Abstract
Panax ginseng was a traditional Chinese medicine with various pharmacological activities and one of its important activities was hypoglycemic activity; therefore, panax ginseng has been used in China as an adjuvant in the treatment of diabetes mellitus. In vivo and in vitro tests have revealed that ginsenosides, which are derived from the roots and rhizomes of panax ginseng have anti-diabetic effects and produce different hypoglycemic mechanisms by acting on some specific molecular targets, such as SGLT1, GLP-1, GLUTs, AMPK, and FOXO1. α-Glucosidase is another important hypoglycemic molecular target, and its inhibitors can inhibit the activity of α-Glucosidase so as to delay the absorption of dietary carbohydrates and finally reduce postprandial blood sugar. However, whether ginsenosides have the hypoglycemic mechanism of inhibiting α-Glucosidase activity, and which ginsenosides exactly attribute to the inhibitory effect as well as the inhibition degree are not clear, which needs to be addressed and systematically studied. To solve this problem, affinity ultrafiltration screening coupled with UPLC-ESI-Orbitrap-MS technology was used to systematically select α-Glucosidase inhibitors from panax ginseng. The ligands were selected through our established effective data process workflow based on systematically analyzing all compounds in the sample and control specimens. As a result, a total of 24 α-Glucosidase inhibitors were selected from panax ginseng, and it was the first time that ginsenosides were systematically studied for the inhibition of α-Glucosidase. Meanwhile, our study revealed that inhibiting α-Glucosidase activity probably was another important mechanism for ginsenosides treating diabetes mellitus. In addition, our established data process workflow can be used to select the active ligands from other natural products using affinity ultrafiltration screening.
1. Introduction
Panax ginseng, which is a traditional medicinal and edible plant, has been widely used in China and Asia for thousands of years. Modern research has proved that panax ginseng has various pharmacological activities, and as well as having anti-tumor [1], anti-aging [2], anti-oxidation [3], anti-fatigue [4], improvement of immune function, intelligence as well as learning ability [5,6], a protective effect on cerebral ischemia, liver as well as kidneys [7,8,9], and regulation of central nervous system [10], panax ginseng also has the effect of decreasing blood sugar and has been used in China as an adjuvant in the treatment of diabetes mellitus. Accumulating evidence has shown that ginsenosides, which are extracted from panax ginseng, exert anti-diabetic effects [11,12,13,14,15]. In vivo and in vitro tests revealed that ginsenosides have anti-diabetic effects mainly because they act on some specific molecular targets. For example, ginsenoside Rg1, Rg3, F2, compound K, and Rh2 could effectively reduce intestinal glucose uptake through the regulation of sodium-glucose cotransporters 1 (SGLT1) gene expression [16,17]; ginsenoside Rg3 reduces blood glucose and increases plasma glucagon-like peptide-1(GLP-1) and plasma insulin through the improvement of insulin resistance, lipid metabolism, energy metabolism, and gut flora metabolism [11]; ginsenoside Rb1 can promote the translocation of glucose transporter to increase glucose uptake in adipocytes, and this reduces fasting glucose through recovery in the expression of glucose transporters (GLUTs) and the phosphorylation of Akt in the adipose tissue of db/db mice [18]; ginsenoside Rg1, Rb3, and compound K can reduce gluconeogenesis through increase AMP-activated protein kinase (AMPK) expression and decreased Forkhead transcription factor 1 (FOXO1) activity [19,20,21], etc. All these modern research results demonstrate that ginsenosides can produce different hypoglycemic mechanisms by acting on different molecule targets. However, besides the above-mentioned pharmacological mechanisms of the anti-diabetic effects of drugs, inhibiting the activity of α-Glucosidase so as to delay the absorption of dietary carbohydrates and finally reduce postprandial blood sugar is another important hypoglycemic mechanism. For ginsenosides, the anti-diabetic effects, whether they have the mechanism of inhibiting α-Glucosidase activity, and which ginsenosides exactly attribute to the inhibitory effect as well as the inhibition degree are not clear and need to be addressed and systematically studied.
In a previous study, the main approach for identifying active ingredients from the roots of panax ginseng was separately testing their biological activity after chemical separation one by one [22]. However, the experiment period is long with a heavy workload, and even some active ingredients with trace amounts are lost during separation. Moreover, it is impossible to achieve high-throughput screening. In order to achieve rapid screening and identification of α-glucosidase inhibitors from natural products, some researchers developed a novel at-line nanofractionation screening platform, in which a time-course bioassay based on high-density well-plates was performed in parallel with high-resolution mass spectrometry (MS), providing a straightforward and rapid procedure to simultaneously obtain chemical and biological information of active compounds, and with this approach, the efficiency and quality of screening can be increased [23]. However, in our study, another much more useful technology for screening α-glucosidase inhibitors, affinity ultrafiltration screening-mass spectrometry, which combined affinity ultrafiltration screening technology with liquid chromatogram-mass spectrometry (LC-MS) technology, was employed. Due to it having the advantage of making the screening of active components from natural products more convenient and effective, it has played an increasingly vital role in early drug discovery [24,25,26,27]. Through affinity ultrafiltration screening technology, the small molecule ligands bound to biological macromolecules can be obtained, and through LC-MS technology, especially through ultra-performance liquid chromatography (UPLC) coupled with ESI-Orbitrap-MS technology which can provide high-resolution MS spectra, the structures of the selected ligands can be rapid analysis and characterization. However, during the selection of ligands from dissociation solution obtained through affinity ultrafiltration screening, some researchers visually compared chromatographic peak intensity on liquid chromatograms of the sample group containing target protein with the control group containing denatured/unadded target protein, and the compounds with higher intensities in the sample group were selected as the ligands [24,25,26,27]. However, when peaks contain many overlapping signals, this approach will lead to false positive or false negative results. Under these circumstances, an effective data process workflow for ligands selection should be established.
Based on the above problems that need to be solved urgently, we used affinity ultrafiltration screening coupled with UPLC-ESI-Orbitrap-MS technology to systematically select α-Glucosidase inhibitors from panax ginseng, in which the major components were ginsenosides, and meanwhile established an effective data process workflow to systematically select the small molecule ligands instead of previous visual comparison of chromatographic peak intensity on liquid chromatograms of the sample and the control specimens. Here, through our integrated affinity ultrafiltration screening-MS and established data process workflow, a total of 24 active ingredients were selected from panax ginseng, including 14 known ginsenosides and 10 potential new ginsenosides. Our study first systematically selected and characterized the α-Glucosidase inhibitors from panax ginseng and revealed that inhibiting α-Glucosidase activity probably was another important mechanism for the hypoglycemic effect of ginsenosides, which will improve cognition in people undergoing ginsenosides treatment of diabetes mellitus.
2. Results and Discussion
2.1. Selection of α-Glucosidase Inhibitors by Affinity Ultrafiltration Screening–LC-UPLC-ESI-Orbitrap-MS
The designed process of selection of α-Glucosidase inhibitors is briefly introduced as follows: when the extract of panax ginseng was incubated with α-Glucosidase, active ligands could bind to the active site of α-Glucosidase forming receptor–ligands complexes, and unbound small molecules were free, which could be separated from receptor–ligands complexes by using an ultrafiltration membrane. Then, the receptor–ligands complexes were disrupted by the addition of dissociation agent, and the released ligands were analyzed by UPLC-ESI-Orbitrap-MS analysis. The full scan data was analyzed by our established data process workflow based on systematic analysis of all compounds in the specimens to select the active ingredients, and the ingredient with the peak area ratio (PAR) value, which was defined as the ratio of peak area of a compound detected in the sample group to that detected in the control group, >1 and p < 0.05 (n = 4) was selected as the ligand.
Figure 1 shows the total ion chromatograms of panax ginseng extract, sample, and control specimens. From their total ion chromatograms, we found that there were many overlapping signals due to ginsenosides in panax ginseng owning similar chemical structures and polarity, leading to their poor separation in the reversed chromatographic column. Even in the optimized chromatographic conditions, it is still difficult to achieve their complete separation. Therefore, directly comparing the intensity of each peak in their total ion chromatograms between the sample and the control groups can lead to false positive or false negative results. In our experiment, we established an effective workflow to systematically analyze all compounds in the sample specimens, and compounds not only with high abundance but also with lower intensity even covered by others were analyzed. Each compound of PAR value and its mean PAR value were calculated according to the obtained peak areas yielded by the compounds-extracting workflow. A two-tailed t-test was used to calculate the significant difference (p value) in the peak area of each compound between the sample and the control group. The compounds, whose mean PAR values >1 and p < 0.05 (n = 4), were selected as the potential α-Glucosidase inhibitors, and as a result, 24 ligands (shown in Table 1 and Supplementary Materials) including 14 known ginsenosides (R1–R14) and 10 unknown ginsenosides (R15–R24) were successfully selected, and the data of affinity ultrafiltration screening was shown in Supplementary Information Data S1.
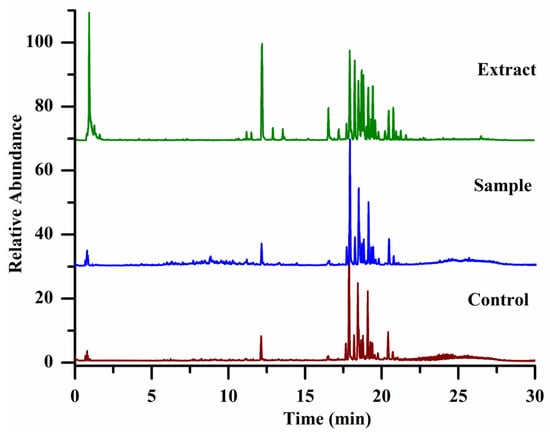
Figure 1.
The total ion chromatograms of specimens, the extract of panax ginseng (green color); the specimens of the sample group (blue color), and the control group (red color).

Table 1.
The α-Glucosidase inhibitors selected from panax ginseng extract.
From Table 1, we found that the PAR values of the selected were all >1, meaning the intensities of these compounds in the sample group were higher than those in the corresponding control group. In order to confirm that there was indeed a difference in peak intensity between the sample and the control group for the selected ligands, we separately extracted their ion chromatograms in the sample and control specimens, and we found all of the selected ligands exhibiting higher intensities in the sample group, meaning our established data process workflow was reliable and effective. Figure 2 showed the PAR values of some representative ligands. The precursor ions of all the selected ligands were then used to perform targeted MS/MS analysis for structure identification. Although we could characterize the selected ligands based on their high-resolution MS and fragmentation ions produced by targeted MS/MS analysis, unambiguous structural elucidation requires strict confirmation with reference standards. To this end, we obtained the 14 reference standards (R1–R14) (shown in Figure 3) through commercial sources. The identification of these ligands is based on matching both retention time deviation (<0.1 min) and accurate mass deviation (<5 ppm) with the corresponding reference standards. The unknown ligands R15–R24 were elucidated according to the diagnostic ions and fragmentation pathways of ginsenosides, and we found that most of the unknown ligands were the isomers of the known ligands. For example, R15 and R18 showed the same precursor ions at m/z 955.4904 with a mass deviation of 0.10 ppm indicating their molecular formula was C48H76O19. In their MS/MS spectra, their diagnostic ions were observed at m/z 455.3534 suggesting that they were oleanolic-type ginsenosides. The fragmentation ions at m/z 793.4376, 731.4376, 613.3737, 569.3847, and 455.3534 were the same as those of ginsenoside Ro (R8). Thus, R15 and R18 were separately deduced as ginsenoside Ro isomers. Similarly, R16 and R22 were separately deduced as ginsenoside Ra1 isomer or ginsenoside Ra2 isomer due to their fragmentation ions being the same as those of ginsenoside Ra1 or ginsenoside Ra2. R24 was deduced as a quinquenoside R1 isomer due to its fragmentation ions were the same as those of quinquenoside R1. In the MS/MS spectra of R17 and R21, after the loss of Ac (42 Da), their remaining fragmentation ions were the same as those of ginsenoside Rd; thus, R17 and R21 were separately deduced as acetyl-ginsenoside Rd. In the same way, R19 was deduced as (E)-but-2-enoyl ginsenoside Rd because after the loss of (E)-but-2-enoyl (68 Da), the remaining fragmentation ions were the same as those of ginsenoside Rd. The precursor ion of R20 was observed at m/z 943.5251 with a mass deviation of −1.59 ppm indicating its molecular formula was C48H80O18. In its MS/MS spectra, its aglycone ion was obtained at m/z 457.3699, which was 18 Da less than the aglycone of protopanaxatriol (m/z 475 in negative-ion mode); thus, we considered the aglycone ion m/z 457.3699 was dehydrated-protopanaxatriol. The fragmentation ions of m/z 781.4766, 619.4214, and 457.3699 suggested that Glc, Glc, Glc were successively eliminated from the precursor ion. Thus, R20 was deduced as dehydrated-protopanaxatriol + 3Glc. The precursor ion of R23 was observed at m/z 1105.5779 with a mass deviation of −1.45 ppm, suggesting its molecular formula was C54H90O23. In its MS/MS spectra, its aglycone ion was obtained at m/z 457.3698, which was formed by successive losses of 4Glc from the precursor ion m/z 1105.5779. Thus, R23 was deduced as dehydrated-protopanaxatriol + 4Glc. The fragmentation ions of all 24 ligands are shown in Table 1.
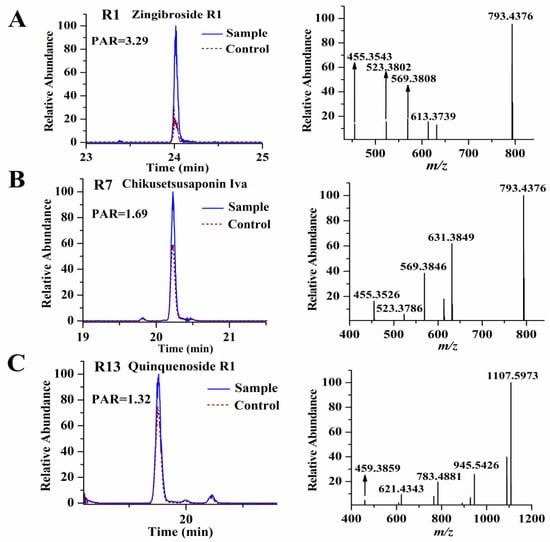
Figure 2.
The PAR values of some representative ligands, zingibroside R1 (A); chikusetsusaponin Iva (B); and quinquenoside R1 (C).
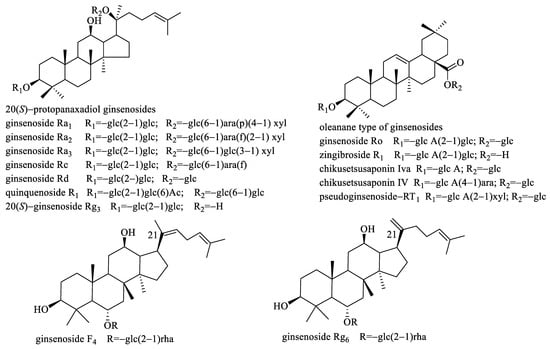
Figure 3.
The structures of the 14 (R1–R14) reference standards.
As we know, there are mainly three types of ginsenosides in panax ginseng, including protopanaxadiol-, protopanaxatriol-, and oleanolic acid-type. Among the selected ligands, seven ginsenosides including R1, R4–R6, R8, R15, and R18 belong to oleanolic acid-type, whereas thirteen ginsenosides including R2, R7, R10–R14, R16, R17, R19, R21, R22 and R24 belong to protopanaxadiol-type. However, only four ginsenosides, including R3, R9, R20, and R23 are protopanaxatriol-type. Thus, it can be seen that protopanaxadiol-type ginsenosides are the main α-Glucosidase inhibitors, and oleanolic acid-type ginsenosides come second. Nevertheless, protopanaxatriol-type ginsenosides which are one of the most important ginsenosides in panax ginseng own the least number of ligands with much lower abundance.
It is worth noting that through our established data process workflow, much more ligands were selected, and not only were the known compounds with higher amounts (R1–R14) selected and characterized as α-Glucosidase inhibitors but also some unknown compounds (R15–R24) with lower abundance covered by other compounds, and these unknown compounds were probably potential new compounds.
2.2. Molecular Docking of α-Glucosidase and Ligands
In order to predict the affinity between the selected ligands and α-Glucosidase, the selected ligands were docked with α-Glucosidase. The compounds (R1–R14) owning the definite structures were downloaded from PubChem and their 3D structures were docked with pre-processed α-Glucosidase. Generally, when the protein–ligand interaction was analyzed using AutoDock vina (version 1.5.6), affinity ≤ −7 kcal/mol indicated the compounds had strong binding with the target protein. After molecular docking with α-Glucosidase, the affinities of R1–R14 were all in the range of −7.2–−9.0 kcal/mol, indicating they all had a strong affinity with α-Glucosidase. In addition, acarbose, which was widely used as an α-Glucosidase inhibitor, was also docked with the pre-processed α-Glucosidase and its affinity was −7.1 kcal/mol. From another aspect, the results mentioned above verified the reliability of the affinity ultrafiltration screening. However, R15–R24 could not be docked with α-Glucosidase due to the fact that they were probably new compounds, and their definite structures were unknown yet.
2.3. α-Glucosidase Inhibitory Activity of Ligands
To further verify the α-Glucosidase inhibitory activity of the selected ligands, in vitro enzyme inhibition assay was performed. Due to only the reference standards of R1–R14 being commercially available, these fourteen compounds were performed in vitro enzyme inhibition assay. However, due to R2 and R7 being insoluble in a 0.1 M phosphate buffer (pH 6.8) even though co-solvent DMSO (2%) was added, their in vitro enzyme inhibition assay was not performed. The remaining twelve compounds were easily soluble in a 0.1 M phosphate buffer (pH 6.8) and their α-Glucosidase inhibitory activities were tested. The results are shown in Table 2. From Table 2, we found that oleanolic acid-type ginsenoside zingibroside R1 (R1) exhibited much stronger α-Glucosidase inhibitory activity with the IC50 value of 3.61 mM, even superior to the positive control acarbose (IC50 value of 5.25 mM). Nevertheless, the other selected oleanolic acid-type ginsenosides displayed weaker α-Glucosidase inhibitory activities than acarbose, such as pseudoginsenoside-RT1 (R4) and chikusetsusaponin Iva (R6) with the IC50 values of 39.30 mM and 17.33 mM, respectively, whereas chikusetsusaponin IV (R5) and ginsenoside Ro (R8) separately exhibited 16.20% and 20.23% inhibition rate at 40 mM. For the tested protopanaxadiol-type ginsenosides, all of them displayed lower α-Glucosidase inhibitory activities, and except for ginsenoside Rc (R14) was with the IC50 value 36.83 mM, the inhibition rate of ginsenoside Ra1 (R10), as well as ginsenoside Ra2 (R11), were <30% at 40 mM whereas the inhibition rate of quinquenoside R1 (R12), as well as ginsenoside Ra3 (R13), were <20% at 24 mM. For the tested protopanaxatriol-type ginsenosides, ginsenoside F4 (R9) displayed stronger α-Glucosidase inhibitory activity than ginsenoside Rg6 (R3). From Figure 3, we found that the difference in their structures is the double bond position at C21, and ginsenoside F4 (R9) owns a non-terminal double bond whereas ginsenoside Rg6 (R3) owns a terminal double bond. This implied that the double bond position of ginsenosides could affect the activation degree of ginsenosides, and the non-terminal double bond was better than the terminal double bond.

Table 2.
The affinity of ligands for α-Glucosidase and their inhibitory activities on α-Glucosidase.
3. Materials and Methods
3.1. Samples, Reference Standards, and Reagents
α-Glucosidase from Saccharomyces cerevisiae was purchased from Sigma (Enzyme Commission number: 3.2.1.20, 100 units, St. Louis, MO, USA), and p-Nitrophenyl-α-d-glucopyranoside (pNPG) was obtained from Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Ammonium acetate buffer (10 mM, pH 6.86) and phosphate buffer (0.1 M, pH 6.8) were obtained from Applygen Technologies Inc. (Beijing, China) LC-MS-grade formic acid was obtained from Fisher-Scientific (Fair Lawn, NJ, USA) whereas LC-MS-grade acetonitrile and methanol were purchased from Merck (Darmstadt, Germany). The distilled water was obtained from Watsons.
The roots and rhizomes of panax ginseng were supplied by the Scientific Research Institute of Beijing Tongrentang Co., Ltd. A total of 14 reference standards (shown in Figure 3), including zingibroside R1 (R1), 20(S)-ginsenoside Rg3 (R2), ginsenoside Rg6 (R3), pseudoginsenoside-RT1 (R4), chikusetsusaponin IV (R5), chikusetsusaponin Iva (R6), ginsenoside Rd (R7), ginsenoside Ro (R8), ginsenoside F4 (R9), ginsenoside Ra1 (R10), ginsenoside Ra2 (R11), quinquenoside R1 (R12), ginsenoside Ra3 (R13), and ginsenoside Rc (R14), were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). The purity of all the reference standards was >98%.
3.2. Sample Preparations
The panax ginseng was pulverized into powder (just like flour). The powder of panax ginseng (15.0 g) was ultrasonically extracted for 30 min with 150 mL 70% methanol at 25 °C. The extracted solution was then filtered through a filter paper. This extraction was repeated twice. The filtrate was combined and evaporated to dryness (3.5 g) using a rotary evaporator at 40 °C. The residue (10 mg) was then dissolved in 4 mL of ammonium acetate buffer (10 mM, pH 6.86) and filtered through a 0.22-μm nylon filter membrane to obtain the sample of affinity ultrafiltration screening.
3.3. Screening of α-Glucosidase Inhibitors from Panax Gingseng by Ultrafiltration-ESI-Orbitrap-MS
3.3.1. Affinity Ultrafiltration Screening
The affinity ultrafiltration procedure was performed according to the modified method of Li [28]. α-Glucosidase was dissolved in 10 mM ammonium acetate buffer (pH 6.86) to obtain the α-Glucosidase solution (40 U/mL). A total of 100 μL of 2.5 mg/mL panax ginseng sample solution was incubated for 30 min at 37 °C with 100 μL α-Glucosidase (40 U/mL). After incubation, the mixture was filtered through an ultrafiltration centrifugal filter (AMICON ULTRA, 0.5 mL, 10 kDa, Millipore, MA, USA) containing a regenerated cellulose ultrafiltration membrane with a 10,000 MW cut-off at 25 °C. The filter was washed with 250 μL ammonium acetate buffer (pH 6.86) by centrifugal force at 14,000 r/min for 10 min to remove the unbound compounds. This process was then repeated four times. Then, 100 μL of methanol–water (50:50; v/v, pH 3.30) was added to release the bound ligands, and centrifugation was followed (14,000 r/min for 15 min). The release process was then repeated twice. All of the dissociation solution was combined and evaporated to dryness using a nitrogen-blowing instrument. The residue was re-dissolved in 50 μL methanol–water (50:50; v/v) for LC-ESI-Orbitrap-MS analysis. The control experiment was carried out with denatured enzyme (in boiling water for 10 min). Each pair of sample and control specimens was prepared in four replicates.
3.3.2. UPLC-ESI-Orbitrap-MS Analysis
The re-dissolved solution was analyzed on a Vanquish™ Flex UPLC system (Thermo Scientific, Waltham, MA, USA) equipped with a binary pump and a thermostated column compartment. Multiple components were separated on a Waters ACQUITY UPLC® BEH C18 column (2.1 × 100 mm, 1.7 μm) (Waters, Milford, MA, USA) coupled with an ACQUITY UPLC® BEH C18 VanGuardTM Pre-Column (2.1 × 5 mm, 1.7 μm) using mobile phase A (0.1% formic acid/water, v/v) and mobile phase B (acetonitrile) by the following gradient elution program: 0–7 min, 2–20% B; 7–10 min, 20–25% B; 10–20 min, 25–40% B; 20–25 min, 40–65% B; 25–30 min, 65–95% B. The temperature was set at 35 °C, and the flow rate was 0.3 mL/min. The injection volume was 2 μL.
An Orbitrap Exploris 240 mass spectrometer (Thermo Scientific, Waltham, MA, USA) equipped with a Heated ESI source was used to acquire the mass spectra and negative-ion mode was adopted. The MS parameters were as follows: ion spray voltage: 2500 V, sheath gas: 5.08 L/min, auxiliary gas: 9.37 L/min, ion transfer tube temperature: 320 °C, vaporizer temperature: 350 °C, scan range (m/z): 150–2000, and collision-energy voltage: 35 V. The full scan was operated at a mass resolution of 60,000 whereas the MS2 scan was operated at a mass resolution of 15,000. An internal calibration source, Thermo Scientific EASY-ICTM (Thermo Scientific, Waltham, MA, USA), was adopted to calibrate the entire mass range.
The re-dissolved solution was first analyzed in a full scan mode to minimize signal loss, and then the precursor ions of the selected α-Glucosidase inhibitors were performed targeted MS/MS analysis for structure characterization.
3.4. Data Process
Screening ligands from multiple compounds requires some strategies, and in our experiment, we established an effective data process workflow that mainly included three steps:
- ①
- Extracting all compounds in each specimen
To comprehensively screen the ligands of α-Glucosidase from the crude extract of panax ginseng, we used a workflow to systematically analyze each compound in the sample and control group. The Compound DiscovererTM software (Thermo ScientificTM, version 3.2.0.421) was used for the data process, and all compounds in each specimen were processed by peak alignment and peak extraction based on our extracting workflow “input files → select spectra → align retention time → detect compounds → group compounds”. In the step of “input files,” all LC/MS data files of sample and control specimens were input, whereas in the step of “select spectra,” the entire run time (0–30 min) of the spectra, as well as the negative-ion mode, was selected for further processing. In the “align retention time” step, the retention times of all LC-MS data files were aligned (mass tolerance: 5 ppm), whereas in the “detect compounds” step, all compounds in LC-MS data files were extracted using component elucidator algorithm (mass tolerance: 5 ppm; intensity tolerance: 30; S/N threshold: 3; minimum peak intensity: 100,000; extracted ions: [M-H]−, [M-H+HAc]−; minimum element composition: CHO, and maximum element composition: C90H190O90. The same substances detected in different addition methods were grouped by molecular weight (mass tolerance: 5 ppm) as well as retention time (RT tolerance: 0.1 min) across all files in the “group compounds” step. Running this extracting workflow, we obtained the information of each compound existing in each specimen, including molecular weight, retention time, peak area, etc., which were used for further data analysis.
- ②
- Calculating the peak area ration of each compound and t-test
Ideally, after specific binding to α-Glucosidase, the peaks of compounds incubated with α-Glucosidase showed higher intensities or bigger peak areas than those of compounds incubated with denatured enzyme, meaning the PAR value for a ligand was >1. In this step, we calculated the PAR value of every compound in each specimen, and then the mean PAR value of each compound was calculated. The significant difference in peak area of each compound between the sample and the control group was determined by a two-tailed t-test. Finally, potential α-Glucosidase inhibitors were selected based on a mean PAR > 1 and p < 0.05 from four replicates.
- ③
- Characterizing the structures of ligands
The precursor ions of the selected potential α-Glucosidase inhibitors were performed targeted MS/MS analysis and the ingredients were characterized according to the MS/MS spectra obtained.
3.5. Molecular Docking of α-Glucosidase and Ligands
The 3D coordinate of α-Glucosidase was retrieved from the Protein Data Bank (PDB code: 5ZCB) [29]. The α-Glucosidase structure was pre-processed by removing solvent and adding hydrogen atoms. The 2D structures of ligands were downloaded from PubChem and then their 3D structures were generated in Chemdraw Ultra (version 14.0, http://www.cambridgesoft.com/, accessed on 13 January 2023). Molecular docking was performed with AutoDock vina (version 1.5.6, https://vina.scripps.edu/, accessed on 13 January 2023), and structural cartoons were prepared using PyMOL (version 2.4.0, https://pymol.org/2/, accessed on 13 January 2023).
3.6. α-Glucosidase Inhibitory Activity Assay
The α-Glucosidase inhibitory activity assay was performed in 96-well plates according to the modified method of Jiang [30]. Both α-glucosidase and pNPG were dissolved in a 0.1 M phosphate buffer (pH 6.8). Each tested compound was also dissolved in 0.1 M phosphate buffer to give solutions of various concentrations. A total of 40 μL of the tested compound solution was mixed with 40 μL α-Glucosidase solution (0.2 U/mL). After incubation for 5 min at 37 °C, 20 μL of pNPG solution (2 mM), which was used as a substrate, was added and then incubated for 30 min at 37 °C. The amount of released nitrophenyl product was measured on an Epoch 2 microplate spectrophotometer (BioTek) at 405 nm. Controls contained the same reaction mixture, except the same volume of phosphate buffer was added instead of a solution of tested compounds. Acarbose was used as the positive control. The inhibition (%) of the tested ligands on α-Glucosidase was calculated as: (Aa − Ab)/Aa × 100%, where Aa was the absorbance of the control, and Ab was the absorbance of the tested compound.
4. Conclusions
In our study, α-Glucosidase inhibitors from panax ginseng were systematically selected and characterized using affinity ultrafiltration screening combined with the UPLC-ESI-Orbitrap-MS method, which has the advantages of shortening the experimental period, reducing the workload, and achieving large-scale and high-throughput screening compared to the traditional active ingredient selection method based on multiple extraction and separation. The ligands were selected through our established data process workflow based on systematic analysis of all compounds in the dissociation solution, and as a result, a total of 24 ligands were selected as α-Glucosidase inhibitors, including 14 known ginsenosides and 10 unknown ginsenosides. The α-Glucosidase inhibitor’s activity of ligands with definite structures were verified by molecular docking and in vitro enzyme inhibition assay. For ginsenosides, our study first systematically selected and characterized the α-Glucosidase inhibitors and revealed that inhibiting α-Glucosidase activity probably was another important mechanism for hypoglycemic effect of ginsenosides. In addition, our established data process workflow has two advantages. First, when samples containing many overlapping signals, such as the extract of panax ginseng, our data process workflow could avoid false positive/negative results due to each compound in the specimens being analyzed. The second advantage was much more ligands were selected including ingredients with lower or much lower intensities which were easily neglected by visual comparison of chromatographic peak intensity on liquid chromatograms or total ion chromatograms mode. Therefore, our established data process workflow can be used to select the active ligands from other natural products using affinity ultrafiltration screening.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28052069/s1, Table S1: The data of affinity ultrafiltration screening of ligands.
Author Contributions
The listed authors contributed to this work as follows: H.-P.W. and Z.-B.W. conceived and designed the experiments, H.-P.W., C.-L.F., Z.-Z.L., Q.Y., C.Z., P.P., R.Z. and Z.-J.W. performed the experiments and analyzed the data, J.D. supervised the execution of the work, H.-P.W. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding, and the APC was funded by Scientific Research Institute of Beijing Tongrentang Co., Ltd.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the first author.
Acknowledgments
This research was supported by the Scientific Research Institute of Beijing Tongrentang Co., Ltd. (Address: A14 Waiguan East St, Chaoyang District, Beijing).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, M.; Wang, X.; Wang, Y.; Bao, S.C.; Chang, Q.; Liu, L.L.; Zhang, S.; Sun, L.W. Strategies for remodeling the tumor microenvironment using active ingredients of ginseng-A promising approach for cancer therapy. Front. Pharmacol. 2021, 12, 797634. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.Q.; Ding, C.B.; Peng, X.J.; Chen, H.Y.; Dong, L.; Zhang, Y.; Chen, X.Y.; Liu, W.C.; Luo, Y.Q. Ginseng under forest exerts stronger anti-aging effects compared to garden ginseng probably via regulating PI3K/AKT/mTOR pathway, SIRT1/NF-κB pathway and intestinal flora. Phytomedicine 2022, 105, 154365. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.H.; Kim, S.W.; Seo, H.W.; Youn, S.H.; Kyung, J.S.; Lee, Y.Y.; In, G.; Park, C.K.; Han, C.K. Physiological and pharmacological features of the non-saponin components in Korean Red Ginseng. J. Ginseng Res. 2020, 44, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.S.; Kang, H.R.; Jung, C.Y.; Park, S.S.; Lee, S.H.; Kim, E.J. Efficacy of Korean red ginseng (Panax ginseng) for middle-aged and moderate level of chronic fatigue patients: A randomized, double-blind, placebo-controlled trial. Complement. Ther. Med. 2020, 48, 102246. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; Qi, Y.L.; Qi, Z.; Gao, K.; Gong, R.Z.; Shao, Z.J.; Liu, S.X.; Li, S.S.; Sun, Y.S. A comparative study on the effects of different parts of panax ginseng on the immune activity of cyclophosphamide-induced immunosuppressed mice. Molecules 2019, 24, 1096. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.X.; Zhang, X.Y.; Zhang, Y.G.; Zhang, X.; Shan, M.Y.; Guan, S.G.; Qiu, Z.D.; Zhu, D.F.; Luo, H.M. Exploring the potential of ginseng glycoprotein to improve learning and memory in mice via Notch signaling pathway and structural analysis using multi-information fusion based on liquid chromatography-mass spectrometry. J. Ethnopharmacol. 2023, 303, 115978. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.Q.; Hu, S.; Zhang, C.X.; Zhou, Q.; Wang, H.; Yang, Y.; Liu, C.; Ding, H.Y. Ginsenoside Rd attenuates cerebral ischemia/reperfusion injury by exerting an anti-pyroptotic effect via the miR-139-5p/FoxO1/Keap1/Nrf2 axis. Int. Immunopharmacol. 2022, 105, 108582. [Google Scholar] [CrossRef]
- Mostafa, R.E.; Shaffie, N.M.; Allam, R.M. Panax Ginseng alleviates thioacetamide-induced liver injury in ovariectomized rats: Crosstalk between inflammation and oxidative stress. PLoS ONE 2021, 16, e0260507. [Google Scholar] [CrossRef]
- Jin, D.; Zhang, Y.Q.; Zhang, Y.H.; Duan, L.Y.; Zhou, R.R.; Duan, Y.Y.; Sun, Y.T.; Lian, F.M.; Tong, X.L. Panax Ginseng C.A. Mey. as Medicine: The Potential Use of Panax Ginseng C.A. Mey. as a Remedy for Kidney Protection from a Pharmacological Perspective. Front. Pharmacol. 2021, 12, 734151. [Google Scholar] [CrossRef]
- Lu, J.; Wang, X.; Wu, A.X.; Cao, Y.; Dai, X.L.; Liang, Y.D.; Li, X.F. Ginsenosides in central nervous system diseases: Pharmacological actions, mechanisms, and therapeutics. Phytother. Res. 2022, 36, 1523–1544. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, W.J.; He, S.B.; Sun, Y.F.; Meng, X.B.; Sun, G.B.; Sun, X.B. Ginsenoside Rb1 as an anti-diabetic agent and its underlying mechanism analysis. Cells 2019, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Nam, J.; Ahn, C.W.; Kim, Y.S. Anti-diabetic properties of different fractions of Korean red ginseng. J. Ethnopharmacol. 2019, 236, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.D.; Quan, H.Y.; Jung, M.S.; Kim, S.J.; Huang, B.; Kim, D.Y.; Chung, S.H. Anti-Diabetic Effect of Pectinase-Processed Ginseng Radix (GINST) in High Fat Diet-Fed ICR Mice. J. Ginseng Res. 2011, 35, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Kim, N.; Lee, K.; Sonn, C.H.; Lee, J.E.; Kim, S.T.; Baeg, I.H.; Lee, K.M. Korean red ginseng (Panax ginseng) ameliorates type 1 diabetes and restores immune cell compartments. J. Ethnopharmacol. 2012, 144, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Wang, L.M.; Su, H.; Zhang, L.; Yang, Y.; Sun, L.; Wu, Y.; Ran, L.; Liu, S.D.; Yin, M.; et al. Ginsenoside Rg1 improved diabetes through regulating the intestinal microbiota in high-fat diet and streptozotocin-induced type 2 diabetes rats. J. Food Biochem. 2022, 46, e14321. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.M.; Tu, Y.K.; Liu, I.M.; Chen, P.F.; Cheng, J.T. Mediation of β-endorphin by ginsenoside Rh2 to lower plasma glucose in streptozotocin-induced diabetic rats. Planta Med. 2006, 72, 9–13. [Google Scholar] [CrossRef]
- Wang, C.W.; Su, S.C.; Huang, S.F.; Huang, Y.C.; Chan, F.N.; Kuo, Y.H.; Hung, M.W.; Lin, H.C.; Chang, W.L.; Chang, T.C. An essential role of cAMP response element binding protein in ginsenoside Rg1-mediated inhibition of Na+/glucose cotransporter 1 gene expression. Mol. Pharmacol. 2015, 88, 1072–1083. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Q.; Wu, X.; Zhao, X.; Zhao, L.; Tong, X. New Insights into the mechanisms of Chinese herbal products on diabetes: A focus on the bacteriamucosal immunity-inflammation-diabetes axis. J. Immunol. Res. 2017, 2017, 1813086. [Google Scholar] [CrossRef]
- Shang, W.B.; Guo, C.; Zhao, J.; Yu, X.Z.; Zhang, H. Ginsenoside Rb1 upregulates expressions of GLUTs to promote glucose consumption in adiopcytes. China J. Chin. Mater. Med. 2014, 39, 4448–4452. [Google Scholar]
- Liu, Q.; Zhang, F.G.; Zhang, W.S.; Pan, A.; Yang, Y.L.; Liu, J.F.; Li, P.; Liu, B.L.; Qi, L.W. Ginsenoside Rg1 inhibits glucagon-induced hepatic gluconeogenesis through Akt-FoxO1 interaction. Theranostics 2017, 7, 4001–4012. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Su, X.; Li, W.; Zheng, Y. Ginsenoside Rb3 Strengthens the hypoglycemic effect through AMPK for inhibition of hepatic gluconeogenesis. Exp. Ther. Med. 2017, 13, 2551–2557. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.P.; Yang, X.B.; Yang, X.W.; Liu, J.X.; Xu, W.; Zhang, Y.B.; Zhang, L.X.; Wang, Y.P. Ginsenjilinol, a new protopanaxatriol-type saponin with inhibitory activity on LPS-activated NO production in macrophage RAW 264.7 cells from the roots and rhizomes of Panax ginseng. J. Asian Nat. Prod. Res. 2013, 15, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.J.; Kool, J.; Jian, J.Y.; Wang, J.C.; Zhao, X.L.; Jiang, Z.J.; Zhang, T.T. Rapid screening α-Glucosidase inhibitors from natural products by at-line nanofractionation with parallel mass spectrometry and bioactivity assessment. J. Chromatogr. A 2021, 1635, 461740. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.F.; Liu, Q.; Cong, D.L.; Zhang, H.; Yu, J.J.; Jiang, Y.; Cui, X.Y.; Sun, J.M. Screening and determination for potential α-glucosidase inhibitory constituents from Dalbergia odorifera T. Chen using ultrafiltration-LC/ESI-MSn. Biomed. Chromatogr. 2013, 27, 1621–1629. [Google Scholar] [CrossRef]
- Zhou, H.; Xing, J.P.; Liu, S.; Song, F.R.; Cai, Z.W.; Pi, Z.F.; Liu, Z.Q.; Liu, S.Y. Screening and determination for potential α-Glucosidase inhibitors from leaves of acanthopanax senticosus Harms by using UF-LC/MS and ESI-MSn. Phytochem. Anal. 2012, 23, 315–323. [Google Scholar] [CrossRef]
- Zhou, X.L.; Liang, J.S.; Zhang, Y.; Zhao, H.D.; Guo, Y.; Shi, S.Y. Separation and purification of α-Glucosidase inhibitors from Polygonatum odoratum by stepwise high-speed counter-current chromatography combined with Sephadex LH-20 chromatography target-guided by ultrafiltration–HPLC screening. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 15, 149–154. [Google Scholar] [CrossRef]
- Wu, B.; Song, H.P.; Zhou, X.; Liu, X.G.; Gao, W.; Dong, X.; Li, H.J.; Li, P.; Yang, H. Screening of minor bioactive compounds from herbal medicines by in silico docking and the trace peak exposure methods. J. Chromatogr. A 2016, 4, 91–99. [Google Scholar] [CrossRef]
- Li, H.L.; Song, F.R.; Xing, J.P.; Tsao, R.; Liu, Z.Q.; Liu, S.Y. Screening and structural characterization of α-Glucosidase inhibitors from Hawthorn leaf flavonoids extract by ultrafiltration LC-DAD-MSn and SORI-CID FTICR MS. J. Am. Soc. Mass Spectrom. 2009, 20, 1496–1503. [Google Scholar] [CrossRef]
- Auiewiriyanukul, W.; Saburi, W.; Kato, K.; Yao, M.; Mori, H. Function and structure of GH13_31 α-glucosidase with high α-(1→4)-glucosidic linkage specificity and transglucosylation activity. FEBS Lett. 2018, 592, 2268–2281. [Google Scholar] [CrossRef]
- Jiang, W.Y.; Kan, H.; Li, P.D.; Liu, S.; Liu, Z.Y. Screening and structural characterization of potential α-glucosidase inhibitors from Radix Astragali flavonoids extract by ultrafiltration LC-DAD-ESI-MSn. Anal. Methods 2015, 7, 123–128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).