens spiked into secondary treated wastewater effluent at low input current. Appl. Catal. B Environ. 2018, 224, 410–418. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Li, B.; Wang, Y.; Wang, T. Preparation of Porous Ti/RuO2-IrO2@Pt, Ti/RuO2-TiO2@Pt and Ti/Y2O3-RuO2-TiO2@Pt Anodes for Efficient Electrocatalytic Decomposition of Tetracycline. Molecules 2023, 28, 2189. https://doi.org/10.3390/molecules28052189
Zhu Y, Li B, Wang Y, Wang T. Preparation of Porous Ti/RuO2-IrO2@Pt, Ti/RuO2-TiO2@Pt and Ti/Y2O3-RuO2-TiO2@Pt Anodes for Efficient Electrocatalytic Decomposition of Tetracycline. Molecules. 2023; 28(5):2189. https://doi.org/10.3390/molecules28052189
Chicago/Turabian StyleZhu, Yunqing, Bingqing Li, Yongming Wang, and Tian Wang. 2023. "Preparation of Porous Ti/RuO2-IrO2@Pt, Ti/RuO2-TiO2@Pt and Ti/Y2O3-RuO2-TiO2@Pt Anodes for Efficient Electrocatalytic Decomposition of Tetracycline" Molecules 28, no. 5: 2189. https://doi.org/10.3390/molecules28052189
APA StyleZhu, Y., Li, B., Wang, Y., & Wang, T. (2023). Preparation of Porous Ti/RuO2-IrO2@Pt, Ti/RuO2-TiO2@Pt and Ti/Y2O3-RuO2-TiO2@Pt Anodes for Efficient Electrocatalytic Decomposition of Tetracycline. Molecules, 28(5), 2189. https://doi.org/10.3390/molecules28052189





