Abstract
It is interesting and meaningful to explore fluorescent probes for novel rapid detection methods. In this study, we discovered a natural fluorescence probe, bovine serum albumin (BSA), for the assay of ascorbic acid (AA). Due to clusterization-triggered emission (CTE), BSA has the character of clusteroluminescence. AA shows an obvious fluorescence quenching effect on BSA, and the quenching effect increases with increasing concentrations of AA. After optimization, a method for the rapid detection of AA is established by the AA-caused fluorescence quenching effect. The fluorescence quenching effect reaches saturation after 5 min of incubation time and the fluorescence is stable within more than one hour, suggesting a rapid and stable fluorescence response. Moreover, the proposed assay method shows good selectivity and a wide linear range. To further study the mechanisms of AA-caused fluorescence quenching effect, some thermodynamic parameters are calculated. The main intermolecular force between BSA and AA is electrostatic, presumably leading to the inhibiting CTE process of BSA. This method also shows acceptable reliability for the real vegetable sample assay. In summary, this work will not only provide an assay strategy for AA, but also open an avenue for the application expansion of CTE effect of natural biomacromolecules.
1. Introduction
Ascorbic acid (AA), generally known as vitamin C, is an essential micronutrient that the human body is unable to synthesize for itself and needs to be obtained from the diet [1]. As one of the water-soluble vitamins, it has been extensively identified in fresh fruits, vegetables and human blood. Many pieces of research have demonstrated that AA is of practical significance in the fields of medical treatment, clinical diagnosis and food production [2,3,4,5,6,7]. For example, it can not only effectively prevent and treat scurvy but also enhance the human body’s immune function [4]. Moreover, AA can also be used in beauty skin protection to prevent skin aging by promoting the synthesis of collagen in the body [8]. The content of AA has also acted as a key biological marker for diagnosing health disorders and the quality control of health products [9,10]. Additionally, AA is a type of unstable substance, which can easily combine with oxygen to passivate metal ions. So, the inherent properties of AA can effectively inhibit the browning of fruits and vegetables and change the flavor of food and AA is often used as an antioxidant in the food industry to prevent discoloration or flavor change during processing [11]. Therefore, the content of AA in food and beverages is also a quality indicator of antioxidant capacity [11]. Besides, AA is a nutritional enhancer, and the appropriate uptake can promote dietary intake [1,12]. It is altogether necessary to accurately detect AA in fruits, vegetables and other food products.
At present, several analysis methods are commonly applied for the detection of AA, such as chromatography [13,14], redox titration [15], spectrophotometry [16,17,18] and electrochemistry [19,20,21,22]. These methods for the determination of AA have their own characteristics [23]. Chromatographic methods, such as high performance liquid chromatography, characteristically possess high sensitivity and accuracy for the determination of AA, but expensive equipment and professional operating personnel are required [23,24]. The redox titration method is simple to operate, but the determination of the titration endpoint is difficult to accurately achieve [23,25]. Although the operation of spectrophotometry has a simple detection process, the results are easily disturbed by the color of the sample itself [23,26]. The electrochemical techniques are sensitive, but cannot obtain accurate results in complex samples [23,27]. Hence, these AA assay methods cannot simultaneously satisfy the need for rapid assays, anti-interference capabilities and high sensitivity. Recently, the fluorescence detection method has been extensively applied in the field of analytical chemistry, due to its fast response, visualization, convenience and high sensitivity [28,29,30]. For the fluorescence detection method, the fluorescence probes play a critical role in the effective recognition of small molecular targets [31]. Although some routine fluorescent probes have been widely employed, a complex design and synthesis were required [32,33]. Compared with synthesized fluorescent probes, natural fluorescent probes show enormous advantages of low-cost and easy accessibility [34]. However, natural fluorescent probes have been rarely reported as being able to recognize AA. Therefore, it is of great significance to explore natural fluorescent probes for the fluorescent detection of AA.
At the beginning of this century, it was found that the luminescence behavior in non-conjugated molecules could be attributed to the properties peculiar to the non-conjugated compounds [35,36,37]. Tang’s team defined this type of luminescence as clusteroluminescence and this process as clusterization-triggered emission (CTE) [35,38,39]. Clusteroluminescence is caused by the aggregation of the unconjugated molecules under UV irradiation, while diluted solutions do not emit light [40,41,42]. Using this photoluminescence phenomenon, a number of applications can be developed, including biosensors and the visualization of biological processes [43,44]. This phenomenon has also been found in many natural compounds, such as starch, cellulose and protein [35,45]. Typically, serum albumin, as the most plentiful protein in plasma, plays an indispensable role in the biological system [46]. Bovine serum albumin (BSA) has generally been applied as a commercial globulin in bovine serum. BSA was widely used in various fields including the synthesis of nanomaterials [47,48], encapsulation materials [49] and as the block agent of biosensors [50]. Due to good biocompatibility and non-specific absorption, BSA was selected as a fluorescent marker for analytical detection [51]. Although the clusteroluminescence of BSA had been reported [52], the effect of interaction with other molecules on fluorescence had rarely been researched. BSA composites were mostly employed in the detection of small molecules, but they only performed auxiliary functions, such as stabilizing nanomaterials, attaching fluorescent probes and so on [53]. There were few studies on BSA clusteroluminescence probes for fluorescence detection. Thus, it is of great importance and interest to find natural CTE probes for realizing low-cost and rapid fluorescence detection.
Here, BSA was used as a natural protein clusteroluminescence probe to realize the fast and sensitive detection of AA. Interestingly, AA can impact on the clusteroluminescence properties of BSA. Not only does AA have a quenching effect on the fluorescence of BSA, but also the decrease in fluorescence intensity has a positive linear relationship with the concentration of AA. Accordingly, considering the fluorescence quenching effect of BSA by AA, a quick AA fluorescence assay was established. The proposed AA sensing platform based on quenching fluorescence effect on BSA realized the simple, rapid, sensitive and selective detection of AA. Further, the mechanism of the AA-caused quenching effect of BSA was judged by the Stern–Volmer equation and the Lineweaver–Burk plot, indicating the static quenching effect of AA on BSA. Moreover, the thermodynamic parameters of fluorescence quenching were calculated by the Van't Hoff equation, showing the presence of electrostatic forces between molecules. Finally, the developed method was successfully applied to detect AA in practical samples, and satisfactory results were obtained for the determination of three different vegetables. This work not only provides insights for the interaction of CTE probes with other molecules, but also opens an avenue for a fluorescence sensing platform based on the quenching effect of natural clusteroluminescence biomacromolecules.
2. Results and Discussions
2.1. Effect of AA on Clusteroluminescence of BSA
To investigate the influence of AA on clusteroluminescence of BSA, the fluorescence intensity of BSA was analyzed by fluorescence spectra [34]. Firstly, BSA (0.1 mol L−1) standard solution was taken into the spectrometer, and different excitation wavelengths were set for emission spectrum scanning to determine the fluorescence emission wavelength of BSA. Figure 1A shows that the maximum emission wavelength of BSA was about 340 nm at different excitation wavelengths. Subsequently, the maximum excitation wavelength was 288 nm at the maximum emission wavelength by scanning the excitation spectrum. Therefore, the maximum emission wavelength of BSA was identified at 340 and the maximum excitation wavelength was determined at 288 nm. Figure 1B showed that the maximum absorption wavelength was obtained at 278 nm from the fluorescence excitation and emission spectra of BSA according to ultraviolet spectrum scanning. In addition, the various concentrations of AA (0, 40, 100, 200 and 400 μM L−1) were added to the BSA, and the fluorescence spectrum scanning was performed to analyze the effect of AA on BSA clusteroluminescence. It can be seen from Figure 1C that the position of the fluorescence emission peak of BSA is generally unchanged after the addition of AA, and the fluorescence intensity of BSA gradually declines with the increase in AA concentration. The results suggest that AA has a quenching effect on BSA.
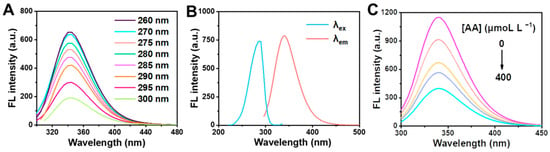
Figure 1.
(A) Fluorescence emission spectra of BSA at different excitation wavelengths. (B) Fluorescence excitation (λem = 340 nm) and emission (λex = 288 nm) spectra of BSA. (C) Fluorescence emission spectra of system containing various concentration of AA (0, 40, 100, 200 and 400 μmol L−1).
2.2. Condition Optimization and Selectivity Assay
To optimize the reaction condition for AA detection, the fluorescence intensity of the AA-BSA system was measured at different pH and reaction times. Based on the factory specification of the BSA, the pH-dependent condition was used in the pH range of 6.5–7.2. A too high or too low level of pH had an influence on the structure and fluorescence of the BSA, affecting the reliability of the study, thus AA was detected at pH 6.6 and 7.2. To further illustrate the optimal level at pH 7.2, the detection of AA was also performed at pH 8.1. Owing to the preparation of the PBS (pH = 6–8.5) comprising a mixture of NaH2PO4 and Na2HPO4 the actual pH could not be accurately controlled, so the used pH value was the actual measured pH value. The pH levels of 6.6, 7.2 and 8.1 were selected based on the above reasons. As shown in Figure 2A, we found that the pH had different influences on the decreasing value of fluorescence intensity (ΔF) after the same reaction time in the AA-BSA system, and ΔF was the largest at pH 7.2. The above-selected pH -already proves that CTE was affected by pH, and the optimal pH (7.2) was applied for the subsequent experiments.
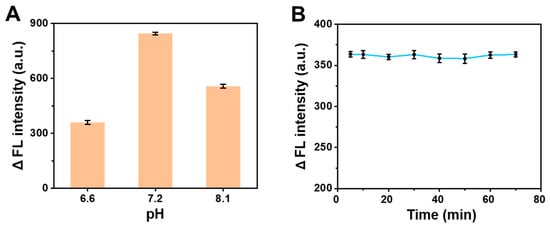
Figure 2.
(A) Optimization of pH on the change in fluorescence intensity of the AA-BSA system. (B) Optimization of reaction time on the change in fluorescence intensity of the AA-BSA system.
The interaction between organic small molecules and macromolecules required a certain time, which can affect their binding sites and binding constants. So, the trend plot of fluorescence intensity at different reaction times was obtained (Figure 2B). The fluorescence intensity of the solution changed slightly and the ΔF remained nearly stable within the range of 5–70 min. To save time, 5 min was selected as the time to determine the interaction between BSA and AA.
To research the selectivity of the AA assay method, some interferents including amino acids (glutamic acid (Glu), phenylalanine (Phe), serine (Ser), aspartic acid (Asp), valine (Val), leucine (Leu), proline (Pro), threonine (Thr)), saccharide (glucose, sucrose, fructose) and glutathione (GSH) were used to replace AA in the same concentration under the same conditions. As shown in Figure 3, only AA could provide significant fluorescence responses signal of the system, while other substances in the experiment had no obviously influence on the fluorescence change of BSA. The results manifest that the BSA fluorescent probe has high selectivity for AA assay. Thus, this proposed method based on the fluorescence quenching of BSA by AA would be potentially feasible for the detection of AA in real samples.
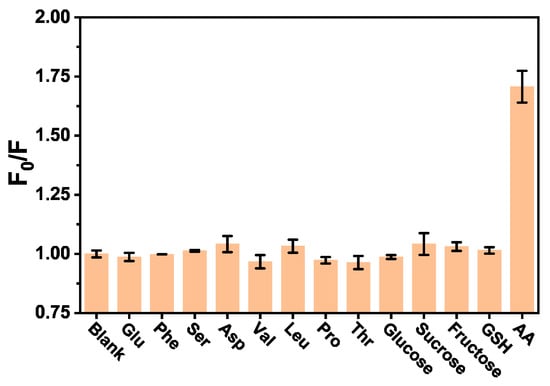
Figure 3.
Selectivity of the fluorescence sensor for AA detection.
2.3. Mechanism Study of Fluorescence Quenching of BSA by AA
Under the optimized pH and reaction time, the influence of the different temperatures on the fluorescence intensity of BSA at different AA concentrations was studied by fluorescence spectra. As illustrated in Figure 4A, the fluorescence intensity of the solution gradually declined with the increase in temperature at the same AA concentration. The results showed that the temperature had an effect on this reaction system, and the fluorescence quenching degree of BSA by AA was enhanced with the increase in temperature.
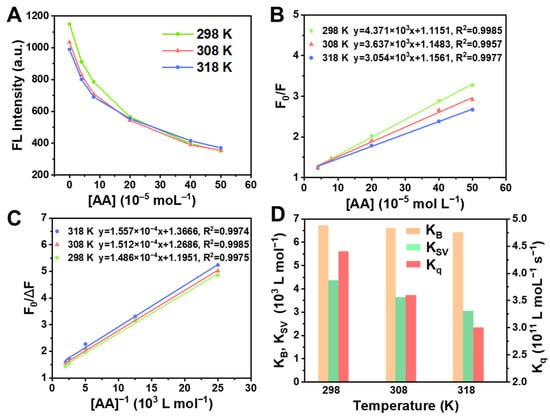
Figure 4.
(A) Effect of temperatures on fluorescence intensity. (B) Stern–Volmer plots of fluorescence quenching of BSA by AA at three different temperatures. (C) Lineweaver–Burk curves of fluorescence quenching of BSA by AA at three different temperatures. (D) KB, KSV and Kq values at three different temperatures.
To research the mechanism of the fluorescence quenching of BSA by AA, the quenching process was further discussed by analyzing the data obtained. Fluorescence quenching, also known as extraction quenching, is a phenomenon whereby the fluorescence intensity and lifetime of fluorescent molecules are decreased for some reason, and the substance that causes the quenching of fluorescent molecules is called quenchers. Dynamic quenching refers to the process of diffusion-related interactions between the quenchers and the excited-state fluorescent molecules. Static quenching is a process in which the quencher and the fluorescent molecules form a non-luminescent complex in the ground state. The processes of the fluorescence quenching, including dynamic quenching and static quenching, which conformed to the Stern–Volmer equation:
where F0 and F are the fluorescence intensity before and after adding quencher; c expresses the quencher concentration (mol L−1); KSV designates Stern–Volmer quenching constants (L moL−1). According to Equation (1), F0/F has a linear relationship with the concentration of the AA. The Stern–Volmer plot at 298, 308 and 318 K is shown in Figure 4B, where F0/F is used as the ordinate and the AA concentration is taken as the abscissa, and the slope of the line is KSV. The curve is linearly fitted, and the slope of the resulting line is quenching constants. Therefore, the values of quenching constants (KSV) at 298, 308 and 318 K were described to be 4.371 × 103, 3.637 × 103 and 3.054 × 103 L moL−1, respectively (Figure 4D). With the increase in temperature, KSV gradually decreased, indicating the static quenching.
F0/F = 1 + KSV c
To further determine the type of quenching between AA and BSA, the bimolecular quenching rate constant (Kq) was calculated according to the equation:
where τ0 is the average lifetime of the fluorescent molecule without the quencher (about 10−8 s). As illustrated in Figure 4D, the Kq values were 4.371 × 1011, 3.637 × 1011 and 3.054 × 1011 L moL−1 s−1 at 298, 308 and 318 K, respectively. According to the judgment method of the fluorescence static quenching and dynamic quenching, the maximum diffusion collision constant (2.0 × 1010 L mol−1 s−1) of the quenching agent on biological macromolecules was the baseline, where a Kq value much larger than 2 represents static quenching [54,55]. The Kq value obtained by the experiment was much larger than 2.0 × 1010 L mol−1 s−1, verifying that the quenching mode between BSA and AA was static, which was consistent with the fluorescence quenching method judged by KSV (Figure 4B).
KSV = Kqτ0
To further research the interaction of AA with BSA, the Lineweaver–Burk plot was analyzed. In static quenching process, the relationship between fluorescence intensity, quencher concentration, and binding constant (KB) can be expressed as the following equation [54]:
1/(F0 − F) = F0−1 + KDF0−1c−1
Equation (3) can be deformed to the following equation:
where KD is the dissociation constant. From Figure 4C, the Lineweaver–Burk plot of AA to BSA fluorescence quenching at 298, 308 and 318 K was obtained. Since the slope of the Lineweaver–Burk equation is KD, those at 298, 308 and 318 K are 1.486 × 10−4, 1.512 × 10−4 and 1.557 × 10−4 L moL−1, respectively. Subsequently, the binding constants (KB) at three different temperatures were calculated to be 6.729 × 103, 6.614 × 103 and 6.423 × 103 L moL−1 (Figure 4D), according to the following equation:
F0/(F0 − F) = 1 + KDc−1
KB = 1/KD
It can be seen that the value of the binding constant is larger, indicating that AA has a strong interaction with BSA.
To further explore the type of acting force between BSA and AA, thermodynamic parameters were calculated (Figure 5), because of the interrelationship between the thermodynamic parameters and the acting forces. In general, the binding forces between small organic molecules and biological macromolecules include hydrogen bonds, van der Waals contacts, electrostatic interaction and hydrophobic forces [56]. The forces between BSA and AA were investigated, using the Van’t Hoff equation:
where K was calculated, and the gas constant was taken as 8.314 J mol−1 K−1. The main types of intermolecular forces were judged by the thermodynamic parameters ΔH and ΔS from the Van’t Hoff equation [57,58,59]. The lnK is plotted against 1/T at three different temperatures by the Van’t Hoff equation, where the slope of the curve is ΔH/R, and the intercept is ΔS/R. In Figure 5, the equation of lnK = 220.45 T−1 + 8.0767 was obtained by plotting the reciprocal of lnK to temperature, the negative value of enthalpy change (ΔH) is 1.832 KJ moL−1 and the positive values of entropy change (ΔS) is 67.15 J moL−1 K−1 according to this equation. The negative value of ΔH indicated that the process was exothermically reactive, and the positive value of the ΔS showed that the chaos of the reaction system was in the direction of the increase. The results indicate that the acting force between AA and BSA was mainly electrostatic force [58].
lnK = −ΔH/RT + ΔS/R
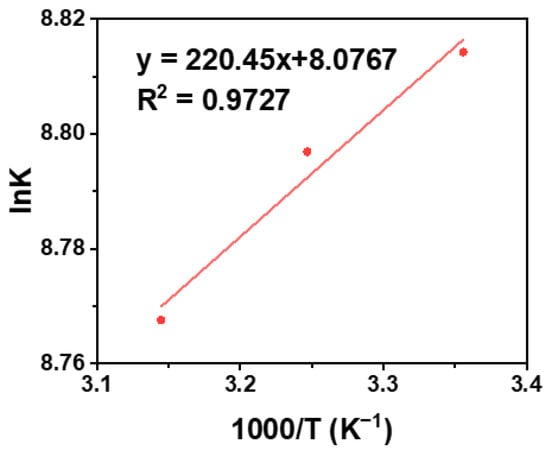
Figure 5.
Fluorescence quenching curve of AA-BSA system.
The tryptophan (Typ) and tyrosine (Tyr) residues in BSA have adsorption peaks at 278 nm. With the conformation of amino acid residue changes, the corresponding microenvironment and absorption spectra of amino acid are also changed, because the microenvironment of amino acid residues in BSA is determined by the conformation of protein molecules [55]. Therefore, the changes in the absorption peaks reflect the conformational changes of BSA after the binding of small molecules [60]. In this work, to further explore the reaction between AA and BSA, the structural changes of BSA by AA were investigated by ultraviolet-visible absorption spectroscopy. As illustrated in the absorption spectra (Figure 6), the characteristic absorption peak of BSA at 278 nm was caused by the heteroaromatic ring π→π* transition of Trp and Tyr on its peptide chain [61]. Interestingly, after the addition of AA, the absorption peak of BSA increased and its maximum absorption wavelength shifted from 278 to 265 nm. This indicated that the spatial structure of BSA changes during binding, exposing the hydrophobic groups of Trp and Tyr residues surrounding the protein molecule, resulting in an enhancement of the hydrophobic interaction between hydrophobic groups, and the blue-shift of the absorption peak [59]. The results show that there was an interaction between the AA and BSA, and BSA might combine with AA to form a complex. It is deduced that the conformation and microenvironment of BSA changed, which affected the clusteroluminescence of BSA [55].
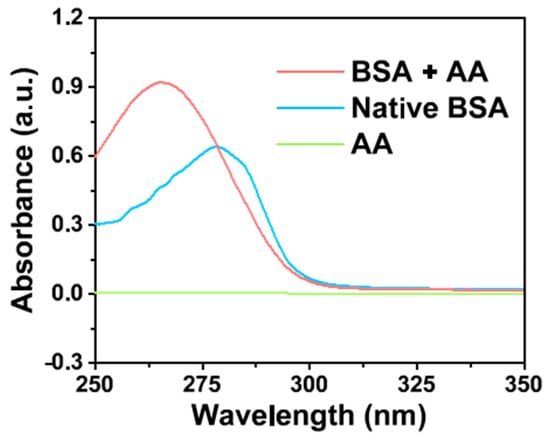
Figure 6.
Ultraviolet-visible absorption spectra of BSA in the presence and absence of AA.
To further study the effect of AA on the conformation of BSA, the surface tension of BSA in the presence or absence of AA was observed by the pendant drop method. As shown in Figure 7A, the volume of the BSA solution drip gradually increased with the increase in AA concentration. Figure 7B indicated that the surface tension of BSA solution with AA was higher than native BSA solution, and the surface tension of BSA gradually increased with the increase in AA concentration. These results suggested that AA decreases the hydrophobicity of BSA [47].
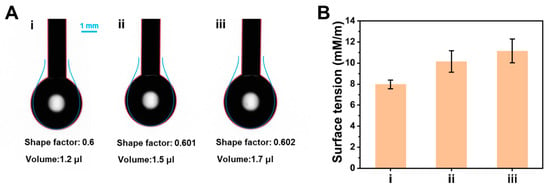
Figure 7.
(A) Dropping photos and (B) corresponding surface tension of different solutions: (i) BSA, (ii) BSA + low concentration of AA (200 μmol L−1), (iii) BSA + high concentration of AA (400 μmol L−1).
Taken together, BSA, a kind of non-conjugated protein, shows a spontaneous CTE process, and fluoresces under UV irradiation. When AA was added to BSA, AA combined BSA by electrostatic force to form an AA-BSA complex. Therefore, the spatial structure and microenvironment of BSA changed, which caused the static quenching of the clusteroluminescence of BSA. On account of the above results (Figure 4, Figure 5, Figure 6 and Figure 7), the quenching mechanism is summarized as follows.
(1) Owing to electrostatic interactions (Figure 4 and Figure 5), AA was embedded in BSA to form an AA-BSA complex, which changes the microenvironment and conformation of BSA. The polarity of the microenvironment around Tyr and Trp residues changed (Figure 6), resulting in a decrease in hydrophobicity (Figure 7) [59,60]. Moreover, AA was closely combined with Trp, which further reduced hydrophobicity [62];
(2) In the meantime, the BSA protein skeleton became loose (Figure 6) due to the embedding of AA into BSA [63];
(3) Because of the decrease in hydrophobicity and the looseness of the protein skeleton, the aggregation degree of BSA non-conjugated molecular clusters was weakened. This caused intense intramolecular motion and weakened the CTE effect, leading to the clusteroluminescence quenching of BSA [64].
2.4. Fluorescent AA Assay Based on BSA
Given the AA-caused quenching effect on the clusteroluminescence of BSA, we developed a simple and fast fluorescent detection for AA. To verify the feasibility, the fluorescent AA assay method was performed for the detection of different concentrations of AA. As illustrated in Figure 8A, with the increase in AA concentration, the corresponding fluorescence intensity of the BSA solution gradually weakened at room temperature (298 K). When the concentration of AA gradually increased from 10 to 500 μmoL L−1, the fluorescence intensity gradually decreased. F0/F shows a good linear relationship with various concentrations of AA within the range from 10 to 500 μmoL L−1 (Figure 8B), and the linear regression equation was y = 0.0045x + 1.0880 (R2 = 0.9986). The limit of detection (LOD) was estimated by the typical formula:
where S/N is the signal-to-noise ratio and is generally set to 3; σ is the standard deviation of blank; and S is the slope of the working curve [65]. Accordingly, the LOD is 6 μmoL L−1 (S/N = 3). Compared with other rapid AA detection methods (Table 1), the fluorescence method developed in this work has a wider linear detection range and a lower detection limit [20,66,67,68,69]. In particular, rapid detection was achieved in a short time.
LOD = S/N × σ/S
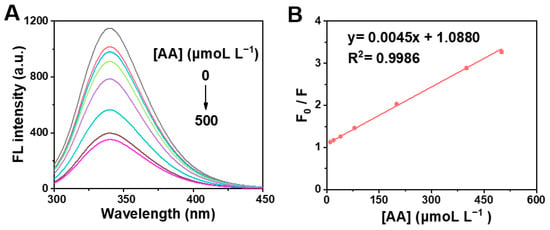
Figure 8.
(A) Fluorescence emission spectra of BSA in the presence of various concentration of AA (0, 10, 20, 40, 80, 200, 400 and 500 μmoL L−1) at 298 K. (B) Corresponding working curve for AA detection.

Table 1.
Comparison of AA sensor detection with other rapid methods.
Owing to the fluorescent quenching of BSA by AA, a fluorescence sensor was constructed to detect AA. BSA as a natural fluorescent probe effectively improved the speed of the fluorescent response, which demonstrated the rapid and low-cost detection of AA. The proposed method based on the AA-induced quenching effect not only has an excellent selectivity, but also displays a wide linear range toward AA. The proposed quenching-based fluorescence biosensor holds great potential to be applied for rapid AA determination.
2.5. AA Analysis in Real Samples
To further evaluate the practical feasibility, we used this method to determine the content of AA in vegetables. As summarized in Table 2, this method attained the fast detection of AA. The usual spiked-recovery experiment was performed to determine the accuracy of the analysis results [70]. The average recovery was in the range of 91.9–95.0% and the relative standard deviation (RSD, n = 5) was within 5.03%, showing the acceptable reproducibility, repeatability of AA detection in the vegetable samples.

Table 2.
Determination of AA in Real Samples.
3. Materials and Methods
3.1. Reagents and Materials
BSA (fraction V, heat shock isolation) was purchased from Shanghai Sangon Biotech Co., Ltd. (Shanghai, China). AA was obtained from Tianjin Kermel Chem reagent Co., Ltd. (Tianjin, China). Phosphate buffer saline (PBS) was prepared in this experiment. Phe was obtained from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Pro was purchased from the Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Glu, Ser, Asp, Val, Leu, Thr, glucose, sucrose, fructose and GSH were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). All amino acids are L-type. All other chemicals were of analytical grade and used without further purification. The ultrapure water (18.2 MΩ cm) was obtained by the Milli-Q purification system (Milipore, Germany).
3.2. Apparatus
The fluorescence assay was performed using a fluorescence spectrophotometer F-2700 (Hitachi, Japan). The corresponding wavelengths were acquired with the excitation and emission slit width of 5 nm for fluorescence detection. The scan speed was set at 300 nm min−1, and the delay time was set to 0 s. The pH of the buffer solution was recorded using PB-10 pH Meter (Beijing Sartorius Scientific Instruments Co., Ltd., Beijing, China) and Electronic Balance Analytique AR124CN (Changzhou Ohaus Instrument Co., Ltd., Changzhou, China). The optimization of the temperature assay was recorded using the Thermostatic Water Bath (Changzhou Yineng Experimental Instrument Co., Ltd., Changzhou, China). The ultraviolet-visible (UV-vis) absorbance signal was obtained by spectrophotometer UV-2600 (Shimazu, Kyoto, Japan), and the silt width of the ultraviolet spectrum was set to 0.2 nm. Surface tensions were measured by a drop shape analyzer DSA25S (Kruss, Germany).
3.3. Fluorescent Spectra and Ultraviolet-Visible Absorption Spectra Measurements
The appropriate amount of AA was added to the BSA solution, and the fluorometric determinations were performed on the F-2700 spectrophotometer at the excitation wavelength of 340 nm.
In the presence and absence of AA, the ultraviolet–visible absorption spectra of BSA was measured on the UV-2600 spectrophotometer over a wavelength range of 250–350 nm at room temperature.
3.4. Surface Tension Measurement
The surface tension of the solution was measured by the pendant drop method. The solution was drawn into the syringe, and the needle tube was fixed on the device for testing. Finally, the surface tension of the solution at the air–water interface and the corresponding photo were obtained by dripping.
3.5. Fluorescent Detection of AA
For typical quantitative analysis of AA, the different concentrations of AA were added to BSA (0.5 g L−1). Then, PBS (pH = 7.2) was used to dilute and fix the volume. The mixture was reacted at three different temperatures for 5 min. Finally, the fluorescence spectrum was scanned and the fluorescence intensity was measured.
The effect of pH and reaction time on the fluorescence quenching of BSA by AA was investigated by the above method, except for changing the pH (pH 6.0–8.5) or reaction time (5–70 min).
To evaluate the specificity of the proposed fluorescence sensor, some common interferents (100 μmol/L), including Glu, Phe, Ser, Asp, Val, Leu, Pro, Thr, glucose, sucrose, fructose and GSH, were used to replace AA in the above fluorescent assay method. The fluorescence signal of BSA after reaction was monitored by fluorescence spectroscopy.
3.6. AA Detection in Real Samples
In order to evaluate the reliability of the proposed fluorescent method based on the AA-caused fluorescent quench effect of BSA, vegetables (Chinese cabbage, Turnip and Zizania latifolia) purchased from a local supermarket (RT-Mart) were employed as the actual samples. Prior to AA detection, different vegetables (100 g) were pretreated by crushing to a homogeneous state, diluting with deionized water and centrifuging at 3000 rpm. After the supernatant was filtered twice by filter membranes (0.45 and 0.22 μm), the filtrate (5 mL) was added into the BSA solution and the mixture was shaken well for testing. Subsequently, the sample solutions were measured by the fluorescence spectrometer (λex = 288 nm, λem = 340 nm) for AA analysis. The experiment of each sample was independently tested for three times.
4. Conclusions
In summary, BSA was used as a natural protein clusteroluminescence probe for detecting AA. The clusteroluminescence properties of BSA were researched, and AA had obvious quenching effect on the clusteroluminescence of BSA. The proposed method of AA detection is very simple and the procedure of fluorescence quenching can be completed rapidly within 5 min. Moreover, the fluorescence intensity of BSA decreases linearly with the concentration of AA in the range of 10–500 μmoL L−1. The LOD of AA achieved 6 μmoL L−1, indicating an acceptable sensitivity. Therefore, the fluorescence AA assay method based on the fluorescence quenching of BSA shows simple operation, fast response, wide linear range, acceptable sensitivity and good selectivity. Furthermore, it was speculated that the BSA-AA binary system was a static quenching process by calculating the Stern–Volmer equation, the Lineweaver–Burk equation and the Van't Hoff equation. Overall, this work exemplifies the promising aspects of the fluorescence quenching effect for AA rapid detection, and pioneers the development of CTE effect of natural biomacromolecules. Looking forwards, this proposed strategy will find widespread uses in the analytical chemistry. The proposed quenching mechanism will provide a reference for studying the interaction between clusteroluminogens and other molecules.
Author Contributions
Conceptualization, L.H.; methodology, J.S. and X.G.; software, J.S.; validation, J.S. and Y.T.; formal analysis, J.S. and Y.T.; investigation, J.S. and X.G.; resources, L.H.; data curation, J.S. and X.G.; writing—original draft preparation, X.G., H.C. and L.H.; writing—review and editing, L.H.; visualization, J.S. and X.G.; supervision, L.H.; project administration, L.H.; funding acquisition, L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (21705087); the Youth Innovation Team Project for Talent Introduction and Cultivation in Universities of Shandong Province (096-1622002); the Research Foundation for Distinguished Scholars of Qingdao Agricultural University (663-1117015); and the Postgraduate Innovation Program of Qingdao Agricultural University (QNYCX21069).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Qi Wu from the College of Chemistry and Pharmaceutical Sciences, Qingdao Agricultural University for providing valuable help. We are grateful to the staff from the Central Laboratory of QAU for providing valuable help.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Fujii, J. Ascorbate is a multifunctional micronutrient whose synthesis is lacking in primates. J. Clin. Biochem. Nutr. 2021, 69, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, N.; Creagan, E.; Witzig, T.; Levine, M. Ascorbic Acid in Cancer Treatment: Let the Phoenix Fly. Cancer Cell. 2018, 34, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Janani, N.; Zare, E.N.; Salimi, F.; Makvandi, P. Antibacterial tragacanth gum-based nanocomposite films carrying ascorbic acid antioxidant for bioactive food packaging. Carbohydr. Polym. 2020, 247, 116678. [Google Scholar] [CrossRef] [PubMed]
- Kressin, C.; Pandya, K.; Woodward, B.M.; Donaldson, C.; Flannery, A.H. Ascorbic acid in the acute care setting. JPEN J. Parenter. Enter. Nutr. 2021, 45, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.R.; Vitorino, C.; Fortuna, A. From antioxidant to neuromodulator: The role of ascorbate in the management of major depression disorder. Biochem. Pharmacol. 2022, 206, 115300. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Chanphai, P.; Tajmir-Riahi, H.A. Conjugation of vitamin C with serum proteins: A potential application for vitamin delivery. Int. J. Biol. Macromol. 2019, 137, 966–972. [Google Scholar] [CrossRef]
- Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef]
- Lykkesfeldt, J. Ascorbate and dehydroascorbic acid as biomarkers of oxidative stress: Validity of clinical data depends on vacutainer system used. Nutr. Res. 2012, 32, 66–69. [Google Scholar] [CrossRef]
- Pandey, I.; Jha, S.S. Molecularly imprinted polyaniline-ferrocene-sulfonic acid-Carbon dots modified pencil graphite electrodes for chiral selective sensing of D-Ascorbic acid and L-Ascorbic acid: A clinical biomarker for preeclampsia. Electrochim. Acta 2015, 182, 917–928. [Google Scholar] [CrossRef]
- Barberis, A.; Spissu, Y.; Fadda, A.; Azara, E.; Bazzu, G.; Marceddu, S.; Angioni, A.; Sanna, D.; Schirra, M.; Serra, P.A. Simultaneous amperometric detection of ascorbic acid and antioxidant capacity in orange, blueberry and kiwi juice, by a telemetric system coupled with a fullerene- or nanotubes-modified ascorbate subtractive biosensor. Biosens. Bioelectron. 2015, 67, 214–223. [Google Scholar] [CrossRef]
- Bowman, G.L. Ascorbic acid, cognitive function, and Alzheimer’s disease: A current review and future direction. BioFactors 2022, 38, 114–122. [Google Scholar] [CrossRef]
- Wimalasiri, P.; Wills, R.B.H. Simultaneous analysis of ascorbic acid and dehydroascorbic acid in fruit and vegetables by high-performance liquid chromatography. J. Chromatogr. 1983, 256, 368–371. [Google Scholar] [CrossRef]
- Oliveira, E.J.; Watson, D.G. Chromatographic techniques for the determination of putative dietary anticancer compounds in biological fluids. J. Chromatogr. B 2001, 764, 3–25. [Google Scholar] [CrossRef]
- Lenghor, N. Sequential injection redox or acid–base titration for determination of ascorbic acid or acetic acid. Talanta 2002, 58, 1139–1144. [Google Scholar] [CrossRef]
- Han, L.; Liu, P.; Zhang, H.; Li, F.; Liu, A. Phage capsid protein-directed MnO2 nanosheets with peroxidase-like activity for spectrometric biosensing and evaluation of antioxidant behaviour. Chem. Commun. 2017, 53, 5216–5219. [Google Scholar] [CrossRef]
- Nguyen, T.H.A.; Nguyen, V.T.M.; Le, V.T.; Doan, V.D.; Chau, T.P.; Nguyen, V.C.; Nguyen, A.T.; Vasseghian, Y. A novel gold nanoparticle-based colorimetric assay for highly sensitive detection of ascorbic acid. Mater. Lett. 2022, 309, 131307. [Google Scholar] [CrossRef]
- Han, Y.R.; Luo, L.P.; Zhang, L.; Kang, Y.; Sun, H.; Dan, J.; Sun, J.; Zhang, W.T.; Yue, T.L.; Wang, J.L. Oxidase-like Fe–Mn bimetallic nanozymes for colorimetric detection of ascorbic acid in kiwi fruit. LWT-Food Sci. Technol. 2022, 154, 112821. [Google Scholar] [CrossRef]
- Siraprapa, P.; Kornkamon, M.; Gasidit, P.; Oratai, J. MWCNT/Ti-doped ZnO nanocomposite as electro-chemical sensor for detecting glutamate and ascorbic acid. Int. J. Appl. Ceram. Technol. 2022, 19, 467–479. [Google Scholar]
- Fernandes, D.S.; Carmo, D.R. Silsesquioxane Modified with PAMAM Dendrimer and a Bimetallic Complex for Electrochemical Detection of Ascorbic Acid. Electroanalysis 2021, 33, 365–374. [Google Scholar] [CrossRef]
- Murugan, N.; Jerome, R.; Murugan, P.; Sundaramurthy, A.; Sundramoorthy, A.K. 2D-titanium carbide (MXene) based selective electrochemical sensor for simultaneous detection of ascorbic acid, dopamine and uric acid. J. Mater. Sci. Technol. 2021, 72, 122–131. [Google Scholar] [CrossRef]
- Virginia, M.V.; Benjamín, N.V.; Alejandro, T.; Griselda, A.E.; Gustavo, A.R.; Pablo, R.D. Ultrasensitive multiwall carbon nanotube-mesoporous MCM-41 hybrid-based platform for the electrochemical detection of ascorbic acid. Analyst 2022, 147, 2130–2140. [Google Scholar]
- Malik, M.; Narwal, V.; Pundir, V. Ascorbic acid biosensing methods: A review. Process Biochem. 2022, 118, 11–23. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Zhang, Y.; Chen, Y.; Yang, X.; Duan, L.; Dharmarajan, R.; Wang, X.; Li, L. Simultaneous determination of 20 disperse dyes in foodstuffs by ultra high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2019, 300, 125183. [Google Scholar] [CrossRef] [PubMed]
- Buriánek, J.D.; Kvicala, J.; Sekerova, L.; Müller, B.H.; Francke, R.; Bystron, T. Determination of Diaryliodonium Species by Reverse Iodometric Titration with Ascorbic Acid. Electroanalysis 2023. [Google Scholar] [CrossRef]
- Songtham, R.; Pattraporn, S.; Chutima, P.; Amornrat, K.; Thawatchai, T. Poly(methacrylic acid)-Stabilized Silver Nanoclusters as Colorimetric Sensors for the Rapid and Sensitive Detection of Ascorbic Acid. ChemistrySelect 2021, 6, 1248–1254. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Cornelia, F. Electrochemical methods for ascorbic acid determination. Electrochim. Acta 2014, 121, 443–460. [Google Scholar] [CrossRef]
- Wu, A.; Ding, H.; Zhang, W.; Rao, H.; Wang, L.; Chen, Y.; Lu, C.; Wang, X. A colorimetric and fluorescence turn-on probe for the detection of ascorbic acid in living cells and beverages. Food Chem. 2021, 363, 130325. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhang, Z.T.; Sheng, M.S.; Xiao, H.Y.; Zhang, F.; Meng, J.; Lai, M.M.; Wu, X.M.; Li, Y. Luminescent carbon nanoclusters for sensitive detection of ascorbic acid and fluorescent printing. ACS Appl. Nano Mater. 2022, 5, 5234–5243. [Google Scholar] [CrossRef]
- Pan, W.; Han, L.; Cao, X.; Shen, S.; Pang, X.; Zhu, Y. Dual-response near-infrared fluorescent probe for detecting cyanide and mitochondrial viscosity and its application in bioimaging. Food Chem. 2023, 407, 135163. [Google Scholar] [CrossRef]
- Mehta, V.N.; Kailasa, S.K.; Wu, H.F. Surface modified quantum dots as fluorescent probes for biomolecule recognition. J. Nanosci. Nanotechnol. 2014, 14, 447–459. [Google Scholar] [CrossRef]
- Huang, L.Y.; Yang, Z.T.; Zhou, Z.L.; Li, Y.Q.; Tang, S.P.; Xiao, W.P.; Hu, M.; Peng, C.; Chen, Y.X.; Gu, B.; et al. A dual colorimetric and near-infrared fluorescent turn-on probe for Hg2+ detection and its applications. Dye. Pigment. 2019, 163, 118–125. [Google Scholar] [CrossRef]
- Liu, R.; Yang, R.; Qu, C.J.; Mao, H.C.; Hu, Y.; Li, J.J.; Qu, L.B. Synthesis of glycine-functionalized graphene quantum dots as highly sensitive and selective fluorescent sensor of ascorbic acid in human serum. Sens. Actuators B Chem. 2017, 241, 644–651. [Google Scholar] [CrossRef]
- Chen, Y.; Li, K.; Zhang, S.; Xu, P.; Song, B. Turn-on fluorescence probe for BSA detection and selective cell imaging. Dye. Pigment. 2022, 202, 110267. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; McGonigal, P.R.; Ye, R.; Liu, S.; Lam, J.W.Y.; Kwok, R.T.K.; Yuan, W.Z.; Xie, J.; Rogach, A.L.; et al. Clusterization-triggered emission: Uncommon luminescence from common materials. Mater. Today 2020, 32, 275–292. [Google Scholar] [CrossRef]
- Zhang, J.; Alam, P.; Zhang, S.; Shen, H.; Hu, L.; Sung, H.H.Y.; Williams, I.D.; Sun, J.; Lam, J.W.Y.; Zhang, H.; et al. Secondary through-space interactions facilitated single-molecule white-light emission from clusteroluminogens. Nat. Commun. 2022, 13, 3492. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, Z.; Chu, B.; Zhang, Z.; Xie, Y.; Wang, L.; Sun, J.Z.; Zhang, H.; Zhang, X.H.; Tang, B.Z. Manipulation of clusteroluminescence in carbonyl-based aliphatic polymers. Aggregate 2022, 3, e278. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Klajnert-Maculewicz, B.; Johnson, K.A.M.; Brinkman, H.F.; Janaszewska, A.; Hedstrand, D.M. Non-traditional intrinsic luminescence: Inexplicable blue fluorescence observed for dendrimers, macromolecules and small molecular structures lacking traditional/conventional luminophores. Prog. Polym. Sci. 2019, 90, 35–117. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Du, L.; Ma, C.; Leung, N.L.C.; Niu, Y.; Qin, A.; Sun, J.; Peng, Q.; Sung, H.H.Y.; et al. Drawing a clear mechanistic picture for the aggregation-induced emission process. Mater. Chem. Front. 2019, 3, 1143–1150. [Google Scholar] [CrossRef]
- Kang, C.; Tao, S.; Yang, F.; Yang, B. Aggregation and luminescence in carbonized polymer dots. Aggregate 2022, 3, e169. [Google Scholar] [CrossRef]
- Kang, M.; Zhang, Z.; Song, N.; Li, M.; Sun, P.; Chen, X.; Wang, D.; Tang, B.Z. Aggregation-enhanced theranostics: AIE sparkles in biomedical field. Aggregate 2020, 1, 80–106. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Z.; Tavakoli, J.; Shan, G.; Zhang, J.; Peng, C.; Xiong, Y.; Zhang, X.; Cheung, T.S.; Tang, Y.; et al. Revisiting an ancient inorganic aggregation-induced emission system: An enlightenment to clusteroluminescence. Aggregate 2021, 2, e36. [Google Scholar] [CrossRef]
- Liang, J.J.; Li, Y.; Yuan, Y.; Li, S.H.; Zhu, X.D.; Barlow, S.; Fung, M.K.; Jiang, Z.Q.; Marder, S.R.; Liao, L.-S. A blue thermally activated delayed fluorescence emitter developed by appending a fluorene moiety to a carbazole donor with meta-linkage for high-efficiency OLEDs. Mater. Chem. Front. 2018, 2, 917–922. [Google Scholar] [CrossRef]
- Zeng, J.; Guo, J.; Liu, H.; Lam, J.W.Y.; Zhao, Z.; Chen, S.; Tang, B.Z. Aggregation-Induced Delayed Fluorescence Luminogens for Efficient Organic Light-Emitting Diodes. Chem. Asian J. 2019, 14, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Yuan, C.; Bai, Y.; He, C.; Long, J.; Tan, H.; Wang, H. Effects of Through-Bond and Through-Space Conjugations on the Photoluminescence of Small Aromatic and Aliphatic Aldimines. Molecules 2022, 27, 8046. [Google Scholar] [CrossRef]
- Song, F.; Xue, Y.; Wang, X.; Wang, J.; Xiong, X.; Peng, X. Ratiometric fluorescent probe based on novel red-emission BODIPY for determination of bovine serum albumin. Chem. Res. Chin. Univ. 2014, 30, 738–742. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, X.; Chen, D.; Bian, X.; Liu, W.; Han, L. Denatured proteins show new vitality: Green synthesis of germanium oxide hollow microspheres with versatile functions by denaturing proteins around bubbles. Aggregate 2023, 4, e204. [Google Scholar] [CrossRef]
- Liu, K.W.; Han, L.; Zhuang, J.Y.; Yang, D.P. Protein-directed gold nanoparticles with excellent catalytic activity for 4-nitrophenol reduction. Mater. Sci. Eng. C 2017, 78, 429–434. [Google Scholar] [CrossRef]
- Gan, S.; Zhou, J.; Smith, T.A.; Su, H.; Luo, W.; Hong, Y.; Zhao, Z.; Tang, B.Z. New AIEgens with delayed fluorescence for fluorescence imaging and fluorescence lifetime imaging of living cells. Mater. Chem. Front. 2017, 1, 2554–2558. [Google Scholar] [CrossRef]
- Han, L.; Wang, D.; Yan, L.; Petrenko, V.A.; Liu, A.H. Specific phages-based electrochemical impedimetric immunosensors for label-free and ultrasensitive detection of dual prostate-specific antigen. Sens. Actuators B Chem. 2019, 297, 126727. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, T.W.; Xu, F.; Li, W.; Tao, S.; Chu, L.Q.; He, Y.; Li, Y.; Iyer, S.S.; Yu, P. Fluorescent Neomannosyl Bovine Serum Albumin as Efficient Probe for Mannose Receptor Imaging and MCF-7 Cancer Cell Targeting. ACS Appl. Nano Mater. 2018, 1, 1058–1065. [Google Scholar] [CrossRef]
- Wang, Q.; Dou, X.; Chen, X.; Zhao, Z.; Chen, X.; Wang, S.; Wang, Y.; Sui, K.; Tan, Y.; Gong, Y.; et al. Reevaluating Protein Photoluminescence: Remarkable Visible Luminescence upon Concentration and Insight into the Emission Mechanism. Angew. Chem. 2019, 58, 12667–12673. [Google Scholar] [CrossRef]
- Niu, Y.; Ding, T.; Liu, J.; Zhang, G.; Tong, L.; Cheng, X.; Yang, Y.; Chen, Z.; Tang, B. Fluorescence switch of gold nanoclusters stabilized with bovine serum albumin for efficient and sensitive detection of cysteine and copper ion in mice with Alzheimer’s disease. Talanta 2021, 223, 121745. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Q.H.; Li, C.R.; Wang, Z.; Ma, J.J.; Zang, X.H.; Qin, N.X. Interaction of tetrandrine with human serum albumin: A fluorescence quenching study. Anal. Sci. 2007, 23, 429–433. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.G.; Zen, B.R.; Kang, Q.L.; Dai, L.Z. Study on the binding of chloroamphenicol with bovine serum albumin by fluorescence and UV–vis spectroscopy. Spectrochim. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 105, 74–79. [Google Scholar] [CrossRef]
- Wang, N.; Ye, L.; Yan, F.F.; Xu, R. Spectroscopic studies on the interaction of azelnidipine with bovine serum albumin. Int. J. Pharm. 2008, 351, 55–60. [Google Scholar] [CrossRef]
- Qi, C.L.; Fu, C.X. Interaction of folic acid and amino acid by fluorescence spectrometry. Chem. Res. 2014, 25, 148–151. [Google Scholar]
- Ross, P.; Subramanian, S. Thermodynamics of Protein Association Reactions: Forces Contributing to Stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef]
- Zhao, P.; Kong, J.; Li, L. Elucidation of Binding Mechanism of Dibutyl Phthalate on Bovine Serum Albumin by Spectroscopic Analysis and Molecular Docking Method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 230, 118044. [Google Scholar]
- Li, G.; Huang, J.; Chen, T.; Wang, X.; Zhang, H.; Chen, Q. Insight into the Interaction between Chitosan and Bovine Serum Albumin. Carbohydr. Polym. 2017, 176, 75–82. [Google Scholar] [CrossRef]
- Wang, Z.; Li, D.J.; Jin, J. Study on the interaction of puerarin with lysozyme by spectroscopic methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 70, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Liang, Q.; Li, Y.; Liu, X.; Zhang, D.; Li, X. Study of the Binding Mechanism between Hydroxytyrosol and Bo-vine Serum Albumin Using Multispectral and Molecular Docking. Food Hydrocoll. 2022, 122, 107072. [Google Scholar] [CrossRef]
- Hussain, I.; Fatima, S.; Ahmed, S.; Tabish, M. Biophysical and Molecular Modelling Analysis of the Binding of β-Resorcylic Acid with Bovine Serum Albumin. Food Hydrocoll. 2023, 135, 108175. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, L.; Zhang, K.; Liu, J.; Li, X.; Wang, H.; Wang, Z.; Sung, H.H.Y.; Williams, I.D.; Zeng, Z.; et al. How to Manipulate Through-Space Conjugation and Clusteroluminescence of Simple AIEgens with Isolated Phenyl Rings. J. Am. Chem. Soc. 2021, 143, 9565–9574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Han, L.; Li, F. A universal one-pot assay strategy based on bio-inorganic cascade catalysts for different analytes by changing pH-dependent activity of enzymes on enzyme mimics. Sens. Actuators B Chem. 2019, 286, 460–467. [Google Scholar] [CrossRef]
- Manivel, P.; Dhakshnamoorthy, M.; Balamurugan, A.; Ponpandian, N.; Mangalaraj, D.; Viswanathan, D. Conducting polyaniline-graphene oxide fibrous nanocomposites: Preparation, characterization and simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. RSC Adv. 2013, 3, 14428–14437. [Google Scholar] [CrossRef]
- Cai, S.F.; Xiao, W.; Duan, H.H.; Liang, X.X.; Wang, C.; Yang, R.; Li, Y.D. Single-layer Rh nanosheets with ultrahigh peroxidase-like activity for colorimetric biosensing. Nano Res. 2018, 11, 6304–6315. [Google Scholar] [CrossRef]
- He, J.; He, D.X.; Yang, L.; Wu, G.L.; Tian, J.M.; Liu, Y.; Wang, W.G. Preparation of urchin-like Pd-Pt-Ir nanozymes and their application for the detection of ascorbic acid and hydrogen peroxide. Mater. Lett. 2022, 314, 131851. [Google Scholar] [CrossRef]
- Zhao, T.; Zhu, C.; Xu, S.; Wu, X.; Zhang, X.; Zheng, Y.; Zhang, K. Fluorescent color analysis of ascorbic acid by ratiometric fluorescent paper utilizing hybrid carbon dots-silica coated quantum dots. Dye. Pigment. 2020, 186, 108995. [Google Scholar] [CrossRef]
- Fan, P.F.; Liu, C.; Hu, C.C.; Li, F.F.; Xi, L.N.; Yang, S.Y.; Xiao, F.B. Green and facile synthesis of iron-doped biomass carbon dots as a dual-signal colorimetric and fluorometric probe for the detection of ascorbic acid. New J. Chem. 2022, 46, 2526–2533. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).