Antitumor Effect of Chalcone Derivatives against Human Prostate (LNCaP and PC-3), Cervix HPV-Positive (HeLa) and Lymphocyte (Jurkat) Cell Lines and Their Effect on Macrophage Functions
Abstract
1. Introduction
2. Results
2.1. Effect of Chalcones on the Metabolic Activity of Tumor Cell Lines
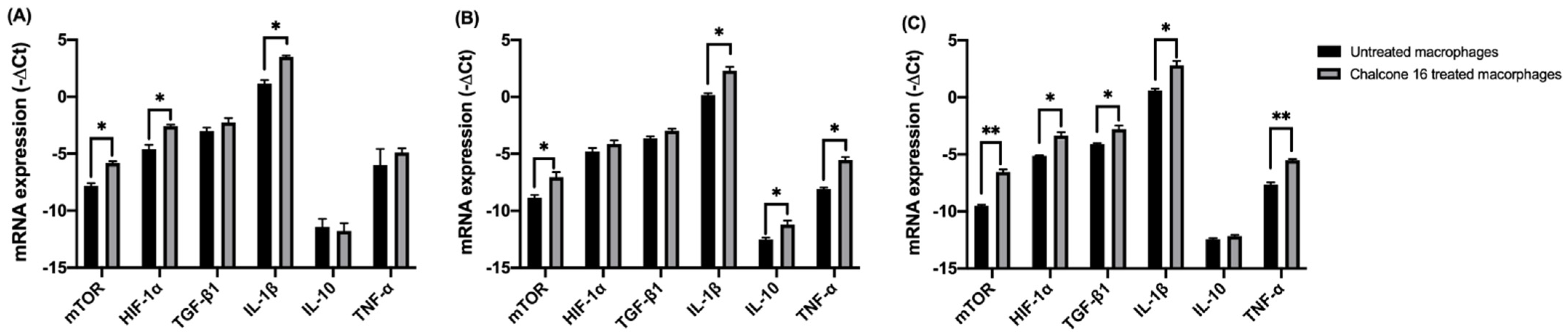
2.2. Effect of Chalcone 16 on Macrophage Functions
2.2.1. Effect of Chalcone 16 on THP-1 Macrophage HIF-1α, mTORC1 and Cytokine Expression
2.2.2. Effect of Chalcone 16 on NO Production by RAW264.7 Macrophages
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Chalcone Derivatives 1–18
4.3. Cell Lines and Cell Culture
4.4. Cytotoxic Assay (MTT) for Adherent Tumor Cell Lines
4.5. Cytotoxic Assay (MTT) for Non-Adherent Tumor Cell Lines
4.6. THP-1 Macrophage-Phenotype Differentiation
4.7. Assay for Quantification of mRNA Expression
4.8. Nitric Oxide Production Assay
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yan, S.; Wan, G. Tumor-associated macrophages in immunotherapy. FEBS J. 2021, 288, 6174–6186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Horta, B.; Pereira, T.; Medeiros, R.; Cerqueira, F. Cervical Cancer Outcome and Tumor-Associated Macrophages. Res. Evidence. Immuno. 2022, 2, 460–468. [Google Scholar] [CrossRef]
- De Nola, R.; Loizzi, V.; Cicinelli, E.; Cormio, G. Dynamic crosstalk within the tumor microenvironment of uterine cervical carcinoma: Baseline network, iatrogenic alterations, and translational implications. Crit. Rev. Oncol. Hematol. 2021, 162, 103343. [Google Scholar] [CrossRef]
- Sousa, S.; Brion, R.; Lintunen, M.; Kronqvist, P.; Sandholm, J.; Mönkkönen, J.; Kellokumpu-Lehtinen, P.L.; Lauttia, S.; Tynninen, O.; Joensuu, H.; et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. BCR 2015, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Huang, Q.; Liang, X.; Ren, T.; Huang, Y.; Zhang, H.; Yu, Y.; Chen, C.; Wang, W.; Niu, J.; Lou, J.; et al. The role of tumor-associated macrophages in osteosarcoma progression—Therapeutic implications. Cell. Oncol. 2021, 44, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Deng, Y.R.; Wang, Z.C.; Wei, W.F.; Zhou, C.F.; Zhang, Y.M.; Yan, R.M.; Liang, L.J.; Zhong, M.; Liang, L.; et al. Hypoxia-induced ZEB1 promotes cervical cancer progression via CCL8-dependent tumour-associated macrophage recruitment. Cell Death Dis. 2019, 10, 508. [Google Scholar] [CrossRef]
- Werno, C.; Menrad, H.; Weigert, A.; Dehne, N.; Goerdt, S.; Schledzewski, K.; Kzhyshkowska, J.; Brüne, B. Knockout of HIF-1α in tumor-associated macrophages enhances M2 polarization and attenuates their pro-angiogenic responses. Carcinogenesis 2010, 31, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kim, S.; Hong, B.J.; Lee, C.J.; Kim, Y.E.; Bok, S.; Oh, J.M.; Gwak, S.H.; Yoo, M.Y.; Lee, M.S.; et al. Tumor-Associated Macrophages Enhance Tumor Hypoxia and Aerobic Glycolysis. Cancer Res. 2019, 79, 795–806. [Google Scholar] [CrossRef]
- Fang, W.-Y.; Ravindar, L.; Rakesh, K.; Manukumar, H.; Shantharam, C.; Alharbi, N.S.; Qin, H.-L. Synthetic approaches and pharmaceutical applications of chloro-containing molecules for drug discovery: A critical review. Eur. J. Med. Chem. 2019, 173, 117–153. [Google Scholar] [CrossRef]
- Zhao, C.; Rakesh, K.; Ravidar, L.; Fang, W.-Y.; Qin, H.-L. Pharmaceutical and medicinal significance of sulfur (SVI)-containing motifs for drug discovery: A critical review. Eur. J. Med. Chem. 2018, 162, 679–734. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Bronikowska, J.; Kłósek, M.; Janeczko, T.; Kostrzewa-Susłow, E.; Czuba, Z.P. The modulating effect of methoxy-derivatives of 2’-hydroxychalcones on the release of IL-8, MIF, VCAM-1 and ICAM-1 by colon cancer cells. Biomed. Pharmacother. 2022, 145, 112428. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.; Marques, S.; Silva, P.; Brandão, P.; Cidade, H.; Pinto, M.; Bousbaa, H. Prenylated chalcone 2 acts as an antimitotic agent and enhances the chemosensitivity of tumor cells to paclitaxel. Molecules 2016, 21, 982. [Google Scholar] [CrossRef] [PubMed]
- Leão, M.; Soares, J.; Gomes, S.; Raimundo, L.; Ramos, H.; Bessa, C.; Queiroz, G.; Domingos, S.; Pinto, M.; Inga, A.; et al. Enhanced cytotoxicity of prenylated chalcone against tumour cells via disruption of the p53–MDM2 interaction. Life Sci. 2015, 142, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Masawang, K.; Pedro, M.; Cidade, H.; Reis, R.M.; Neves, M.P.; Corrêa, A.G.; Sudprasert, W.; Bousbaa, H.; Pinto, M.M. Evaluation of 2′,4′-dihydroxy-3,4,5-trimethoxychalcone as antimitotic agent that induces mitotic catastrophe in MCF-7 breast cancer cells. Toxicol. Lett. 2014, 229, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.P.; Cravo, S.; Lima, R.T.; Vasconcelos, M.H.; Nascimento, M.S.J.; Silva, A.S.; Pinto, M.; Cidade, H.; Corrêa, A.G. Solid-phase synthesis of 2’-hydroxychalcones. Effects on cell growth inhibition, cell cycle and apoptosis of human tumor cell lines. Bioorganic Med. Chem. 2012, 20, 25–33. [Google Scholar] [CrossRef]
- Neves, M.P.; Lima, R.T.; Choosang, K.; Pakkong, P.; Nascimento, M.S.J.; Vasconcelos, M.H.; Pinto, M.; Silva, A.M.S.; Cidade, H. Synthesis of a natural chalcone and its prenyl analogues—Evaluation of tumor cell growth inhibitory activity and effects on cell cycle and apoptosis. Chem. Biodivers 2012, 9, 1133–1143. [Google Scholar] [CrossRef]
- Pinto, P.; Machado, C.M.; Moreira, J.; Almeida, J.D.; Silva, P.M.; Henriques, A.C.; Soares, J.X.; Salvador, J.A.; Afonso, C.; Pinto, M.; et al. Chalcone derivatives targeting mitosis: Synthesis, evaluation of antitumor activity and lipophilicity. Eur. J. Med. Chem. 2019, 184, 111752. [Google Scholar] [CrossRef]
- Pedro, M.; Cerqueira, F.; Sousa, M.E.; MSJ; Pinto, M. Xanthones as inhibitors of growth of human cancer cell lines and Their effects on the proliferation of human lymphocytes In Vitro. Bioorg. Med. Chem. 2002, 10, 3725–3730. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.; Cerqueira, F.; Barbosa, C.; Nascimento, M.S.J.; Pinto, M.M.M. Improvement of the inhibitory effect of xanthones on NO production by encapsulation in PLGA nanocapsules. J. Drug Target. 2005, 13, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Cerqueira, F.; Nazareth, N.; Medeiros, R.; Sarmento, A.; Sousa, E.; Pinto, M. 1,2-Dihydroxyxanthone: Effect on A375-C5 Melanoma Cell Growth Associated with Interference with THP-1 Human Macrophage Activity. Pharmaceuticals 2019, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Horta, B.; Freitas-Silva, J.; Silva, J.; Dias, F.; Sousa, E.; Pinto, M.; Cerqueira, F. Effect of 1-Carbaldehyde-3,4-dimethoxyxanthone on Prostate and HPV-18 Positive Cervical Cancer Cell Lines and on Human THP-1 Macrophages. Molecules 2021, 26, 3721. [Google Scholar] [CrossRef]
- Orzechowska, E.J.; Girstun, A.; Staron, K.; Trzcinska-Danielewicz, J. Synergy of BID with doxorubicin in the killing of cancer cells. Oncol. Rep. 2015, 33, 2143–2150. [Google Scholar] [CrossRef]
- Henslee, E.A.; Torcal Serrano, R.M.; Labeed, F.H.; Jabr, R.I.; Fry, C.H.; Hughes, M.P.; Hoettges, K.F. Accurate quantification of apoptosis progression and toxicity using a dielectrophoretic approach. Analyst 2016, 141, 6408–6415. [Google Scholar] [CrossRef]
- Petrova, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The hypoxic tumour microenvironment. Oncogenesis 2018, 7, 10. [Google Scholar] [CrossRef]
- Fu, L.Q.; Du, W.L.; Cai, M.H.; Yao, J.Y.; Zhao, Y.Y.; Mou, X.Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Li, Y.; Zhao, X. Research Progress on Tumor-Associated Macrophages and Inflammation in Cervical Cancer. Biomed. Res. Int. 2020, 2020, 6842963. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Zhu, Y.; Li, H.; Zhu, H.; Liu, T. Docetaxel-loaded M1 macrophage-derived exosomes for a safe and efficient chemoimmunotherapy of breast cancer. J. Nanobiotechnology 2022, 20, 359. [Google Scholar] [CrossRef]
- Williamson, S.C.; Hartley, A.E.; Heer, R. A review of tasquinimod in the treatment of advanced prostate cancer. Drug Des. Devel. Ther. 2013, 7, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Byles, V.; Covarrubias, A.J.; Ben-Sahra, I.; Lamming, D.W.; Sabatini, D.M.; Manning, B.D.; Horng, T. The TSC-mTOR pathway regulates macrophage polarization. Nat. Commun. 2013, 4, 2834. [Google Scholar] [CrossRef] [PubMed]
- Kashfi, K.; Kannikal, J.; Nath, N. Macrophage Reprogramming and Cancer Therapeutics: Role of iNOS-Derived NO. Cells 2021, 10, 3194. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Gabrani, R.; Jain, R.; Sharma, A.; Sarethy, I.P.; Dang, S.; Gupta, S. Antiproliferative Effect of Solanum nigrum on Human Leukemic Cell Lines. Indian J. Pharm. Sci. 2012, 74, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Chanput, W.; Reitsma, M.; Kleinjans, L.; Mes, J.J.; Savelkoul, H.F.J.; Wichers, H.J. β-Glucans are involved in immune-modulation of THP-1 macrophages. Mol. Nutr. Food Res. 2012, 56, 822–833. [Google Scholar] [CrossRef]
- He, X.; Shu, J.; Xu, L.; Lu, C.; Lu, A. Inhibitory Effect of Astragalus Polysaccharides on Lipopolysaccharide-Induced TNF- and IL-1β Production in THP-1 Cells. Molecules 2012, 17, 3155–3164. [Google Scholar] [CrossRef]
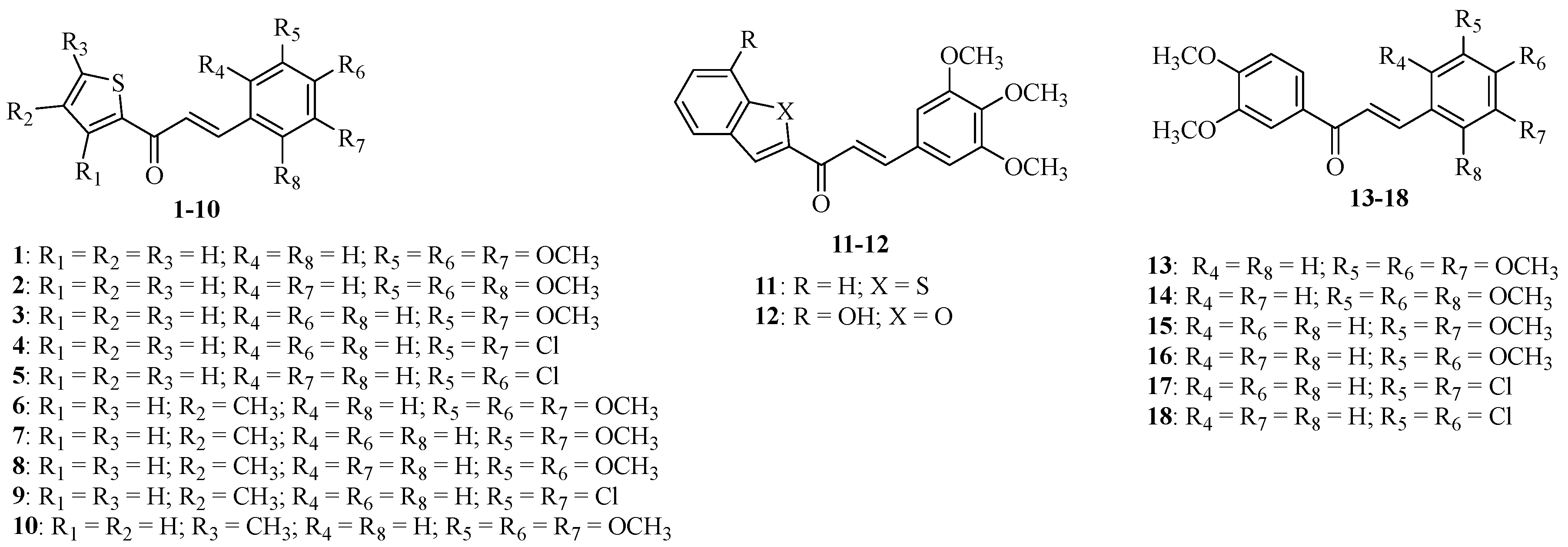
| Compound | Concentration (µM) | Inhibition of Metabolic Viability (% of Control) | |||
|---|---|---|---|---|---|
| HeLa | LNCaP | PC-3 | Jurkat | ||
| 1 | 5 | 39.1 ± 0.1 | 16.0 ± 5.4 | 23.5 ± 7.2 | 58.6 ± 13.6 |
| 10 | 33.5 ± 5.6 | 33.5 ± 6.9 | 33.6 ± 10.7 | 90.8 ± 4.2 | |
| 20 | 43.6 ± 5.7 | 39.1 ± 1.1 | 47.6 ± 10.5 | T.I. | |
| 2 | 5 | 24.0 ± 12.5 | 24.3 ± 1.9 | 31.2 ± 4.3 | 6.8 ± 3.9 |
| 10 | 22.4 ± 7.5 | 39.2 ± 5.3 | 41.9 ± 4.0 | 20.8 ± 1.6 | |
| 20 | 29.5 ± 4.2 | 45.3 ± 4.1 | 45.4 ± 8.7 | 81.8 ± 7.3 | |
| 3 | 5 | 29.4 ± 10.4 | 23.1 ± 8.5 | 33.4 ± 5.8 | 55.3 ± 15.8 |
| 10 | 20.3 ± 5.7 | 40.9 ± 5.9 | 32.0 ± 5.0 | 87.8 ± 5.6 | |
| 20 | 61.1 ± 10.1 | 74.4 ± 0.8 | 84.6 ± 2.7 | T.I. | |
| 4 | 5 | 21.9 ± 3.8 | 30.1 ± 4.9 | 33.3 ± 4.7 | 29.1 ± 11.5 |
| 10 | 29.3 ± 9.7 | 40.5 ± 2.7 | 27.0 ± 2.8 | 66.1 ± 10.9 | |
| 20 | 26.8 ± 4.9 | 56.3 ± 2.4 | 52.3 ± 3.9 | 91.6 ± 5.9 | |
| 5 | 5 | 31.3 ± 4.7 | 31.2 ± 2.1 | 28.4 ± 9.2 | 60.2 ± 9.5 |
| 10 | 33.7 ± 3.4 | 49.1 ± 2.4 | 50.5 ± 4.0 | 57.1 ± 2.4 | |
| 20 | 45.8 ± 8.6 | 58.5 ± 3.7 | 63.6 ± 3.7 | 88.9 ± 4.0 | |
| 6 | 5 | 25.0 ± 7.7 | 30.4 ± 8.5 | 26.6 ± 5.4 | 35.6 ± 1.1 |
| 10 | 52.0 ± 7.8 | 37.0 ± 8.3 | 32.2 ± 3.1 | 30.7 ± 7.6 | |
| 20 | 74.8 ± 7.5 | 63.4 ± 7.1 | 78.7 ± 2.3 | 79.2 ± 3.7 | |
| 7 | 5 | 25.7 ± 4.0 | 23.6 ± 0.8 | 19.6 ± 5.1 | 47.0 ± 8.0 |
| 10 | 45.8 ± 5.9 | 34.4 ± 5.0 | 38.5 ± 5.7 | 89.0 ± 7.4 | |
| 20 | 60.2 ± 14.5 | 78.4 ± 2.6 | 62.2 ± 7.5 | T.I. | |
| 8 | 5 | 11.4 ± 3.5 | 24.4 ± 1.5 | 18.3 ± 0.6 | 28.0 ± 8.2 |
| 10 | 35.7 ± 7.1 | 28.4 ± 8.2 | 33.6 ± 9.1 | 45.7 ± 8.4 | |
| 20 | 51.6 ± 6.0 | 47.8 ± 5.1 | 45.5 ± 6.3 | 91.6 ± 6.9 | |
| 9 | 5 | 26.4 ± 7.1 | 23.5 ± 4.8 | 32.0 ± 1.0 | 32.2 ± 9.8 |
| 10 | 34.4 ± 3.0 | 36.9 ± 2.1 | 33.4 ± 10.7 | 70.6 ± 13.2 | |
| 20 | 55.8 ± 7.1 | 51.5 ± 7.5 | 56.3 ± 11.2 | T.I. | |
| 10 | 5 | 14.0 ± 5.5 | 29.4 ± 6.0 | 47.0 ± 5.5 | 45.1 ± 10.0 |
| 10 | 56.4 ± 8.6 | 41.4 ± 1.4 | 33.9 ± 11.6 | 93.6 ± 6.4 | |
| 20 | 70.2 ± 7.9 | 60.0 ± 5.9 | 84.7 ± 2.9 | T.I. | |
| 11 | 5 | 29.2 ± 8.9 | 28.6 ± 5.0 | 18.4 ± 1.9 | 18.1 ± 2.2 |
| 10 | 31.9 ± 8.9 | 31.5 ± 5.0 | 30.0 ± 2.2 | 40.5 ± 2.5 | |
| 20 | 51.5 ± 3.3 | 49.9 ± 2.1 | 60.4 ± 3.4 | 99.0 ± 1.6 | |
| 12 | 5 | 35.8 ± 4.4 | 27.4 ± 6.0 | 15.4 ± 3.8 | 10.8 ± 2.0 |
| 10 | 42.6 ± 8.3 | 39.2 ± 4.8 | 30.0 ± 2.7 | 47.4 ± 11.7 | |
| 20 | 85.8 ± 3.7 | 69.1 ± 7.9 | 80.9 ± 5.1 | T.I. | |
| 13 | 5 | 46.8 ± 3.7 | 37.9 ± 12.2 | 22.1 ± 6.6 | 48.9 ± 9.2 |
| 10 | 40.5 ± 13.5 | 58.9 ± 11.2 | 44.5 ± 2.5 | 77.7 ± 10.9 | |
| 20 | 68.0 ± 10.6 | 75.1 ± 6.2 | 73.1 ± 2.8 | T.I. | |
| 14 | 5 | 29.7 ± 11.6 | 18.6 ± 2.2 | 16.1 ± 1.1 | 57.0 ± 11.1 |
| 10 | 63.7 ± 8.6 | 43.4 ± 9.1 | 37.9 ± 11.8 | 97.0 ± 6.7 | |
| 20 | 74.5 ± 9.1 | 69.5 ± 5.3 | 57.4 ± 15.4 | T.I. | |
| 15 | 5 | 33.3 ± 6.2 | 24.3 ± 6.4 | 17.2 ± 4.4 | 14.6 ± 6.5 |
| 10 | 24.5 ± 11.2 | 44.0 ± 7.1 | 37.7 ± 2.4 | 13.0 ± 2.3 | |
| 20 | 33.6 ± 2.4 | 39.8 ± 10.1 | 37.0 ± 2.3 | 77.3 ± 6.3 | |
| 16 | 5 | 37.3 ± 12.8 | 30.6 ± 4.4 | 40.8 ± 4.8 | 69.4 ± 13.7 |
| 10 | 57.8 ± 8.9 | 52.4 ± 6.9 | 64.7 ± 1.8 | 81.6 ± 15.1 | |
| 20 | 78.3 ± 3.0 | 76.9 ± 4.6 | 87.6 ± 3.7 | T.I. | |
| 17 | 5 | 19.5 ± 6.8 | 21.3 ± 6.7 | 19.6 ± 3.0 | 32.1 ± 10.2 |
| 10 | 30.3 ± 5.2 | 29.3 ± 3.0 | 28.0 ± 1.4 | 44.2 ± 8.6 | |
| 20 | 47.0 ± 8.8 | 37.0 ± 3.9 | 38.9 ± 3.6 | T.I. | |
| 18 | 5 | 9.8 ± 3.2 | 23.0 ± 9.3 | 28.7 ± 1.5 | 34.6 ± 11.3 |
| 10 | 21.5 ± 14.8 | 33.0 ± 1.2 | 43.1 ± 2.9 | 84.1 ± 13.2 | |
| 20 | 26.8 ± 3.9 | 45.1 ± 15.3 | 59.9 ± 7.2 | T.I. | |
| Doxorubicin | 5 | 82.8 ± 2.5 | 62.5 ± 1.8 | 73.2 ± 2.8 | 111.4 ± 2.8 |
| Compound | Concentration (µM) | Time | ||
|---|---|---|---|---|
| 0 h | 6 h | 14 h | ||
| Chalcone 16 | 1.3 | 52.4 ± 10.1 | 19.0 ± 0.7 * | 11.0 ± 1.1 * |
| 2.5 | 66.4 ± 3.6 | 24.8 ± 4.0 * | 12.3 ± 2.0 * | |
| Dexamethasone | 5 | 58.1 ± 7.8 | 31.6 ± 1.8 * | 5.9 ± 3.2 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horta, B.; Freitas-Silva, J.; Silva, J.; Dias, F.; Teixeira, A.L.; Medeiros, R.; Cidade, H.; Pinto, M.; Cerqueira, F. Antitumor Effect of Chalcone Derivatives against Human Prostate (LNCaP and PC-3), Cervix HPV-Positive (HeLa) and Lymphocyte (Jurkat) Cell Lines and Their Effect on Macrophage Functions. Molecules 2023, 28, 2159. https://doi.org/10.3390/molecules28052159
Horta B, Freitas-Silva J, Silva J, Dias F, Teixeira AL, Medeiros R, Cidade H, Pinto M, Cerqueira F. Antitumor Effect of Chalcone Derivatives against Human Prostate (LNCaP and PC-3), Cervix HPV-Positive (HeLa) and Lymphocyte (Jurkat) Cell Lines and Their Effect on Macrophage Functions. Molecules. 2023; 28(5):2159. https://doi.org/10.3390/molecules28052159
Chicago/Turabian StyleHorta, Bruno, Joana Freitas-Silva, Jani Silva, Francisca Dias, Ana Luísa Teixeira, Rui Medeiros, Honorina Cidade, Madalena Pinto, and Fátima Cerqueira. 2023. "Antitumor Effect of Chalcone Derivatives against Human Prostate (LNCaP and PC-3), Cervix HPV-Positive (HeLa) and Lymphocyte (Jurkat) Cell Lines and Their Effect on Macrophage Functions" Molecules 28, no. 5: 2159. https://doi.org/10.3390/molecules28052159
APA StyleHorta, B., Freitas-Silva, J., Silva, J., Dias, F., Teixeira, A. L., Medeiros, R., Cidade, H., Pinto, M., & Cerqueira, F. (2023). Antitumor Effect of Chalcone Derivatives against Human Prostate (LNCaP and PC-3), Cervix HPV-Positive (HeLa) and Lymphocyte (Jurkat) Cell Lines and Their Effect on Macrophage Functions. Molecules, 28(5), 2159. https://doi.org/10.3390/molecules28052159











