1. Introduction
Despite the advent of DNA profiling, fingerprints remain a primary form of biometric identification informing both investigations and judicial debates. A suspect identification is typically made by comparing a crime scene mark to a fingerprint record held in national databases (provided that the suspect has had a previous arrest) or to a fingerprint taken from a person of interest. Whilst three levels of details are available to fingerprint experts to compare a crime scene mark to a fingerprint, the composition and distribution of local characteristics of the ridge pattern called
minutiae (level 2 details) are particularly important for identification, after having ascertained that the less specific level 1 details are present (e.g., arch, loop, delta etc.). Whilst generally successful, fingerprinting becomes difficult for smudged, faint, and very partial marks. Additionally, overlapping fingermarks pose a further challenge given by the difficulty to distinguish two or more ridge patterns to assign these impressions to the individuals that have generated them. The ability to separate overlapping fingermarks would be very important for both eliminating irrelevant marks and distinguishing, for example, between the victim’s and the offender’s fingermarks. In the last 15 years, the analytical community has offered spectroscopic imaging [
1,
2,
3] and mass spectrometric imaging solutions to this problem (in combination with various chemometric approaches) [
4,
5,
6,
7,
8,
9,
10]. All the approaches proposed are based on the principle that, as hundreds to thousands of molecules can be detected in fingermarks and as this composition varies all the time, it is possible to pick out those compounds that are uniquely present in each of the marks analysed and can be selected to reconstruct the fingermark ridge pattern. In terms of the mass spectrometric approach, a few techniques have been reported to successfully separate fingermark impressions, namely Desorption Electrospray Ionisation Mass Spectrometry imaging (DESI MSI) [
4], Matrix-Sssisted Laser Desorption Ionisation Mass Spectrometry Imaging (MALDI MSI) [
5,
6,
7], (nanoparticle-assisted) Laser Desorption Ionisation [
8,
9] and time-of-flight Secondary Ion Mass Spectrometry (ToF-SIMS) [
10].
Whilst these techniques have been used to separate ridge patterns from two or more individuals, to the authors’ knowledge, no study has been reported to date attempting separation of fingermarks left on a surface by the same individual.
The only studies that are relevant to this subject are from Lauzon et al. [
11] and Gorka et al. [
12,
13]. In their report [
11], the former group suggests the possibility to use the chemical profile of the fingermark composition to connect fingermarks of the same individual left in different areas of a crime scene. For example, should a smudged mark be unidentifiable in one area of the crime scene (for example the kitchen), it could be assigned to a certain individual, should their identifiable mark be found there or in a different area (for example the living room). This link would provide the opportunity to reconstruct the movements of the offender during the crime based on the principle that the molecular profiles of the smudged and identifiable marks are the same. This assumption would be much easier to accept for marks that have been deposited at the same time. However, despite the long-standing common understanding and literature-supported knowledge that the fingermark composition varies according to metabolism, pharmacological and pathological states, stress, physical activity, diet, etc., Becue’s group has been challenging the fingerprint community [
12,
13] with the idea that the intra-donor fingermark chemical composition remains fairly constant over time; as a result, it is potentially distinctive of an individual (some sort of biological passport) or permits categorisation of a group of individuals. In their recent MALDI MS-based work, Gorka et al. [
13] analysed natural fingerprints from 13 individuals over 12 months, concluding that overall “the composition of the fingermarks provided by the 13 donors is quite consistent over the months and the year”. Specifically, they report that
(i) the compositions at different times of the year (for example January vs. July) are closely linked in the heatmaps and
(ii) the compositional consistency observed over the year is also reproducible between the thirteen donors given that percentage of the
m/
z features retained between each selection is similar for every participant to the study. Whilst the heatmaps are sound, donors’ habits, medications, diet, etc. at the time of each fingermark deposition does not appear to have been recorded. Additionally, donors were only asked to not wash their hands 45 min prior to each deposition and thus reducing the chance of picking up different contaminants. Importantly, whilst the composition may be constant, the abundance of those components may be different, and the resulting ion intensity should be considered a discriminant of the intra-donor chemical profile. Finally, due to lower mass range chosen (100–2000 Da) and the fact that only some species may ionise in positive mode or are not masked by the presence of the matrix selected, the vast majority of the anti-microbial peptides and small proteins [
14] as well as amino acids are excluded from detection. Therefore, whilst certainly a very interesting paper, these uncertainties still justify the investigation into the separation of overlapping fingermarks deposited by the same individual at different times by MALDI MSI. The possibility to separate these types of marks is extremely relevant in a forensic context as it may inform on the legitimate access or lack thereof of the suspect to the scene. If same-day fingermark compositions cannot be distinguished, their separation must mean they were deposited at different times, whilst linking the suspect to the scene (through the provision of biometric identification). There remains the issue of dating a crime fingermark with some notable efforts from the mass spectrometry community [
15,
16,
17,
18]. The issue of establishing the age of a fingermark is not extensively addressed here as it needs, in the authors’ opinion, a much larger, concerted, and systematic approach to be appropriately tackled. However, a proof-of-concept verification of the hypothesis that it is possible to separate the same person’s fingermarks deposited at different times will both contribute to add complementary knowledge to that provided by Gorka et al. [
13] and to the global fingermark-dating problem. Results from the present study have shown it is possible to separate overlapping fingermarks from the same donor in most cases, although it becomes more difficult to observe
minutiae for some marks older than 15 days (often due to sub-optimal contact pressure resulting in ridge merging). As only one donor was used, capabilities and limitations will need to be verified with a larger cohort of donors.
2. Results and Discussion
The separation of overlapping fingermarks and fingermark age determination by mass spectrometry-based approaches are topics that have been previously investigated and reported in the scientific literature [
4,
5,
6,
7,
8,
9,
10]. However, to date, no study has been published on the separation of
overlapping fingermarks from the
same donor and
deposited at different times.
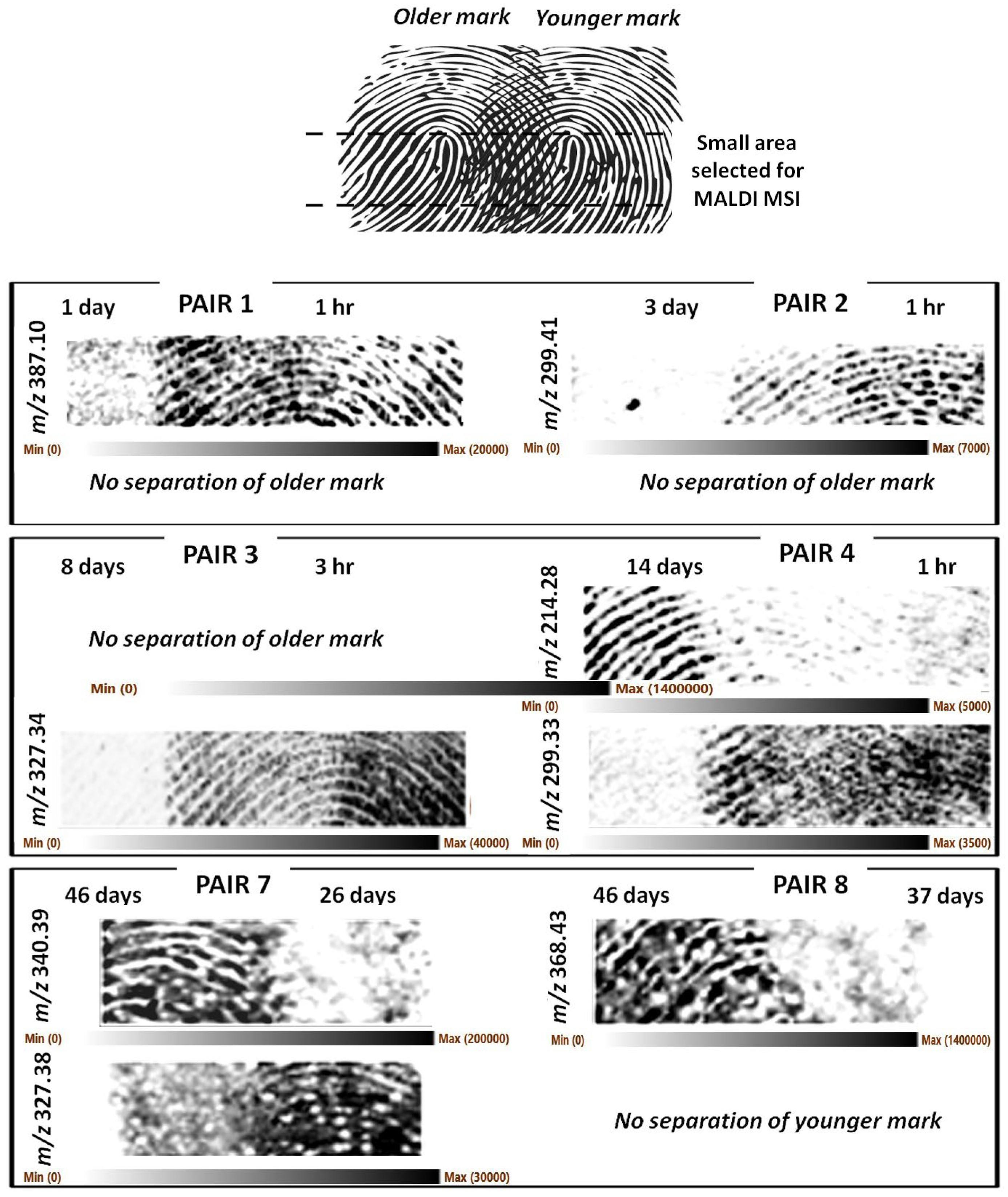
Preliminarily, groomed fingermarks were analysed to address the hypothesis that the molecular composition of fingermarks from the same donor, but of different ages, is different enough to permit separation of the ridge pattern. Partially overlapping groomed fingermarks of different ages were therefore generated by the same donor in a total of eight pairs, as shown in
Table 1.
Only a small section of the mark was imaged to reduce the run time and ensure all the intended analyses could be completed in the time available for this study.
Figure 1 shows representative
m/
z ions responsible for the separation of the marks.
As it can be observed, it was generally possible to achieve separation of the marks albeit with some exceptions. These exceptions were mainly due to occasional lack of clarity of the ridge detail, likely resulting from a combination of sub-optimal matrix application and occasional sub-optimal fingermark deposition leading to ridge merging due to excessive contact pressure. The latter circumstance was particularly deleterious for pairs 5 and 6, which did not generate any useful images and were not reported in
Figure 1.
Figure S1 reports the optical images of the groomed set showing instances of sub-optimal fingermark deposition.
In other circumstances, the image of the younger fingermark could be separated/recovered but not that of the older mark (pair 1- 1 d vs. 1 h, pair 2- 3 d vs. 1 h, pair 3- 8 d vs. 3 h). However, this circumstance does not mean fingermarks as old as 1 day and older cannot be imaged; in fact, pairs 4 and 8 have yielded images of 14- and 46-day-old marks. In one case only, it was the younger (37 d) fingermark image that could not be separated/recovered (pair 8- 46 d vs. 37 d).
It was reasonable to hypothesise that for groomed marks, endogenous species would be the main separating ions, given that marks were generated by rubbing the fingertips on the forehead and cheeks prior to subsequent deposition of the marks. However, it is important to note that for the scope of this paper, which is the attainment of biometric information by separating two impressions, knowing the identity of the ions that separate the two fingermarks is not essential. Whichever is the ion fit for this purpose, in any given instance, can be used without further investigating its identity. In any case, we have attempted a putative identification of the separating ions. It is likely the ions at
m/
z 340.394 and 368.426 are exogenous quaternary ammonium ions (C
23H
50N
+, mass accuracy −2.3 ppm and C
25H
54N
+, mass accuracy −3.8 ppm, respectively). While the ion
m/
z 327.337 (separating the younger mark of pair 3) could not be putatively identified, the ion at
m/
z 327.382 (partially separating the younger mark of pair 7) may be the C-13 isotope of the didecyldimethylammonium ion discussed later in this paper. As for the other ions separating the fingermarks, a quick lipid map search (
https://www.lipidmaps.org, accessed on 17 March 2023) using a mass tolerance of +/−0.001 Th yielded a putative identification only for the ion at
m/
z 387.099 as a potassiated phospholipid derivative, lysophosphatidic acid LPA 12:3 of formula C
15H
25O
7PK (mass accuracy −5.7 ppm), reported as involved to train the skin to repair and strengthen the epidermis [
19].
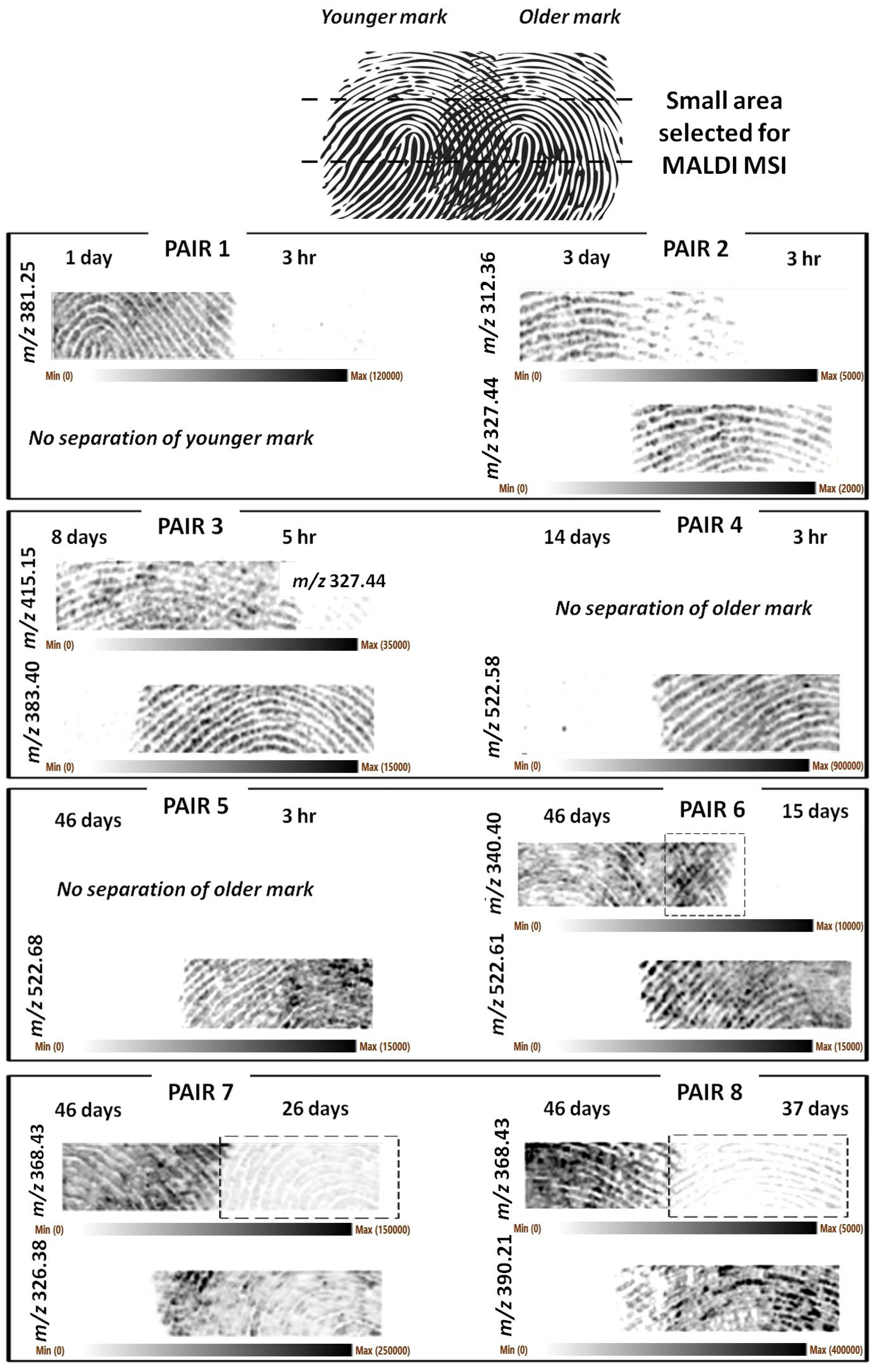
To investigate applicability in real case scenarios, these analyses were repeated using natural marks, which are impressions made without grooming or any prior preparation of the fingertip. However, the marks were aged for slightly different duration with respect to the groomed marks (
Table 1), generating another set of eight pairs of partially overlapping fingermarks (
Table 2), though employing the same donor and environmental aging conditions.
The slight difference in the age of the marks was dictated by instrumental availability. This natural mark set was then analysed by MALDI MSI upon optimisation of the matrix deposition conditions that are reported in the Methods
Section 3.2.5.
In this case too,
Figure 2 shows representative
m/
z ions responsible for the separation of the marks.
A higher image resolution and clarity of the fingermark ridge detail was achieved in this instance. However, it was clear that for natural marks too, in some cases, excessive pressure had occasionally been applied, or too little chemical content was deposited (pair 1 younger mark), resulting in marks that were not recovered or that do not show clear ridge detail.
Figure S2 reports the optical images of the natural sets showing instances of sub-optimal fingermark deposition.
Generally, the separation of the ridge pattern was successful, and the number of older marks that could not be separated was lower than the groomed set. However, some additional observations need to be made. Older fingermark ridge patterns could not be separated for pair 4 (14 d vs. 3 h) and pair 5 (46 d vs. 3 h). However, as in the case of groomed pairs, the ridge detail for the marks of 26, 37, and 46 days of age could be separated/recovered in other instances (pairs 7 and 8).
In the case of pair 1 (1 d vs. 3 h), it was the younger fingermark’s ridge pattern (3 h) that could not be recovered. However, 3 hr old fingermarks were successfully imaged for the other pairs in this set of impressions (pairs 2–5). For pair 6, the squared frame highlights an area of the separated older mark, which possibly also exhibits some level of overlapping with the younger mark. Pairs 7 and 8 are interesting in that the squared frames highlight the fact that “separating ions” are not necessarily molecules unique to one fingermark, but they can be present in both, though in very different abundances such that separation can still occur. For pairs 7 and 8, the marks at 26 and 37 days are still visible, and whilst separated from the 46-day-old mark, the 26-day-old mark exhibits an even better clarity of the ridge detail.
As for the groomed fingermarks (
Figure 1), the ions
m/
z 340.399 and 368.429 could be putatively assigned the formulas of C
23H
50N
+ (mass accuracy −2.35 ppm) and C
25H
54N
+ (mass accuracy −3.8 ppm), respectively. The ion at
m/
z 326.379 is also a contaminant and has previously been detected and identified in fingermarks as the didecyldimethylammonium ion [
20]. The ions at nominal
m/
z 523 were suspected to be the external contaminant previously detected [
21,
22] of formula C
36H
76N
+. However, the relative error is too high to permit this assignment (>20 ppm). A lipid maps search permitted the ion at
m/
z 381.247 to be putatively identified as a sterol lipid (M+H-H
2O]+) of formula C
22H
38O
4S (mass accuracy −2.8 ppm). The ion at
m/
z 415.15 could be assigned to another sodiated lysophosphatidic acid derivative (LPA 14:3;O) of formula C
17H
29O
8PNa; however, the relative error of −12.5 ppm casts stronger doubts on this ion’s identity. The lipid maps search did not allow any other univocal putative identification for any of the remaining separating ions shown in
Figure 2, and MS/MS experiments are necessary to establish their identity.
Regions of interest were drawn from the natural set of marks from the non-overlapping areas; spectral variability reflecting variable molecular composition could be observed in the mass range investigated even by manual inspection (
Figure 3), either in terms of absence/presence of signals or in terms of the relative intensity.
As expected, the spectral profiles change dramatically with the age of the mark. Visually, the spectral profiles of marks of 3 h, 3 days, and 8 days of age differ significant from those with ages of 15 days, 26 days, and 46 days. In particular, the lower molecular range exhibits signals of higher intensity, as highlighted by the zoomed in spectra (for example for the 3h and 3 days vs. 8 days), possibly reflecting the breakdown/degradation of complex lipids with time. Though more donors and replicates would strengthen the reliability of this observation in a follow up study, this spectral variability explains the fingermark image separation achieved. The zoomed in spectra for the marks at 15, 26, and 46 days have a different (higher relative intensity) y scale compared to the other marks to better highlight any differences between these older marks; the ions at nominal
m/
z 304, 326, and 332 decrease in intensity as the age increases. The ions at
m/
z 304 and 332 are exogenous species and have been putatively identified as dodecylbenzyldimethylammonium (nominal
m/
z 304) with two extra CH
2 units, respectively, also previously detected in fingermarks [
20]. As reported earlier in this paper, the ion at nominal
m/z 326 is also a contaminant and has previously been detected and/or identified in fingermarks as didecyldimethylammonium ion [
21,
22]. These ions are common species found in toiletries (e.g., hair gels) and antibacterial products, such as hand sanitizers. They are observed for all marks up to 46 days of age. Their constant presence in all the marks examined indicate these are persistent substances and can be used to obtain MALDI molecular images of fingermarks recovered at crime scenes that are not accessed immediately (up to 46 days of age in this study) due to their high ionisation efficiency. The mass spectral profiles of the 26- and 46-day-old marks appear to exhibit a higher ion population in the higher mass range (
m/
z 690–900) compared to the 15-day-old mark. Again, this is possibly reflecting the breakdown/degradation of high molecular weight compounds (beyond the mass range measured in this study) with time.
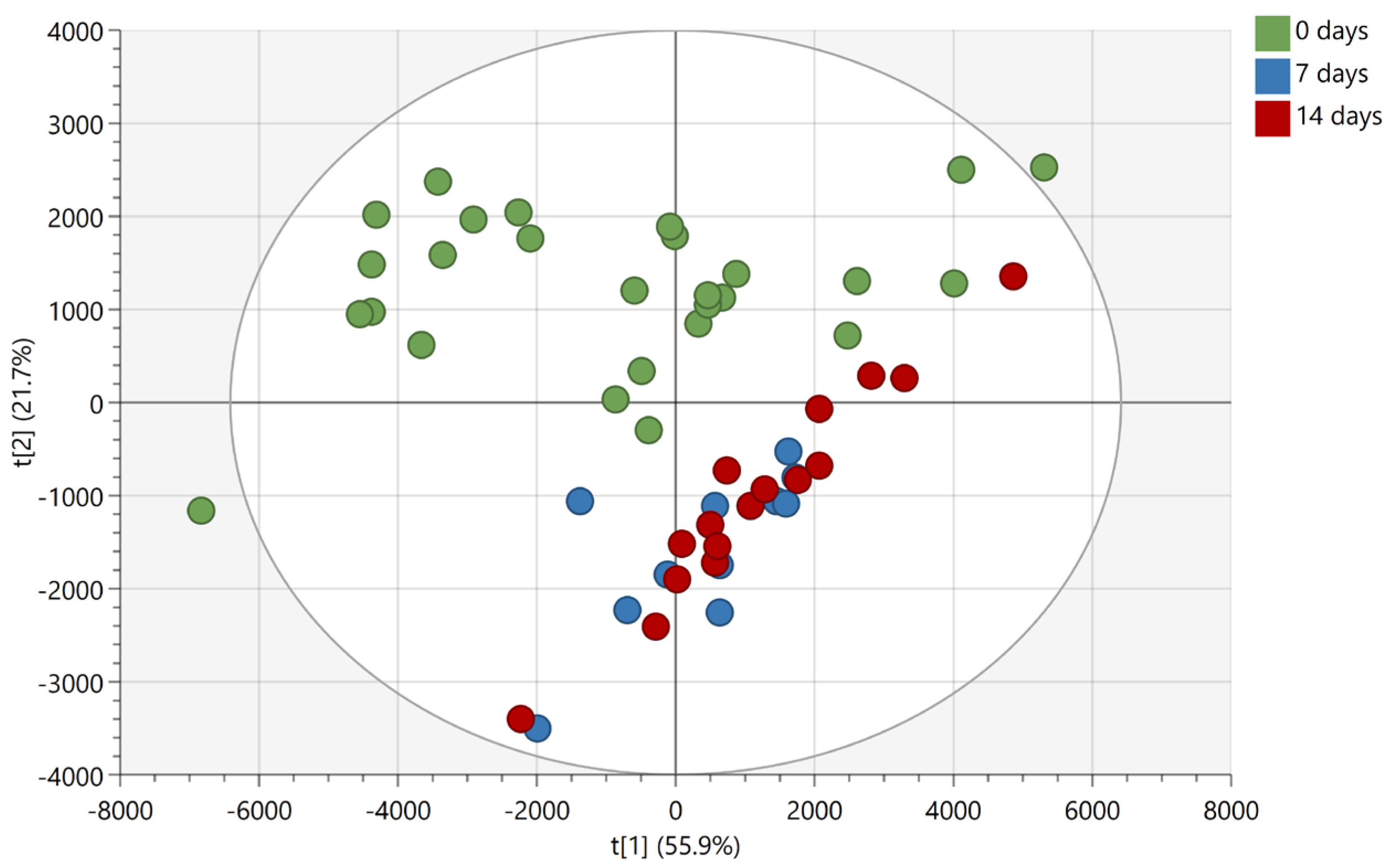
Based on the findings reported in
Figure 2 and
Figure 3, another experiment was conducted whereby the same donor deposited non-overlapping natural fingermarks, which were aged for 0, 7, and 14 days, matrix-spray-coated under the same conditions as for the overlapping fingermarks and subjected to manual firing of the laser in discrete locations (MALDI MS Profiling (MALDI MSP)). The 7- and 14-days-aging points for the chemical profiling were selected based on the more dramatic difference in spectral profiles observed for the overlapping fingermarks of 8 and 15 days of age (
Figure 3). Mass spectra were subsequently processed for submission to multivariate statistical analysis. Principle component analysis (PCA) shows clear separation between fresh marks (0 days) and marks of older age points (7 and 14 days) (
Figure 4).
In contrast, group overlap was observed between the 7- and 14-days-age points indicating that, overall, the compositional change of marks between 7 and 14 days old is not enough to permit differentiation between 1-week- and 2-weeks-old marks. Importantly, however, the inability under these experimental conditions to differentiate between 7- and 14-day-old marks does not mean that unique molecules could not be found, allowing a separation of the ridge pattern for these two age points.
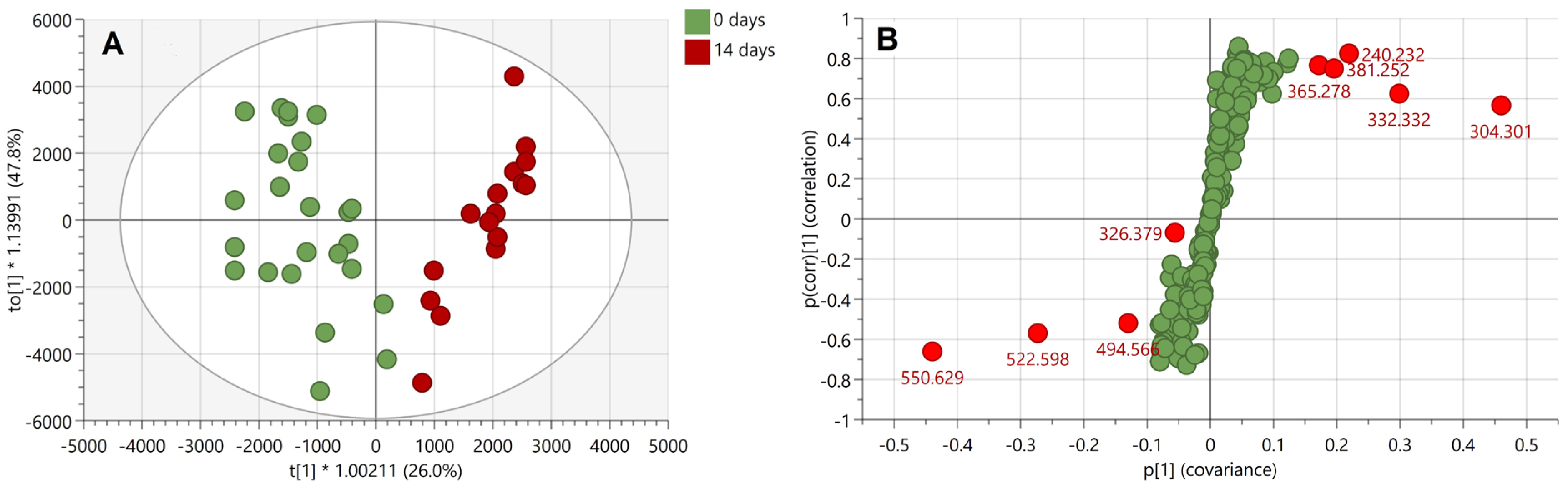
A subsequent linear discriminant analysis was performed using OPLS-DA with a seven-fold cross validation on the marks of 0 and 14 days of age. The two 0-day old outliers observed in the PCA score plot (
Figure 4) were excluded from data before performing the analysis. The OPLS-DA model could describe all included data (R
2X(cum) = 0.829), showing clear separation of the two age groups (R
2Y(cum) = 0.833) as well as robustness in the ability to classify and distinguish fresh from 14-days-old marks (Q
2(cum) = 0.785) (
Figure 5A).
An S-plot based on the OPLS-DA model was used to reveal the most discriminant ions for the two age groups (
Figure 5B). The most discriminating ions are
m/
z 304.301, 550.629, 332.332, and 522.598 with VIP scores of 6.3, 5.6, 3.9, and 3.7, respectively. Interestingly, despite the ion at
m/
z 326.379 displaying a high VIP score (4.5), suggesting a high contribution to the separation of the two groups of marks in the OPLS-DA model, the S-plot indicates instead a low reliability in the model, i.e., high risk of spurious correlation. Similarly to the ions at nominal
m/
z 304, 326 and 332, the ion at nominal
m/
z 551 is also an exogenous species and is putatively identified as the dimethyloctadecylammonium ion from previous published work [
5,
20,
22]. In addition, the discriminating ion at
m/
z 499.566 may also putatively be identified as ditallowdimethylammonium ion, likely resulting from the use of personal and household products, as reported by Manier et al. [
22]. This time the ion at nominal
m/
z 523 could be putatively identified as another ditallowdimethylammonium ion (C
36H
76N
+, mass accuracy −1.9 ppm) belonging to the same family as the ion at
m/
z 494.566. Therefore, within this profiling experiment and based on the statistical analysis, the most age-discriminant ions are exogenous contaminants. However, as shown in
Figure 2, endogenous compounds may also contribute to the separation between overlapping fingermarks.
In conclusion, this study indicates it is possible for this donor to separate overlapping fingermarks deposited at different times as a result of a different enough molecular composition. This result is supported especially by the use of natural marks (not artificially enriched). However, a multi-donor study is required to extend this observation to the general population. The output from this study is not in contrast with the work published by Gorka et al. [
12]; intra-donor variability may still, as they conclude from their study, be very low. However, to separate overlapping fingermarks, even just one molecule per mark out of the thousands detected is needed to be unique to obtain a separate image of the two impressions. In the future, this capability will be very useful when supported by a method that definitively established the age of a fingermark to establish
(i) one person has deposited both overlapping marks,
(ii) the identity of the individual (through the separated ridge detail obtained), and
(iii) the time at which the scene was accessed on both occasions (in the case of two overlapping fingermarks) to establish legitimate access.