Analysis of Drugs in Saliva of US Military Veterans Treated for Substance Use Disorders Using Supported Liquid Extraction and Surface-Enhanced Raman Spectral Analysis
Abstract
1. Introduction
2. Results
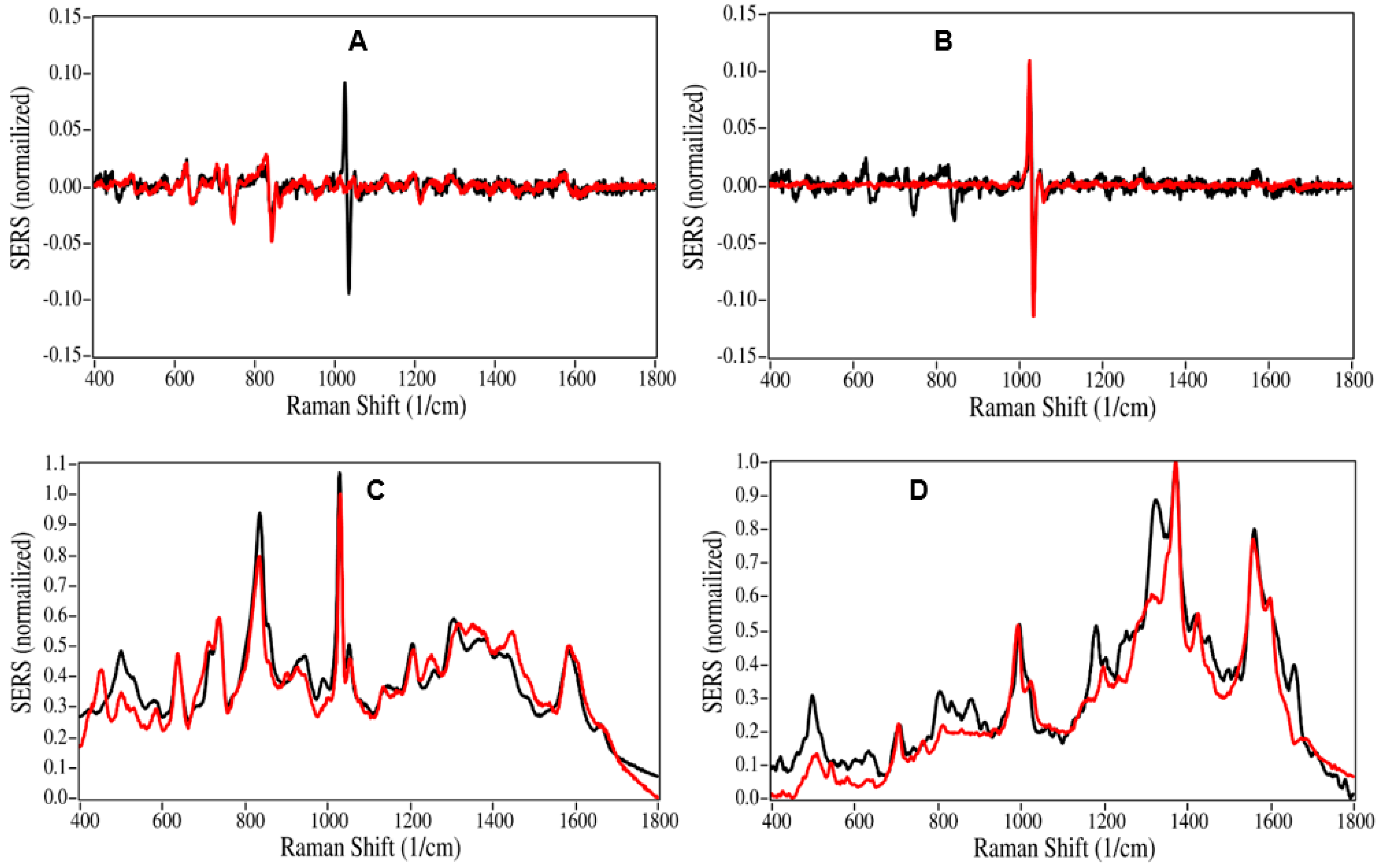
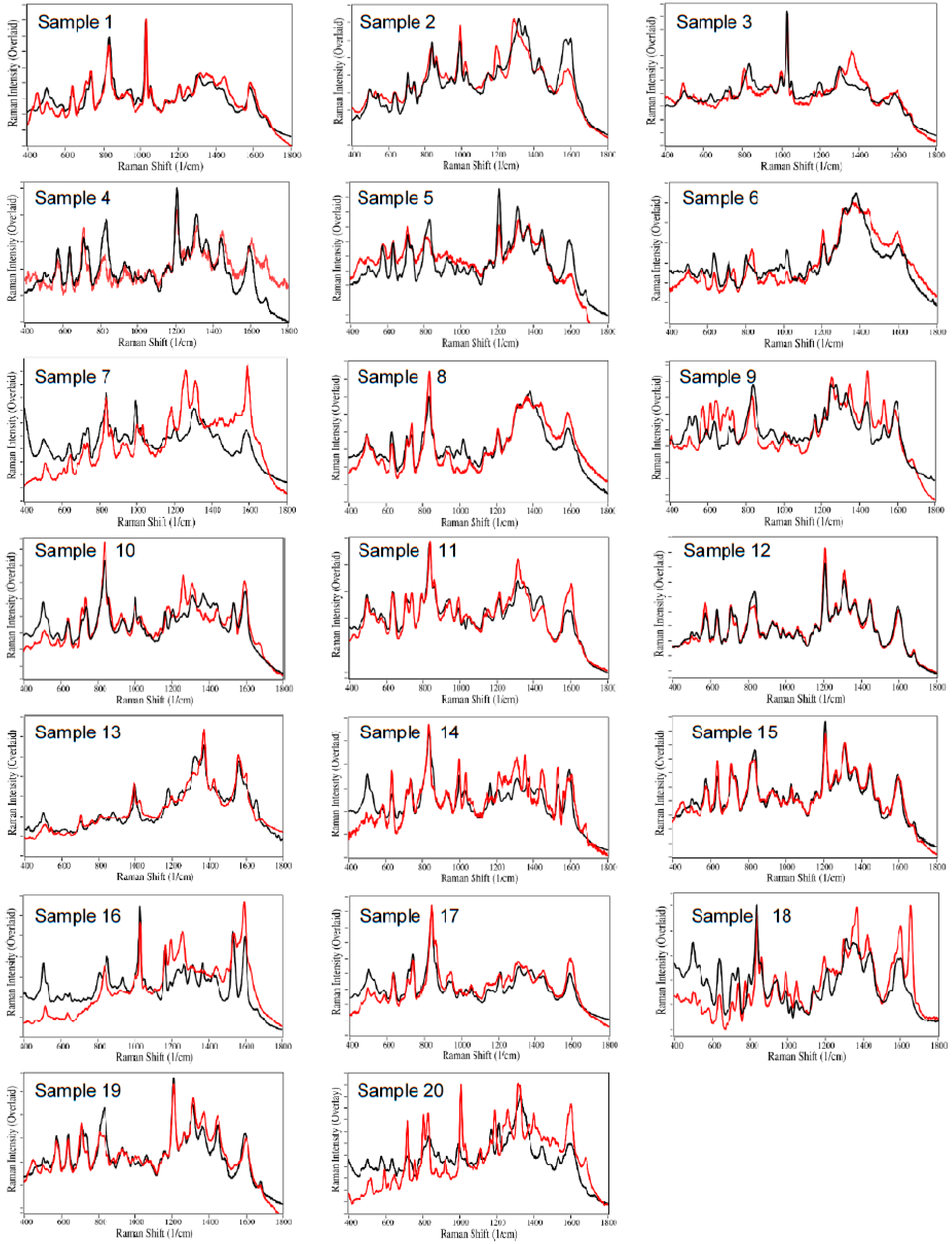
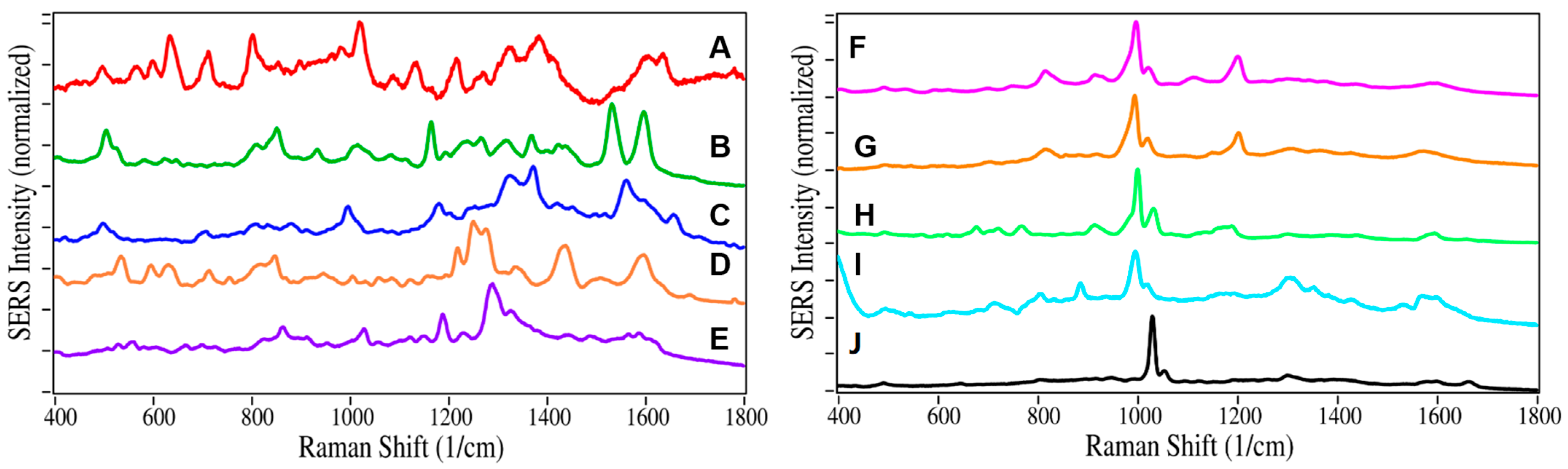
2.1. Drug Identification
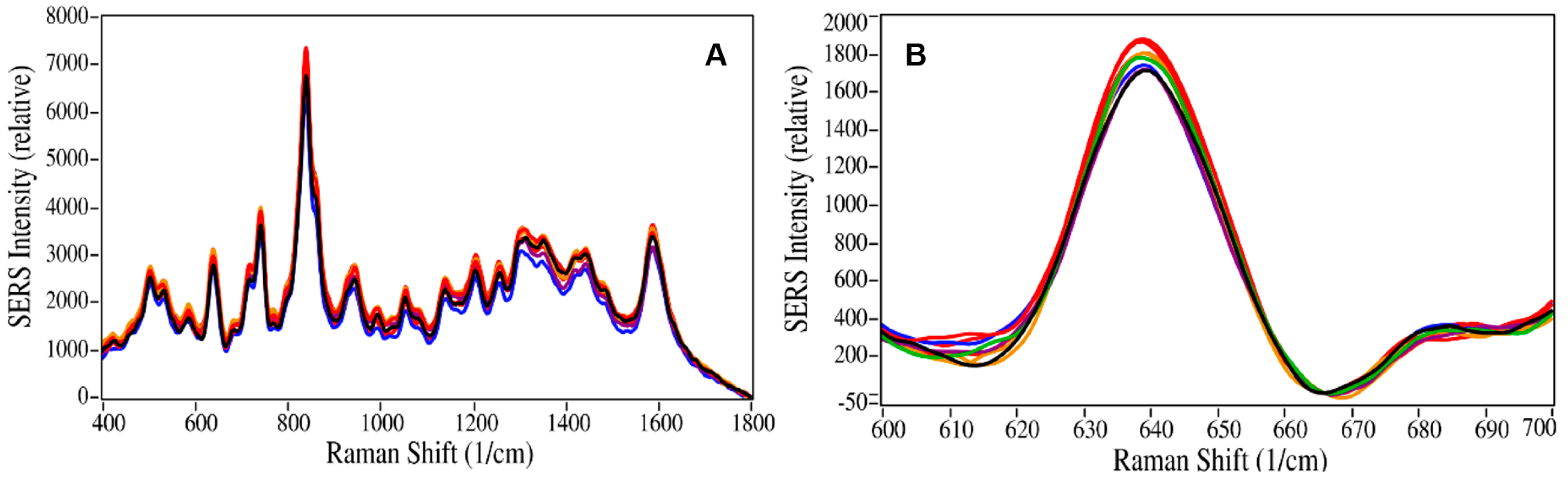
2.2. Buprenorphine Quantitation
2.3. Analytical Figures of Merit
3. Discussion
4. Materials and Methods
4.1. Materials 1: Purchased Materials
4.2. Materials 2: Prepared Materials
4.3. Materials 3: Patient Samples
4.4. Method 1: Urine Toxicology
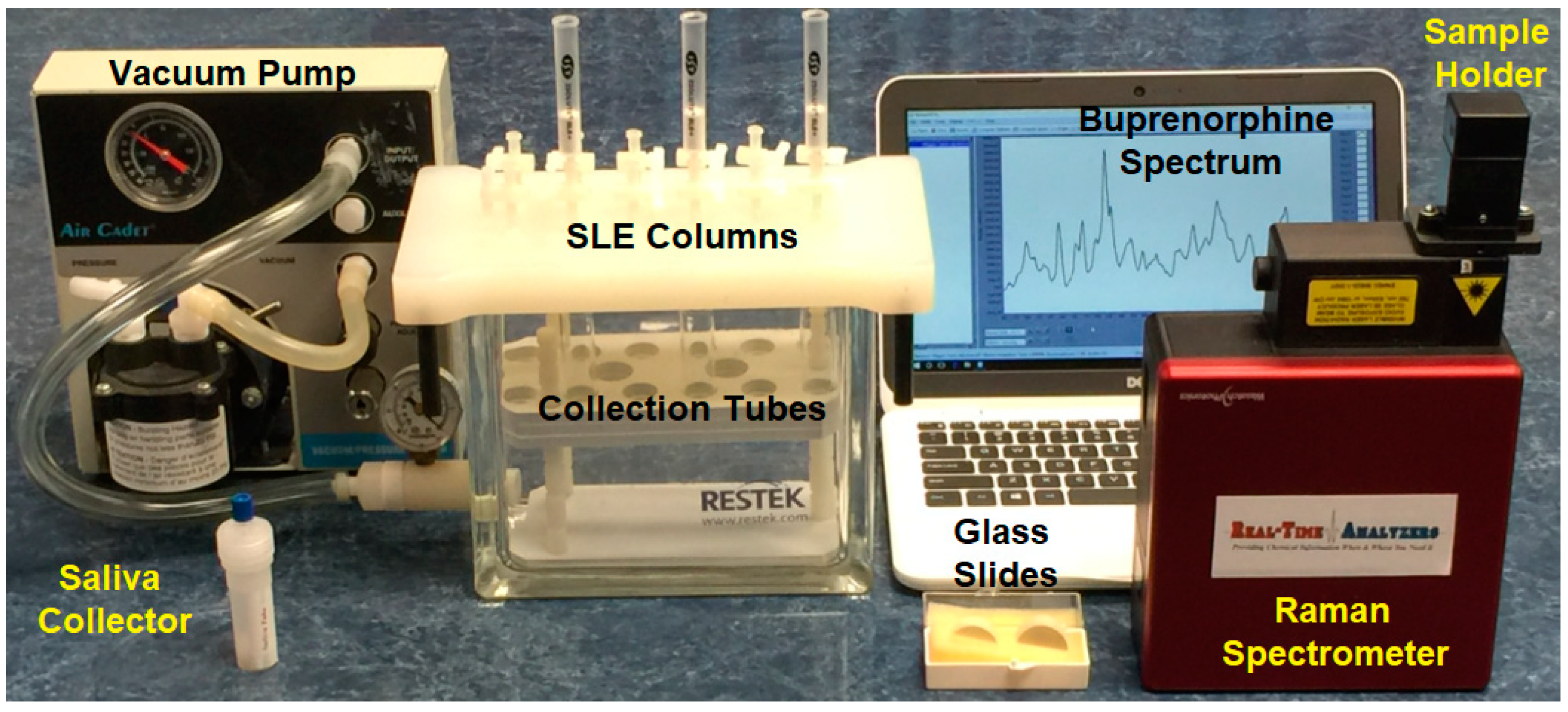
4.5. Method 2: Liquid Extraction
4.6. Method 3: Raman Spectroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- U.S. Overdose Deaths In 2021 Increased Half as Much as in 2020–But Are Still Up 15%. Available online: www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm#print (accessed on 11 November 2022).
- Ahmad, F.B.; Cisewski, J.A.; Rossen, L.M.; Sutton, P. Provisional Drug Overdose Death Counts. National Center for Health Statistics. 2022. Available online: www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm (accessed on 11 November 2022).
- Daigh, J., Jr.; Department of Veterans Affairs Office of Inspector General. Healthcare Inspection–VA Patterns of Dispensing Take-Home Opioids and Monitoring Patient on Opioid Therapy; Report No. 14–00895–163; Office of Healthcare Inspections: Washington, DC, USA, 14 May 2014. [Google Scholar]
- Arias, A.J.; Kranzler, H. Treatment of co-occurring alcohol and other drug use disorders. Alcohol Res. Health 2008, 31, 155–167. [Google Scholar]
- Dahl, N.H.; Department of Veterans Affairs Office of Inspector General. Independent Review of VA’s FY 2016 Detailed Accounting Submission of the Office of National Drug Control Policy; Report No. 17–00976–176; Office of Healthcare Inspections: Washington, DC, USA, 2017. [Google Scholar]
- Office of Public and Intergovernmental Affairs. VA Reduces Prescription Opioid Use by 64% during Past Eight Years. Available online: www.va.gov/opa/pressrel/pressrelease.cfm?id=5492 (accessed on 11 November 2022).
- Teeters, J.B.; Lancaster, C.; Brown, D.; Back, S. Substance use disorders in military veterans: Prevalence and treatment challenges. Subst. Abuse Rehab. 2017, 8, 69–77. [Google Scholar] [CrossRef]
- Childress, S. Veterans Face Greater Risks Amid Opioid Crisis. Public Broadcasting System-Front Line. Available online: www.pbs.org/wgbh/frontline/article/veterans-face-greater-risks-amid-opioid-crisis/ (accessed on 11 November 2022).
- Bennett, A.S.; Elliott, L.; Golub, A. Veterans’ health and opioid safety-contexts, risks, and outreach implications. Fed. Pract. 2015, 32, 4–7. [Google Scholar]
- Mattick, R.P.; Breen, C.; Kimber, J.; Davoli, M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2014, 6, CD002207. [Google Scholar] [CrossRef]
- Heel, R.C.; Brodgen, R.; Speight, T.; Avery, G. Buprenorphine: A review of its pharmacological properties and therapeutic effects. Drugs 1979, 17, 81–110. [Google Scholar] [CrossRef]
- Lintzeris, N.; Leung, S.; Dunlop, A.; Larance, B.; White, N.; Rivas, G.; Holland, R.M.; Degenhardt, L.; Muhleisen, P.; Hurley, M.; et al. A randomised controlled trial of sublingual buprenorphine-naloxone film versus tablets in the management of opioid dependence. Drug Alcohol Depend. 2013, 131, 119–126. [Google Scholar] [CrossRef]
- Suboxone® Sublingual Film. Richmond: Reckitt Benckiser Pharmaceuticals Inc. Available online: www.suboxone.com/patients/about_suboxone/about_suboxone_film.aspx#10 (accessed on 16 July 2013).
- Lofwall, M.R.; Walsh, S. A review of buprenorphine diversion and misuse: The current evidence base and experiences from around the world. J. Addict. Med. 2014, 8, 315–326. [Google Scholar] [CrossRef]
- Monte, A.A.; Mandell, T.; Wilford, B.; Tennyson, J.; Boyer, E. Diversion of buprenorphine/naloxone coformulated tablets in a region with high prescribing prevalence. J. Addict. Dis. 2009, 28, 226–231. [Google Scholar] [CrossRef]
- Graham, R.L. Buprenorphine for opioid dependence: Are there really differences between the formulations? Mental Health Clin. 2014, 4, 17–21. [Google Scholar] [CrossRef]
- Hull, M.J.; Bierer, M.; Griggs, D.; Long, W.; Nixon, A.; Flood, J. Urinary buprenorphine concentrations in patients treated with suboxone as determined by liquid chromatography-mass spectrometry and CEDIA immunoassay. J. Anal. Toxicol. 2008, 32, 516–521. [Google Scholar] [CrossRef]
- Suzuki, J.; Zinser, J.; Issa, M.; Rodriguez, C. Quantitative testing of buprenorphine and norbuprenorphine to identify urine sample spiking during office-based opioid treatment. Subst. Abus. 2017, 38, 504–507. [Google Scholar] [CrossRef]
- Cirimele, V.; Etienne, S.; Villain, M. Ludes, P. Kintz. Evaluation of the One-Step TM ELIZA kit for the detection of buprenorphine in urine, blood, and hair. Forensic Sci. Int. 2004, 143, 153–156. [Google Scholar] [CrossRef]
- Miller, E.I.; Torrance, H.; Oliver, J. Validation of the Immunanlysis® microplate ELISA for the detection of buprenorphine and its metabolite norbuprenorphine in urine. J. Anal. Toxicol. 2006, 30, 115–119. [Google Scholar] [CrossRef]
- Berg, J.; Schjøtt, J.; Fossan, K.; Riedel, B. Cross-reactivity of the CEDIA buprenorphine assay in drugs-of-abuse screening: Influence of dose and metabolites of opioids. Subst. Abuse Rehabil. 2015, 6, 131–139. [Google Scholar] [CrossRef]
- Gervais, J.R.; Hobbs, G.A. Use of an acetyl derivative to improve GC-MS determination of norbuprenorphine in the presence of high concentrations of buprenorphine in urine. J. Anal. Toxicol. 2016, 40, 208–212. [Google Scholar] [CrossRef]
- Truver, M.T.; Swortwood, M. Quantitative analysis of novel synthetic opioids, morphine and buprenorphine in oral fluid by LC-MS-MS. J. Anal. Toxicol. 2018, 42, 554–561. [Google Scholar] [CrossRef]
- Agostini, M.; Renzoni, C.; Pierini, E.; Piergiovanni, M.; Termopoli, V.; Famiglini, G.; Cappiello, A. Rapid, hydrolysis-free, dilute-and-shoot method for the determination of buprenorphine, norbuprenorphine and their glucuronides in urine samples using UHPLC-MS/MS. J. Pharm. Biomed. Anal. 2019, 166, 236–243. [Google Scholar] [CrossRef]
- Mariottini, C.; Gergov, M.; Ojanperä, I. Determination of buprenorphine, norbuprenorphine, naloxone, and their glucuronides in urine by liquid chromatography-tandem mass spectrometry. Drug Test Anal. 2021, 13, 1658–1667. [Google Scholar] [CrossRef]
- Farquharson, S.; Shende, C.; Inscore, F.; Maksymiuk, P.; Gift, A. Analysis of 5-fluorouracil in saliva using surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2005, 36, 208–212. [Google Scholar] [CrossRef]
- Inscore, F.; Shende, C.; Sengupta, A.; Huang, H.; Farquharson, S. Detection of Drugs of Abuse in Saliva by SERS. Appl. Spectrosc. 2011, 65, 1004–1008. [Google Scholar] [CrossRef]
- Shende, C.; Farquharson, A.; Brouillette, C.; Smith, W.; Farquharson, S. Quantitative Measurements of Codeine and Fentanyl on a Surface-Enhanced Raman-Active Pad. Molecules 2019, 24, 2578–2585. [Google Scholar] [CrossRef]
- Shende, C.; Farquharson, S. Detection of Tacrolimus in Saliva using a Lateral Flow Assay and Surface-Enhanced Resonance Raman Scattering. J. Anal. Bioanal. Tech. 2022, 13, 3. [Google Scholar]
- Weaver, M.J.; Farquharson, S.; Tadayyoni, M. Surface-enhancement factors for Raman scattering at silver electrodes. Role of adsorbate-surface interactions and electrode structure. J. Chem. Phys. 1985, 82, 4867–4874. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Farquharson, S.; Brouillette, C.; Smith, W.; Shende, C. A surface-enhanced Raman spectral library of important drugs associated with point-of-care and field applications. Front. Chem. 2019, 7, 706–721. [Google Scholar] [CrossRef]
- Cone, E.J. Saliva testing for drugs of abuse. Ann. N. Y. Acad. Sci. 2007, 694, 91–127. [Google Scholar] [CrossRef]
- Farquharson, S.; Dana, K.; Shende, C.; Gladding, Z.; Newcomb, J.; Dascher, J.; Petrakis, I.; Rapid, A.A. Identification of Buprenorphine in Patient Saliva. J. Anal. Bioanal. Tech. 2017, 8, 368. [Google Scholar] [CrossRef]
- Gemperline, P. Practical Guide to Chemometrics, 2nd ed.; Taylor & Francis: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Farquharson, S.; Shende, C.; Sengupta, A.; Huang, H.; Inscore, F. Rapid detection and identification of overdose drugs in saliva by surface-enhanced Raman scattering using fused gold colloids. Pharmaceutics 2011, 3, 425–439. [Google Scholar] [CrossRef]
- Orman, J.S.; Keating, G.M. Buprenorphine/naloxone: A review of its use in the treatment of opioid dependence. Drugs 2009, 69, 577–607. [Google Scholar] [CrossRef]
- Farquharson, S.; Gift, A.; Shende, C.; Inscore, F.; Ordway, B.; Farquharson, C.; Murran, J. Surface-enhanced Raman Spectral Measurements of 5-Fluorouracil in Saliva. Molecules 2008, 13, 2608–2627. [Google Scholar] [CrossRef]
- Mendelson, J.; Upton, R.; Everhart, E.; Jacob, P., III; Jones, R. Bioavailability of sublingual buprenorphine. J. Clin. Pharmacol. 1997, 37, 31–317. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]



| Sample and mg/day | Urinalysis | SERS Analysis (ID and %) | Saliva-SERS ng/mL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BUPa | CAN | COC | OPI | Other | BUP | CAN | OPI/Other | NOR | NIC | ACE | UNK | |||
| 1 | 24 | Y | 53 | 47 | 111 | |||||||||
| 2 | 20 | Y | Y | 53 | 24b | MAMP 23 | 164 | |||||||
| 3 | 8 | Y | Y | AMP | 23 | 24b | AMP 7 | 46 | 1 | |||||
| 4 | 24 | Y | 100 | 34 | ||||||||||
| 5 | 20 | Y | 89 | 11 | 35 (39) | |||||||||
| 6 | 16 | Y | 68 | 32 | 18 (26) | |||||||||
| 7 | 20 | Y | 61 | COC-ET 39 | UNK | 75 | ||||||||
| 8 | 20 | Y | 92 | 8 | 48 (52) | |||||||||
| 9 | 16 | Y | Y | 56 | 10c | COD 15 | 19 | 45 (80) | ||||||
| 10 | 8 | Y | Y | 65 | MDON 21 | 14 | 121 | |||||||
| 11 | 24 | Y | 100 | 88 | ||||||||||
| 12 | 8 | Y | Y | MDON | 100 | 55 | ||||||||
| 13 | 24 | Y | Y | 0 | IBU 95 | 5 | UNK | 0 (6) | ||||||
| 14 | 24 | Y | 61 | MDON 23 | 11 | 5 | 147 | |||||||
| 15 | 8 | Y | 85 | 15 | 206 | |||||||||
| 16 | 8 | NO | Y | 0 | 66 | 34 | 0 | |||||||
| 17 | 20 | Y | Y | 100 | 55 | |||||||||
| 18 | 8 | Y | Y | >70 | UNK | 38 | ||||||||
| 19 | 24 | Y | 100 | 98 | ||||||||||
| 20 | 12 | Y | Y | Y | 38 | 56c | COC-ET 6 | UNK | 20 | |||||
| acamprosate | bupropion | codeine | ibuprofen | methadone | norbuprenorphine |
| acetaminophen | caffeine | Δ-THC | LSD | methamphetamine | nordiazepam |
| amphetamine | cannabidiol | diazepam | MDA | methylphenidate | oxazepam |
| aspirin | cannabinol | fentanyl | MDMA | morphine | oxycodone |
| benzoylecgonine | cocaethylene | heroin | meperidine | naloxone | PCP |
| buprenorphine | cocaine | hydrocodone | mescaline | nicotine | secobarbital |
| Repeat SLE-SERS | ||
|---|---|---|
| Sample | 638 cm−1 Ht | Calc. Conc. |
| 1 | 1754 | 51.1 |
| 2 | 1755 | 51.1 |
| 3 | 1682 | 49.0 |
| 4 | 1609 | 46.9 |
| 5 | 1634 | 47.6 |
| 6 | 1649 | 48.0 |
| 7 | 1727 | 50.3 |
| 8 | 1709 | 49.8 |
| 9 | 1606 | 46.8 |
| AVE | 1680.6 | 48.9 |
| STD DEV | 58.9 | 1.7 |
| % STD DEV | 3.5 | 3.5 |
| Patient Demographic Information | ||||||||
|---|---|---|---|---|---|---|---|---|
| African American | American Indian and Alaskan | Asian | Caucasian | Hispanic | Native Hawaiian and Pacific Islander | Other | Total | |
| Male | 5 | 0 | 0 | 12 | 2 | 0 | 1 | 20 |
| Female | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 5 | 0 | 0 | 12 | 2 | 0 | 1 | 20 |
| Drug | Calibrant | Positive Detection Cut-Off Value |
|---|---|---|
| Buprenorphine | Buprenorphine | 10 ng/mL |
| Amphetamines | d-methamphetamine | 1000 ng/mL |
| Barbiturates | Secobarbital | 200 ng/mL |
| Benzodiazepines | Oxazepam | 200 ng/mL |
| Cannabinoids | Delta-9-THC | 50 ng/mL |
| Cocaine | Benzoylecgonine | 300 ng/mL |
| Opiates | Morphine | 300 ng/mL |
| Methadone | Methadone | 300 ng/mL |
| Oxycodone | Oxycodone | 100 ng/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farquharson, S.; Shende, C.; Newcomb, J.; Petrakis, I.L.; Arias, A.J. Analysis of Drugs in Saliva of US Military Veterans Treated for Substance Use Disorders Using Supported Liquid Extraction and Surface-Enhanced Raman Spectral Analysis. Molecules 2023, 28, 2010. https://doi.org/10.3390/molecules28052010
Farquharson S, Shende C, Newcomb J, Petrakis IL, Arias AJ. Analysis of Drugs in Saliva of US Military Veterans Treated for Substance Use Disorders Using Supported Liquid Extraction and Surface-Enhanced Raman Spectral Analysis. Molecules. 2023; 28(5):2010. https://doi.org/10.3390/molecules28052010
Chicago/Turabian StyleFarquharson, Stuart, Chetan Shende, Jenelle Newcomb, Ismene L. Petrakis, and Albert J. Arias. 2023. "Analysis of Drugs in Saliva of US Military Veterans Treated for Substance Use Disorders Using Supported Liquid Extraction and Surface-Enhanced Raman Spectral Analysis" Molecules 28, no. 5: 2010. https://doi.org/10.3390/molecules28052010
APA StyleFarquharson, S., Shende, C., Newcomb, J., Petrakis, I. L., & Arias, A. J. (2023). Analysis of Drugs in Saliva of US Military Veterans Treated for Substance Use Disorders Using Supported Liquid Extraction and Surface-Enhanced Raman Spectral Analysis. Molecules, 28(5), 2010. https://doi.org/10.3390/molecules28052010







