Polygonati Rhizoma Polysaccharide Prolongs Lifespan and Healthspan in Caenorhabditis elegans
Abstract
1. Introduction
2. Results
2.1. Analysis of the Monosaccharide Composition of RPRP and PPRP
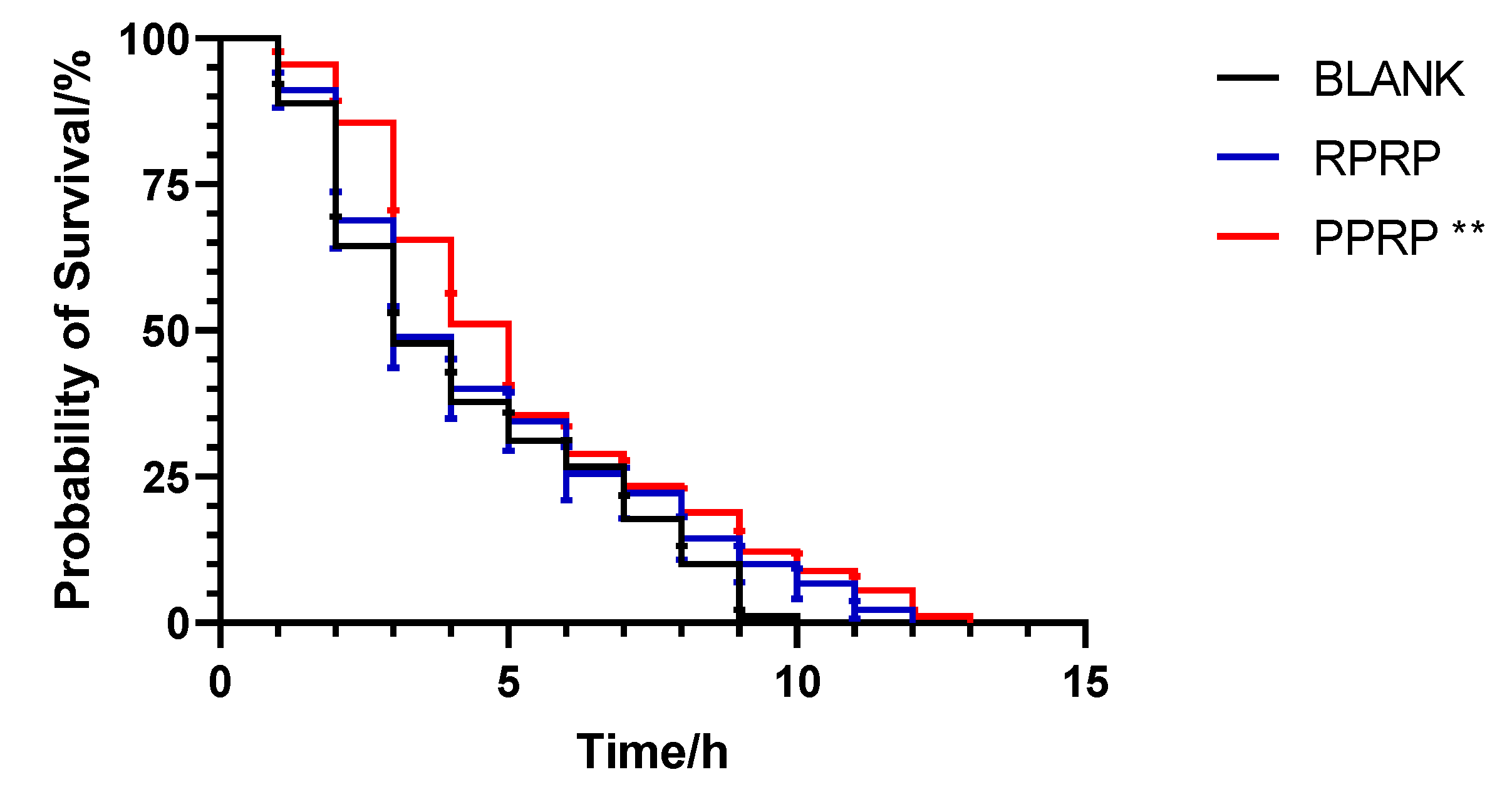
2.2. Effect of RPRP and PPRP on the Longevity of N2
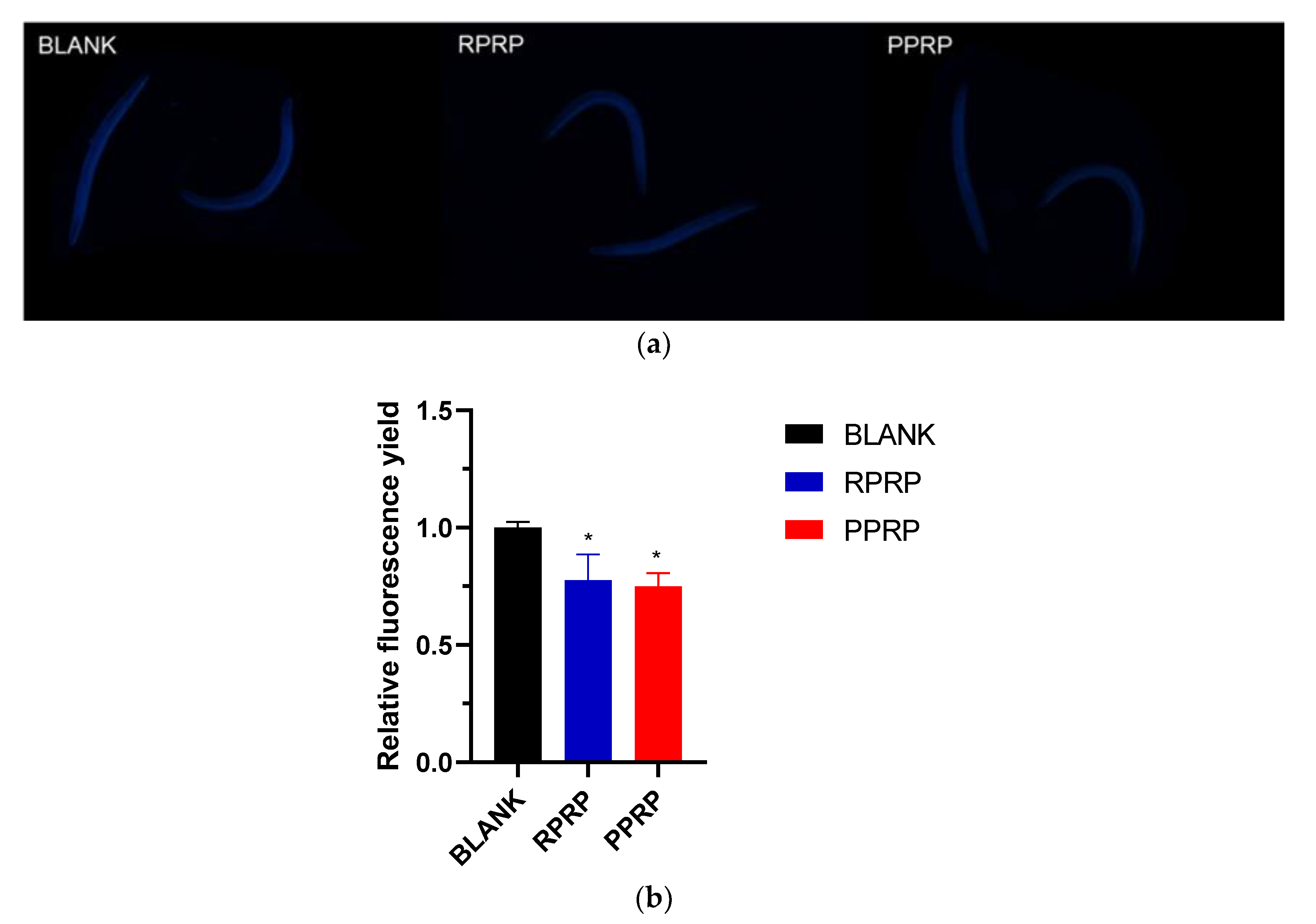
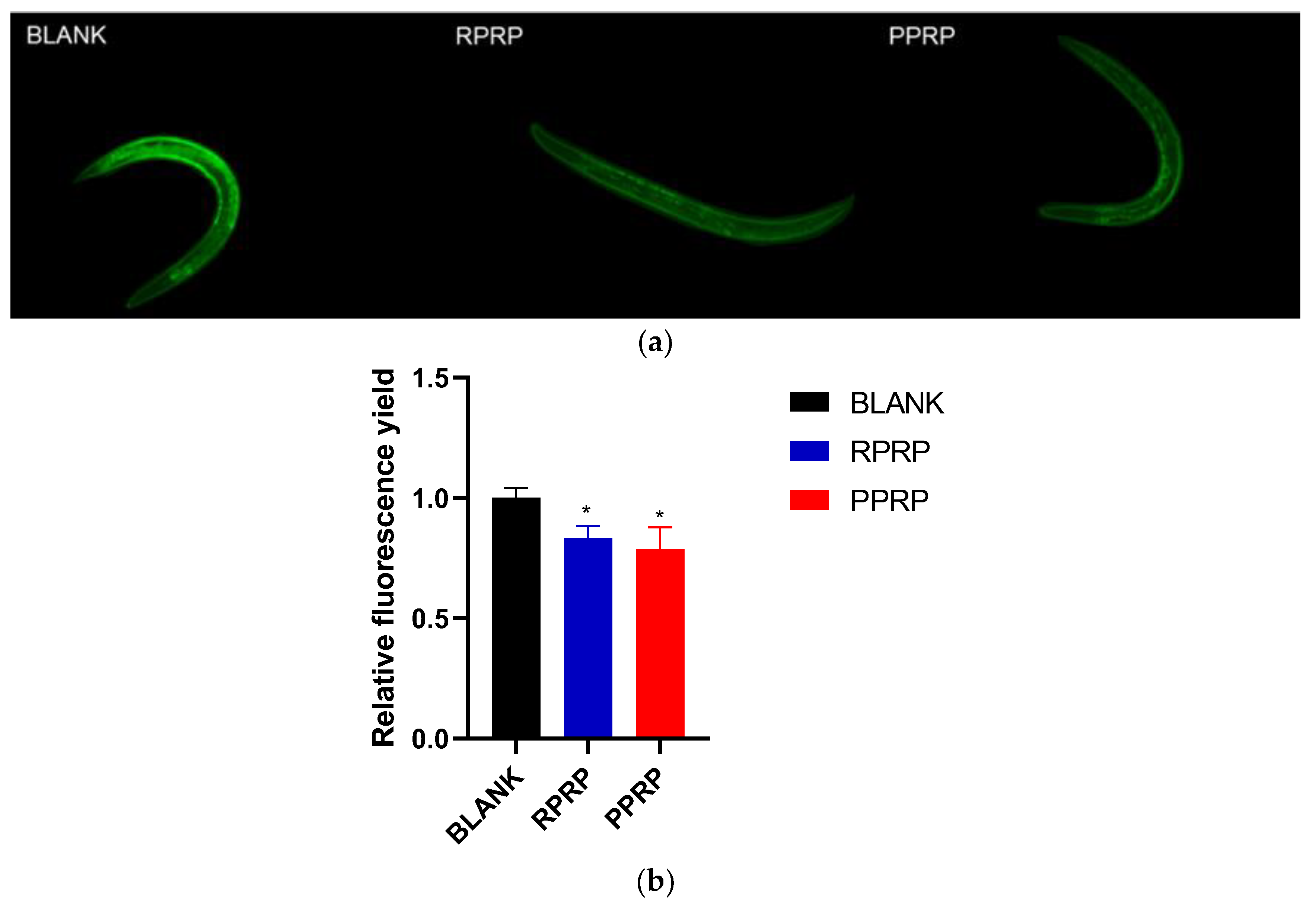
2.3. Effect of RPRP and PPRP on Lipofuscin Accumulation in N2
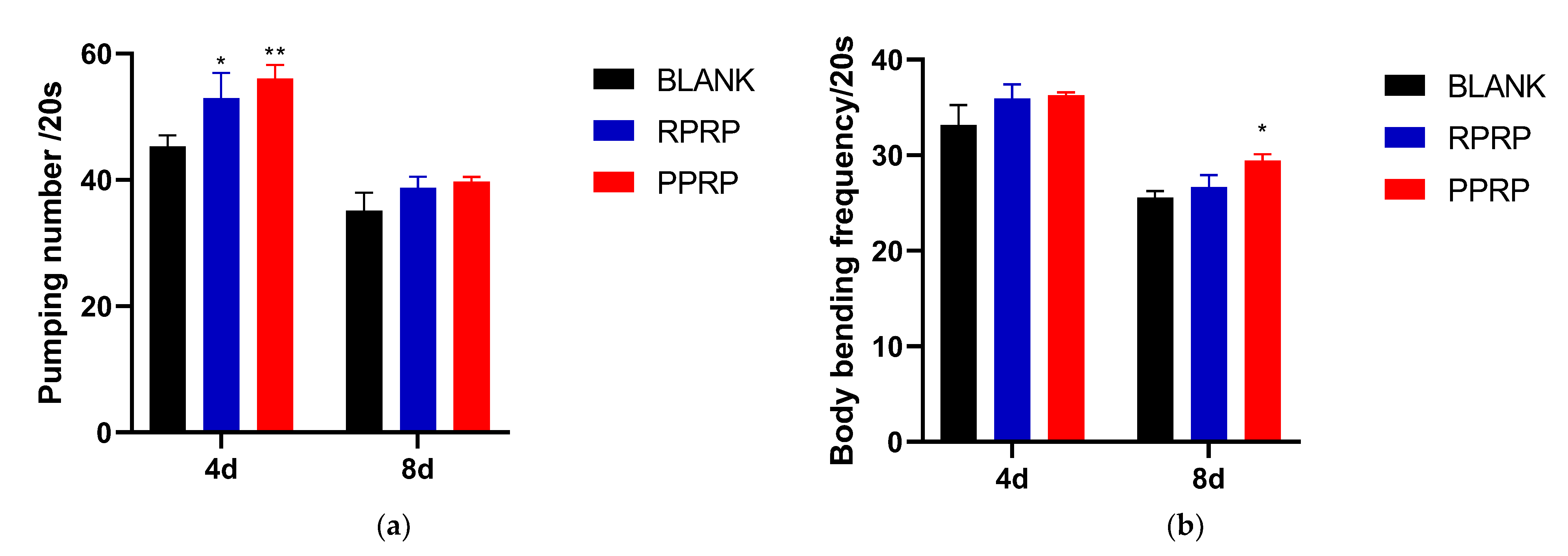
2.4. Effect of RPRP and PPRP on Pharyngeal Pump Frequency and Locomotion Frequency of N2
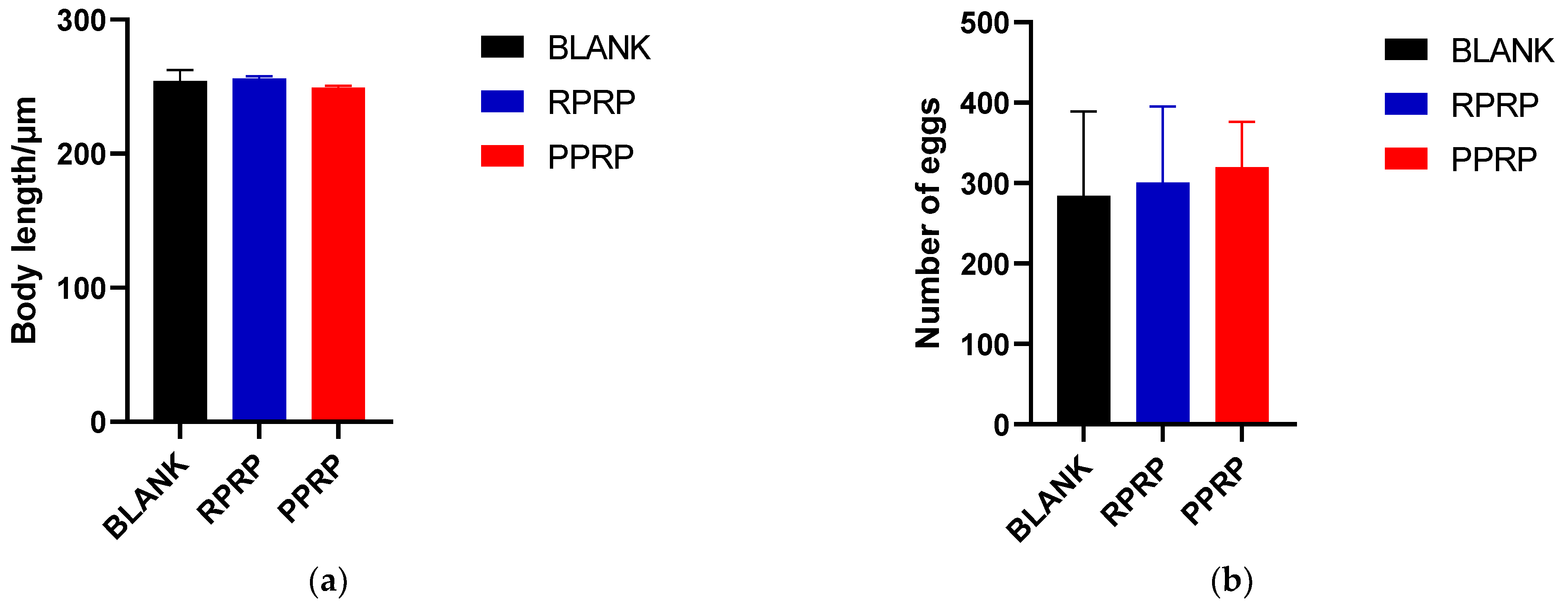
2.5. Effect of RPRP and PPRP on the Growth and Reproduction of N2
2.6. Effect of RPRP and PPRP on the Resistance to Oxidative Stress in N2
2.7. Effect of RPRP and PPRP on Antioxidant Enzymes of N2
2.8. Effect of RPRP and PPRP on Reactive Oxygen Species (ROS) in N2
2.9. Effect of RPRP and PPRP on the Longevity of daf-16 Mutant
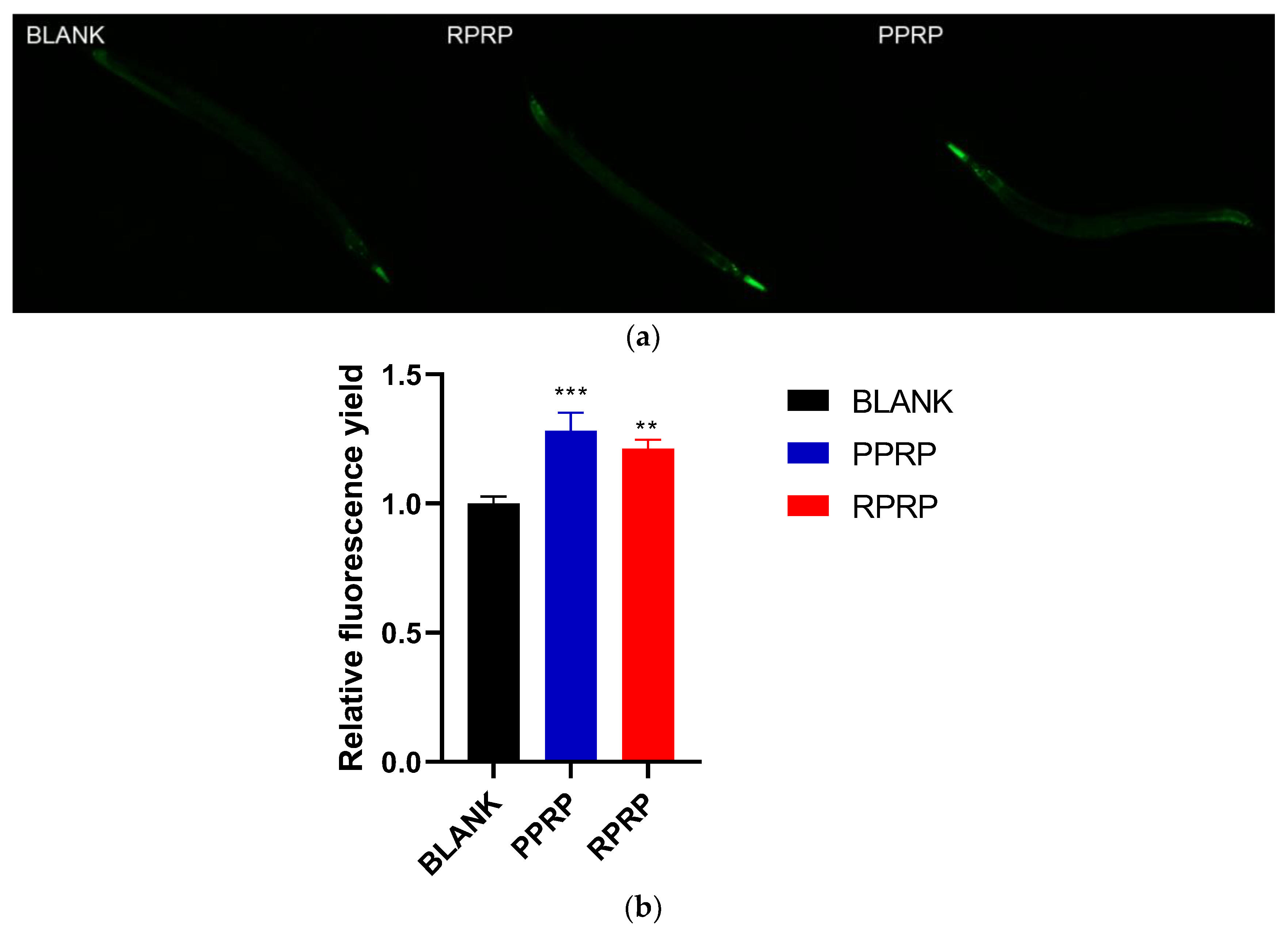
2.10. Effect of RPRP and PPRP on sod-3 Expression
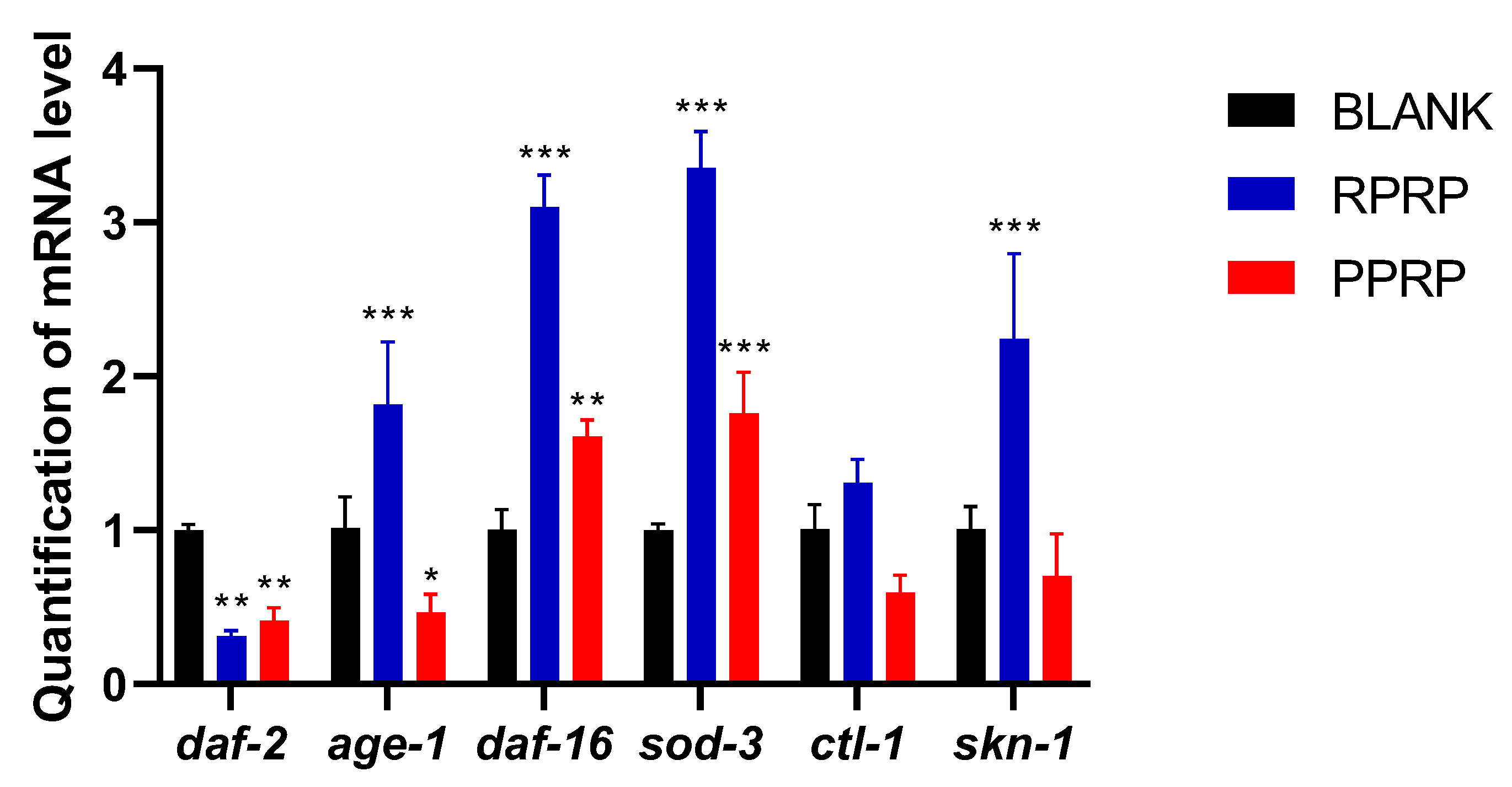
2.11. Effect of RPRP and PPRP on mRNA in N2
2.12. Effect of RPRP and PPRP on the Longevity of daf-2 Mutant
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. C. elegans
4.3. Preparation of RPRP and PPRP
4.4. Analysis of The Monosaccharide Composition of RPRP and PPRP
4.5. Exposure Experiments
4.6. Longevity Experiments
4.7. Lipofuscin Experiment
4.8. Pharyngeal Pump Assay and Locomotion Assay
4.9. Body Length Experiment
4.10. Reproduction Experiments
4.11. Anti-oxidative Stress Assay
4.12. Antioxidant Enzyme Assay
4.13. Determination of ROS
4.14. sod-3 Fluorescence Expression Assay
4.15. RT-PCR Experiment
- daf-2, F 5′-CGACTGAAGTGAATGGTGGA-3′, R 5′-CGCCGTTACTGAGACAAAATA-3′;
- age-1, F 5′-CGCTGGCATCAAAATCTACA-3′, R 5′-ATTGGCAGTCGGTTCAGGAG-3′;
- daf-16, F 5′-GGAGCCAAGAAGAGGATAAAGG-3′, R 5′-GGAGAAACACGAGACGACGAT-3′;
- sod-3, F 5′-AGCATCATGCCACCTACGTGA-3′, R 5′-CACCACCATTGAATTTCAGCG-3′;
- ctl-1, F 5′-TCCTACACGGACACGCATTAC-3′, R 5′-CGGAAACTGTTCGGGAAGTAA-3′;
- skn-1, F 5′-TAGCCGACGACGAAGAAGAG-3′, R 5′-AGGTGTTGGACGATGGTGAA-3′;
- actin, F 5′-GTCATGGTCGGTATGGGACA-3′, R 5′-TTGTAGAAGGTGTGATGCCAGA-3′.
4.16. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Niccoli, T.; Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Loos, R.J.; Marshall, S.M.; Zierath, J.R. Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care. Cell 2021, 184, 1530–1544. [Google Scholar] [CrossRef] [PubMed]
- Lacolley, P.; Regnault, V.; Segers, P.; Laurent, S. Vascular smooth muscle cells and arterial stiffening: Relevance in development, aging, and disease. Physiol. Rev. 2017, 97, 1555–1617. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Sun, T.T.; Liu, Y.W.; Fan, G.T.; Zhang, W.J.; Liu, C.Y. Protective effect of Polygonatum sibiricum polysaccharides on gentamicin-induced acute kidney injury in rats via inhibiting p38 MAPK/ATF2 pathway. Int. J. Biol. Macromol. 2020, 151, 595–601. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Zhang, Y.-S.; Zhang, F.; Zhang, Y.-Y.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Methyl protodioscin from Polygonatum sibiricum inhibits cervical cancer through cell cycle arrest and apoptosis induction. Food Chem. Toxicol. 2019, 132, 110655. [Google Scholar] [CrossRef]
- Singh, S.K.; Patra, A. Evaluation of phenolic composition, antioxidant, anti-inflammatory and anticancer activities of Polygonatum verticillatum (L.). J. Integr. Med. 2018, 16, 273–282. [Google Scholar] [CrossRef]
- Sun, L.-R.; Li, X.; Wang, S.-X. Two new alkaloids from the rhizome of Polygonatum sibiricum. J. Asian Nat. Prod. Res. 2005, 7, 127–130. [Google Scholar] [CrossRef]
- Zhao, X.; Patil, S.; Qian, A.; Zhao, C. Bioactive compounds of Polygonatum sibiricum—Therapeutic effect and biological activity. Endocrine, Metab. Immune Disord.-Drug Targets 2022, 22, 26–37. [Google Scholar] [CrossRef]
- Shen, W.-D.; Li, X.-Y.; Deng, Y.-Y.; Zha, X.-Q.; Pan, L.-H.; Li, Q.-M.; Luo, J.-P. Polygonatum cyrtonema Hua polysaccharide exhibits anti-fatigue activity via regulating osteocalcin signaling. Int. J. Biol. Macromol. 2021, 175, 235–241. [Google Scholar] [CrossRef]
- Li, L.; Thakur, K.; Cao, Y.-Y.; Liao, B.-Y.; Zhang, J.-G.; Wei, Z.-J. Anticancerous potential of polysaccharides sequentially extracted from Polygonatum cyrtonema Hua in Human cervical cancer Hela cells. Int. J. Biol. Macromol. 2020, 148, 843–850. [Google Scholar] [CrossRef]
- Markaki, M.; Tavernarakis, N. Caenorhabditis elegans as a model system for human diseases. Curr. Opin. Biotechnol. 2020, 63, 118–125. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Zheng, R.-Q.; Wang, Y.; Liu, Y.-H.; Jiang, S.; Wang, X.-Z.; He, K.; Pan, X.; Zhou, T.; Li, T.; et al. The Endogenous metabolite glycerophosphocholine promotes longevity and fitness in Caenorhabditis elegans. Metabolites 2022, 12, 177. [Google Scholar] [CrossRef]
- Bansal, A.; Zhu, L.J.; Yen, K.; Tissenbaum, H.A. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc. Natl. Acad. Sci. USA 2015, 112, 277–286. [Google Scholar] [CrossRef]
- Chen, W.; Sudji, I.R.; Wang, E.; Joubert, E.; van Wyk, B.-E.; Wink, M. Ameliorative effect of aspalathin from rooibos (Aspalathus linearis) on acute oxidative stress in Caenorhabditis elegans. Phytomedicine 2013, 20, 380–386. [Google Scholar] [CrossRef]
- Youssef, F.S.; Sobeh, M.; Dmirieh, M.; Bogari, H.A.; Koshak, A.E.; Wink, M.; Ashour, M.L.; Elhady, S.S. Metabolomics-Based profiling of Clerodendrum speciosum (Lamiaceae) leaves using LC/ESI/MS-MS and in vivo evaluation of its antioxidant activity using Caenorhabditis elegans model. Antioxidants 2022, 11, 330. [Google Scholar] [CrossRef]
- Kwak, M.S.; Rhee, W.J.; Lee, Y.J.; Kim, H.S.; Kim, Y.H.; Kwon, M.K.; Shin, J.-S. Reactive oxygen species induce Cys106-mediated anti-parallel HMGB1 dimerization that protects against DNA damage. Redox Biol. 2021, 40, 101858. [Google Scholar] [CrossRef]
- Monickaraj, F.; Aravind, S.; Nandhini, P.; Prabu, P.; Sathishkumar, C.; Mohan, V.; Balasubramanyam, M. Accelerated fat cell aging links oxidative stress and insulin resistance in adipocytes. J. Biosci. 2013, 38, 113–122. [Google Scholar] [CrossRef]
- Yuan, Y.; Kang, N.; Li, Q.; Zhang, Y.; Liu, Y.; Tan, P. Study of the effect of neutral polysaccharides from rehmannia glutinosa on lifespan of caenorhabditis elegans. Molecules 2019, 24, 4592. [Google Scholar] [CrossRef]
- Rangsinth, P.; Prasansuklab, A.; Duangjan, C.; Gu, X.; Meemon, K.; Wink, M.; Tencomnao, T. Leaf extract of Caesalpinia mimosoides enhances oxidative stress resistance and prolongs lifespan in Caenorhabditis elegans. BMC Complement. Altern. Med. 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Wu, C.; Liu, J.; Ma, J.; Yan, Q.; Jiang, Z. Neoagarotetraose extends the lifespan of Caenorhabditis elegans through AMPK mediated signaling pathways and activation of autophagy. J. Funct. Foods 2021, 77, 104341. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.H.; Lam, S.M.; Shui, G. Rebaudioside a enhances resistance to oxidative stress and extends lifespan and healthspan in caenorhabditis elegans. Antioxidants 2021, 10, 262. [Google Scholar] [CrossRef]
- Wolff, S.; Dillin, A. The trifecta of aging in Caenorhabditis elegans. Exp. Gerontol. 2006, 41, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.D.; Kennedy, B.K.; Kaeberlein, M. Genome-wide identification of conserved longevity genes in yeast and worms. Mech. Ageing Dev. 2007, 128, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.K. The genetics of ageing: Insight from genome-wide approaches in invertebrate model organisms. J. Intern. Med. 2008, 263, 142–152. [Google Scholar] [CrossRef]
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 1993, 366, 461–464. [Google Scholar] [CrossRef]
- Slack, C.; Giannakou, M.E.; Foley, A.; Goss, M.; Partridge, L. dFOXO-independent effects of reduced insulin-like signaling in Drosophila. Aging Cell 2011, 10, 735–748. [Google Scholar] [CrossRef]
- Zhang, J.F.; He, L.Z.; Wang, A.L.; Wu, B.Q.; Zhang, P.X.; Zhu, Y.; Jiang, Y.; Bai, J.; Xiao, X. Responses of bitter melon saponins to oxidative stress and aging via the IIS pathway linked with sir-2.1 and hlh-30. J. Food Biochem. 2022, 46, 14456. [Google Scholar] [CrossRef]
- Jiang, S.; Jiang, C.-P.; Cao, P.; Liu, Y.-H.; Gao, C.-H.; Yi, X.-X. Sonneradon A Extends Lifespan of Caenorhabditis elegans by Modulating Mitochondrial and IIS Signaling Pathways. Mar. Drugs 2022, 20, 59. [Google Scholar] [CrossRef]
- Kang, N.; Luan, Y.; Jiang, Y.; Cheng, W.; Liu, Y.; Su, Z.; Liu, Y.; Tan, P. Neuroprotective effects of oligosaccharides in rehmanniae radix on transgenic caenorhabditis elegans models for alzheimer’s disease. Front. Pharmacol. 2022, 13, 878631. [Google Scholar] [CrossRef]

| Group | Maximum Lifespan/d | Average Lifespan/d |
|---|---|---|
| BLANK | 23 | 15.32 ± 0.43 |
| RPRP | 24 | 16.27 ± 0.28 |
| PPRP | 25 | 16.92 ± 0.21 * |
| Group | SOD (U/mg) | CAT (U/mg) |
|---|---|---|
| BLANK | 4.71 ± 0.86 | 2.54 ± 0.69 |
| RPRP | 8.10 ± 1.85 * | 4.02 ± 0.52 |
| PPRP | 9.00 ± 1.86 * | 5.41 ± 0.98 * |
| Group | Maximum Lifespan/d | Average Lifespan/d |
|---|---|---|
| BLANK | 20 | 13.61 ± 0.53 |
| RPRP | 20 | 14.14 ± 0.32 |
| PPRP | 20 | 14.23 ± 1.04 |
| Group | Maximum Lifespan/d | Average Lifespan/d |
|---|---|---|
| BLANK | 21 | 14.40 ± 0.83 |
| RPRP | 22 | 15.09 ± 0.25 |
| PPRP | 22 | 15.09 ± 1.01 |
| Group | Maximum Lifespan/d | Average Lifespan/d |
|---|---|---|
| BLANK | 30 | 19.58 ± 1.16 |
| RPRP | 30 | 20.21 ± 0.11 |
| PPRP | 30 | 19.87 ± 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, Y.; Jiang, Y.; Huang, R.; Wang, X.; He, X.; Liu, Y.; Tan, P. Polygonati Rhizoma Polysaccharide Prolongs Lifespan and Healthspan in Caenorhabditis elegans. Molecules 2023, 28, 2235. https://doi.org/10.3390/molecules28052235
Luan Y, Jiang Y, Huang R, Wang X, He X, Liu Y, Tan P. Polygonati Rhizoma Polysaccharide Prolongs Lifespan and Healthspan in Caenorhabditis elegans. Molecules. 2023; 28(5):2235. https://doi.org/10.3390/molecules28052235
Chicago/Turabian StyleLuan, Yage, Yu Jiang, Rong Huang, Xuan Wang, Xiujuan He, Yonggang Liu, and Peng Tan. 2023. "Polygonati Rhizoma Polysaccharide Prolongs Lifespan and Healthspan in Caenorhabditis elegans" Molecules 28, no. 5: 2235. https://doi.org/10.3390/molecules28052235
APA StyleLuan, Y., Jiang, Y., Huang, R., Wang, X., He, X., Liu, Y., & Tan, P. (2023). Polygonati Rhizoma Polysaccharide Prolongs Lifespan and Healthspan in Caenorhabditis elegans. Molecules, 28(5), 2235. https://doi.org/10.3390/molecules28052235






