GC-MS Analysis of Methadone and EDDP in Addicted Patients under Methadone Substitution Treatment: Comparison of Urine and Plasma as Biological Samples
Abstract
1. Introduction
2. Results
2.1. Characterization of the Study Group
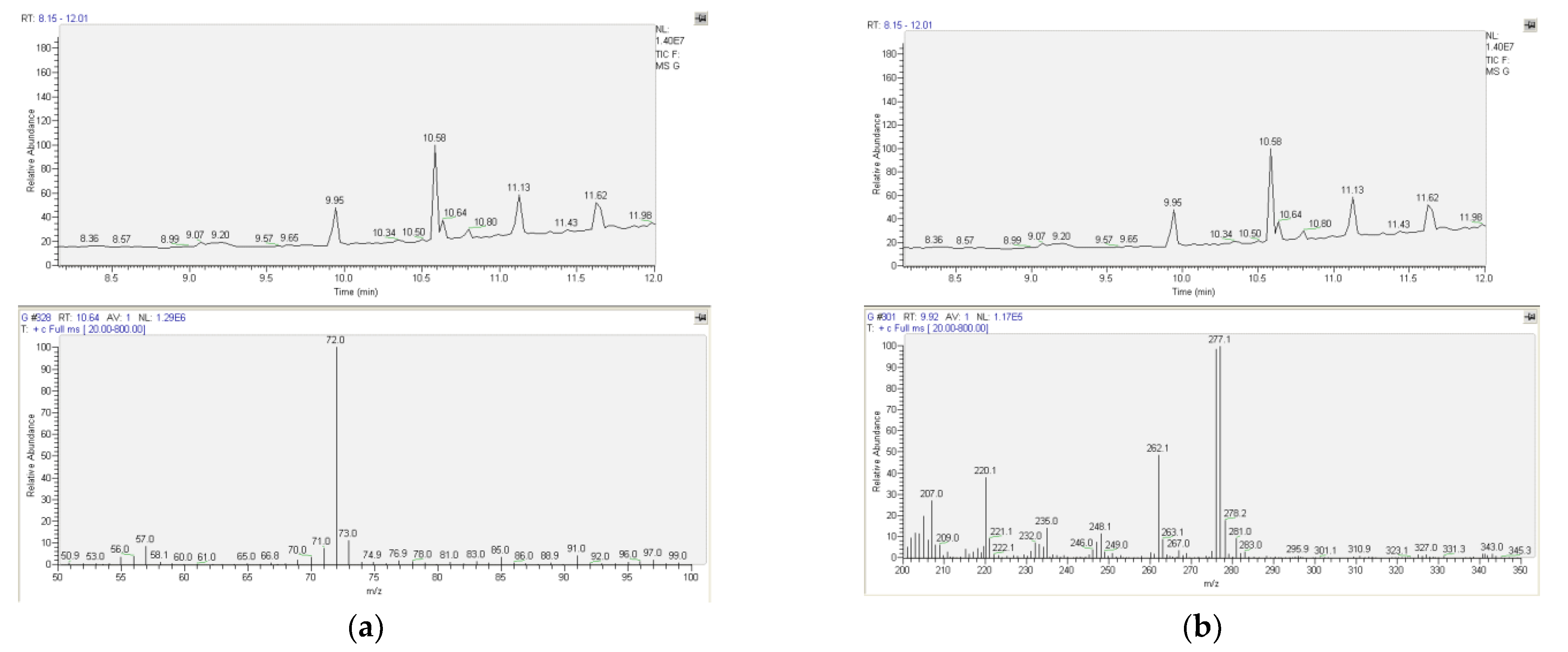
2.2. Qualitative and Quantitative Analyses: Determination of Methadone and Comparison between Urine and Plasma Samples
3. Discussion
3.1. Characterization of the Study Group
3.2. Qualitative and Quantitative Analyses: Determination of Methadone and Comparison between Urine and Plasma Samples
4. Materials and Methods
4.1. Study Group
4.2. Statistical Analysis
4.3. Biological Samples
4.4. Chemicals
4.5. Preparation of Standard Solution
4.6. Liquid–liquid Extraction Procedure
4.7. Instrumentation
4.8. Chromatographic Separation and Mass Spectrometric Detection Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Anderson, I.B.; Kearney, T.E. Use of methadone. West J. Med. 2000, 172, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Durrani, M.; Bansal, K. Methadone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Baconi, L.D.; Ciobanu, A.M.; Vasile, R.D.; Vlăsceanu, A.; Nedelescu, M.; Stan, M. Methadone treatment for heroin dependence. In Drug Addiction; Zhao, F., Li, M., Eds.; IntechOpen: London, UK, 2018; pp. 117–133. [Google Scholar]
- Loimer, N.; Schmid, R. The use of plasma levels to optimize methadone maintenance treatment. Drug Alcohol. Depend. 1992, 30, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.J. Methadone is methadone. Can. Fam Physician 2021, 67, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Favia, M.; Santoiemma, I.; Mita, Q.; Strisciullo, G.; Introna, F.; De Donno, A. Methadone overdose in patients following methadone maintenance treatment: A three years overview in the district of Bari (South-Italy). Clin. Ter. 2021, 172, 247–249. [Google Scholar] [PubMed]
- Cristea, A.N. Tratat de farmacologie, 1st ed.; Editura Medicală: Bucharest, Romania, 2011; p. 181. [Google Scholar]
- Methadone. Drugs and Lactation Database (LactMed); National Library of Medicine: Bethesada, MD, USA, 2022. [Google Scholar]
- Vasile, R.D.; Baconi, D.; Hudiţă, C.; Bârcă, M.; Bălălău, C.; Ciobanu, A.M. Methadone plasma levels in heroin addict patients during substitution therapy. Farmacia 2014, 62, 1202–1213. [Google Scholar]
- Iwersen-Bergmann, S.; Plattner, S.; Hischke, S.; Müller, A.; Andresen-Streichert, H.; Jungen, H.; Erb, R.; Beer-Sandner, B. Brain/blood ratios of methadone and ABCB1 polymorphisms in methadone-related deaths. Int. J. Legal. Med. 2021, 135, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Kharasch, E.D. Current concepts in methadone metabolism and transport. Clin. Pharmacol. Drug Dev. 2017, 6, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Metzger, I.F.; Thomas, A.E.; Evrard, C.A.; Jones, D.R.; Masters, A.R.; Haas, D.M.; Haneline, L.S.; Quinney, S.K. Stereoselective analysis of methadone and EDDP in laboring women and neonates in plasma and dried blood spots and association with neonatal abstinence syndrome. Am. J. Perinatol. 2021, 38, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Feld, K.; Dahm, P.; Kieliba, T.; Klee, A.; Rothschild, M.A.; Andresen-Streichert, H.; Beike, J. Evidence for the transfer of methadone and EDDP by sweat to children’s hair. Int. J. Legal. Med. 2021, 135, 1799–1811. [Google Scholar] [CrossRef] [PubMed]
- Bratinčević, M.; Visković, T.; Sutlović, D. Comparison of the solid phase and liquid-liquid extraction methods for methadone determination in human serum and whole blood samples using gas chromatography/mass spectrometry. Arh. Hig. Rada Toksikol. 2017, 68, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.E.; Goldstein, A.; Poklis, J.L.; Poklis, A. Evaluation of an enzyme immunoassay for the detection of methadone metabolite EDDP [2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine] in urine. J. Clin. Lab. Anal. 2014, 28, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Larson, M.E.M.; Richards, T.M. Quantification of a methadone metabolite (EDDP) in urine: Assessment of compliance. Clin. Med. Res. 2009, 7, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, A.M.; Baconi, D.L.; Bârcă, M.; Negrei, C.; Cristescu, I.; Balalau, D. GC–MS method for methadone quantification in plasma. Toxicol. Lett. 2009, 164S, S312. [Google Scholar] [CrossRef]
- Moffat, A.C.; Osselton, M.D.; Widdop, B. Clarke’s Analysis of Drugs and Poisons, 4th ed.; Pharmaceutical Press: London, UK; Chicago, IL, USA, 2011; pp. 1648–1651. [Google Scholar]
- Zhu, Y.; Mooney, L.J.; Yoo, C.; Evans, E.A.; Kelleghan, A.; Saxon, A.J.; Curtis, M.E.; Hser, Y.I. Psychiatric comorbidity and treatment outcomes in patients with opioid use disorder: Results from a multisite trial of buprenorphine-naloxone and methadone. Drug Alcohol. Depend. 2021, 228, 108996. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Braithwaite, R.A. A pilot study to determine the usefulness of the urinary excretion of methadone and its primary metabolite (EDDP) as potential markers of compliance in methadone detoxification programs. J. Anal. Toxicol. 1999, 23, 81–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baconi, D.; Popescu, G.; Ciobanu, A.M.; Stan, M.; Vlăsceanu, A.M.; Bălălău, C. EDDP metabolite as biomarker for monitoring of methadone substitution treatment. Farmacia 2016, 64, 521–527. [Google Scholar]



| Matrix | Analyte | Internal Standard | Method of Extraction | Method of Analysis | Reference |
|---|---|---|---|---|---|
| Cadaveric blood | MTD EDDP | No IS | LLE Organic mixture solvents at pH 9.0 | GC-MS | [6] |
| Plasma Dried blood spots (DBS) | MTD LOQ 0.05 ng/mL (plasma): 1 ng/mL (DBS); EDDP LOQ 0.025 ng/mL (plasma): 0.5 ng/mL (DBS) | Diphenhydramine | LLE, Ethyl acetate | HPLC-MS/MS, Acetonitrile (gradient from 10–34%) in 0.1% formic acid (pH 6.5), Chiral-AGP 150 × 4.6 mm, 5 μm | [12] |
| Hair Skin | d,l-MTD LOQ 25 µg/L (skin) LOQ of 50 pg/mg (hair); EDDP LOQ 25 µg/L (skin) LOQ of 50 pg/mg (hair) | d,l-Methadone-D9 EDDP-D3 | SPE, Chromabond HR-XC | GC-MS/MS (hair), LC-MS/MS (serum and sweat patches), Water with 0.1% formic acid and acetonitrile with 0.1% formic acid, RP 18 EC 150 × 2.0 mm, 2.7 µm | [13] |
| Femoral venous blood Brain (medulla) | MTD LOQ 0.01 mg/L | Methadone-D9 | SPE, Bond Elut Certify | GC-MS | [10] |
| Whole blood | MTD LOQ 25 ng/mL | No IS | SPE, Bond Elut Nexus, Supelco ENVI Florisil, Supelco LC-18, Amberlite XAD2, LLE, Ethyl acetate–dichloromethane 1:3 v/v | GC-MS | [14] |
| Urine | MTD EDDP LOQ: 25 ng/mL | Not mentioned | Not mentioned | HPLC-MS/MS, Water/acetonitrile (20:80 v/v) with 1% formic acid, C18 100 × 3.2 mm, 3 µm | [15] |
| Plasma | MTD EDDP | Diphenylamine | LLE, Hexane–2-propanol 97:3, v/v, pH 9 | GC-MS | [9] |
| Urine | EDDP LOQ 40 ng/mL | EDDP-D3 | LLE, Chloroform–2-propanol 90:10, v/v | GC-MS | [16] |
| Plasma | MTD LOQ 0.5 ng/mL | Diphenylamine | LLE, Tertbutyl methyl ether | GC-MS | [17] |
| Parameter | Group Characteristics | |
|---|---|---|
| Total cases | 14 | |
| Sex | 14.29% females (2); 85.71% males (12) | |
| Male/female ratio | 6 | |
| Age (years) (mean ± SD) | 32.23 ± 9.90 (range 19–47 years) Males 32.73 ± 10.71 Females 29.5 ± 3.54 | |
| Frequency of age groups | <20 years 7.14% (1/14); 20–30 years 42.86% (6/14); 30–40 years 21.43% (3/14); >40 years 28.57% (4/14) | |
| History of opiate use | 78.57% (11/14) | |
MMT history
| 50% (7/14) R = 0.8822, p-value is 0.00003 | |
| Methadone dose (mg/day) (mean ± SD) | 95.71 ± 20.70 (range 50–110) | |
| Associated medication | % (no. of reports/total reports) | |
| Antidepressants (AD) | Escitalopram 7.14 (1/14) Trazodone 14.29 (2/14) Mirtazepine 28.57 (4/14) | |
| Anxiolytics/hypnotics (AX) | Cinolazepam 7.14 (1/14) Diazepam 28.57 (4/14) Zolpidem 14.29 (2/14) | |
| Antipsychotics (AP) | Risperidone 21.43 (3/14) Haloperidol 14.29 (2/14) Quetiapine 14.29 (2/14) Flupentixol 7.14 (1/14) Olanzapine 21.43 (3/14) Levomepromazine 7.14 (1/14) | |
| Anticonvulsants (AC) | Valproic acid 57.15 (8/14) Carbamazepine 28.57 (4/14) Gabapentine 42.86 (6/14) Levetiracetam 7.14 (1/14) | |
| Pattern of treatment (drug, %, count/total count) | MTD 14. 29 (2/14) MTD + AD + AX + AP + AC 35.71 (5/14) MTD + AD + AC (7.14 (1/14) MTD + AC + AP 14.29 (2/14) MTD + AP + AC + AX 14.29 (2/14) MTD + AC + AD + AX 7.14 (1/14) AC + AD 7.14 (1/14) | |
| No. | MTD (mg) | U | P | q-MTD m/z = 72 tR = 10.64 | q-EDDP m/z = 277 tR = 9.92 | Q-Conc. MTD (µg/mL) |
|---|---|---|---|---|---|---|
| 1 | − | Yes | − | − | − | − |
| 2 | 100 | Yes | − | + | − | 0.072 |
| 3 | − | Yes | − | − | − | − |
| 4 | 100 | Yes | − | + | − | 1.06 |
| 5 | 100 | Yes | − | + | + | 4.336 |
| 6 | 110 | Yes | − | + | + | 3.012 |
| 7 | 110 | Yes | − | + | + | 6.697 |
| 8 | − | Yes | − | − | − | − |
| 9 | − | − | Yes | − | − | − |
| 10 | − | − | Yes | − | − | − |
| 11 | − | − | Yes | − | − | − |
| 12 | 50 | − | Yes | − | − | − |
| 13 | − | − | Yes | − | − | − |
| 14 | 100 | − | Yes | + | − | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciucă Anghel, D.-M.; Ciobanu, A.-M.; Guțu, C.M.; Stan, M.; Tudor, G.; Baconi, D.L. GC-MS Analysis of Methadone and EDDP in Addicted Patients under Methadone Substitution Treatment: Comparison of Urine and Plasma as Biological Samples. Molecules 2022, 27, 8360. https://doi.org/10.3390/molecules27238360
Ciucă Anghel D-M, Ciobanu A-M, Guțu CM, Stan M, Tudor G, Baconi DL. GC-MS Analysis of Methadone and EDDP in Addicted Patients under Methadone Substitution Treatment: Comparison of Urine and Plasma as Biological Samples. Molecules. 2022; 27(23):8360. https://doi.org/10.3390/molecules27238360
Chicago/Turabian StyleCiucă Anghel, Daniela-Mădălina, Anne-Marie Ciobanu, Claudia Maria Guțu, Miriana Stan, Gheorghe Tudor, and Daniela Luiza Baconi. 2022. "GC-MS Analysis of Methadone and EDDP in Addicted Patients under Methadone Substitution Treatment: Comparison of Urine and Plasma as Biological Samples" Molecules 27, no. 23: 8360. https://doi.org/10.3390/molecules27238360
APA StyleCiucă Anghel, D.-M., Ciobanu, A.-M., Guțu, C. M., Stan, M., Tudor, G., & Baconi, D. L. (2022). GC-MS Analysis of Methadone and EDDP in Addicted Patients under Methadone Substitution Treatment: Comparison of Urine and Plasma as Biological Samples. Molecules, 27(23), 8360. https://doi.org/10.3390/molecules27238360










