Quantitative Analysis of Camellia oleifera Seed Saponins and Aqueous Two-Phase Extraction and Separation
Abstract
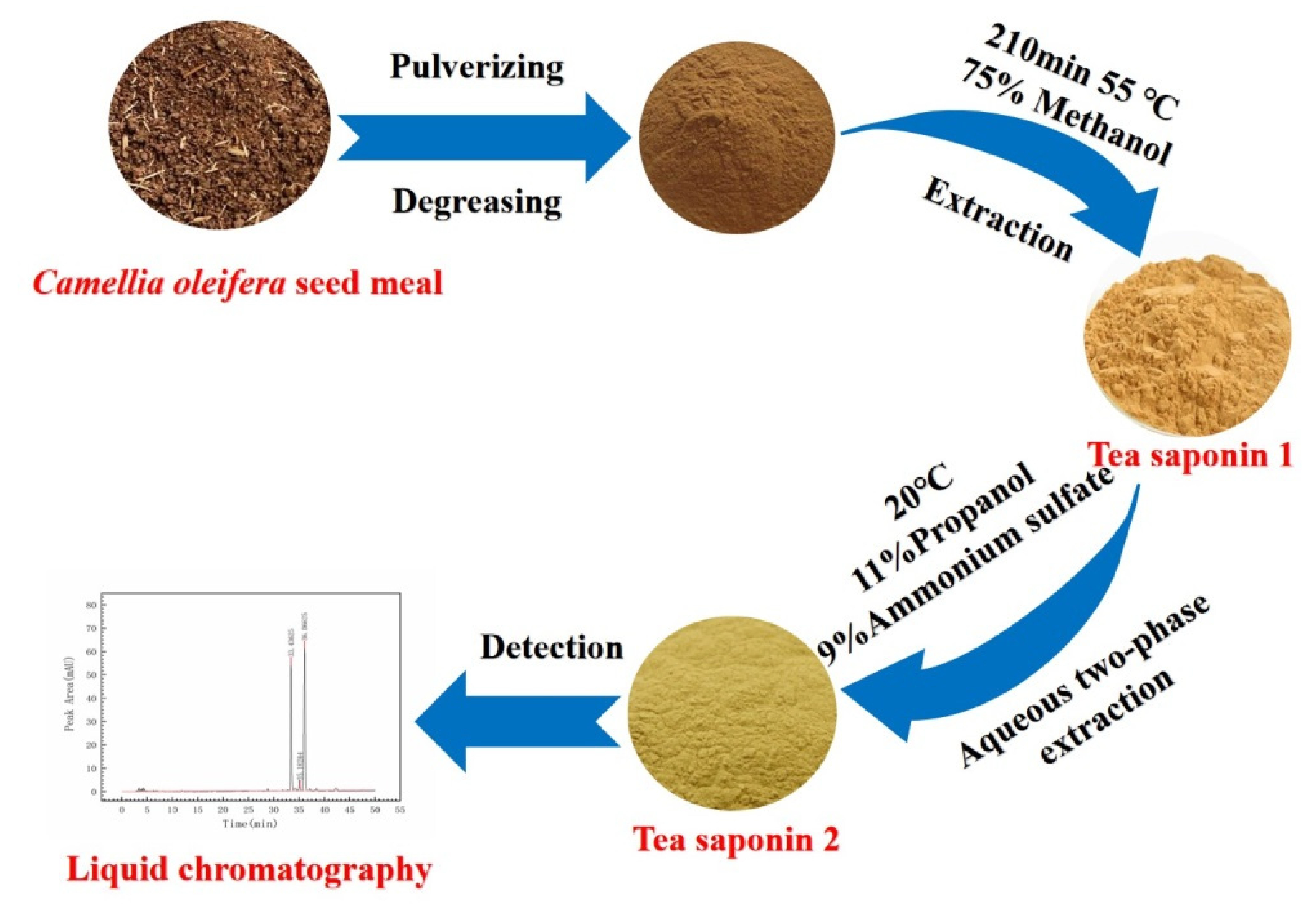
1. Introduction
2. Results and Discussion
2.1. Quantitative Analysis of C. oleifera Saponins
2.1.1. UV Spectrophotometry
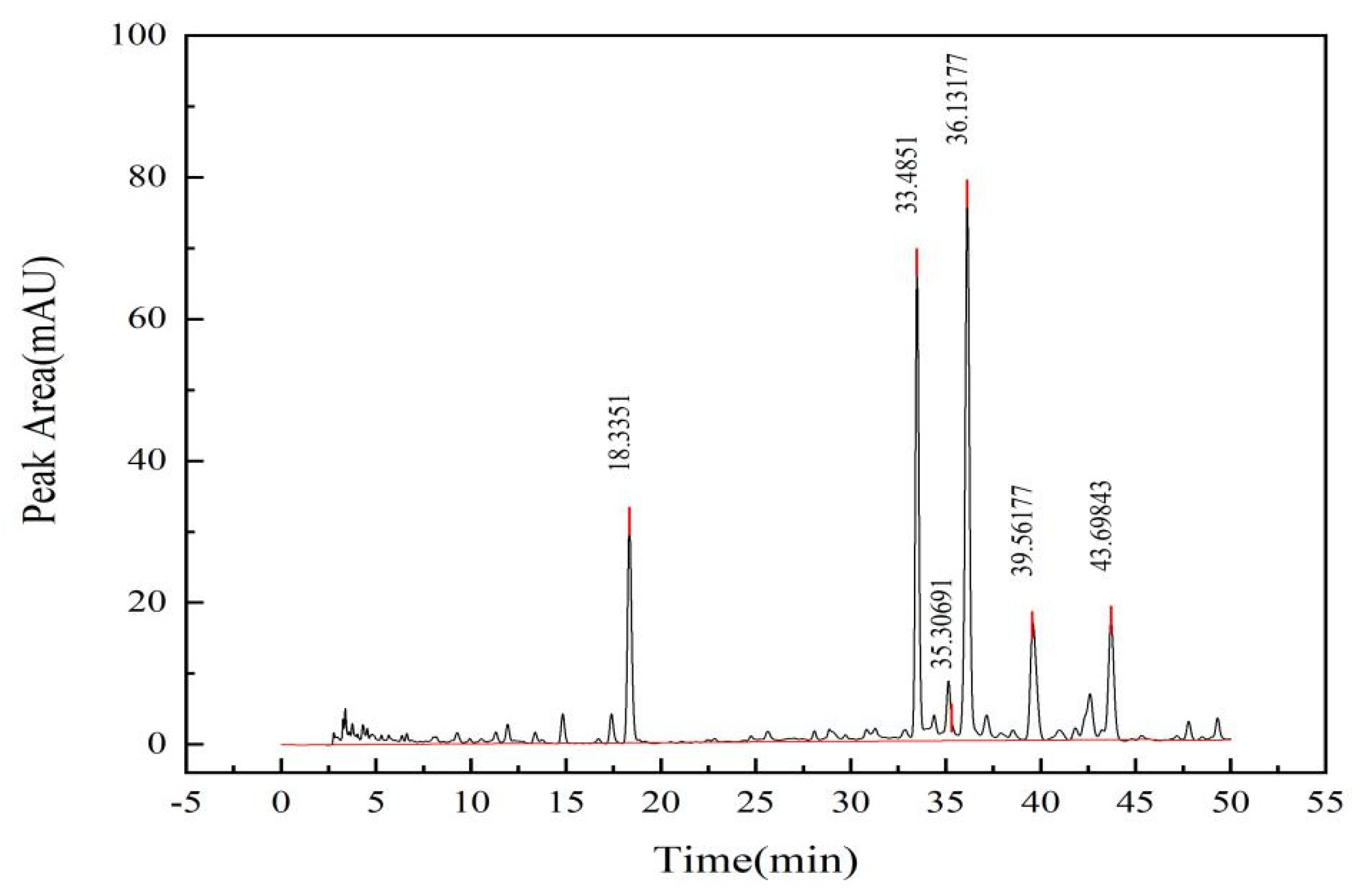
2.1.2. Analysis of Detection Results by Liquid Chromatography
Standard Curve Based on Liquid Chromatography
Precision Test
Repeatability Test
Average Spike Recovery Test
2.1.3. Analysis of Weight Test Results
2.2. Results of Alcoholic Extraction of C. oleifera Saponins
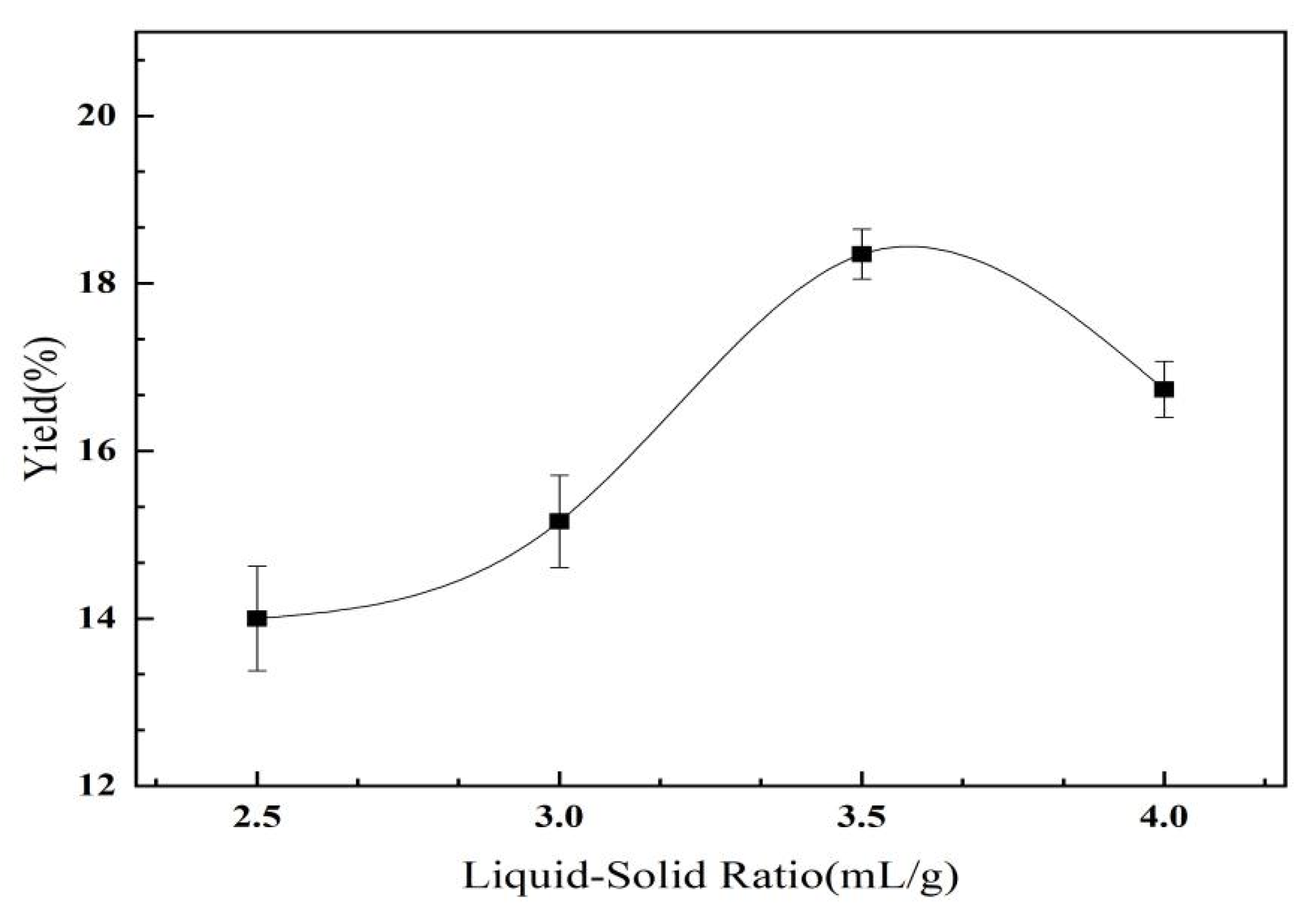
2.2.1. The Effect of the Extracted Liquid–Solid Ratio on the Yield of C. oleifera Saponins
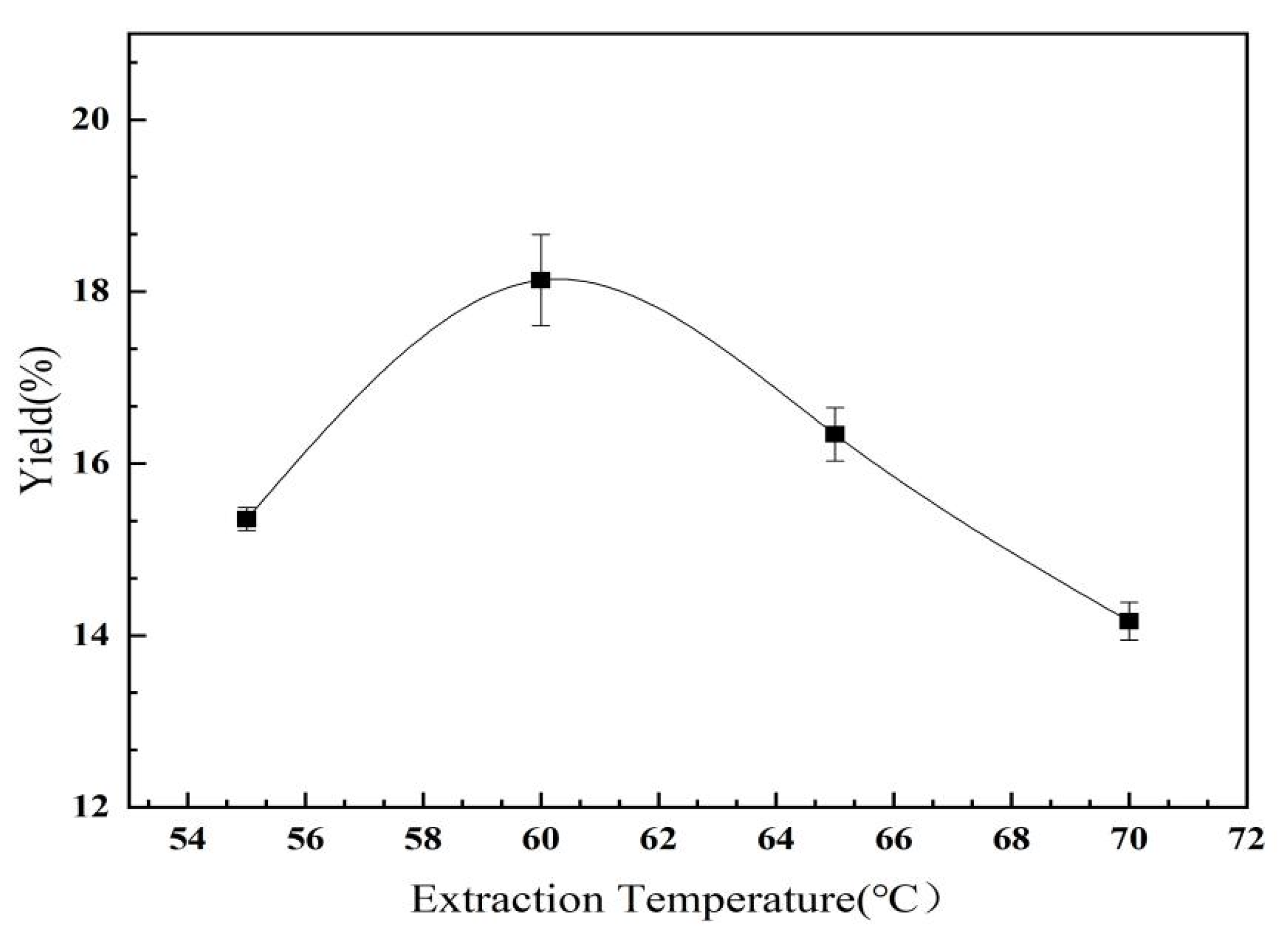
2.2.2. The Effect of Extraction Temperature on the Yield of C. oleifera Saponins
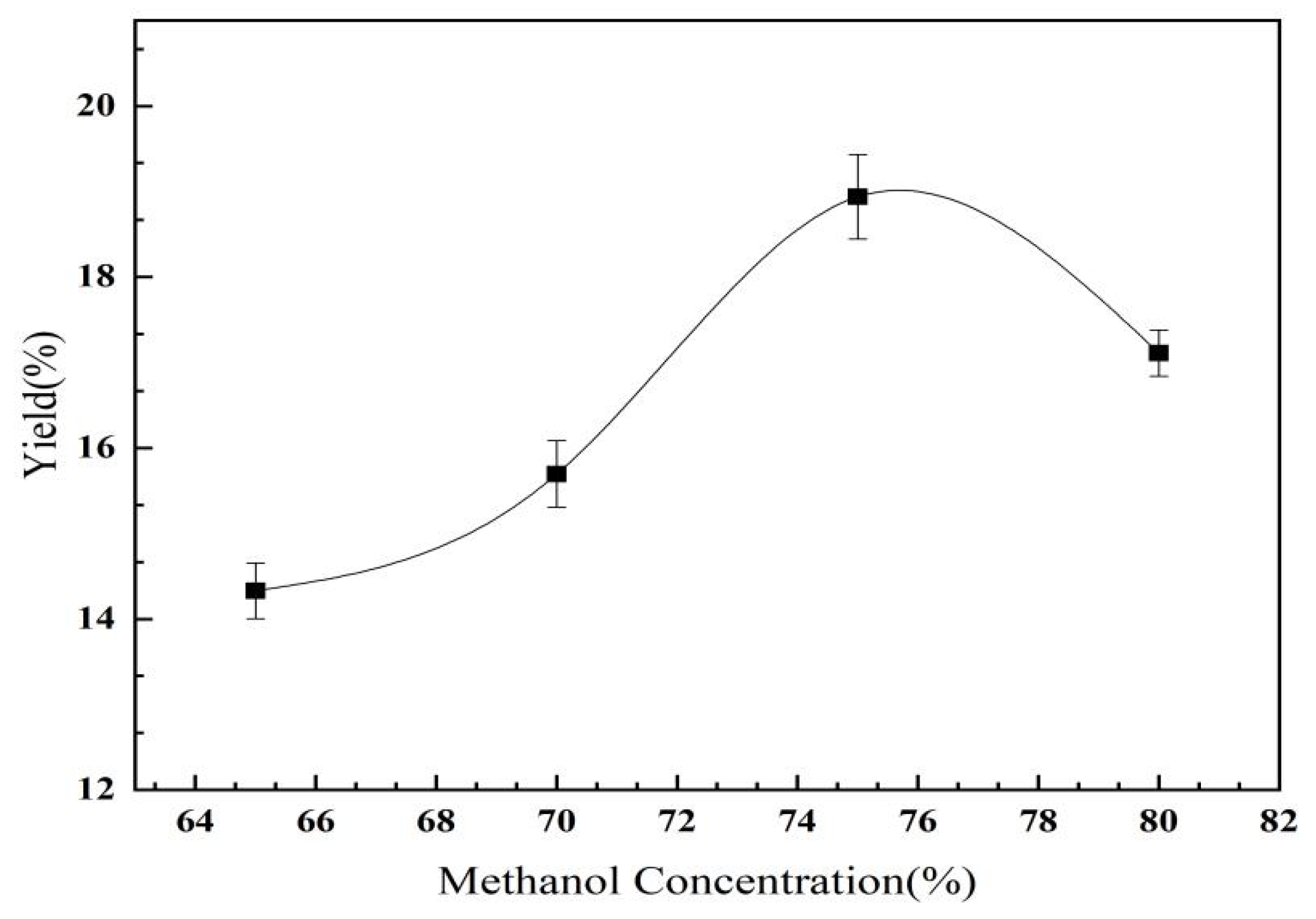
2.2.3. The Effect of Methanol Concentration on the Yield of C. oleifera Saponins
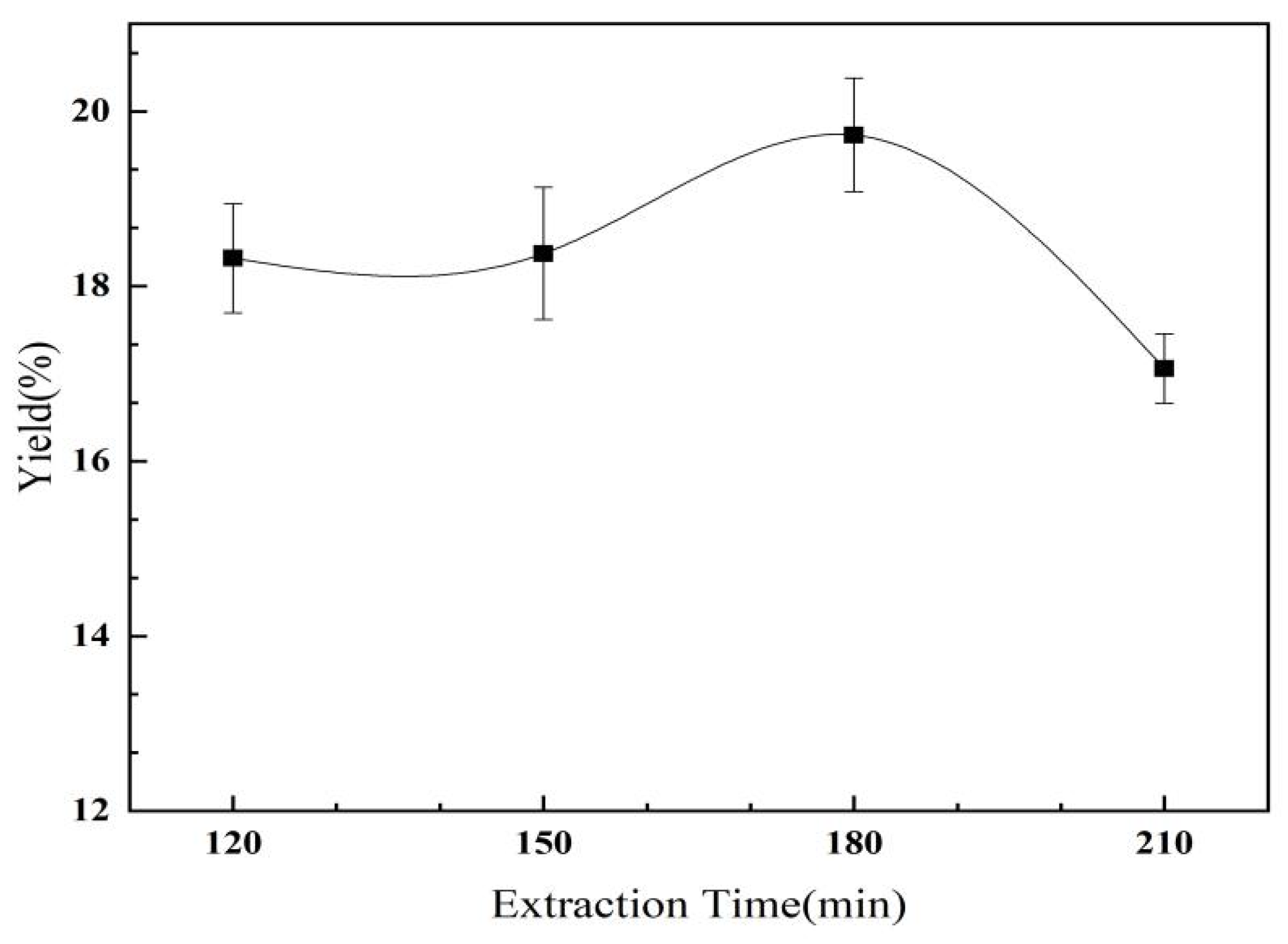
2.2.4. The Effect of Extraction Time on the Yield of C. oleifera Saponins
2.2.5. Orthogonal Test and Analysis of C. oleifera Saponins Extraction
2.3. Analysis of C. oleifera Saponins via Aqueous Two-Phase Extraction Results
2.3.1. Effect of Extraction Temperature on Two-Phase Extraction and Purification of C. oleifera Saponins
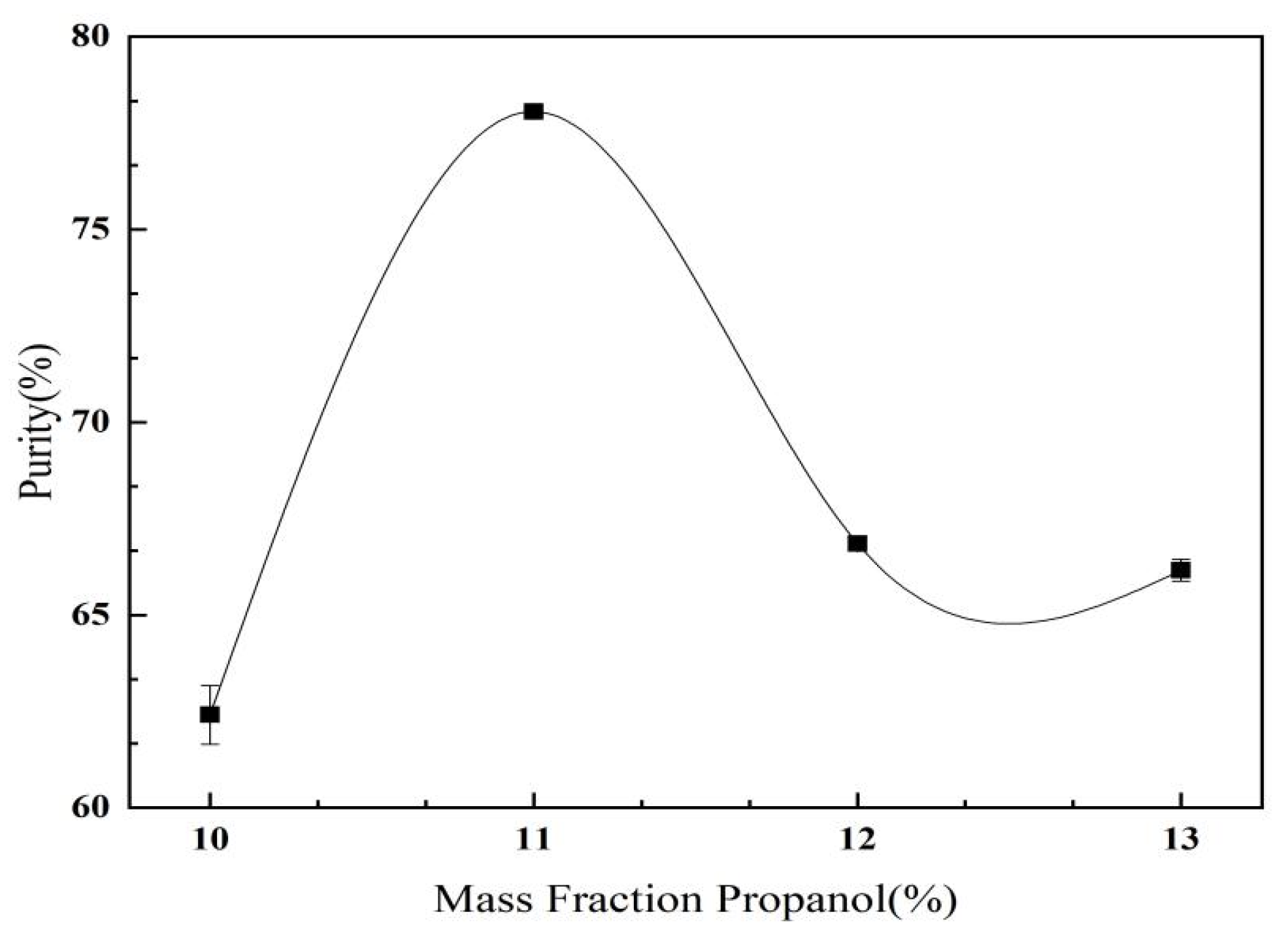
2.3.2. The Effect of Propanol Mass Fraction on C. oleifera Saponins Aqueous Two-Phase Extraction and Purification
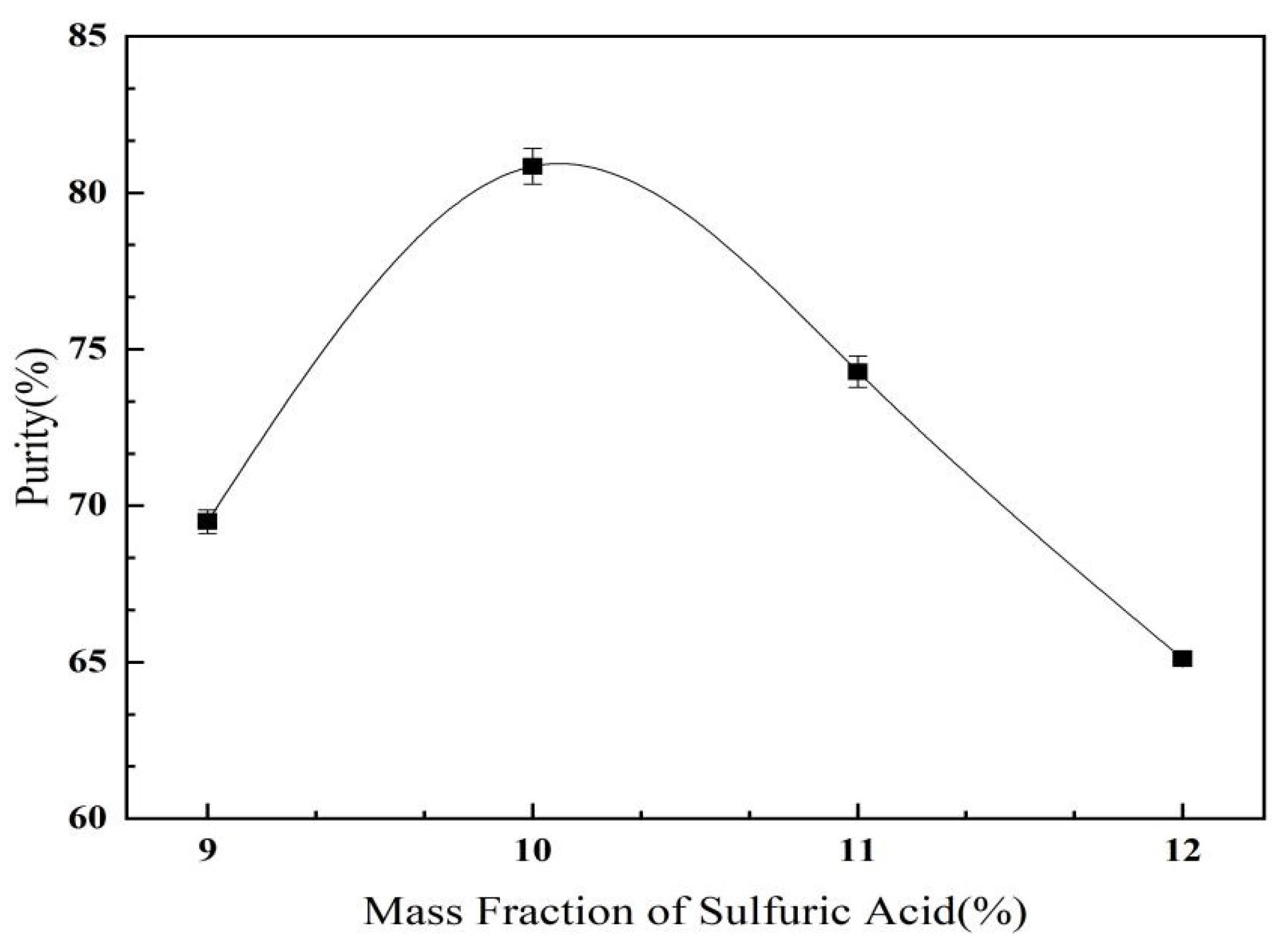
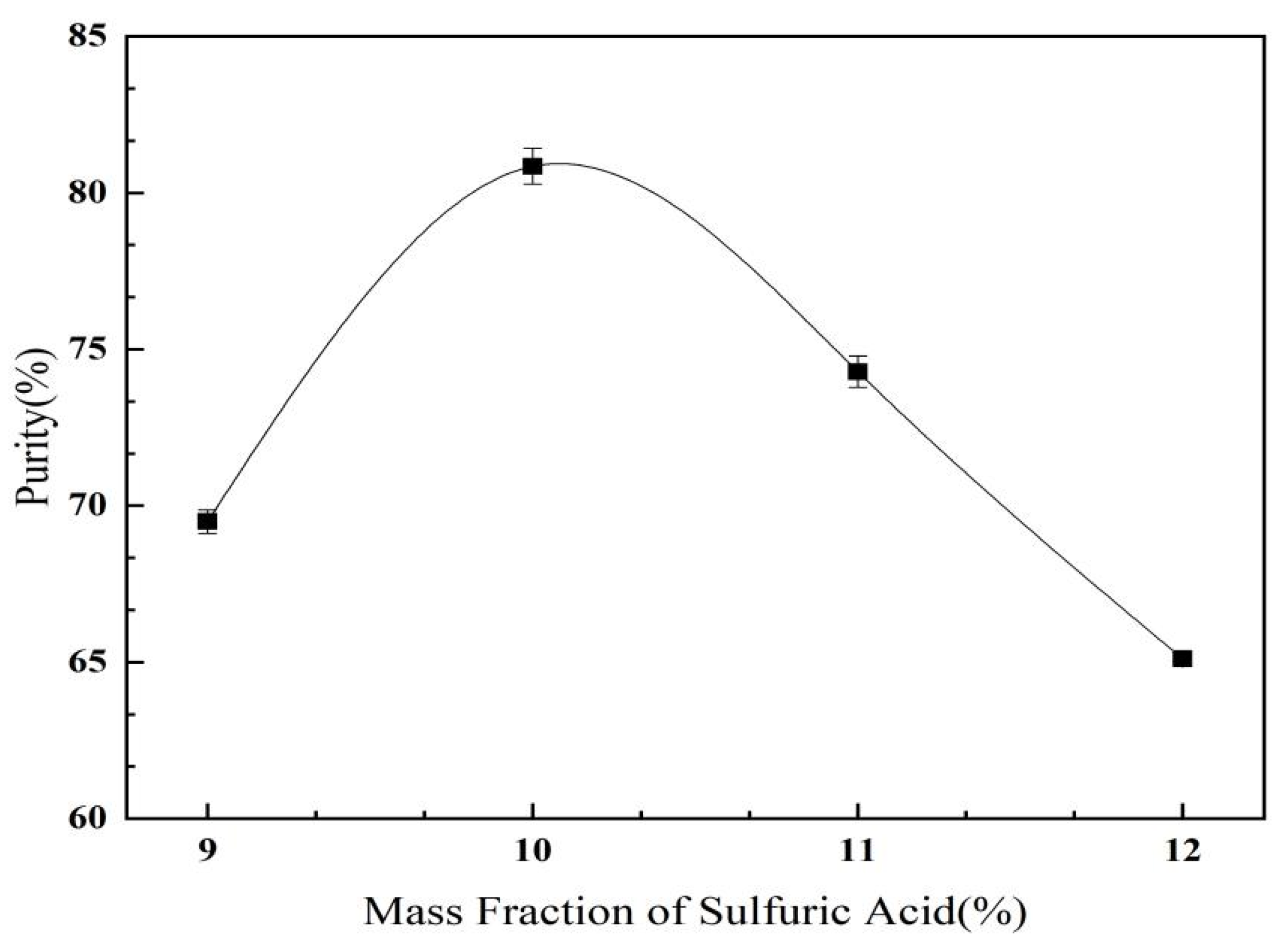
2.3.3. Effect of Ammonium Sulfate Mass Fraction on C. oleifera Saponins Aqueous Two-Phase Extraction and Purification
2.3.4. Orthogonal Test and Analysis of C. oleifera Saponins Aqueous Two-Phase Extraction and Purification
3. Materials and Methods
3.1. Materials
3.2. Experimental Method
3.2.1. Quantitative Analysis of C. oleifera Saponins
UV Spectrophotometry
- Preparation of C. oleifera saponins standard solution and sample solutions
- 2.
- Selection of the maximum absorption wavelength
- 3.
- Drawing of standard curve
- 4.
- Calculation method
- (1)
- Calculation of yield
- (2)
- Calculation of purity
- (3)
- Limit of detection and limit of quantitation of UV spectrophotometry
- (4)
- Limit of detection and limit of quantitation of liquid chromatography
- (5)
- Matrix effect
Liquid Chromatography Detection Method
Weight Detection
3.2.2. Alcohol Extraction of C. oleifera Saponins
3.2.3. Aqueous Two-Phase Extraction of C. oleifera Saponins
3.2.4. Statistics and Analyses
Statistical Analysis of C. oleifera Saponins Alcohol Extraction
Statistical Analysis of C. oleifera Saponins Aqueous Two-Phase Extraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, H.; Li, C.Z.; Li, Z.M.; Liu, R.K.; Zhang, A.H.; Xiao, Z.H.; Ma, L.; Li, J.K.; Deng, S.G. Simultaneous extraction of oil and tae saponin from Camellia oleifera Abel. seeds under subcritical water conditions. Fuel Process. Technol. 2018, 174, 88–94. [Google Scholar] [CrossRef]
- Chen, Y.F.; Yang, C.H.; Chang, M.S.; Huang, Y.C. Foam properties and detergent abilities of the saponins from Camellia oleifera. Int. J. Mol. Sci. 2010, 11, 4417–4425. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Gao, D.F.; Xu, M.; Shi, Z.G.; Wang, D.; Yang, C.R.; Zhang, Y.J. Triterpenoid saponins from the genus Camellia. Chem. Biodivers. 2011, 8, 1931–1942. [Google Scholar] [CrossRef]
- Xia, H.L.; Chi, X.Y.; Yan, Z.J.; Cheng, W.W. Enhancing plant uptake of polychlorinated biphenyls and cadmium using tea saponin. Bioresour. Technol. 2009, 100, 4649–4653. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.Y.; Liu, Y.C.; Yuan, Z.H.; Wang, Z. Formation and Physical Stability of Zanthoxylum bungeanum Essential Oil Based Nanoemulsions Co-Stabilized with Tea Saponin and Synthetic Surfactant. Molecules 2021, 26, 7464. [Google Scholar] [CrossRef]
- Yan, J.; Wu, Z.L.; Zhao, Y.L.; Jiang, C.S. Separation of tea saponin by two–stage foam fractionation. Sep. Purif. Technol. 2011, 80, 300–305. [Google Scholar] [CrossRef]
- Bao, N.N.; Ding, F.C.; Hu, Q.Y. Extraction and bacteriostasis effect of Camellia oleifera saponins in oil-tea Camellia seed cake. J. Nat. Prod. Res. Dev. 2018, 30, 127. [Google Scholar] [CrossRef]
- Yu, X.L.; He, Y. Development of a rapid and simple method for preparing tea-leaf saponins and investigation on their surface tension differences compared with tea–seed Saponins. Molecules 2018, 23, 1796. [Google Scholar] [CrossRef]
- Zhang, X.F.; Yang, S.L.; Han, Y.Y.; Zhao, L.; Lu, G.L.; Xia, T.; Guo, L.P. Qualitative and quantitative analysis of triterpene saponins from tea seed pomace (Camellia oleifera Abel.) and their activities against bacteria and fungi. Molecules 2014, 19, 7568–7580. [Google Scholar] [CrossRef]
- Kuźniar, A.; Włodarczyk, K.; Sadok, I.; Staniszewska, M.; Wozniak, M.; Furtak, K.; Grzadziel, J.; Galazka, A.; Skorzynska-Polit, E.; Wolinska, A. A Comprehensive Analysis Using Colorimetry, Liquid Chromatography-Tandem Mass Spectrometry and Bioassays for the Assessment of Indole Related Compounds Produced by Endophytes of Selected Wheat Cultivars. Molecules 2021, 26, 1394. [Google Scholar] [CrossRef]
- Pandey, S. Analytical applications of room-temperature ionic liquids: A review of recent efforts. Anal. Chim. Acta 2006, 556, 38–45. [Google Scholar] [CrossRef]
- Bronsema, K.J.; Bischoff, R.; van de Merbel, N.C. Internal standards in the quantitative determination of protein biopharmaceuticals using liquid chromatography coupled to mass spectrometry. J. Chromatogr. B 2012, 893, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Benito-Peña, E.; Urraca, J.L.; Moreno-Bondi, M.C. Quantitative determination of penicillin V and amoxicillin in feed samples by pressurised liquid extraction and liquid chromatography with ultraviolet detection. J. Pharm. Biomed. Anal. 2009, 49, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Knol, W.C.; Pirok, B.W.; Peters, R.A. Detection challenges in quantitative polymer analysis by liquid chromatography. J. Sep. Sci. 2021, 44, 63–87. [Google Scholar] [CrossRef]
- Wu, X.J.; Jia, L.Y.; Wu, J.F.; Liu, Y.W.; Kang, H.; Liu, X.B.; Li, P.; He, P.M.; Tu, Y.Y.; Li, B. Simultaneous determination and quantification of triterpene saponins from Camellia sinensis seeds using UPLC-PDA-QTOF-MS/MS. Molecules 2019, 24, 3794. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; He, Q.; Xiao, P.G.; Muhammad, I.; Cheng, Y.Y. Simultaneous quantification of five triterpenoid saponins in Clematis L. spp. by high–performance liquid chromatography with evaporative light scattering detection. Phytochem. Anal. 2008, 19, 40–45. [Google Scholar] [CrossRef]
- Ganzera, M.; Gampenrieder, J.; Pawar, R.S.; Khan, I.A.; Stuppner, H. Separation of the major triterpenoid saponins in Bacopa monnieri by high-performance liquid chromatography. Anal. Chim. Acta 2004, 516, 149–154. [Google Scholar] [CrossRef]
- Aarabi, A.; Mizani, M.; Honarvar, M.; Faghihian, H.; Guerami, A. Extraction of ferulic acid from sugar beet pulp by alkaline hydrolysis and organic solvent methods. J. Food Meas. Charact. 2016, 10, 42–47. [Google Scholar] [CrossRef]
- Wang, Q.F.; Chen, R.J.; Shatner, W.; Bai, Y. State-of-the-art on the technique of dispersive liquid-liquid microextraction. Ultrason. Sonochemistry 2019, 51, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, W.N.; Ma, X.X.; Fan, D.D. Enzymatic hydrolysis and extraction of ginsenoside recovered from deep eutectic solvent-salt aqueous two–phase system. J. Biosci. Bioeng. 2020, 130, 390–396. [Google Scholar] [CrossRef]
- Zhao, R.T.; Pei, D.; Yu, P.L.; Wei, J.T.; Wang, N.L.; Di, D.L.; Liu, Y.W. Aqueous two-phase systems based on deep eutectic solvents and their application in green separation processes. J. Sep. Sci. 2020, 43, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.C.; Liu, J.C. Removal of phosphate and fluoride from wastewater by a hybrid precipitation–microfiltration process. Sep. Purif. Technol. 2010, 74, 329–335. [Google Scholar] [CrossRef]
- Tan, Z.J.; Wang, C.Y.; Yi, Y.J.; Wang, H.Y.; Li, M.; Zhou, W.L.; Tan, S.Y.; Li, F.F. Extraction and purification of chlorogenic acid from ramie (Boehmeria nivea L. Gaud) leaf using an ethanol/salt aqueous two-phase system. Sep. Purif. Technol. 2014, 132, 396–400. [Google Scholar] [CrossRef]
- Dang, Y.Y.; Zhang, H.; Xiu, Z.L. Microwave-assisted aqueous two–phase extraction of phenolics from grape (Vitis vinifera) seed. J. Chem. Technol. Biotechnol. 2014, 89, 1576–1581. [Google Scholar] [CrossRef]
- Liu, X.L.; Mu, T.H.; Sun, H.N.; Zhang, M.; Chen, J.W. Optimisation of aqueous two-phase extraction of anthocyanins from purple sweet potatoes by response surface methodology. Food Chem. 2013, 141, 3034–3041. [Google Scholar] [CrossRef] [PubMed]
- Cláudio, A.F.M.; Ferreira, A.M.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G.; Coutinho, J.A.P. Optimization of the gallic acid extraction using ionic-liquid-based aqueous two–phase systems. Sep. Purif. Technol. 2012, 97, 142–149. [Google Scholar] [CrossRef]
- Amid, M.; Shuhaimi, M.; Sarker, M.Z.I.; Manap, M.Y.A. Purification of serine protease from mango (Mangifera Indica Cv. Chokanan) peel using an alcohol/salt aqueous two phase system. Food Chem. 2012, 132, 1382–1386. [Google Scholar] [CrossRef]
- Tan, Z.J.; Wang, C.Y.; Yang, Z.Z.; Yi, Y.J.; Wang, H.Y.; Zhou, W.L.; Li, F.F. Ionic liquid–based ultrasonic-assisted extraction of secoisolariciresinol diglucoside from flaxseed (Linum usitatissimum L.) with further purification by an aqueous two-phase system. Molecules 2015, 20, 17929–17943. [Google Scholar] [CrossRef]
- Wei, Y.L.; Chen, X.; Yang, T.; Wan, J.F.; Cao, X.J. Partition of tea saponin with a novel recyclable thermo-pH aqueous two-phase systems. Appl. Biochem. Biotechnol. 2021, 193, 3062–3078. [Google Scholar] [CrossRef]
- Motlagh, A.H.; Nasirpour, A.; Saeidy, S.; Keramat, J. Physicochemical and sensory properties of malt beverage containing sugar beet saponins. J. Food Sci. Technol.-Mysore 2022, 59, 4380–4389. [Google Scholar] [CrossRef]
- Guo, X.F.; Lu, Y.A.; Cui, H.P.; Jia, X.X.; Bai, H.C.; Na, Y.X. Factors affecting the physical properties of edible composite film prepared from zein and wheat gluten. Molecules 2012, 17, 3794–3804. [Google Scholar] [CrossRef] [PubMed]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Chaicharoenpong, C.; Petsom, A. Quantitative thin layer chromatographic analysis of the saponins in tea seed meal. Phytochem. Anal. 2009, 20, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Yang, J.B.; Yang, J. Optimization of ultrasound–assisted aqueous two phase extraction of polyphenols from olive leaves. Prep. Biochem. Biotechnol. 2020, 51, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Wu, H.Y.; Liau, B.C.; Chang, C.M.J.; Jong, T.T.; Wu, L.C. Identification and evaluation of antioxidants defatted Camellia oleifera seeds by isopropanol salting-out pretreatment. Food Chem. 2010, 121, 1246–1254. [Google Scholar] [CrossRef]
- He, A.; Dong, B.; Feng, X.T.; Yao, S. Extraction of bioactive ginseng saponins using aqueous two–phase systems of ionic liquids and salts. Sep. Purif. Technol. 2018, 196, 270–280. [Google Scholar] [CrossRef]
- Pehlivan, E.; Cetin, S. Sorption of Cr (VI) ions on two Lewatit–anion exchange resins and their quantitative determination using UV–visible spectrophotometer. J. Hazard. Mater. 2009, 163, 448–453. [Google Scholar] [CrossRef]
- Han, J.; Ye, M.; Guo, H.; Yang, M.; Wang, B.R.; Guo, D.A. Analysis of multiple constituents in a Chinese herbal preparation Shuang–Huang–Lian oral liquid by HPLC-DAD-ESI-MSn. J. Pharm. Biomed. Anal. 2007, 44, 430–438. [Google Scholar] [CrossRef]
- Matsui, Y.; Kobayashi, K.; Masuda, H.; Kigoshi, H.; Akao, M.; Sakurai, H.; Kumagai, H. Quantitative analysis of saponins in a tea-leaf extract and their antihypercholesterolemic activity. Biosci. Biotechnol. Biochem. 2009, 73, 1513–1519. [Google Scholar] [CrossRef]
- Yang, X.B.; Zhao, Y.; Wang, Q.W.; Wang, H.F. Analysis of the monosaccharide components in Angelica polysaccharides by high performance liquid chromatography. Anal. Sci. 2005, 21, 1177–1180. [Google Scholar] [CrossRef]
- He, J.; Wu, Z.Y.; Zhang, S.; Zhou, Y.; Zhao, F.; Peng, Z.Q.; Hu, Z.W. Optimization of microwave-assisted extraction of tea saponin and its application on cleaning of historic silks. J. Surfactants Deterg. 2014, 17, 919–928. [Google Scholar] [CrossRef]
- Zhang, T.J.; Xiong, D.L.; Chen, C.; Xu, G.H.; Chen, J.Z. Separation and Purification of tea saponin with Propyl Alcohol/Ammonium Sulphate Aqueous Two-Phase System. Fine Chem. 2015, 32, 1009–1013. [Google Scholar]



| Serial Number | Concentration (mg/mL) | Injection Pressure (bar) | Peak Area (mAU) |
|---|---|---|---|
| 1 | 10 | 109.36 | 14,556.5 0 ± 0.25 |
| 2 | 5 | 110.98 | 6970.40 ± 0.61 |
| 3 | 2.5 | 108.69 | 3657.0 0 ± 0.44 |
| 4 | 1.25 | 107.96 | 1448.58 ± 0.34 |
| 5 | 0.625 | 108.79 | 637.77 ± 0.48 |
| 6 | 0.3125 | 109.46 | 295.68 ± 0.52 |
| Serial Number | Injection Pressure (bar) | Peak Area (mAU) |
|---|---|---|
| 1 | 107.65 | 822.70 ± 0.84 |
| 2 | 106.96 | 824.30 ± 0.91 |
| 3 | 107.88 | 829.90 ± 0.74 |
| 4 | 105.75 | 830.00 ± 0.23 |
| 5 | 108.96 | 830.00 ± 0.43 |
| 6 | 108.96 | 830.10 ± 0.65 |
| Serial Number | Sample Mass (mg) | Injection Pressure (bar) | Sum of Peak Areas (mAU) |
|---|---|---|---|
| 1 | 10.01 | 107.96 | 1715.10 ± 0.82 |
| 2 | 9.96 | 105.69 | 1708.80 ± 0.64 |
| 3 | 10.01 | 108.46 | 1710.27 ± 0.72 |
| 4 | 9.99 | 107.65 | 1706.46 ± 0.44 |
| 5 | 9.99 | 107.88 | 1708.00 ± 0.52 |
| 6 | 10.00 | 106.44 | 1703.80 ± 0.48 |
| Serial Number | Spiked Amount (mg) | Recovery Amount (mg) | Recovery Rate (%) |
|---|---|---|---|
| 1 | 5.00 | 5.12 | 102.40 ± 0.33 |
| 2 | 5.00 | 4.89 | 97.80 ± 0.45 |
| 3 | 5.00 | 4.86 | 97.20 ± 0.38 |
| 4 | 10.00 | 10.13 | 101.30 ± 0.42 |
| 5 | 10.00 | 10.35 | 103.50 ± 0.22 |
| 6 | 10.00 | 9.98 | 99.80 ± 0.64 |
| 7 | 15.00 | 14.79 | 98.60 ± 0.55 |
| 8 | 15.00 | 15.68 | 104.50 ± 0.43 |
| 9 | 15.00 | 14.81 | 98.70 ± 0.28 |
| Experimental Number Factors | A | B | C | D | Yield/% |
|---|---|---|---|---|---|
| 1 | 55 °C | 150 min | 70% | 3:1 | 21.80 ± 0.21 |
| 2 | 55 °C | 180 min | 75% | 3.5:1 | 22.21 ± 0.32 |
| 3 | 55 °C | 210 min | 80% | 4:1 | 23.42 ± 0.14 |
| 4 | 60 °C | 150 min | 75% | 4:1 | 24.07 ± 0.22 |
| 5 | 60 °C | 180 min | 80% | 3:1 | 19.55 ± 0.24 |
| 6 | 60 °C | 210 min | 70% | 3.5:1 | 17.46 ± 0.33 |
| 7 | 65 °C | 150 min | 80% | 3.5:1 | 17.09 ± 0.25 |
| 8 | 65 °C | 180 min | 70% | 4:1 | 21.13 ± 0.16 |
| 9 | 65 °C | 210 min | 75% | 3:1 | 25.17 ± 0.20 |
| K1 | 67.43 | 62.96 | 60.39 | 66.52 | |
| K2 | 61.08 | 62.89 | 71.45 | 56.76 | |
| K3 | 63.39 | 66.05 | 60.06 | 68.62 | |
| k1 | 22.48 | 20.99 | 20.13 | 22.17 | |
| k2 | 20.36 | 20.96 | 23.82 | 18.92 | |
| k3 | 21.13 | 22.02 | 20.02 | 22.87 | |
| Rj | 2.12 | 1.06 | 3.8 | 3.95 |
| Experimental Number Factors | A | B | C | Purity/% |
|---|---|---|---|---|
| 1 | 20 °C | 9% | 10% | 76.62 ± 0.23 |
| 2 | 20 °C | 10% | 12% | 57.21 ± 0.14 |
| 3 | 20 °C | 11% | 11% | 75.32 ± 0.12 |
| 4 | 30 °C | 9% | 11% | 83.80 ± 0.28 |
| 5 | 30 °C | 10% | 10% | 64.57 ± 0.44 |
| 6 | 30 °C | 11% | 12% | 55.42 ± 0.32 |
| 7 | 40 ℃ | 9% | 12% | 66.73 ± 0.36 |
| 8 | 40 ℃ | 10% | 11% | 65.43 ± 0.33 |
| 9 | 40 °C | 11% | 10% | 73.10 ± 0.21 |
| K1 | 209.15 | 227.15 | 214.29 | |
| K2 | 203.79 | 187.21 | 224.55 | |
| K3 | 205.26 | 203.84 | 179.36 | |
| k1 | 69.72 | 75.72 | 71.43 | |
| k2 | 67.93 | 62.40 | 74.85 | |
| k3 | 68.42 | 67.95 | 59.79 | |
| Rj | 1.79 | 13.32 | 15.06 |
| Level | A | B | C | D |
|---|---|---|---|---|
| 1 | 55 °C | 150 min | 70% | 3:1 |
| 2 | 60 °C | 180 min | 75% | 3.5:1 |
| 3 | 65 °C | 210 min | 80% | 4:1 |
| Level | A | B | C |
|---|---|---|---|
| 1 | 20 °C | 9% | 10% |
| 2 | 30 °C | 10% | 11% |
| 3 | 40 °C | 11% | 12% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Wang, S.; Wan, F.; Zhou, Y.; Wang, Z.; Fan, G.; Wang, P.; Luo, H.; Liao, S.; He, L.; et al. Quantitative Analysis of Camellia oleifera Seed Saponins and Aqueous Two-Phase Extraction and Separation. Molecules 2023, 28, 2132. https://doi.org/10.3390/molecules28052132
Zhu L, Wang S, Wan F, Zhou Y, Wang Z, Fan G, Wang P, Luo H, Liao S, He L, et al. Quantitative Analysis of Camellia oleifera Seed Saponins and Aqueous Two-Phase Extraction and Separation. Molecules. 2023; 28(5):2132. https://doi.org/10.3390/molecules28052132
Chicago/Turabian StyleZhu, Lifang, Shanshan Wang, Faling Wan, Yihong Zhou, Zongde Wang, Guorong Fan, Peng Wang, Hai Luo, Shengliang Liao, Lu He, and et al. 2023. "Quantitative Analysis of Camellia oleifera Seed Saponins and Aqueous Two-Phase Extraction and Separation" Molecules 28, no. 5: 2132. https://doi.org/10.3390/molecules28052132
APA StyleZhu, L., Wang, S., Wan, F., Zhou, Y., Wang, Z., Fan, G., Wang, P., Luo, H., Liao, S., He, L., Yang, Y., Li, X., Zou, X., Chen, S., & Zhang, J. (2023). Quantitative Analysis of Camellia oleifera Seed Saponins and Aqueous Two-Phase Extraction and Separation. Molecules, 28(5), 2132. https://doi.org/10.3390/molecules28052132






