Click-Functionalization of Silanized Carbon Nanotubes: From Inorganic Heterostructures to Biosensing Nanohybrids
Abstract
1. Introduction
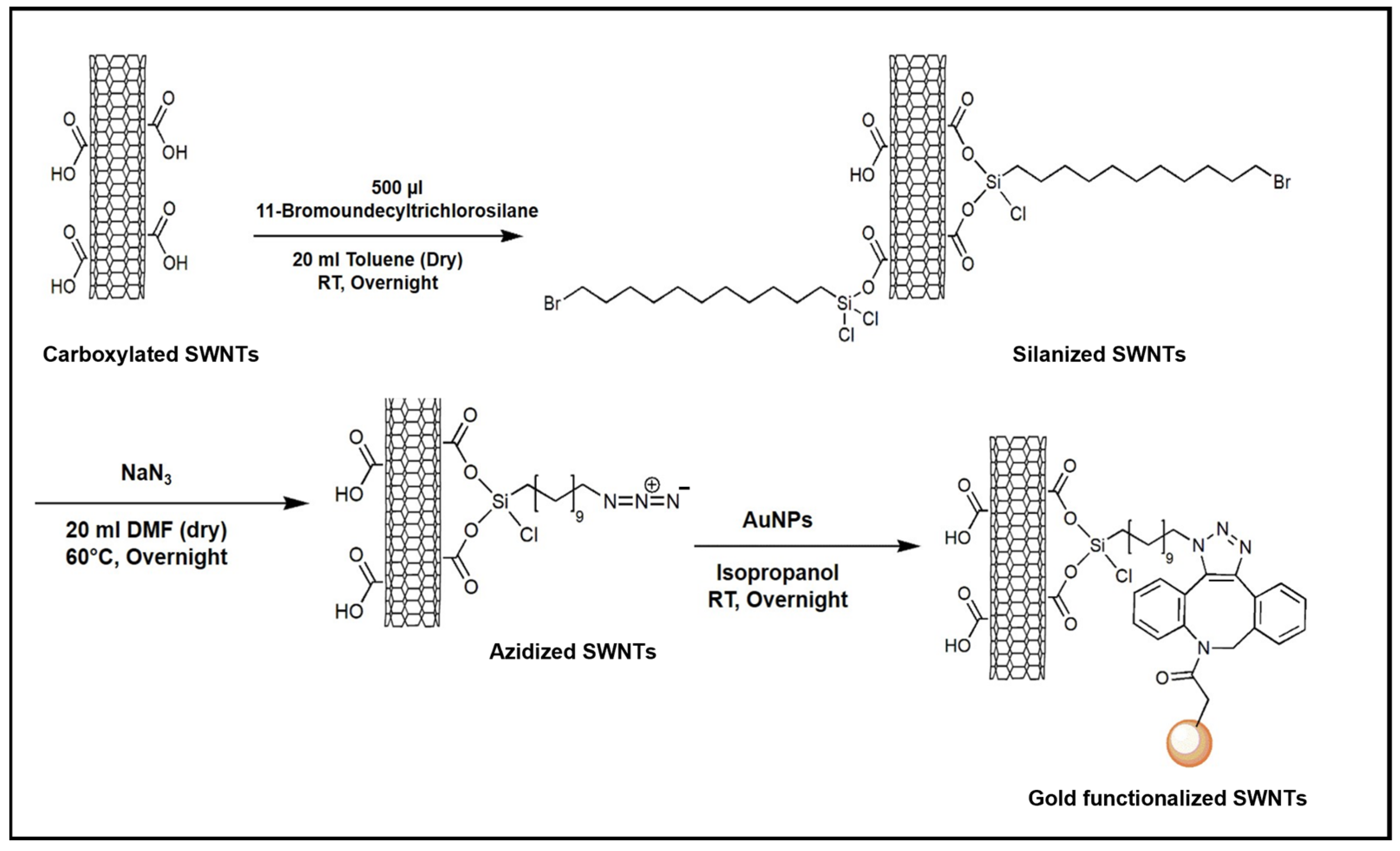
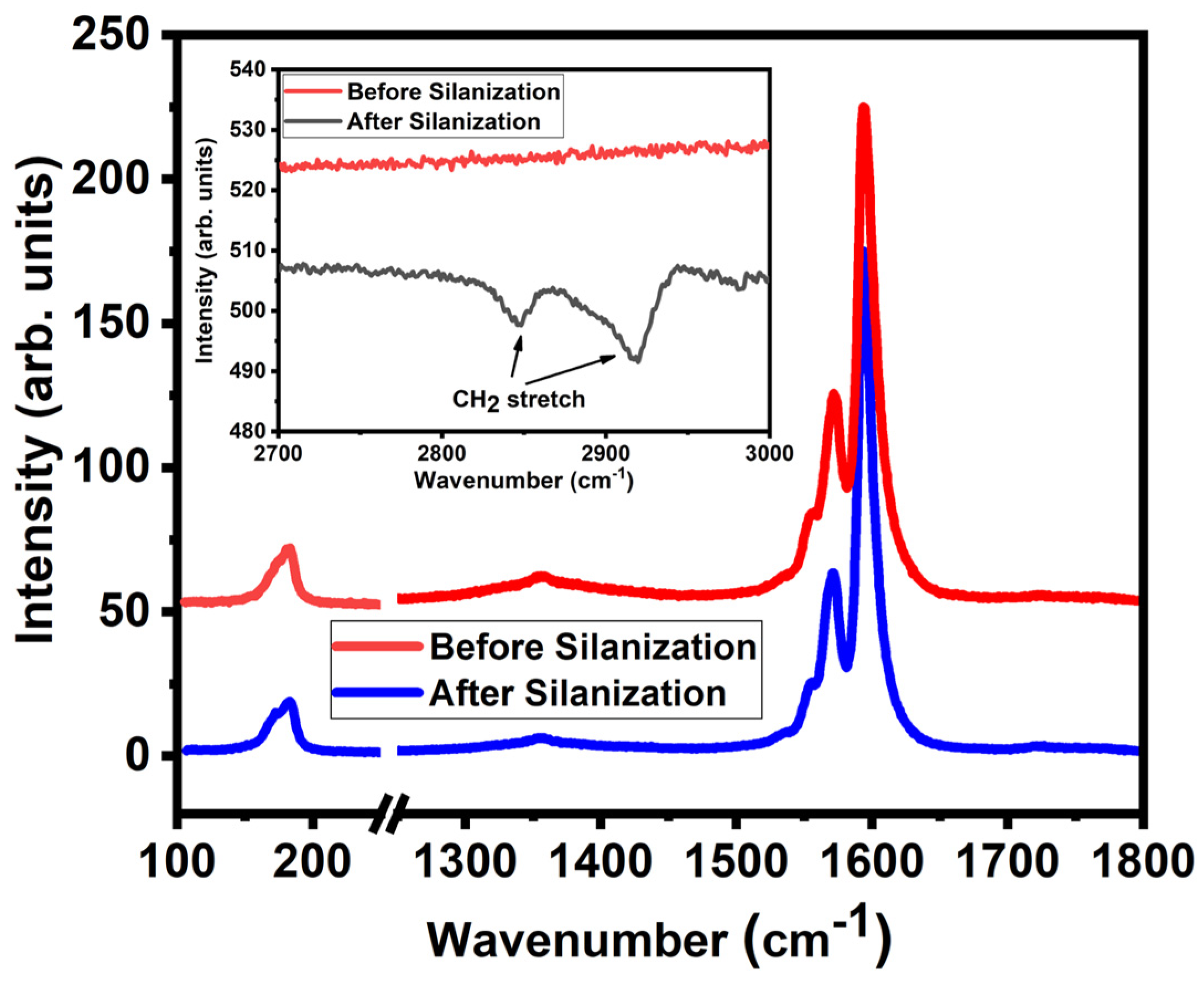
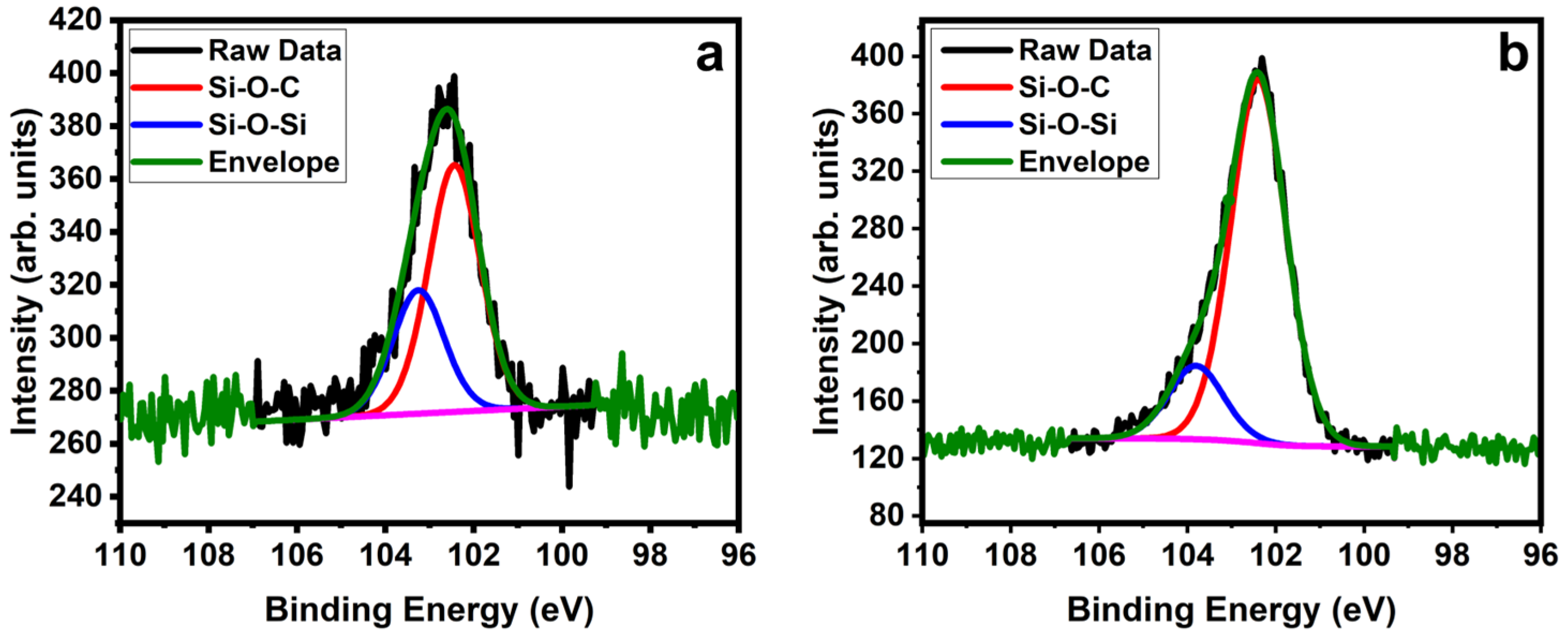
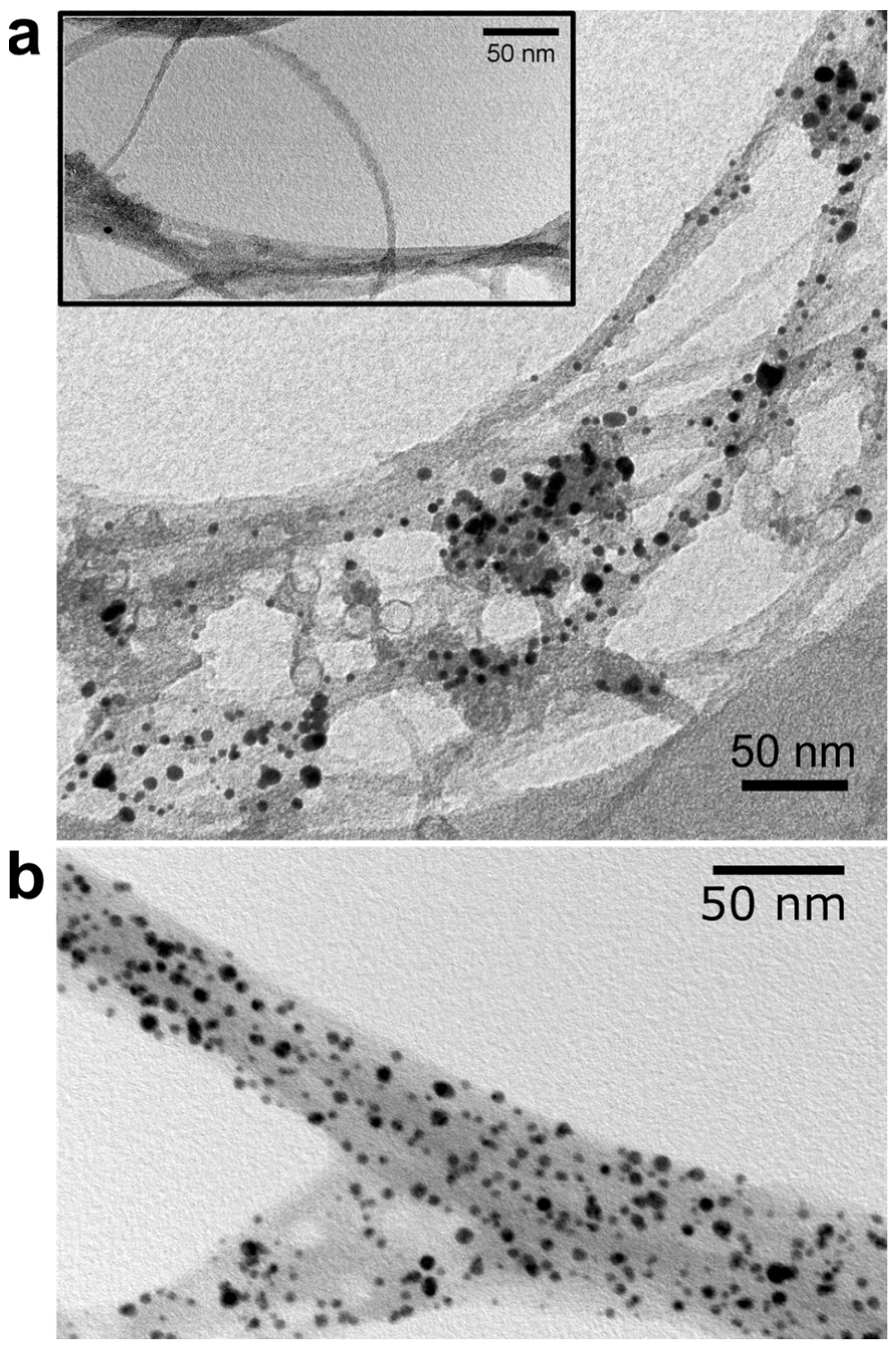
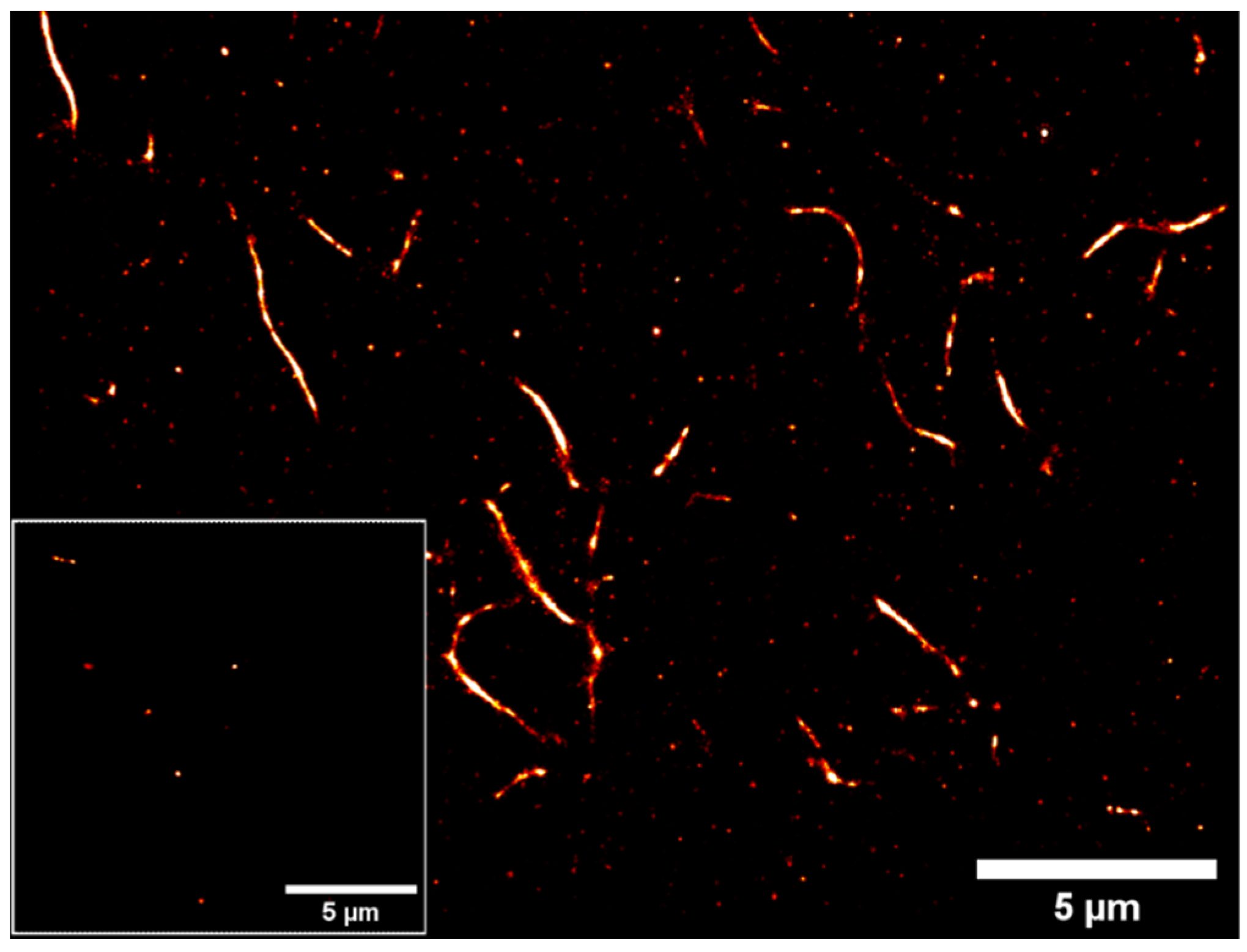
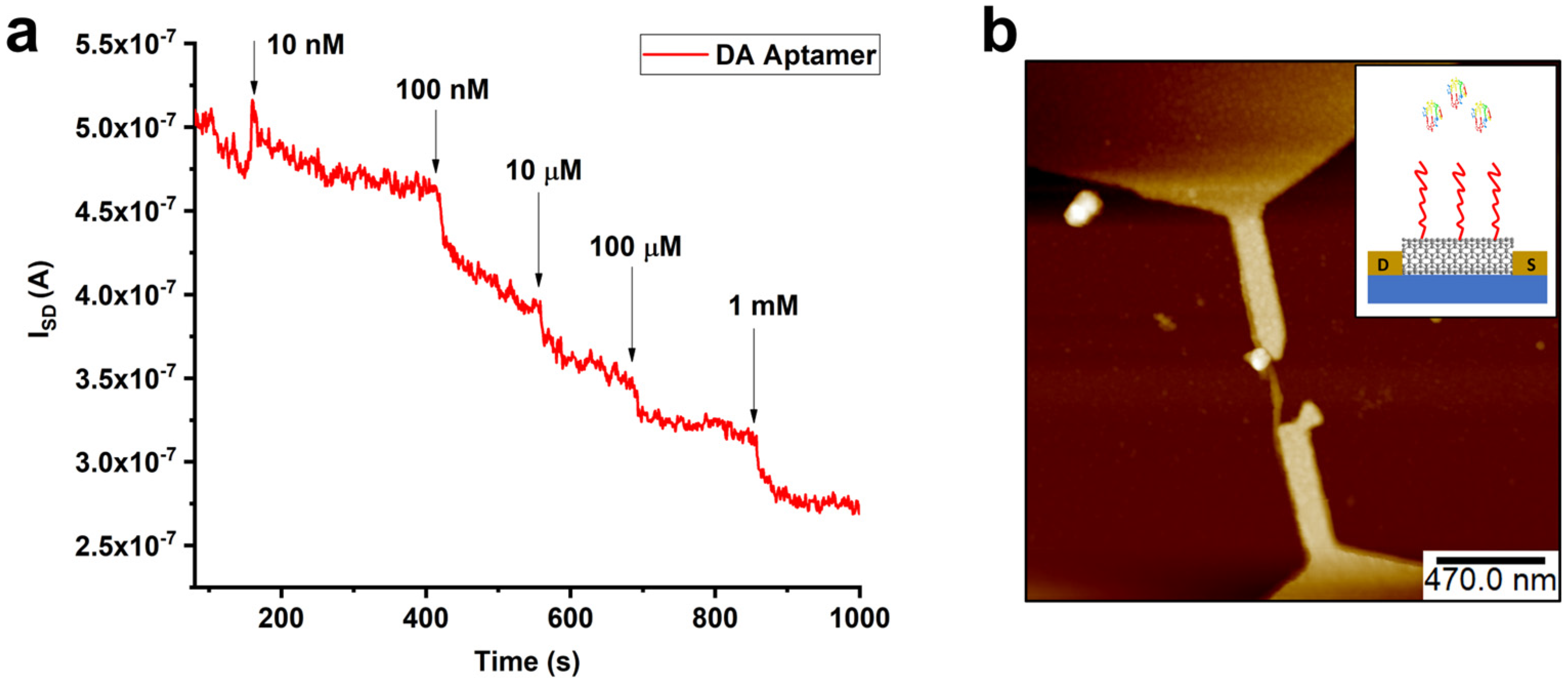
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iijima, S.; Ichihashi, T. Single-Shell Carbon Nanotubes of 1-Nm Diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Avouris, P. Molecular Electronics with Carbon Nanotubes. Acc. Chem. Res. 2002, 35, 1026–1034. [Google Scholar] [CrossRef]
- Collins, P.G.; Avouris, P. Nanotubes for Electronics. Sci. Am. 2000, 283, 62–69. [Google Scholar] [CrossRef]
- Franklin, A.D.; Hersam, M.C.; Wong, H.-S.P. Carbon Nanotube Transistors: Making Electronics from Molecules. Science 2022, 378, 726–732. [Google Scholar] [CrossRef]
- Maruyama, S.; Arnold, M.S.; Krupke, R.; Peng, L.-M. Physics and Applications of Nanotubes. J. Appl. Phys. 2022, 131, 80401. [Google Scholar] [CrossRef]
- Danné, N.; Kim, M.; Godin, A.G.; Kwon, H.; Gao, Z.; Wu, X.; Hartmann, N.F.; Doorn, S.K.; Lounis, B.; Wang, Y.; et al. Ultrashort Carbon Nanotubes That Fluoresce Brightly in the Near-Infrared. ACS Nano 2018, 12, 6059–6065. [Google Scholar] [CrossRef]
- Zhang, A.; Lieber, C.M. Nano-Bioelectronics. Chem. Rev. 2016, 116, 215–257. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Gwyther, R.E.A.; Freeley, M.; Jones, D.; Palma, M. Fabrication and Functionalisation of Nanocarbon-Based Field-Effect Transistor Biosensors. ChemBioChem 2022, 23, e202200282. [Google Scholar] [CrossRef]
- Ajayan, P.M. Nanotubes from Carbon. Chem. Rev. 1999, 99, 1787–1800. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Sabaruddin, F.A.; Harussani, M.M.; Kamarudin, S.H.; Rayung, M.; Asyraf, M.R.M.; Aisyah, H.A.; Norrrahim, M.N.F.; Ilyas, R.A.; Abdullah, N.; et al. Mechanical Performance and Applications of CNTs Reinforced Polymer Composites—A Review. Nanomaterials 2021, 11, 2186. [Google Scholar] [CrossRef]
- Qian, H.; Greenhalgh, E.S.; Shaffer, M.S.P.; Bismarck, A. Carbon Nanotube-Based Hierarchical Composites: A Review. J. Mater. Chem. 2010, 20, 4751–4762. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Biomolecule-Functionalized Carbon Nanotubes: Applications in Nanobioelectronics. ChemPhysChem 2004, 5, 1084–1104. [Google Scholar] [CrossRef]
- Kotov, N.A.; Winter, J.O.; Clements, I.P.; Jan, E.; Timko, B.P.; Campidelli, S.; Pathak, S.; Mazzatenta, A.; Lieber, C.M.; Prato, M.; et al. Nanomaterials for Neural Interfaces. Adv. Mater. 2009, 21, 3970–4004. [Google Scholar] [CrossRef]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef]
- Ménard-Moyon, C.; Kostarelos, K.; Prato, M.; Bianco, A. Functionalized Carbon Nanotubes for Probing and Modulating Molecular Functions. Chem. Biol. 2010, 17, 107–115. [Google Scholar] [CrossRef]
- Bati, A.S.R.; Yu, L.; Batmunkh, M.; Shapter, J.G. Synthesis, Purification, Properties and Characterization of Sorted Single-Walled Carbon Nanotubes. Nanoscale 2018, 10, 22087–22139. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of Carbon Nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Ye, Q.; Xu, X.; Paghi, A.; Bamford, T.; Horrocks, B.R.; Houlton, A.; Barillaro, G.; Dimitrov, S.; Palma, M. Solution-Processable Carbon Nanotube Nanohybrids for Multiplexed Photoresponsive Devices. Adv. Funct. Mater. 2021, 31, 2105719. [Google Scholar] [CrossRef]
- Xu, X.; Clément, P.; Eklöf-Österberg, J.; Kelley-Loughnane, N.; Moth-Poulsen, K.; Chávez, J.L.; Palma, M. Reconfigurable Carbon Nanotube Multiplexed Sensing Devices. Nano Lett. 2018, 18, 4130–4135. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Jin, W.; Hu, Y.; Cui, Y. Carbon Nanotube Field-Effect Transistor-Based Chemical and Biological Sensors. Sensors 2021, 21, 995. [Google Scholar] [CrossRef]
- Tang, J.; Cao, Q.; Tulevski, G.; Jenkins, K.A.; Nela, L.; Farmer, D.B.; Han, S.-J. Flexible CMOS Integrated Circuits Based on Carbon Nanotubes with Sub-10 Ns Stage Delays. Nat. Electron. 2018, 1, 191–196. [Google Scholar] [CrossRef]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon Nanotube—A Review on Synthesis, Properties and Plethora of Applications in the Field of Biomedical Science. Sens. Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Burdanova, M.G.; Kharlamova, M.V.; Kramberger, C.; Nikitin, M.P. Applications of Pristine and Functionalized Carbon Nanotubes, Graphene, and Graphene Nanoribbons in Biomedicine. Nanomaterials 2021, 11, 3020. [Google Scholar] [CrossRef] [PubMed]
- Eder, D. Carbon Nanotube—Inorganic Hybrids. Chem. Rev. 2010, 110, 1348–1385. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Fu, C.; Lu, Q. Advanced Technology for Functionalization of Carbon Nanotubes. Prog. Nat. Sci. 2009, 19, 801–810. [Google Scholar] [CrossRef]
- Hirsch, A.; Vostrowsky, O. Functionalization of Carbon Nanotubes BT—Functional Molecular Nanostructures; Schlüter, A.D., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2005; pp. 193–237. ISBN 978-3-540-31474-5. [Google Scholar]
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized Carbon Nanotubes: Synthesis, Properties and Applications in Water Purification, Drug Delivery, and Material and Biomedical Sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef]
- Clavé, G.; Campidelli, S. Efficient Covalent Functionalisation of Carbon Nanotubes: The Use of “Click Chemistry”. Chem. Sci. 2011, 2, 1887–1896. [Google Scholar] [CrossRef]
- Fort, E.H.; Donovan, P.M.; Scott, L.T. Diels-Alder Reactivity of Polycyclic Aromatic Hydrocarbon Bay Regions: Implications for Metal-Free Growth of Single-Chirality Carbon Nanotubes. J. Am. Chem. Soc. 2009, 131, 16006–16007. [Google Scholar] [CrossRef]
- Song, W.; Wang, Y.; Qu, J.; Lin, Q. Selective Functionalization of a Genetically Encoded Alkene-Containing Protein via “Photoclick Chemistry” in Bacterial Cells. J. Am. Chem. Soc. 2008, 130, 9654–9655. [Google Scholar] [CrossRef]
- Blackman, M.L.; Royzen, M.; Fox, J.M. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels-Alder Reactivity. J. Am. Chem. Soc. 2008, 130, 13518–13519. [Google Scholar] [CrossRef]
- Huisgen, R. Cycloadditions—Definition, Classification, and Characterization. Angew. Chem. Int. Ed. Engl. 1968, 7, 321–328. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-Catalyzed Azide–Alkyne Cycloaddition (CuAAC) and beyond: New Reactivity of Copper(i) Acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide-Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Padwa, A. 1,3-Dipolar Cycloaddition Chemistry. Volumes 1 and 2. Edited by Albert Padwa. John Wiley and Sons. New York, 1984. Volume 1: XIII + 817 Pages. Volume 2: XIII + 704 Pages. ISBN 0–471–08364-X (Set). $295.00 for the Two-Volume Set. J. Heterocycl. Chem. 1986, 23, 1899. [Google Scholar] [CrossRef]
- Han, J.; Gao, C. Functionalization of Carbon Nanotubes and Other Nanocarbons by Azide Chemistry. Nano Micro Lett. 2010, 2, 213–226. [Google Scholar] [CrossRef]
- Tiwari, V.K.; Mishra, B.B.; Mishra, K.B.; Mishra, N.; Singh, A.S.; Chen, X. Cu-Catalyzed Click Reaction in Carbohydrate Chemistry. Chem. Rev. 2016, 116, 3086–3240. [Google Scholar] [CrossRef]
- Campidelli, S. Click Chemistry for Carbon Nanotubes Functionalization. Curr. Org. Chem. 2012, 15, 1151–1159. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Fatou, M.A.G.; Martínez, G.; Marco, C.; Ellis, G.J. Click Functionalization of Carbon Nanotubes and Graphene with Polymers. In Chemical Functionalization of Carbon Nanomaterials; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-1-4822-5396-2. [Google Scholar]
- Huang, J.; Ng, A.L.; Piao, Y.; Chen, C.-F.; Green, A.A.; Sun, C.-F.; Hersam, M.C.; Lee, C.S.; Wang, Y. Covalently Functionalized Double-Walled Carbon Nanotubes Combine High Sensitivity and Selectivity in the Electrical Detection of Small Molecules. J. Am. Chem. Soc. 2013, 135, 2306–2312. [Google Scholar] [CrossRef]
- Qi, H.; Ling, C.; Huang, R.; Qiu, X.; Shangguan, L.; Gao, Q.; Zhang, C. Functionalization of Single-Walled Carbon Nanotubes with Protein by Click Chemistry as Sensing Platform for Sensitized Electrochemical Immunoassay. Electrochim. Acta 2012, 63, 76–82. [Google Scholar] [CrossRef]
- Bouilly, D.; Cabana, J.; Meunier, F.; Desjardins-Carrière, M.; Lapointe, F.; Gagnon, P.; Larouche, F.L.; Adam, E.; Paillet, M.; Martel, R. Wall-Selective Probing of Double-Walled Carbon Nanotubes Using Covalent Functionalization. ACS Nano 2011, 5, 4927–4934. [Google Scholar] [CrossRef]
- Gierlich, J.; Burley, G.A.; Gramlich, P.M.E.; Hammond, D.M.; Carell, T. Click Chemistry as a Reliable Method for the High-Density Postsynthetic Functionalization of Alkyne-Modified DNA. Org. Lett. 2006, 8, 3639–3642. [Google Scholar] [CrossRef]
- Wang, Q.; Chan, T.R.; Hilgraf, R.; Fokin, V.V.; Sharpless, K.B.; Finn, M.G. Bioconjugation by Copper(I)-Catalyzed Azide-Alkyne [3 + 2] Cycloaddition. J. Am. Chem. Soc. 2003, 125, 3192–3193. [Google Scholar] [CrossRef]
- Manova, R.; van beek, T.A.; Zuilhof, H. Surface Functionalization by Strain-Promoted Alkyne-Azide Click Reactions. Angew. Chem. Int. Ed. 2011, 50, 5428–5430. [Google Scholar] [CrossRef]
- Wu, D.; Yang, K.; Zhang, Z.; Feng, Y.; Rao, L.; Chen, X.; Yu, G. Metal-Free Bioorthogonal Click Chemistry in Cancer Theranostics. Chem. Soc. Rev. 2022, 51, 1336–1376. [Google Scholar] [CrossRef]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A Strain-Promoted [3 + 2] Azide-Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z. Development and Applications of the Copper-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) as a Bioorthogonal Reaction. Molecules 2016, 21, 1393. [Google Scholar] [CrossRef]
- Chauhan, N.; Singh, A.; Narang, J.; Dahiya, S.; Pundir, C.S. Development of Amperometric Lysine Biosensors Based on Au Nanoparticles/Multiwalled Carbon Nanotubes/Polymers Modified Au Electrodes. Analyst 2012, 137, 5113–5122. [Google Scholar] [CrossRef]
- Huang, J.; Xing, X.; Zhang, X.; He, X.; Qing, L.; Lian, W.; Zhu, H. A Molecularly Imprinted Electrochemical Sensor Based on Multiwalled Carbon Nanotube-Gold Nanoparticle Composites and Chitosan for the Detection of Tyramine. Food Res. Int. 2011, 44, 276–281. [Google Scholar] [CrossRef]
- Cai, D.; Yu, Y.; Lan, Y.; Dufort, F.J.; Xiong, G.; Paudel, T.; Ren, Z.; Wagner, D.J.; Chiles, T.C. Glucose Sensors Made of Novel Carbon Nanotube-Gold Nanoparticle Composites. BioFactors 2007, 30, 271–277. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, S.; Fang, Y.; Dong, S. Gold Nanoparticle/Carbon Nanotube Hybrids as an Enhanced Material for Sensitive Amperometric Determination of Tryptophan. Electrochim. Acta 2010, 55, 3927–3931. [Google Scholar] [CrossRef]
- Farah, J.; Gravel, E.; Doris, E.; Malloggi, F. Direct Integration of Gold-Carbon Nanotube Hybrids in Continuous-Flow Microfluidic Chips: A Versatile Approach for Nanocatalysis. J. Colloid Interface Sci. 2022, 613, 359–367. [Google Scholar] [CrossRef]
- Ferrier, D.C.; Honeychurch, K.C. Carbon Nanotube (CNT)-Based Biosensors. Biosensors 2021, 11, 486. [Google Scholar] [CrossRef]
- Villalonga, R.; Díez, P.; Eguílaz, M.; Martínez, P.; Pingarrón, J.M. Supramolecular Immobilization of Xanthine Oxidase on Electropolymerized Matrix of Functionalized Hybrid Gold Nanoparticles/Single-Walled Carbon Nanotubes for the Preparation of Electrochemical Biosensors. ACS Appl. Mater. Interfaces 2012, 4, 4312–4319. [Google Scholar] [CrossRef]
- Tîlmaciu, C.-M.; Morris, M.C. Carbon Nanotube Biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef]
- Duc Chinh, V.; Speranza, G.; Migliaresi, C.; Van Chuc, N.; Minh Tan, V.; Phuong, N.T. Synthesis of Gold Nanoparticles Decorated with Multiwalled Carbon Nanotubes (Au-MWCNTs) via Cysteaminium Chloride Functionalization. Sci. Rep. 2019, 9, 5667. [Google Scholar] [CrossRef]
- So, H.-M.; Won, K.; Kim, Y.H.; Kim, B.-K.; Ryu, B.H.; Na, P.S.; Kim, H.; Lee, J.-O. Single-Walled Carbon Nanotube Biosensors Using Aptamers as Molecular Recognition Elements. J. Am. Chem. Soc. 2005, 127, 11906–11907. [Google Scholar] [CrossRef]
- Freeley, M.; Gwyther, R.E.A.; Dafydd Jones, D.; Palma, M. DNA-Directed Assembly of Carbon Nanotube-Protein Hybrids. Biomolecules 2021, 11, 955. [Google Scholar] [CrossRef]
- Xu, X.; Bowen, B.J.; Gwyther, R.E.A.; Freeley, M.; Grigorenko, B.; Nemukhin, A.V.; Eklöf-Österberg, J.; Moth-Poulsen, K.; Jones, D.D.; Palma, M. Tuning Electrostatic Gating of Semiconducting Carbon Nanotubes by Controlling Protein Orientation in Biosensing Devices. Angew. Chem. Int. Ed. 2021, 60, 20184–20189. [Google Scholar] [CrossRef]
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon Nanotubes and Related Nanomaterials: Critical Advances and Challenges for Synthesis toward Mainstream Commercial Applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef]
- Antonucci, A.; Kupis-Rozmysłowicz, J.; Boghossian, A.A. Noncovalent Protein and Peptide Functionalization of Single-Walled Carbon Nanotubes for Biodelivery and Optical Sensing Applications. ACS Appl. Mater. Interfaces 2017, 9, 11321–11331. [Google Scholar] [CrossRef]
- Gwyther, R.E.A.; Nekrasov, N.P.; Emelianov, A.V.; Nasibulin, A.G.; Ramakrishnan, K.; Bobrinetskiy, I.; Jones, D.D. Differential Bio-Optoelectronic Gating of Semiconducting Carbon Nanotubes by Varying the Covalent Attachment Residue of a Green Fluorescent Protein. Adv. Funct. Mater. 2022, 32, 2112374. [Google Scholar] [CrossRef]
- Roy, S.; Das, T.; Ming, Y.; Chen, X.; Yue, C.Y.; Hu, X. Specific Functionalization and Polymer Grafting on Multiwalled Carbon Nanotubes to Fabricate Advanced Nylon 12 Composites. J. Mater. Chem. A 2014, 2, 3961–3970. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ham, S.R.; Kim, J.H.; Yoo, T.H.; Kim, S.R.; Lee, Y.T.; Hwang, D.K.; Angadi, B.; Seo, W.S.; Ju, B.K.; et al. Highly Dispersible Buckled Nanospring Carbon Nanotubes for Polymer Nano Composites. Sci. Rep. 2018, 8, 4851. [Google Scholar] [CrossRef]
- Wang, W.-H.; Huang, B.-C.; Wang, L.-S.; Ye, D.-Q. Oxidative Treatment of Multi-Wall Carbon Nanotubes with Oxygen Dielectric Barrier Discharge Plasma. Surf. Coat. Technol. 2011, 205, 4896–4901. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical Oxidation of Multiwalled Carbon Nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Ma, P.C.; Kim, J.-K.; Tang, B.Z. Functionalization of Carbon Nanotubes Using a Silane Coupling Agent. Carbon 2006, 44, 3232–3238. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Saito, R. Characterizing Graphene, Graphite, and Carbon Nanotubes by Raman Spectroscopy. Annu. Rev. Condens. Matter Phys. 2010, 1, 89–108. [Google Scholar] [CrossRef]
- Jubete, E.; Żelechowska, K.; Loaiza, O.A.; Lamas, P.J.; Ochoteco, E.; Farmer, K.D.; Roberts, K.P.; Biernat, J.F. Derivatization of SWCNTs with Cobalt Phthalocyanine Residues and Applications in Screen Printed Electrodes for Electrochemical Detection of Thiocholine. Electrochim. Acta 2011, 56, 3988–3995. [Google Scholar] [CrossRef]
- Fantini, C.; Usrey, M.L.; Strano, M.S. Investigation of Electronic and Vibrational Properties of Single-Walled Carbon Nanotubes Functionalized with Diazonium Salts. J. Phys. Chem. C 2007, 111, 17941–17946. [Google Scholar] [CrossRef]
- Vennerberg, D.; Rueger, Z.; Kessler, M.R. Effect of Silane Structure on the Properties of Silanized Multiwalled Carbon Nanotube-Epoxy Nanocomposites. Polymer 2014, 55, 1854–1865. [Google Scholar] [CrossRef]
- Velasco-Santos, C.; Martínez-Hernández, A.L.; Lozada-Cassou, M.; Alvarez-Castillo, A.; Castaño, V.M. Chemical Functionalization of Carbon Nanotubes through an Organosilane. Nanotechnology 2002, 13, 495–498. [Google Scholar] [CrossRef]
- Yan, Y.H.; Cui, J.; Chan-Park, M.B.; Wang, X.; Wu, Q.Y. Systematic Studies of Covalent Functionalization of Carbon Nanotubes via Argon Plasma-Assisted UV Grafting. Nanotechnology 2007, 18, 115712. [Google Scholar] [CrossRef]
- Gobbo, P.; Mossman, Z.; Nazemi, A.; Niaux, A.; Biesinger, M.C.; Gillies, E.R.; Workentin, M.S. Versatile Strained Alkyne Modified Water-Soluble AuNPs for Interfacial Strain Promoted Azide-Alkyne Cycloaddition (I-SPAAC). J. Mater. Chem. B 2014, 2, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Dementjev, A.P.; de Graaf, A.; van de Sanden, M.C.M.; Maslakov, K.I.; Naumkin, A.V.; Serov, A.A. X-Ray Photoelectron Spectroscopy Reference Data for Identification of the C3N4 Phase in Carbon–Nitrogen Films. Diam. Relat. Mater. 2000, 9, 1904–1907. [Google Scholar] [CrossRef]
- Gobbo, P.; Novoa, S.; Biesinger, M.C.; Workentin, M.S. Interfacial Strain-Promoted Alkyne-Azide Cycloaddition (I-SPAAC) for the Synthesis of Nanomaterial Hybrids. Chem. Commun. 2013, 49, 3982–3984. [Google Scholar] [CrossRef]
- Islam, M.R.; Bach, L.G.; Nga, T.T.; Lim, K.T. Covalent Ligation of Gold Coated Iron Nanoparticles to the Multi-Walled Carbon Nanotubes Employing Click Chemistry. J. Alloy. Compd. 2013, 561, 201–205. [Google Scholar] [CrossRef]
- Saito, N.; Usui, Y.; Aoki, K.; Narita, N.; Shimizu, M.; Hara, K.; Ogiwara, N.; Nakamura, K.; Ishigaki, N.; Kato, H.; et al. Carbon Nanotubes: Biomaterial Applications. Chem. Soc. Rev. 2009, 38, 1897–1903. [Google Scholar] [CrossRef]
- Nakayama-Ratchford, N.; Bangsaruntip, S.; Sun, X.; Welsher, K.; Dai, H. Noncovalent Functionalization of Carbon Nanotubes by Fluorescein- Polyethylene Glycol: Supramolecular Conjugates with PH-Dependent Absorbance and Fluorescence. J. Am. Chem. Soc. 2007, 129, 2448–2449. [Google Scholar] [CrossRef]
- Berlier, J.E.; Rothe, A.; Buller, G.; Bradford, J.; Gray, D.R.; Filanoski, B.J.; Telford, W.G.; Yue, S.; Liu, J.; Cheung, C.-Y.; et al. Quantitative Comparison of Long-Wavelength Alexa Fluor Dyes to Cy Dyes: Fluorescence of the Dyes and Their Bioconjugates. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2003, 51, 1699–1712. [Google Scholar] [CrossRef]
- Bala, K.; Sharma, D.; Gupta, N. Carbon-Nanotube-Based Materials for Electrochemical Sensing of the Neurotransmitter Dopamine. ChemElectroChem 2019, 6, 274–288. [Google Scholar] [CrossRef]
- Kong, J.; Soh, H.T.; Cassell, A.M.; Quate, C.F.; Dai, H. Synthesis of Individual Single-Walled Carbon Nanotubes on Patterned Silicon Wafers. Nature 1998, 395, 878–881. [Google Scholar] [CrossRef]
- Frielinghaus, R.; Besson, C.; Houben, L.; Saelhoff, A.K.; Schneider, C.M.; Meyer, C. Controlled Covalent Binding of Antiferromagnetic Tetramanganese Complexes to Carbon Nanotubes. RSC Adv. 2015, 5, 84119–84124. [Google Scholar] [CrossRef]
- Schnee, M.; Besson, C.; Frielinghaus, R.; Lurz, C.; Kögerler, P.; Schneider, C.M.; Meyer, C. Quantum Transport in Carbon Nanotubes Covalently Functionalized with Magnetic Molecules. Phys. Status Solidi B Basic Res. 2016, 253, 2424–2427. [Google Scholar] [CrossRef]
- Hou, Y.; Hou, J.; Liu, X. Comparison of Two DNA Aptamers for Dopamine Using Homogeneous Binding Assays. ChemBioChem 2021, 22, 1948–1954. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manoharan, G.; Bösel, P.; Thien, J.; Holtmannspötter, M.; Meingast, L.; Schmidt, M.; Eickmeier, H.; Haase, M.; Maultzsch, J.; Steinhart, M.; et al. Click-Functionalization of Silanized Carbon Nanotubes: From Inorganic Heterostructures to Biosensing Nanohybrids. Molecules 2023, 28, 2161. https://doi.org/10.3390/molecules28052161
Manoharan G, Bösel P, Thien J, Holtmannspötter M, Meingast L, Schmidt M, Eickmeier H, Haase M, Maultzsch J, Steinhart M, et al. Click-Functionalization of Silanized Carbon Nanotubes: From Inorganic Heterostructures to Biosensing Nanohybrids. Molecules. 2023; 28(5):2161. https://doi.org/10.3390/molecules28052161
Chicago/Turabian StyleManoharan, Gririraj, Petra Bösel, Jannis Thien, Michael Holtmannspötter, Laura Meingast, Mercedes Schmidt, Henning Eickmeier, Markus Haase, Janina Maultzsch, Martin Steinhart, and et al. 2023. "Click-Functionalization of Silanized Carbon Nanotubes: From Inorganic Heterostructures to Biosensing Nanohybrids" Molecules 28, no. 5: 2161. https://doi.org/10.3390/molecules28052161
APA StyleManoharan, G., Bösel, P., Thien, J., Holtmannspötter, M., Meingast, L., Schmidt, M., Eickmeier, H., Haase, M., Maultzsch, J., Steinhart, M., Wollschläger, J., Palma, M., & Meyer, C. (2023). Click-Functionalization of Silanized Carbon Nanotubes: From Inorganic Heterostructures to Biosensing Nanohybrids. Molecules, 28(5), 2161. https://doi.org/10.3390/molecules28052161





