Study of Interactions between Saponin Biosurfactant and Model Biological Membranes: Phospholipid Monolayers and Liposomes
Abstract
1. Introduction
2. Results
2.1. Interactions with the Monolayer Membrane
2.1.1. The Surface Pressure-Area and Surface Potential-Area Isotherms
2.1.2. Brewster Angle Microscopy Images
2.1.3. Relaxation/Penetration Studies
2.2. Interaction with a Spherical Model Membrane
3. Discussion
4. Materials and Methods
4.1. Research Regarding Monolayer Membranes
4.2. Research Regarding Spherical Membranes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kregiel, D.; Berlowska, J.; Witonska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-Based, Biological-Active Surfactants from Plants. In Application and Characterization of Surfactants; Reza Najjar, R., Ed.; Intech Open: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Liao, Y.; Li, Z.; Zhou, Q.; Sheng, M.; Qu, Q.; Shi, Y.; Yanga, J.; Lv, L.; Dai, X.; Shi, X. Saponin surfactants used in drug delivery systems: A new application for natural medicine components. Int. J. Pharm. 2021, 603, 120709. [Google Scholar] [CrossRef] [PubMed]
- Liwa, A.C.; Barton, E.N.; Cole, W.C.; Nwokocha, C.R. Chapter 15—Bioactive Plant Molecules, Sources and Mechanism of Action in the Treatment of Cardiovascular Disease. In Pharmacognosy Fundamentals, Applications and Strategies; Academic Press: Boston, MA, USA, 2017; pp. 15–336. [Google Scholar]
- Vincken, J.-P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, L.; Yuan, B.; Liu, Y. The Pharmacological Activities of Licorice. Planta Med. 2015, 81, 1654–1669. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Beatriz, M.; Oliveira, M. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef] [PubMed]
- Al-Turki, A.I.; El-Ziney, M.G.; Abdel-Salam, A.M. Chemical and anti-bacterial characterization of aqueous extracts of oregano, marjoram, sage and licorice and their application in milk and labneh. J. Food Agric. Environ. 2008, 6, 39–44. [Google Scholar]
- Zhou, T.Z.; Deng, X.M.; Qiu, J.Z. Antimicrobial activity of licochalcone E against Staphylococcus aureus and its impact on the production of staphylococcal alpha-toxin. J. Microbiol. Biotechnol. 2012, 22, 800–805. [Google Scholar] [CrossRef]
- Messier, C.; Grenier, D. Effect of licorice compounds licochalcone A, glabridin and glycyrrhizic acid on growth and virulence properties of Candida albicans. Mycoses 2011, 54, e801–e806. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef]
- Güçlü-Ustündağ, O.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Rojewska, M.; Smułek, W.; Prochaska, K.; Kaczorek, E. Combined Effect of Nitrofurantoin and Plant Surfactant on Bacteria Phospholipid Membrane. Molecules 2020, 25, 2527. [Google Scholar] [CrossRef]
- de Groot, C.; Müller-Goymann, C.C. Saponin Interactions with Model Membrane Systems—Langmuir Monolayer Studies, Hemolysis and Formation of ISCOMs. Planta Med. 2016, 82, 1496–1512. [Google Scholar] [CrossRef]
- Orczyk, M.; Wojciechowski, K. Comparison of the effect of two Quillaja bark saponin extracts on DPPC and DPPC/cholesterol Langmuir monolayers. Colloids Surf. B 2015, 136, 291–299. [Google Scholar] [CrossRef]
- Korchowiec, B.; Gorczyca, M.; Wojszko, K.; Janikowska, M.; Henry, M.; Rogalska, E. Impact of two different saponins on the organization of model lipid membranes. Biochim. Biophys. Acta 2015, 1848, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Stępnik, K. Biomimetic Chromatographic Studies Combined with the Computational Approach to Investigate the Ability of Triterpenoid Saponins of Plant Origin to Cross the Blood-Brain Barrier. Int. J. Mol. Sci. 2021, 22, 3573. [Google Scholar] [CrossRef] [PubMed]
- Geisler, R.; Dargel, C.; Hellweg, T. The biosurfactant β-aescin: A review on the physico-chemical properties and its interaction with lipid model membranes and Langmuir monolayers. Molecules 2019, 25, 117. [Google Scholar] [CrossRef]
- Aguilar-Pérez, K.; Avilés-Castrillo, J.; Medina, D.I.; Parra-Saldivar, R.; Iqbal, H. Insight into Nanoliposomes as Smart Nanocarriers for Greening the Twenty-First century Biomedical Settings. Front. Bioeng. Biotechnol. 2021, 8, 1441. [Google Scholar] [CrossRef] [PubMed]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef]
- Szcześ, A.; Jurak, M.; Chibowski, E. Stability of binary model membranes—Prediction of the liposome stability by the Langmuir monolayer study. J. Colloid Interface Sci. 2012, 372, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, M.; Smułek, W.; Kaczorek, E.; Prochaska, K. Langmuir Monolayer Techniques for the Investigation of Model Bacterial Membranes and Antibiotic Biodegradation Mechanisms. Membranes 2021, 11, 707. [Google Scholar] [CrossRef] [PubMed]
- Elderdfi, M.; Sikorski, A.F. Langmuir-monolayer methodologies for characterizing protein-lipid interactions. Chem. Phys. Lipids 2018, 212, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Dopierała, K.; Weiss, M.; Krajewska, M.; Błońska, J. Towards understanding the binding affinity of lipid drug carriers to serum albumin. Chem. Phys. Lipids 2022, 250, 105271. [Google Scholar] [CrossRef] [PubMed]
- Otzen, D.E. Biosurfactants and surfactants interacting with membranes and proteins: Same but different? Biochim. Biophys. Acta Biomembr. 2017, 1859, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; Brockman, H.L. Using monomolecular films to characterize lipid lateral interactions. Methods Mol. Biol. 2007, 398, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Przykaza, K.; Woźniak, K.; Jurak, M.; Wiącek, A.E.; Mroczka, R. Properties of the Langmuir and Langmuir–Blodgett monolayers of cholesterol-cyclosporine A on water and polymer support. Adsorption 2019, 25, 923–936. [Google Scholar] [CrossRef]
- Schmid, C.; Dawid, C.; Peter, V.; Hofmann, T. Saponins from European Licorice Roots (Glycyrrhiza glabra). J. Nat. Prod. 2018, 81, 1734–1744. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Z.; Hua, W.; Castada, H.; Allen, H.C. Reorganization and Caging of DPPC, DPPE, DPPG, and DPPS Monolayers Caused by Dimethylsulfoxide Observed Using Brewster Angle Microscopy. Langmuir 2010, 26, 18902–18908. [Google Scholar] [CrossRef]
- Davies, J.T.; Rideal, K. Interfacial Phenomena, 2nd ed.; Academic Press: New York, NY, USA, 1963; p. 265. [Google Scholar]
- Dynarowicz–Łątka, P.; Dhanabalan, A.; Oliveira, O.N., Jr. Modern physicochemical research on Langmuir monolayers. Adv. Colloid Interface Sci. 2001, 91, 221–293. [Google Scholar] [CrossRef]
- Jurak, M.; Szafran, K.; Cea, P.; Martin, S. Analysis of Molecular Interactions between Components in Phospholipid-Immunosuppressant-Antioxidant Mixed Langmuir Films. Langmuir 2021, 37, 5601–5616. [Google Scholar] [CrossRef]
- Nowotarska, S.; Nowotarski, K.; Friedman, M.; Situ, C. Effect of Structure on the Interactions between Five Natural Antimicrobial Compounds and Phospholipids of Bacterial Cell Membrane on Model Monolayers. Molecules 2014, 19, 7497–7515. [Google Scholar] [CrossRef]
- Costa, A.P.; Xu, X.; Khan, M.; Burgess, D.J. Liposome Formation Using a Coaxial Turbulent Jet in Co-Flow. Pharmaceutical Research 2016, 33, 404–416. [Google Scholar] [CrossRef]

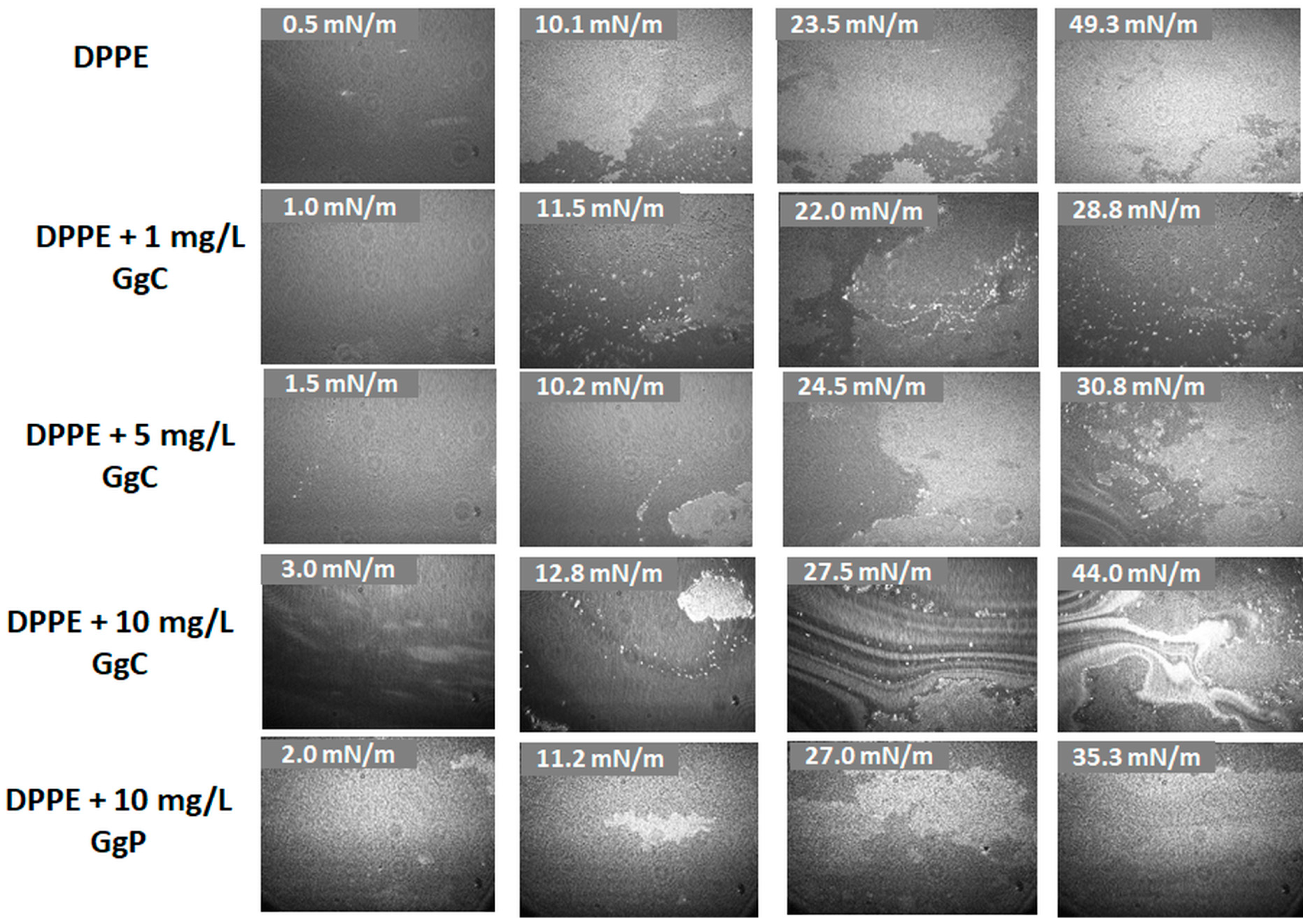

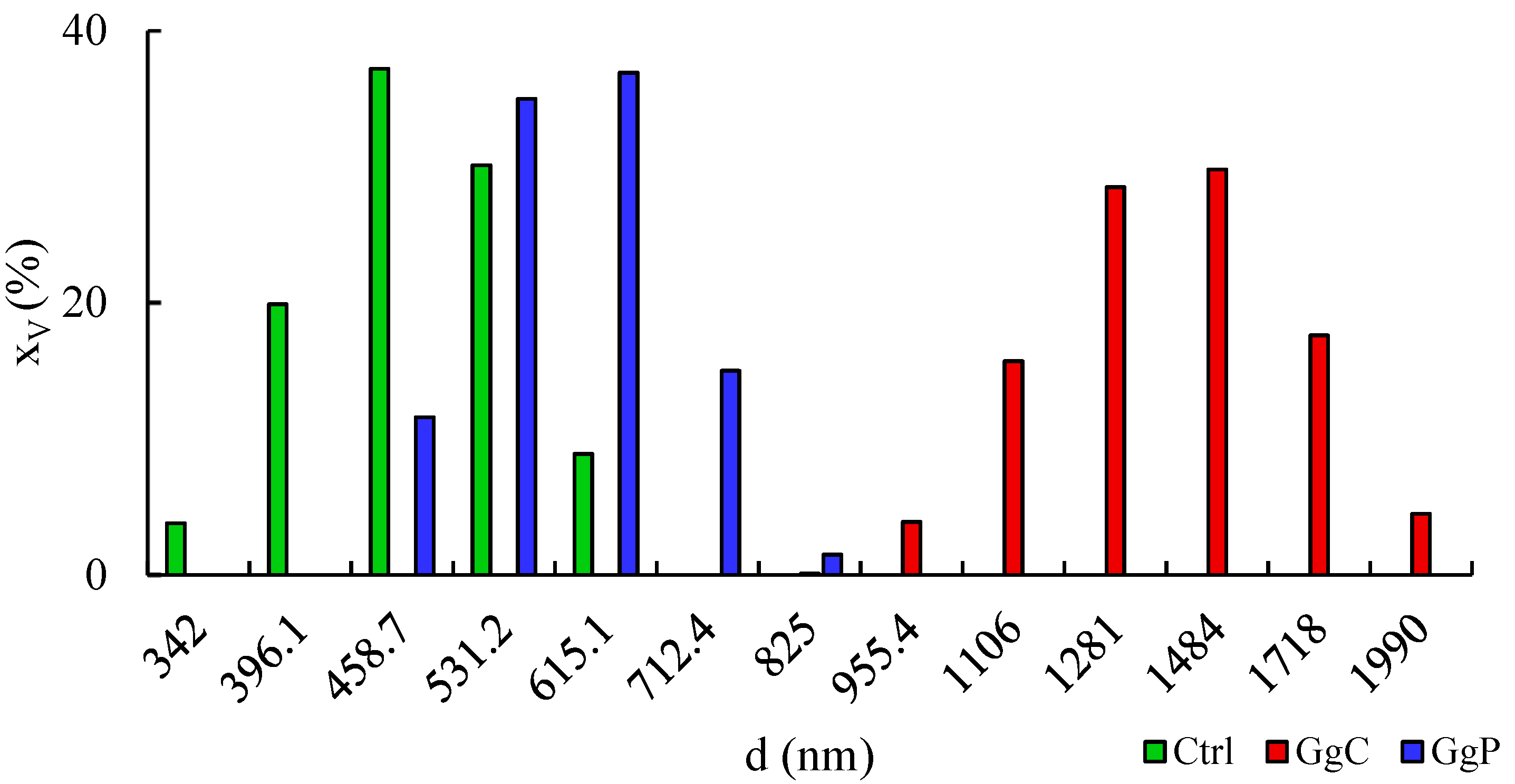
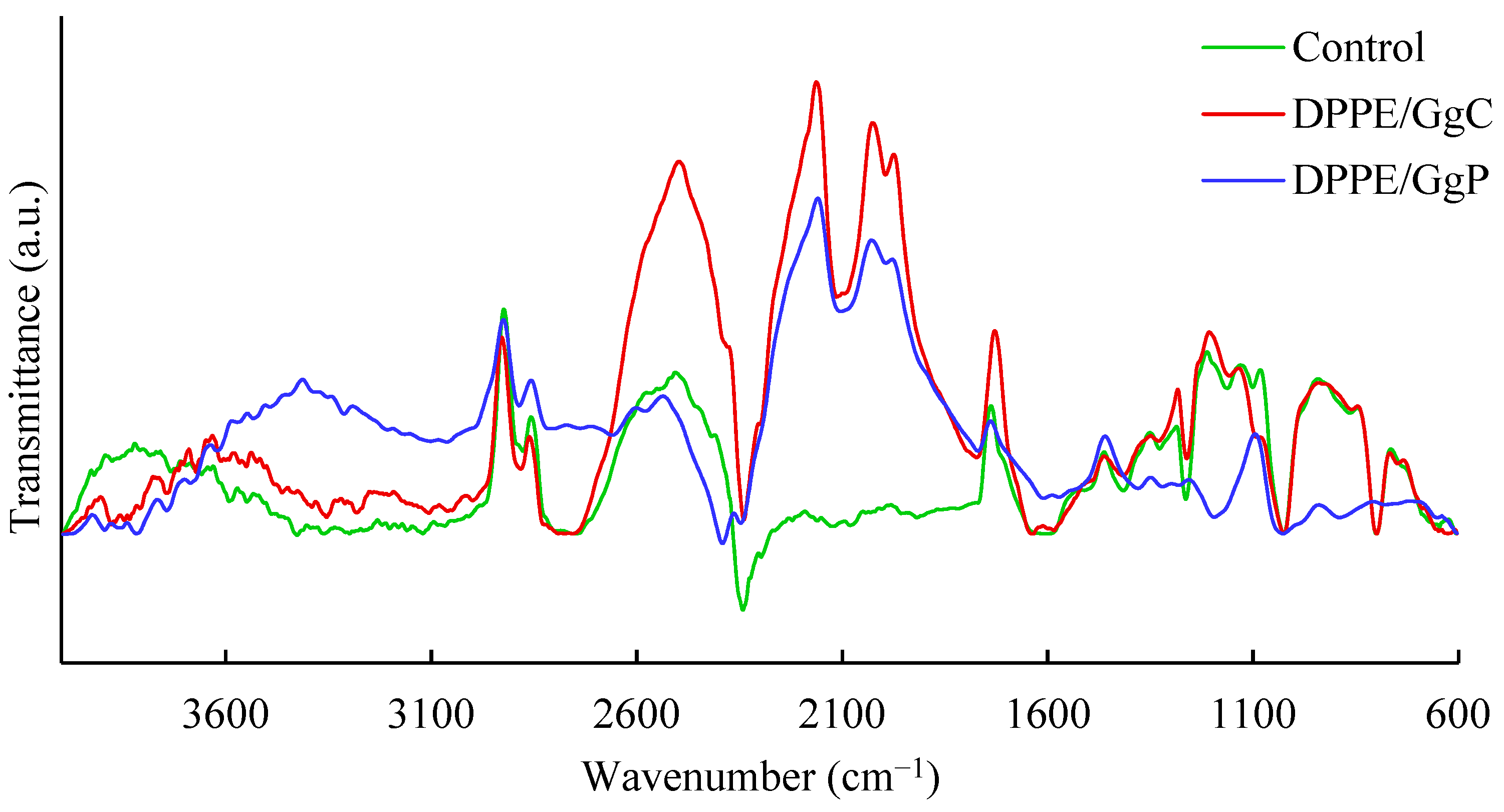
| Alift-off (Å2/molec.) | Acollapse (Å2/molec.) | πcollapse (mN/m) | Amax (Å2/molec.) | πmax (mN/m) | Cs−1max (mN/m) | |
|---|---|---|---|---|---|---|
| DPPE | 42.4 | 17.1 | 56.8 | 24.2 | 37.5 | 89.3 |
| DPPE/1 mg/L GgC | 50.2 | 24.2 | 28.1 | 31.6 | 18.9 | 51.2 |
| DPPE/5 mg/L GgC | 56.8 | 24.9 | 30.7 | 30.8 | 22.5 | 53.4 |
| DPPE/10 mg/L GgC | 61.2 | 21.8 | 48.3 | 29.2 | 24.3 | 49.3 |
| DPPE/1 mg/L GgP | 43.5 | 18.8 | 55.3 | 27.6 | 28.6 | 107.3 |
| DPPE/5mg/L GgP | 57.2 | 28.6 | 53.9 | 32.8 | 40.7 | 120.3 |
| DPPE/10 mg/L GgP | 64.9 | 29.8 | 53.3 | 36.3 | 40.6 | 127.3 |
| Sample | Zeta Potential ζ (mV) | PDI (-) |
|---|---|---|
| Control | −46.94 ± 3.06 a | 0.800 ± 0.141 a’ |
| GgC | −51.40 ± 0.89 b | 0.797 ± 0.143 a’ |
| GgP | −42.74 ± 1.77 c | 0.537 ± 0.153 a’ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojewska, M.; Smułek, W.; Grzywaczyk, A.; Kaczorek, E.; Prochaska, K. Study of Interactions between Saponin Biosurfactant and Model Biological Membranes: Phospholipid Monolayers and Liposomes. Molecules 2023, 28, 1965. https://doi.org/10.3390/molecules28041965
Rojewska M, Smułek W, Grzywaczyk A, Kaczorek E, Prochaska K. Study of Interactions between Saponin Biosurfactant and Model Biological Membranes: Phospholipid Monolayers and Liposomes. Molecules. 2023; 28(4):1965. https://doi.org/10.3390/molecules28041965
Chicago/Turabian StyleRojewska, Monika, Wojciech Smułek, Adam Grzywaczyk, Ewa Kaczorek, and Krystyna Prochaska. 2023. "Study of Interactions between Saponin Biosurfactant and Model Biological Membranes: Phospholipid Monolayers and Liposomes" Molecules 28, no. 4: 1965. https://doi.org/10.3390/molecules28041965
APA StyleRojewska, M., Smułek, W., Grzywaczyk, A., Kaczorek, E., & Prochaska, K. (2023). Study of Interactions between Saponin Biosurfactant and Model Biological Membranes: Phospholipid Monolayers and Liposomes. Molecules, 28(4), 1965. https://doi.org/10.3390/molecules28041965








