Crystal Structure, Photophysical Study, Hirshfeld Surface Analysis, and Nonlinear Optical Properties of a New Hydroxyphenylamino Meldrum’s Acid Derivative
Abstract
1. Introduction
2. Results and Discussion
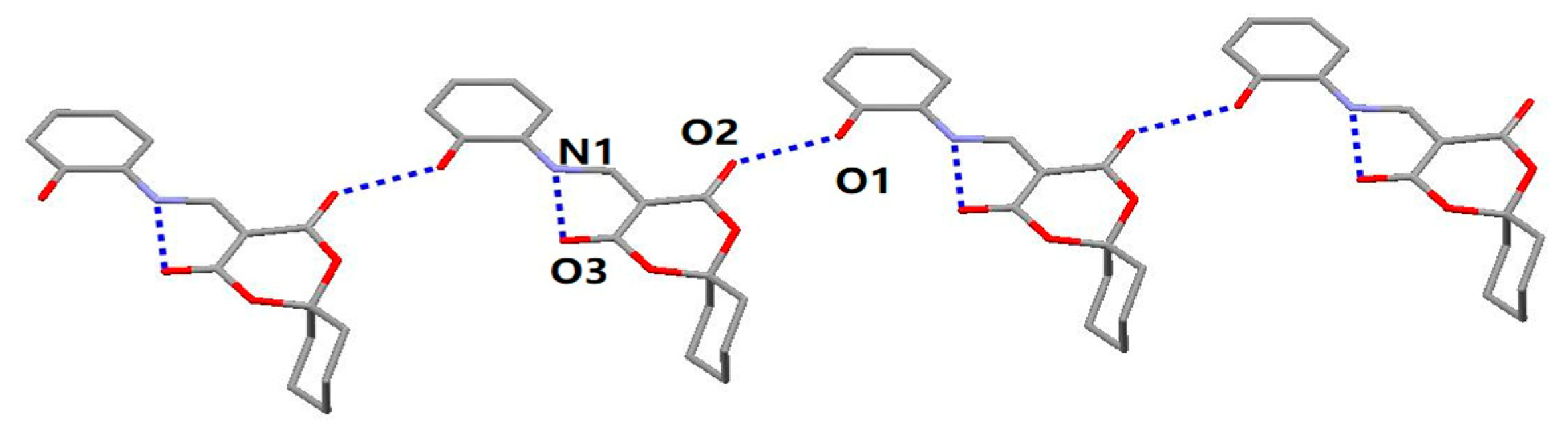
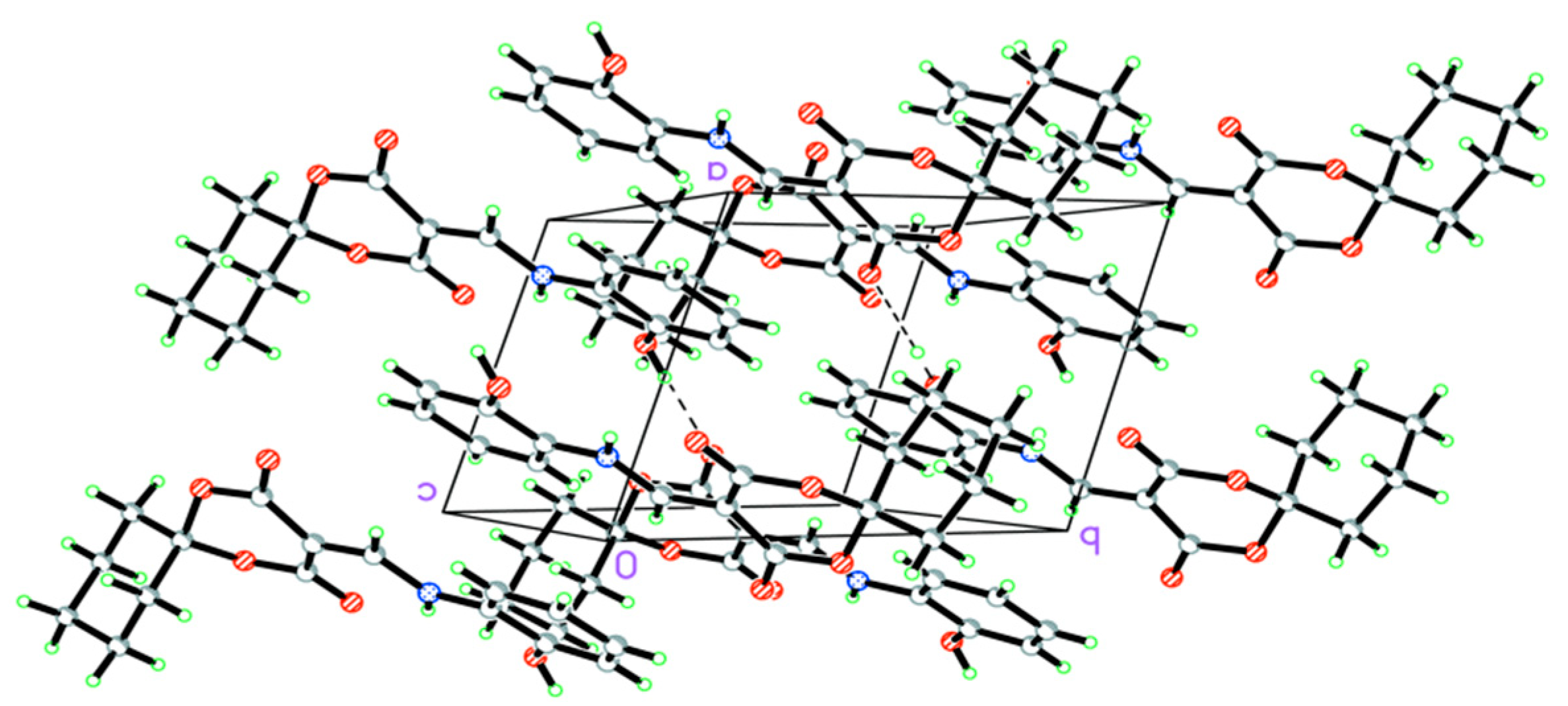
2.1. Structural Characteristic for HMD
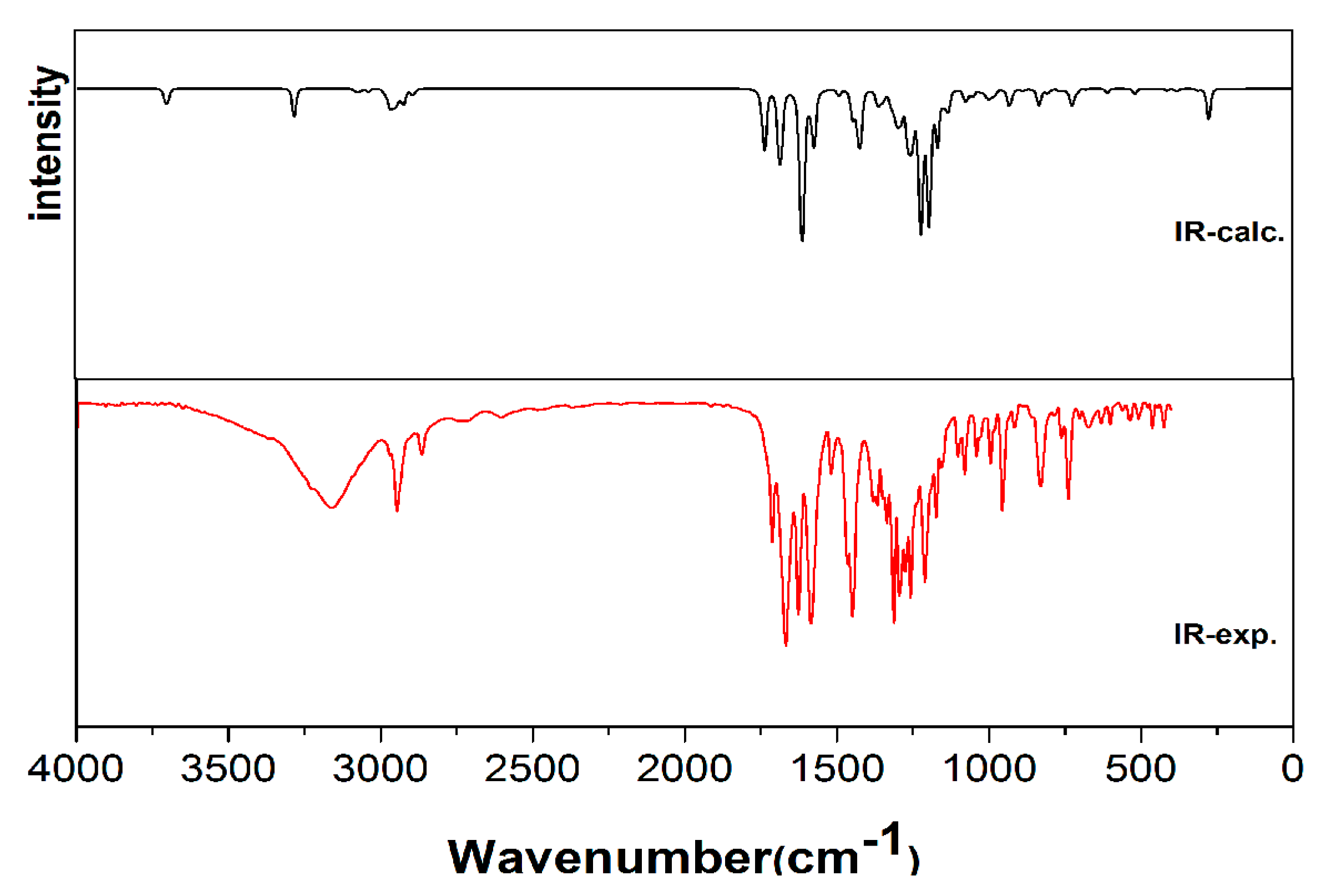
2.2. IR Spectroscopy
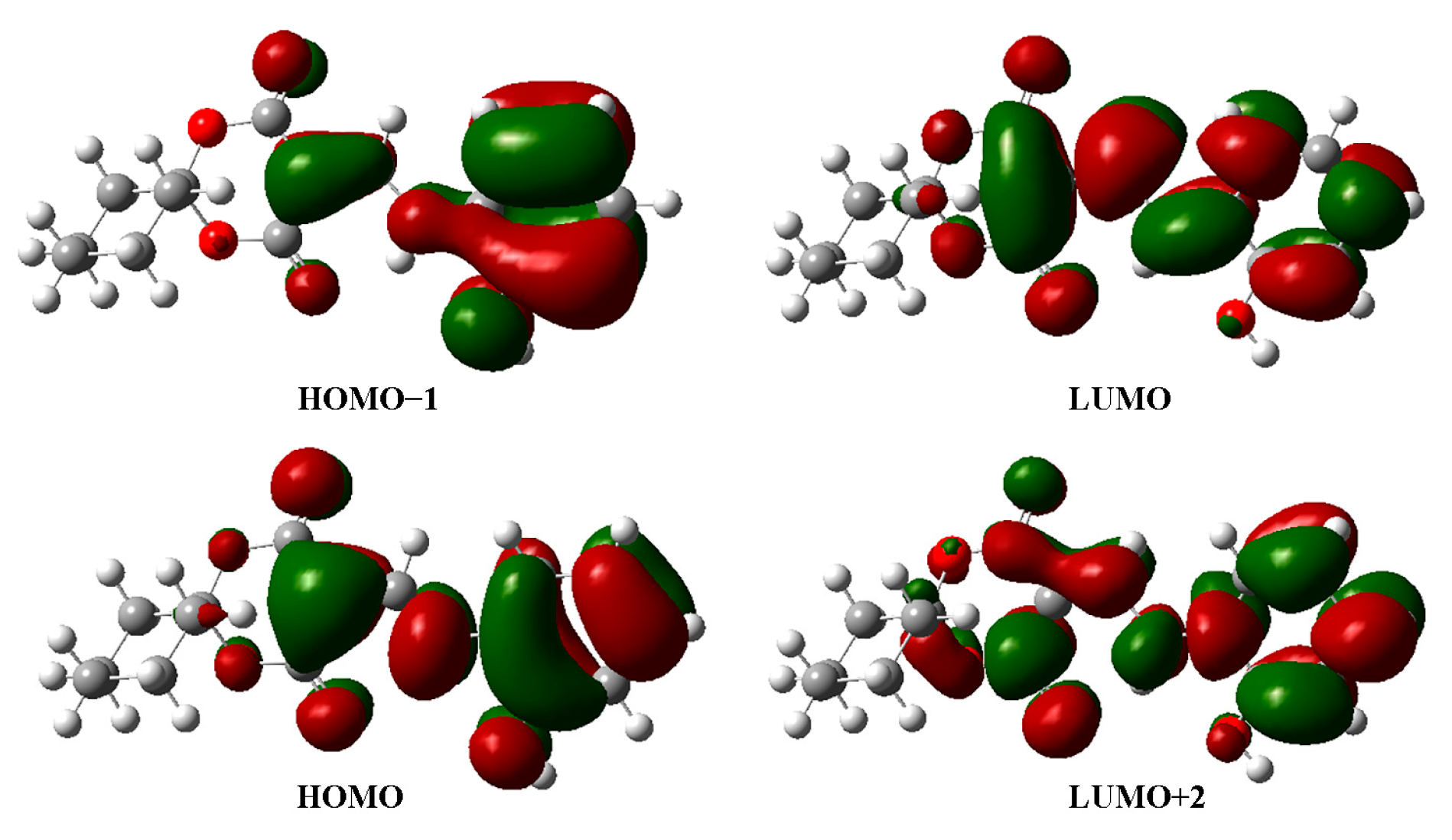
2.3. Electronic Analysis
2.4. Mulliken Population
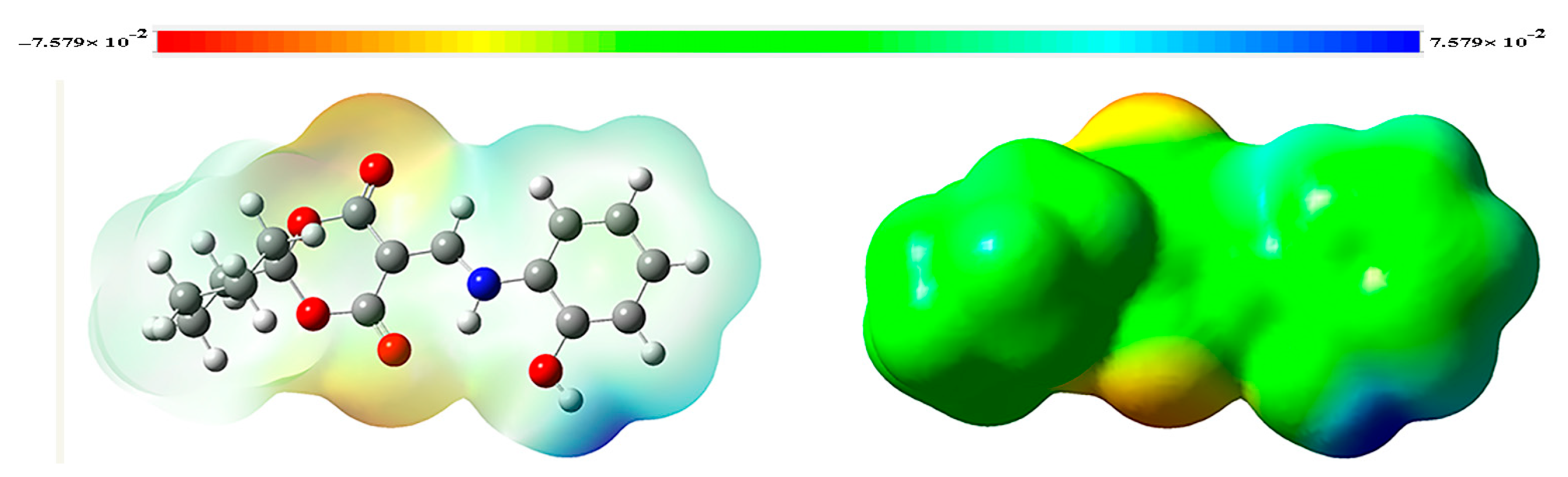
2.5. Molecular Electrostatic Potential (MEP)
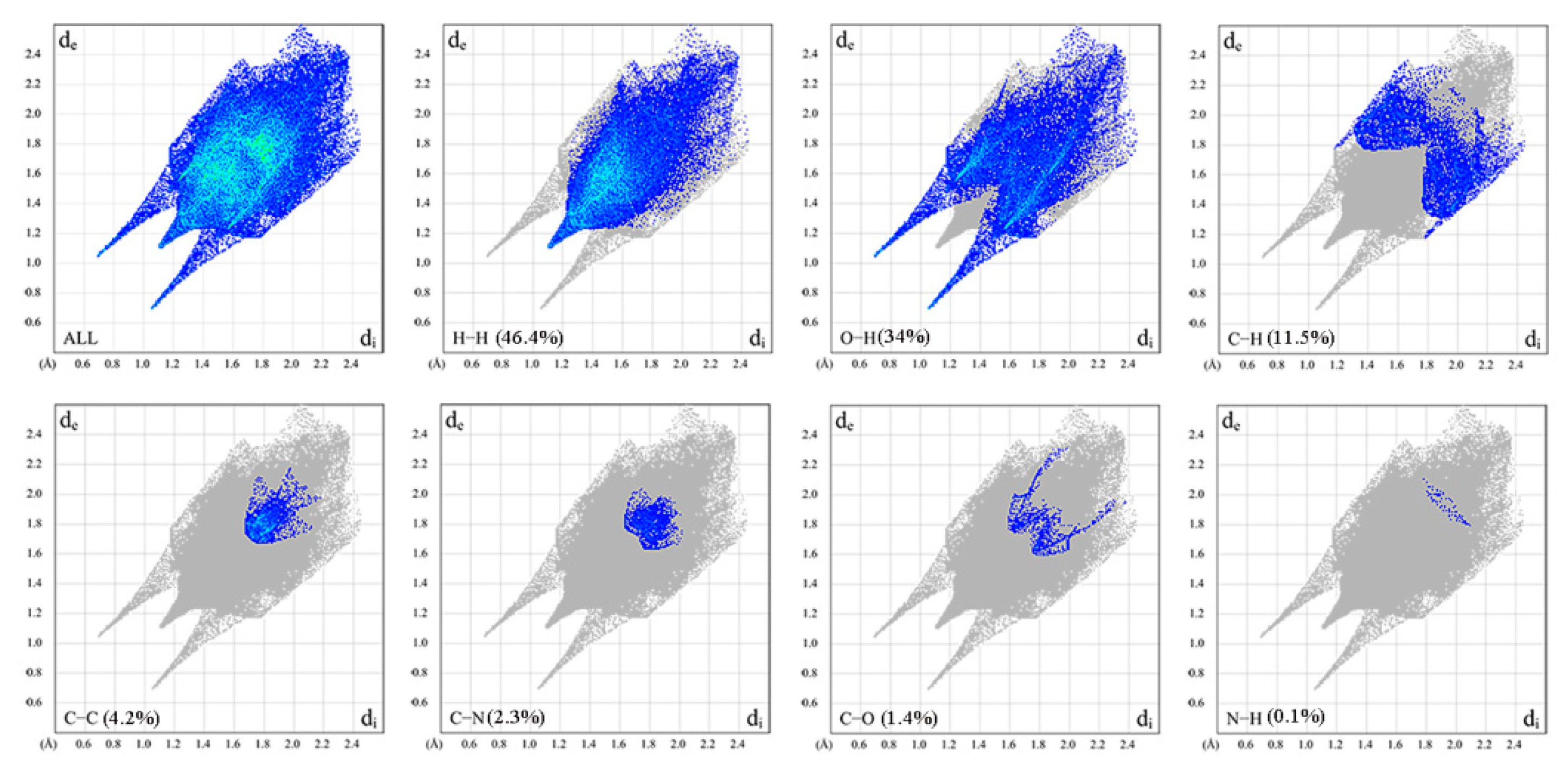
2.6. Hirshfeld Surface Analysis
2.7. Natural Bond Analysis (NBO)
2.8. Non-Linear Properties (NLO)
2.9. TG and DSC Analysis
3. Experimental Procedure
3.1. General Method
3.2. Synthesis
3.3. Single Crystal Studies
3.4. Theoretical Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Arcadi, A.; Calcaterra, A.; Fabrizi, G.; Fochetti, A.; Goggiamani, A.; Iazzetti, A.; Marrone, F.; Mazzoccanti, G.; Serraiocco, A. One-pot synthesis of dihydroquinolones by sequential reactions of o-aminobenzyl alcohol derivatives with Meldrum’s acids. Org. Biomol. Chem. 2022, 20, 3160–3173. [Google Scholar] [CrossRef]
- Johnson, T.C.; Marsden, S.P. Three-component synthesis of pyridylacetic acid derivatives by arylation/decarboxylative substitution of Meldrum’s acids. J. Org. Chem. 2022, 87, 13891–13894. [Google Scholar] [CrossRef]
- Yuan, L.; He, L.; Zhang, X.; Liu, J.; Zhang, D.; Udayabhaskararao, T.; Yang, F.; Zhao, Y.; Wang, D.; Zhao, H. Dynamic postpolymerization modification based on knoevenagel adducts of Meldrum’s Acid. Macromolecules 2022, 55, 6102–6109. [Google Scholar] [CrossRef]
- Cavalli, E.S.; Mies, T.; Rzepa, H.S.; White, A.J.P.; Parsons, P.J.; Barrett, A.G.M. Pyrimidine nucleosides syntheses by late-stage base heterocyclization reactions. Org. Lett. 2022, 24, 8931–8935. [Google Scholar] [CrossRef] [PubMed]
- Komogortsev, A.N.; Melekhina, V.G.; Lichitsky, B.V. Multicomponent protocol for the synthesis of substituted methyl 3-(3-hydroxy-4-oxo-4H-chromen-2-yl)propanoates from 3-hydroxy-4H-chromen-4-one. Synthetic Commun. 2022, 52, 1664–1671. [Google Scholar] [CrossRef]
- Brosge, F.; Singh, P.; Almqvist, F.; Bolm, C. Selected applications of Meldrum’s acid—A tutorial. Org. Biomol. Chem. 2021, 19, 5014–5027. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Biju, S.; Sadasivan, V. Synthesis, structure characterization and biological studies on a new aromatic hydrazone, 5-(2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)hydrazono)-2,2-dimethyl-1,3-dioxane-4,6-dione, and its transition metal complexes. J. Mol. Struct. 2018, 1156, 201–209. [Google Scholar] [CrossRef]
- Mierina, I.; Jure, M.; Zeberga, S.; Makareviciene, V.; Zicane, D.; Tetere, Z.; Ravina, I. Novel type of carbon-centered antioxidants arylmethyl Meldrum’s acids—Inhibit free radicals. Eur. J. Lipid Sci. Technol. 2022, 119, 1700172. [Google Scholar] [CrossRef]
- Kenchappa, R.; Bodke, Y.D.; Telkar, S.; Sindhe, M.A.; Giridhar, M. Synthesis, characterization, and antimicrobial activity of new benzofuran derivatives. Russ. J. Gen. Chem. 2016, 86, 2827–2836. [Google Scholar] [CrossRef]
- Silva, M.M.C.; Araújo-Neto, J.B.; Araújo, A.C.J.; Freitas, P.R.; Oliveira-Tintino, C.D.M.; Begnini, I.M.; Rebelo, R.A.; Silva, L.E.d.; Mireski, S.L.; Nasato, M.C.; et al. Potentiation of antibiotic activity by a Meldrum’s acid arylamino methylene derivative against multidrug-resistant bacterial strains. Indian J. Microbiol. 2021, 61, 100–103. [Google Scholar] [CrossRef]
- Mehfooz, H.; Saeed, A.; Faisal, M.; Larik, F.A.; Muqadar, U.; Khatoon, S.; Channar, P.A.; Ismail, H.; Bilquees, S.; Rashid, S.; et al. Facile one-pot synthesis, butyrylcholinesterase and α-glucosidase inhibitory activities, structure–activity relationship, molecular docking and DNA–drug binding analysis of Meldrum’s acid derivatives. Res. Chem. Intermediat. 2020, 46, 243–2456. [Google Scholar] [CrossRef]
- Liu, X.; Wen, S.; You, W.; Wang, X.; Li, Q.X.; Bian, Q.; Lv, P.; Hua, R. Efficient total synthesis and herbicidal activity of 3-acyltetramic acids: Endogenous abscisic acid synthesis regulators. J. Agr. Food Chem. 2022, 70, 13510–13517. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Wang, X.; Li, Y.; Li, X.; Zhang, Y. Structural, spectroscopic and computational studies of two new spiro compounds containing 2,3,4-trimethoxybenzyl group. J. Mol. Struct. 2022, 1269, 133806. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, X.; Jiang, J. Design and crystal structures of two new compounds fused with 3, 4, 5-trimethoxybenzyl group and 6, 10-dioxaspiro group. Crystals 2018, 8, 146. [Google Scholar] [CrossRef]
- Jiang, J.; Zeng, W. Synthesis and crystal structures of two new oxaspirocyclic compounds. Crystals 2016, 6, 134. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, X. Crystal structures of spiro derivatives including 6,10-dioxaspiro[4.5] decane-7,9-dione group and their spectral studies. J. Chem. Crystallogr. 2019, 49, 139–145. [Google Scholar] [CrossRef]
- Zeng, W.; Jiang, J.; Jiang, G.; Li, Y. Synthesis, characterization, and fluorescence properties of two new heterocyclic compounds containing 1,5-Dioxaspiro group. Crystals 2018, 8, 269. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, X.; Zhang, Y. Synthesis, crystal structures, and density functional theory studies of two salt cocrystals containing Meldrum’s acid group. ACS Omega 2022, 7, 25132–25139. [Google Scholar] [CrossRef]
- Zeng, W.; Jiang, J. Synthesis and crystal structure of a new hydrated benzimidazolium salt containing spiro structure. Crystals 2017, 7, 303. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, X.; Zhang, Y. Crystal Structure, thermodynamic properties and DFT studies of 5,6-dimethyl-1H-benzo[d]imidazol-3-ium 3-((2,4-dioxo-1,5-dioxaspiro[5.5]undecan-3-ylidene)methyl) -2,4-dioxo-1,5-dioxaspiro[5.5]undecane hydrate. Crystals 2021, 11, 1393. [Google Scholar] [CrossRef]
- Antunes, J.A.; Da Silva, L.E.; De Faria, J.L.B.; De Toledo, T.A.; Teixeira, A.M.R.; Ramos, R.J.; Freire, P.T.C.; Bento, R.R.F. Study on optical, electrochemical and thermal properties of the Meldrum acid 5-aminomethylene derivative. Vib. Spectrosc. 2021, 112, 103188. [Google Scholar] [CrossRef]
- Oueslati, Y.; Kansız, S.; Valkonen, A.; Sahbani, T.; Dege, N.; Smirani, W. Synthesis, crystal structure, DFT calculations, Hirshfeld surface, vibrational and optical properties of a novel hybrid non-centrosymmetric material (C10H15N2)2H2P2O7. J. Mol. Struct. 2019, 1196, 499–507. [Google Scholar] [CrossRef]
- Kalai, F.E.; Çınar, E.B.; Lai, C.H.; Daoui, S.; Chelfi, T.; Allali, M.; Dege, N.; Karrouchif, K.; Benchat, N. Synthesis, spectroscopy, crystal structure, TGA/DTA study, DFT and molecular docking investigations of (E)-4-(4-methylbenzyl)-6-styrylpyridazin-3(2H)-on. J. Mol. Struct. 2021, 1228, 129435. [Google Scholar] [CrossRef] [PubMed]
- Bülbül, H.; Köysal, Y.; Yıldırım, S.Ö.; Ünlüer, D.; Soylu, M.S.; Butcher, R.J. Crystal structure, computational study and Hirshfeld surface analysis of 4-(4-Methoxyphenethyl)-5-(p-tolyl)-2,4-Dihydro-3H-1,2,4-Triazol-3- One. C18H19N3O2. J. Chem. Crystallogr. 2022, 52, 440–449. [Google Scholar] [CrossRef]
- Garg, U.; Azim, Y.; Kar, A.; Pradeep, C.P. Cocrystals/salt of 1-naphthalene acetic acid and utilizing Hirshfeld surface calculations for acid-aminopyrimidine synthon. CrystEngComm. 2020, 22, 2978–2989. [Google Scholar] [CrossRef]
- Garg, U.; Azim, Y.; Alam, M.; Kar, A.; Pradeep, C.P. Extensive analyses on expanding the scope of acid−aminopyrimidine synthons for the design of molecular solids. Cryst. Growth Des. 2022, 22, 4316–4331. [Google Scholar] [CrossRef]
- Ersanli, C.C.; Kantar, G.K.; Şaşmaz, S.J. Crystallographic, spectroscopic (FTIR and NMR) and quantum computational calculation studies on bis (2-methoxy-4-((E)-prop-1-enyl) phenyl) oxalate. Mol. Struct. 2017, 1143, 318–327. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect C Cryst. Struct. Commun. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, UK, 2013. [Google Scholar]
- Becke, A.D. A new mixing of HartreeeFock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colic-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Scalmani, G.; Frisch, M.J.; Mennucci, B.; Tomasi, J.; Cammi, R.; Barone, V. Geometries and properties of excited states in the gas phase and in solution: Theory and application of a time-dependent density functional theory polarizable continuum model. J. Chem. Phys. 2006, 124, 094107. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer (Version 3.1); University of Western Australia: Perth, Australia, 2007. [Google Scholar]




| Formula | C16H17NO5 |
|---|---|
| CCDC | 2233681 |
| Mr | 303.30 |
| Color/shape | Yellow/block |
| Temperature | 293(2) K |
| Crystal system, space group | Triclinic, P-1 |
| a | 6.9815(14) Å |
| b | 8.8545(18) Å |
| c | 12.707(3) Å |
| α | 81.96(3)° |
| β | 85.49(3)° |
| γ | 70.39(3)° |
| V | 732.2(3) Å3 |
| Z | 2 |
| Dcalc | 1.376 Mg·m−3 |
| μ | 0.103 mm−1 |
| F(000) | 320 |
| θ | 3.448° to 25.00° |
| Ranges/indices (h, k, l) | −8 ≤ h ≤ 8, −9≤ k ≤ 10, −13 ≤ l ≤ 15 |
| No. of reflections collected/unique | 4931/2547 [Rint = 0.0359] |
| No. of parameters | 199 |
| GOF | 1.031 |
| R1 [I > 2σ(I)] | 0.0571 |
| wR2 [I > 2σ(I)] | 0.1345 |
| R1 [all data] | 0.0896 |
| wR2 (all data) | 0.1628 |
| Largest diff. peak and hole | 0.217 e. Å−3and −0.245 e. Å−3 |
| Geometrical Parameters | Exp | B3LYP |
|---|---|---|
| Bond length (Å) | ||
| N(1)–C(10) | 1.321(3) | 1.333 |
| N(1)–C(11) | 1.413(3) | 1.408 |
| C(8)–C(10) | 1.385(3) | 1.380 |
| C(16)–O(1) | 1.354(3) | 1.365 |
| O(3)–C(7) | 1.220(3) | 1.217 |
| O(2)–C(9) | 1.221(3) | 1.206 |
| Bond angle (°) | ||
| C(10)–N(1)–C(11) | 125.90(2) | 126.85 |
| N(1)–C(10)–C(8) | 125.70(2) | 125.38 |
| C(10)–C(8)–C(7) | 122.40(2) | 121.22 |
| C(10)–C(8)–C(9) | 117.00(2) | 117.39 |
| C(12)–C(11)–N(1) | 124.00(2) | 124.12 |
| C(16)–C(11)–N(1) | 116.50(2) | 116.87 |
| Torsion angle (°) | ||
| C(11)–N(1)–C(10)–C(8) | −175.50(2) | −178.03 |
| C(7)–C(8)–C(10)–N(1) | −2.30(3) | −0.88 |
| C(9)–C(8)–C(10)–N(1) | −175.60(2) | −175.85 |
| D–H···A | Symmetry | D–H (Å) | H…A(Å) | D…A(Å) | ∠D–H···A(°) |
|---|---|---|---|---|---|
| N(1)–H(1)···O(3) | Intra | 0.86 | 2.1333 | 2.772(3) | 130.68 |
| O(1)–H(1A)···O(2) | −1 + x, 1 + y, z | 0.82 | 1.8974 | 2.714(2) | 173.65 |
| Exp. | Calc. (TD-DFT) | ||
|---|---|---|---|
| Wavelength (nm) | Wavelength (nm) | Oscillator Strength (f) | Electronic Transition Orbits |
| 206 | 191 | 0.1113 | 79HOMO−1→83LUMO+2 (40.64%) |
| 234 | 226 | 0.1123 | 80HOMO→83LUMO+2 (48.30%) |
| 346 | 321 | 0.6683 | 80HOMO→81LUMO (97.0%) |
| Parameters (eV) | HMD |
|---|---|
| EHOMO | −6.10 |
| ELUMO | −1.87 |
| Energy gap ΔE | 4.23 |
| Ionization potential (I = −EHOMO) | 6.10 |
| Electron affinity (A = −ELUMO) | 1.87 |
| Global hardness (η = (I − A)/2) | 2.11 |
| Global softness (ζ = 1/2η) | 0.236 |
| Chemical potential (μ = − (I + A)/2) | −4.285 |
| Global electrophilicity (ω = μ2/2η) | 4.645 |
| Electron negativity (χ = (I + A)/2) | 4.285 |
| Maximum charge transfer index (ΔNmax. = −μ/η) | 2.031 |
| Atom | Charge | Atom | Charge | Atom | Charge | Atom | Charge |
|---|---|---|---|---|---|---|---|
| O5 | −0.313277 | O1 | −0.352739 | C12 | −0.057529 | C2 | −0.207575 |
| O4 | −0.316627 | H1A | 0.254086 | H12 | 0.100675 | H2A | 0.110945 |
| N1 | −0.385915 | C11 | 0.160787 | C14 | −0.066608 | H2B | 0.121645 |
| H1 | 0.249818 | C9 | 0.441547 | H13 | 0.087573 | C6 | −0.188236 |
| C8 | −0.485979 | C15 | −0.062515 | C4 | 0.097509 | H6A | 0.117361 |
| O3 | −0.364607 | H15 | 0.085894 | C14 | −0.085738 | H6B | 0.111042 |
| O2 | −0.348109 | C3 | −0.097413 | H13 | 0.087020 | C1 | −0.216698 |
| C16 | 0.090027 | H3A | 0.121878 | C5 | −0.115944 | H1A | 0.110428 |
| C10 | 0.269087 | H38 | 0.117636 | H5A | 0.126228 | H1C | 0.102233 |
| H10 | 0.104773 | C7 | 0.484310 | H5B | 0.113007 |
| Donor NBO (i)→Acceptor NBO (j) | E(2) kcal/mol | Donor NBO (i)→Acceptor NBO (j) | E(2) kcal/mol |
|---|---|---|---|
| π(C10–C8)→π* (O3–C7) | 28.30 | n (2) O1→π* (C15–C16) | 27.74 |
| π(C10–C8)→π* (O2–C9) | 25.49 | n (2) O3→σ*(O4–C5) | 31.10 |
| π(C11–C12)→π* (C15–C16) | 20.62 | n (2) O2→σ* (O5–C9) | 33.73 |
| π (C11–C12)→π* (C13–C14) | 18.64 | n (2) N1→π* (C10–C8) | 62.54 |
| π (C13–C14)→π* (C15–C16) | 19.26 | n (2) N1→π* (C11–C12) | 33.92 |
| n (1) O3→σ* (N1–H1) | 1.98 | n (2) O5→π* (O2–C9) | 29.40 |
| n (2) O3→σ* (N1–H1) | 6.10 | n (2) O4→π* (O3–C7) | 33.74 |
| n (1) O1→σ* (N1–H1) | 0.88 |
| Parameters | Value (esu) |
|---|---|
| μx | 3.3257 |
| μy | −2.4746 |
| μz | 1.7942 |
| μ | 4.5170 |
| αxx | 501.183 × 10−24 |
| αyy | 278.439 × 10−24 |
| αzz | 181.569 × 10−24 |
| αxy | −0.42461 × 10−24 |
| αxz | 2.12344 × 10−24 |
| αyz | 1.70112 × 10−24 |
| α | 320.397 × 10−24 |
| Δα | 276.361 × 10−24 |
| βx | 1.297049 × 10−30 |
| βy | 0.903273 × 10−30 |
| βz | 1.125 × 10−30 |
| β | 1.94064 × 10−30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, W.; Wang, X.; Zhou, T.; Zhang, Y. Crystal Structure, Photophysical Study, Hirshfeld Surface Analysis, and Nonlinear Optical Properties of a New Hydroxyphenylamino Meldrum’s Acid Derivative. Molecules 2023, 28, 2181. https://doi.org/10.3390/molecules28052181
Zeng W, Wang X, Zhou T, Zhang Y. Crystal Structure, Photophysical Study, Hirshfeld Surface Analysis, and Nonlinear Optical Properties of a New Hydroxyphenylamino Meldrum’s Acid Derivative. Molecules. 2023; 28(5):2181. https://doi.org/10.3390/molecules28052181
Chicago/Turabian StyleZeng, Wulan, Xia Wang, Tao Zhou, and Yunju Zhang. 2023. "Crystal Structure, Photophysical Study, Hirshfeld Surface Analysis, and Nonlinear Optical Properties of a New Hydroxyphenylamino Meldrum’s Acid Derivative" Molecules 28, no. 5: 2181. https://doi.org/10.3390/molecules28052181
APA StyleZeng, W., Wang, X., Zhou, T., & Zhang, Y. (2023). Crystal Structure, Photophysical Study, Hirshfeld Surface Analysis, and Nonlinear Optical Properties of a New Hydroxyphenylamino Meldrum’s Acid Derivative. Molecules, 28(5), 2181. https://doi.org/10.3390/molecules28052181





