Strawberry Fragaria x ananassa cv. Festival: A Polyphenol-Based Phytochemical Characterization in Fruit and Leaf Extracts
Abstract
1. Introduction
| Cultivar | Total Polyphenols (mg GAE g−1 DW) a | Ref. | |
|---|---|---|---|
| Fruit | Leaf | ||
| Allstar | 19.71 ± 0.20 | 55.2 ± 3.6 | [28] |
| Amaou | 15.6 ± 3.7 | 117.1 ± 2.7 | [22] |
| Camino Real | 1.8 ± 0.11 (FW) | 6.14 b | [4,36] |
| Diana | - | 14.76 | [18] |
| Elsanta | 24.11 | 3.73 c | [37] |
| Festival | 20 ± 0.1 | 62.37 ± 0.1 | [19] |
| Mount Everest | - | 293.63 ± 1.95 | [27] |
| Polka | 2.57 ± 0.02 (FW) | 81.15 ± 0.64 c | [21,38] |
| Red Merlin | 33.33 ± 0.1 | 72.1 ± 0.1 | [19] |
| San Andreas | - | 8.93 b | [4] |
| Senga Sengana | 15.2 (FW) | 326.57 ± 0.82 | [27,39] |
| Suzana | 35.24 ± 0.3 | 72.63 ± 0.1 | [19] |
| Tamar | 21.1 ± 0.5 | 42.63 ± 0.2 | [19] |
| Tochiotome | - | 122.3 | [40] |
| Winter Dawn | 31.42 ± 0.1 | 66.58 ± 0.2 | [19] |
2. Results
2.1. Preliminary Phytochemical Analysis of Total Polyphenol Fruit and Leaf Extracts
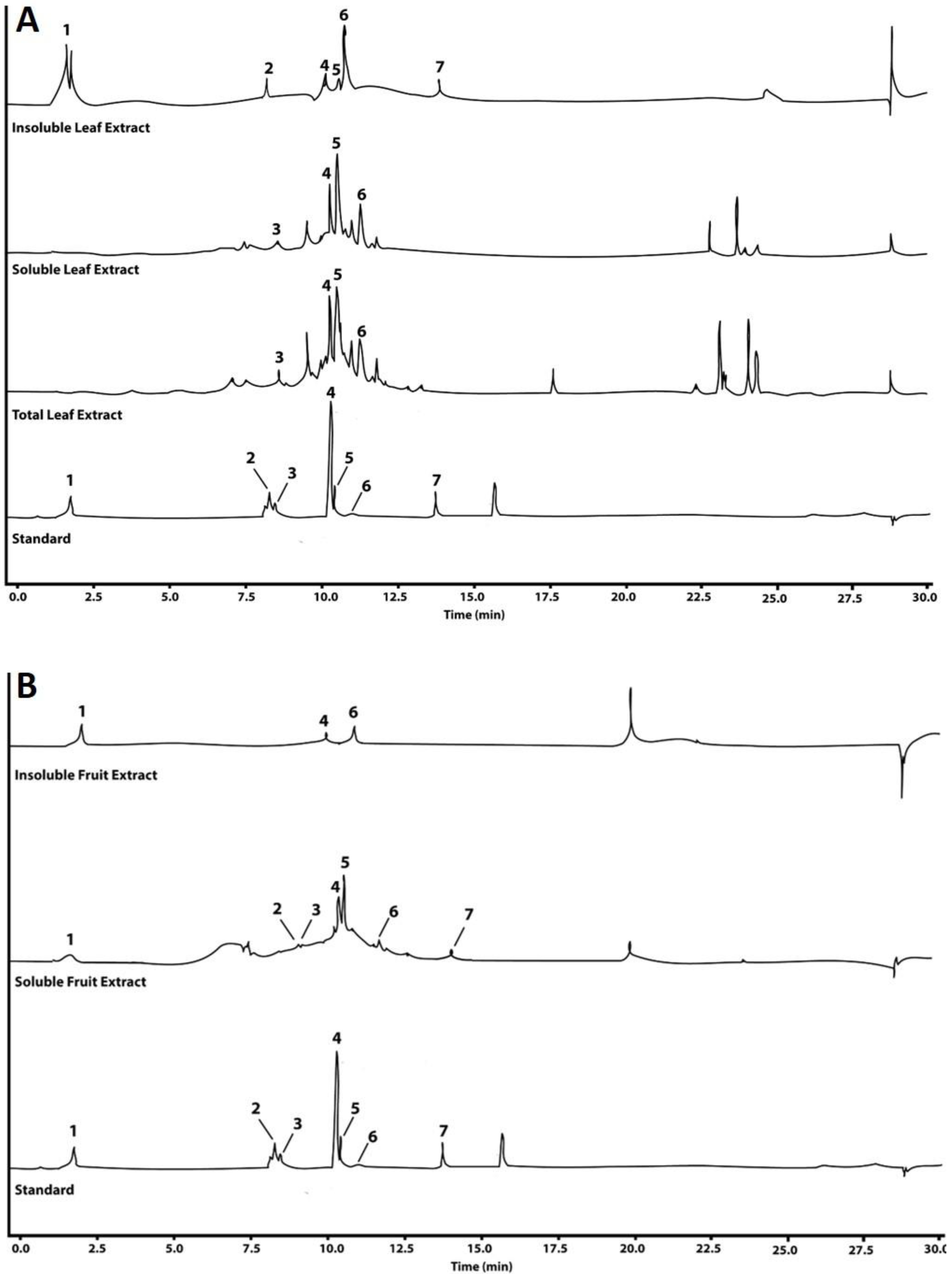
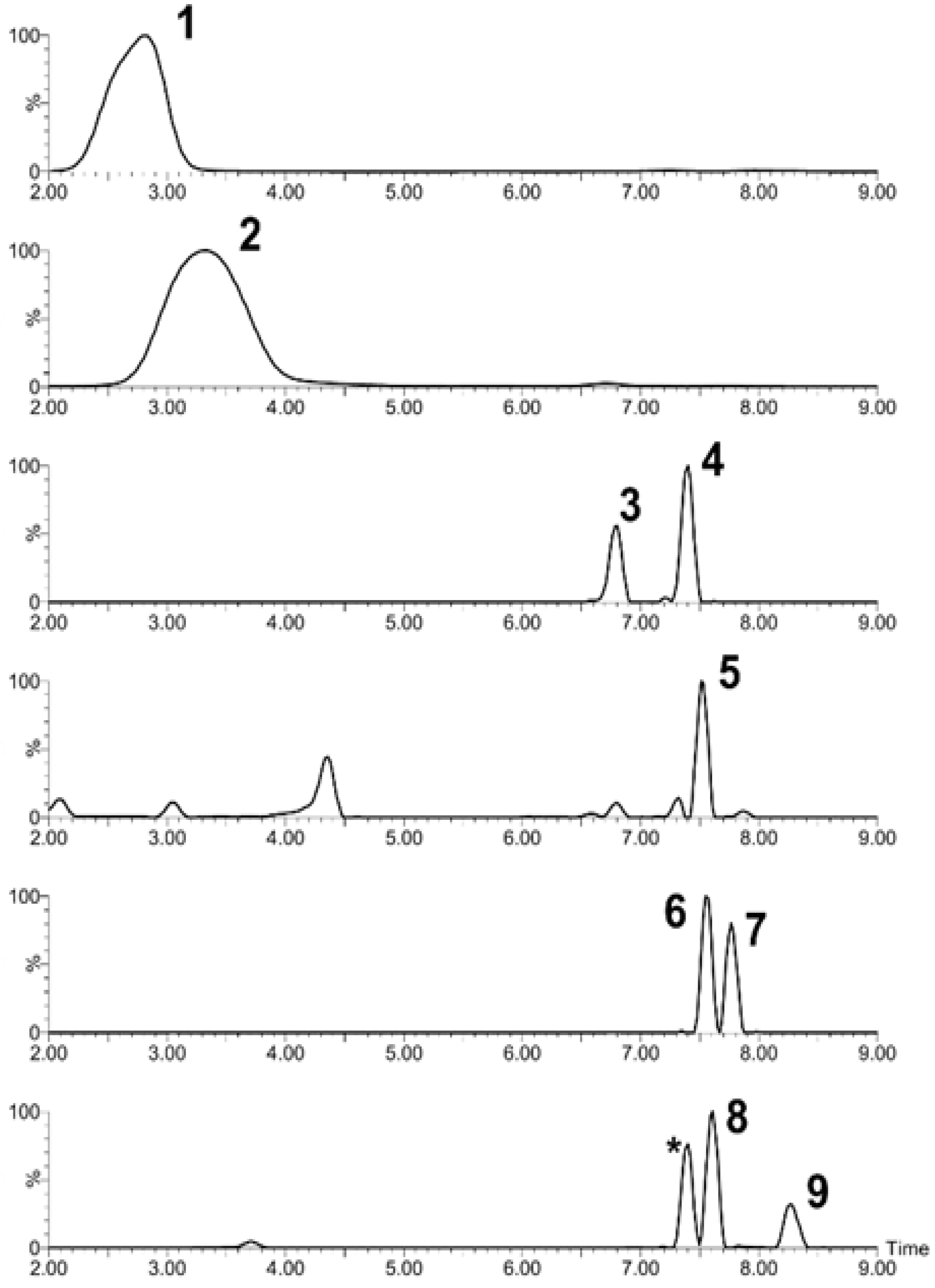
2.2. Chromatographic Analysis
2.3. Cytotoxic Activity
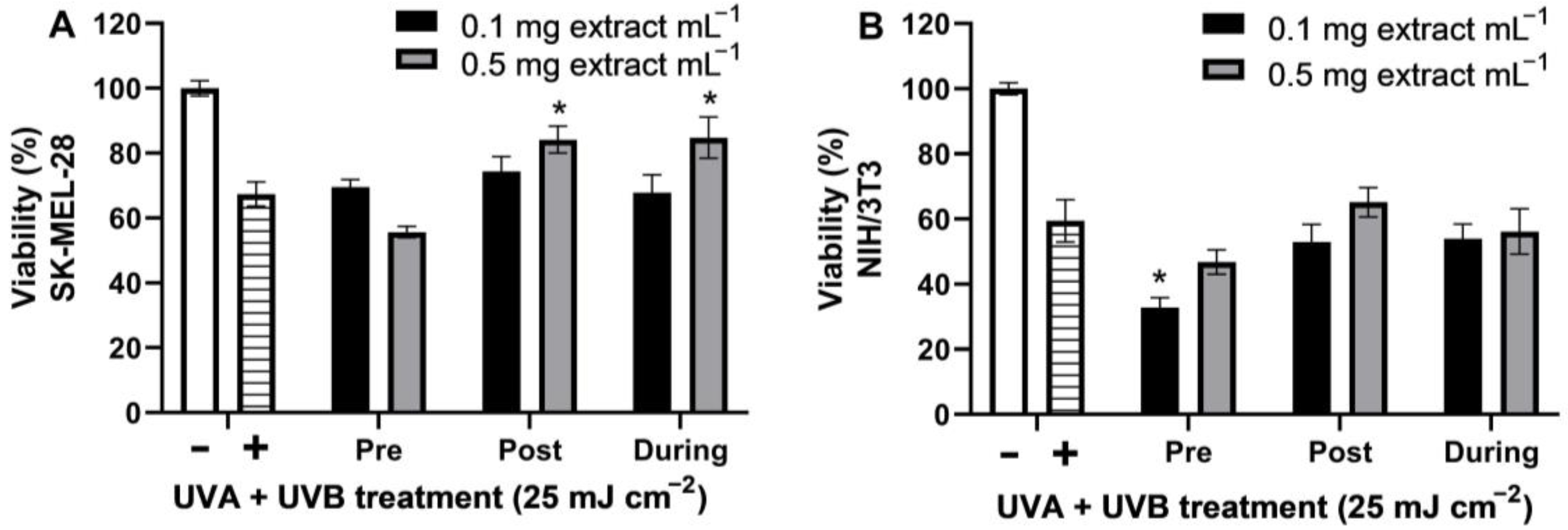
2.4. UVA + UVB Photoprotection
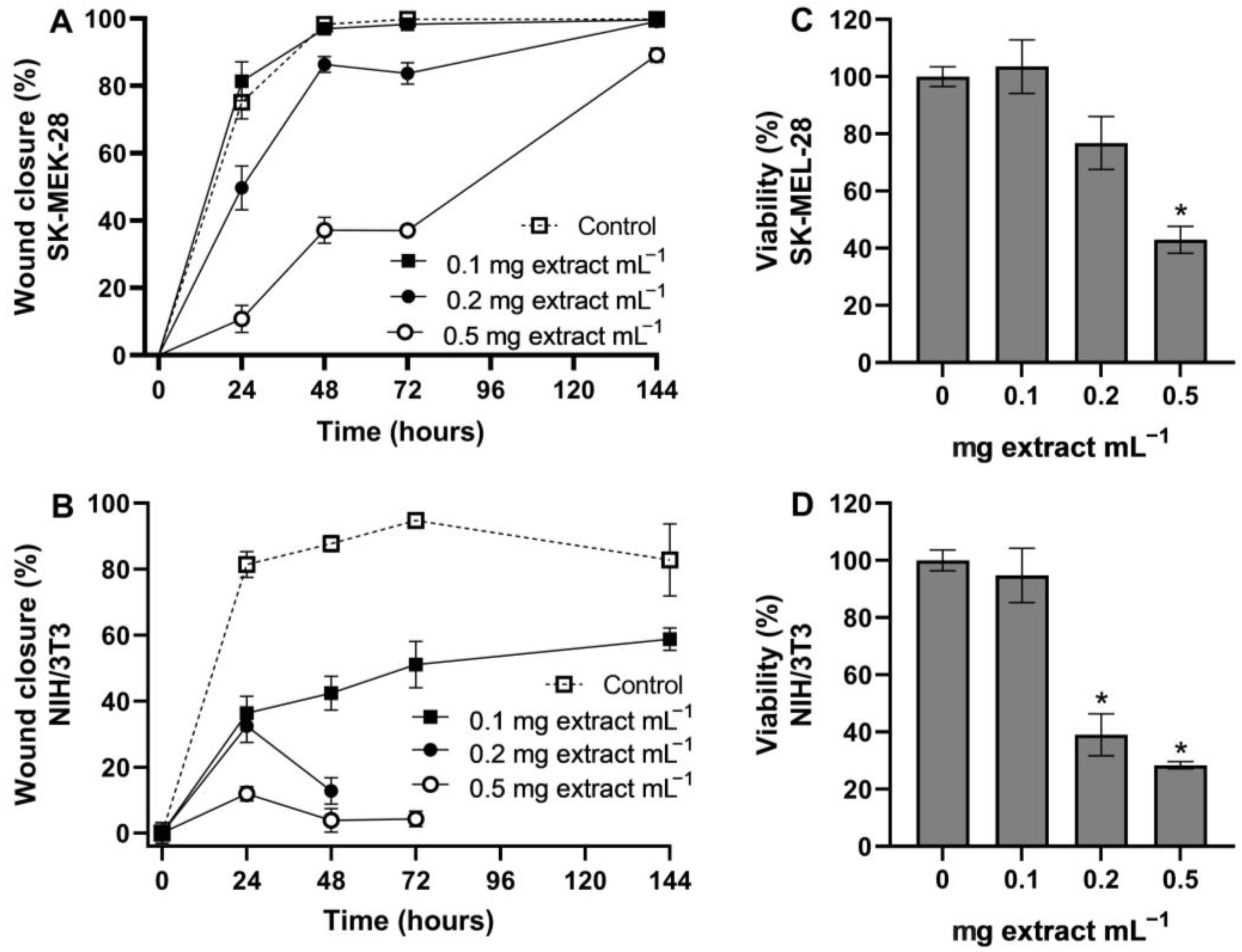
2.5. Scratch Wound Healing Assay
3. Materials and Methods
3.1. Extract Preparation
3.2. Phytochemical Analysis
3.3. Chromatographic Analysis
3.4. Cell Viability Assay
3.5. Photoprotection Experiment
3.6. Scratch Wound Healing Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- FAO, FAOStat. Organización de las Naciones Unidas para la Alimentación y la Agricultura. 2021. Available online: https://www.fao.org (accessed on 25 May 2022).
- Simpson, D. The Economic Importance of Strawberry Crops. In Genomes Rosaceous Berries Their Wild relatives; Compend. Plant Genomes.; Hytönen, T., Graham, J., Harrison, R., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 1–7. [Google Scholar] [CrossRef]
- Kirschbaum, D.; Jerez, E.; Salazar, S.; Borquez, A.; Meneguzzi, N.; Agüero, J.; Conci, V.; Conci, L.; Salame, T.; Santos, B. Causes of non-marketable fruit production throughout the strawberry harvest season in subtropical environments. Acta Hortic. 2014, 1049, 887–892. [Google Scholar] [CrossRef]
- Villamil-Galindo, E.; Van de Velde, F.; Piagentini, A. Strawberry agro-industrial by-products as a source of bioactive compounds: Effect of cultivar on the phenolic profile and the antioxidant capacity. Bioresour. Bioprocess. 2021, 8, 61. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, G.; Cardoso, J.C.; Rubio-Senent, F.; Serrano, A.; Borja, R.; Fernández-Bolaños, J.; Fermoso, F. Thermally-treated strawberry extrudate: A rich source of antioxidant phenols and sugars. Innov. Food Sci. Emerg. Technol. 2019, 51, 186–193. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Fernández-Prior, Á.; Oria, A.B.; Rodríguez-Juan, E.; Pérez-Rubio, A.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Utilization of strawberry and raspberry waste for the extraction of bioactive compounds by deep eutectic solvents. LWT Food Sci. Technol. 2020, 130, 109645. [Google Scholar] [CrossRef]
- Šaponjac, V.T.; Gironés-Vilaplana, A.; Djilas, S.; Mena, P.; Ćetković, G.; Moreno, D.; Čanadanović-Brunet, J.; Vulić, J.; Stajčić, S.; Vinčić, M. Chemical composition and potential bioactivity of strawberry pomace. RSC Adv. 2015, 5, 5397–5405. [Google Scholar] [CrossRef]
- Cubero-Cardoso, J.; Serrano, A.; Trujillo-Reyes, Á.; Villa-Gómez, D.K.; Borja, R.; Fermoso, F. Valorization Options of Strawberry Extrudate Agro-Waste. A Review. In Innovation in the food Sector through Valorization Food Agro-Food by-Products; de Barros, A., Gouvinhas, I., Eds.; IntechOpen: London, UK, 2021; pp. 1–20. [Google Scholar] [CrossRef]
- Afrin, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Reboredo-Rodriguez, P.; Mezzetti, B.; Varela-López, A.; Giampieri, F.; Battino, M. Promising Health Benefits of the Strawberry: A Focus on Clinical Studies. J. Agric. Food Chem. 2016, 64, 4435–4449. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry As a Functional Food: An Evidence-Based Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 790–806. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, R.C.; Temocico, G.; Fierascu, I.; Ortan, A.; Babeanu, N.E. Fragaria Genus: Chemical Composition and Biological Activities. Molecules 2020, 25, 498. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Bompadre, S.; Mezzetti, B.; Quiles, J.L.; Giampieri, F.; Battino, M. The Healthy Effects of Strawberry Polyphenols: Which Strategy behind Antioxidant Capacity? Crit. Rev. Food Sci. Nutr. 2016, 56, S46–S59. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Reboredo-Rodriguez, P.; Varela-Lopez, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. The healthy effects of strawberry bioactive compounds on molecular pathways related to chronic diseases. Ann. N. Y. Acad. Sci. 2017, 1398, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and Human Health: Effects beyond Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive Compounds of Strawberry and Blueberry and Their Potential Health Effects Based on Human Intervention Studies: A Brief Overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef] [PubMed]
- Kårlund, A.; Hanhineva, K.; Lehtonen, M.; McDougall, G.J.; Stewart, D.; O Karjalainen, R. Non-targeted metabolite profiling highlights the potential of strawberry leaves as a resource for specific bioactive compounds. J. Sci. Food Agric. 2017, 97, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Carlen, C.; Heritier, J.; Andlauer, W. Profiles of bioactive compounds in fruits and leaves of strawberry cultivars. J. Berry Res. 2017, 7, 71–84. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Mohammed, R.; El-Din, M.E.; Hassan, H.M.; Ali, Z.Y.; Rateb, M.E.; El Naggar, E.M.B.; Othman, E.M.; Abdelmohsen, U.R. Comparative phytochemical analysis of five Egyptian strawberry cultivars (Fragaria × ananassa Duch.) and antidiabetic potential of Festival and Red Merlin cultivars. RSC Adv. 2021, 11, 16755–16767. [Google Scholar] [CrossRef]
- Karlińska, E.; Masny, A.; Cieślak, M.; Macierzyński, J.; Pecio, Ł.; Stochmal, A.; Kosmala, M. Ellagitannins in roots, leaves, and fruits of strawberry (Fragaria × ananassa Duch.) vary with developmental stage and cultivar. Sci. Hortic. 2021, 275, 109665. [Google Scholar] [CrossRef]
- Kårlund, A.; Salminen, J.-P.; Koskinen, P.; Ahern, J.R.; Karonen, M.; Tiilikkala, K.; Karjalainen, R.O. Polyphenols in Strawberry (Fragaria × ananassa) Leaves Induced by Plant Activators. J. Agric. Food Chem. 2014, 62, 4592–4600. [Google Scholar] [CrossRef]
- Zhu, Q.; Nakagawa, T.; Kishikawa, A.; Ohnuki, K.; Shimizu, K. In vitro bioactivities and phytochemical profile of various parts of the strawberry (Fragaria × ananassa var. Amaou). J. Funct. Foods 2015, 13, 38–49. [Google Scholar] [CrossRef]
- Ferlemi, A.-V.; Lamari, F.N. Berry Leaves: An Alternative Source of Bioactive Natural Products of Nutritional and Medicinal Value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef]
- Fecka, I.; Bednarska, K.; Włodarczyk, M. Fragaria × ananassa cv. Senga Sengana Leaf: An Agricultural Waste with Antiglycation Potential and High Content of Ellagitannins, Flavonols, and 2-Pyrone-4,6-dicarboxylic Acid. Molecules 2022, 27, 5293. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agengy, Wild Strawberry leaf Fragaria. 2019. Available online: https://www.ema.europa.eu/en/medicines/herbal/fragariae-folium (accessed on 20 November 2022).
- Skupień, K.; Oszmiański, J.; Kostrzewa-Nowak, D.; Tarasiuk, J. In vitro antileukaemic activity of extracts from berry plant leaves against sensitive and multidrug resistant HL60 cells. Cancer Lett. 2006, 236, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, D.J.; Stanojević, L.P.; Stanković, M.Z.; Cakić, M.D.; Savić, S.R.; Miljković, M.D. Antioxidant activity of strawberry (Fragaria × ananassa Duch.) leaves. Sep. Sci. Technol. 2017, 52, 1039–1051. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.-S. Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry, and Strawberry Varies with Cultivar and Developmental Stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Al-Deen, A.; Al-Naqeb, G.; Al-Maqtari, Q. Investigation of antioxidant and antibacterial effects of Dodonaea viscose, Fragaria x ananassa duch and Vernonia amygdalina leaves. Aden Univ. J. 2013, 17, 1–14. [Google Scholar]
- Takács, I.; Szekeres, A.; Takács, Á.; Rakk, D.; Mézes, M.; Polyák, Á.; Lakatos, L.; Gyémánt, G.; Csupor, D.; Kovács, K.J.; et al. Wild Strawberry, Blackberry, and Blueberry Leaf Extracts Alleviate Starch-Induced Hyperglycemia in Prediabetic and Diabetic Mice. Planta Medica 2020, 86, 790–799. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Q.; Zhou, Y. Strawberry Leaf Extract Treatment Alleviates Cognitive Impairment by Activating Nrf2/HO-1 Signaling in Rats With Streptozotocin-Induced Diabetes. Front. Aging Neurosci. 2020, 12, 201. [Google Scholar] [CrossRef]
- Moustafa, N.; El-Mallah, M.; Hamdy, H. Protective Effect of Strawberry Leaves Against Nephrotoxicity of Male Rats. Egypt. J. Appl. Sci. 2021, 36, 121–129. [Google Scholar] [CrossRef]
- Mohamed, N.E.; Ashour, S.E. Influence of ethanolic extract of strawberry leaves for abrogating bromate hazards in male rats. J. Basic Appl. Zoöl. 2019, 80, 19. [Google Scholar] [CrossRef]
- Duru, M. Effects of dietary strawberry (Fragaria x ananassa Duch.) leaf powder on egg yield, quality and egg yolk cholesterol in laying hens. J. Food Agric. Environ. 2013, 11, 477–480. [Google Scholar]
- Chandler, C.; Legard, D.; Dunigan, D.D.; Crocker, T.; Sims, C. ‘Strawberry Festival’ Strawberry. Hortscience 2000, 35, 1366–1367. [Google Scholar] [CrossRef]
- Pineli, L.D.L.D.O.; Moretti, C.L.; dos Santos, M.S.; Campos, A.B.; Brasileiro, A.V.; Córdova, A.C.; Chiarello, M.D. Antioxidants and other chemical and physical characteristics of two strawberry cultivars at different ripeness stages. J. Food Compos. Anal. 2011, 24, 11–16. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Oszmiański, J. Effect of Drying Methods with the Application of Vacuum Microwaves on the Bioactive Compounds, Color, and Antioxidant Activity of Strawberry Fruits. J. Agric. Food Chem. 2009, 57, 1337–1343. [Google Scholar] [CrossRef]
- Klopotek, Y.; Otto, K.; Böhm, V. Processing Strawberries to Different Products Alters Contents of Vitamin C, Total Phenolics, Total Anthocyanins, and Antioxidant Capacity. J. Agric. Food Chem. 2005, 53, 5640–5646. [Google Scholar] [CrossRef]
- Sharma, R.R.; Singh, D. Phenolic Content Pattern, Polyphenol Oxidase and Lipoxygenase Activity in Relation to Albinism, Fruit Malformation and Nubbins Production in Strawberry (Fragaria x ananassa Duch). J. Plant Biochem. Biotechnol. 2010, 19, 67–72. [Google Scholar] [CrossRef]
- Sato, T.; Ikeya, Y.; Adachi, S.-I.; Yagasaki, K.; Nihei, K.-I.; Itoh, N. Extraction of strawberry leaves with supercritical carbon dioxide and entrainers: Antioxidant capacity, total phenolic content, and inhibitory effect on uric acid production of the extract. Food Bioprod. Process. 2019, 117, 160–169. [Google Scholar] [CrossRef]
- Calvo-Castro, L.A.; Lobo-Vázquez, M.; Gómez-González, J.C.; Arnáez-Serrano, E.; Zamora-Fallas, G.; Sánchez-Zúñiga, K.; Centeno-Cerdas, C. Bioactive potential of tropical highland apple (Malus domestica cv. Anna) crude extract: Opportunities for food waste revalorization. Futur. J. Pharm. Sci. 2022, 8, 57. [Google Scholar] [CrossRef]
- Lux, P.E.; Freiling, M.; Stuetz, W.; von Tucher, S.; Carle, R.; Steingass, C.B.; Frank, J. (Poly)phenols, Carotenoids, and Tocochromanols in Corn (Zea mays L.) Kernels As Affected by Phosphate Fertilization and Sowing Time. J. Agric. Food Chem. 2020, 68, 612–622. [Google Scholar] [CrossRef]
- Harborne, J. Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis; Springer: Dordrecht, The Netherlands, 1984; Available online: https://link.springer.com/book/10.1007/978-94-009-5570-7 (accessed on 3 May 2021). [CrossRef]
- Krishnaswamy, N.R. Chemistry of Natural Products: A Laboratory Handbook; Universities Press: Hyderabad, India, 2003. [Google Scholar]
- Rojas-Garbanzo, C.; Pérez, A.M.; Vaillant, F.; Pineda-Castro, M.L. Physicochemical and antioxidant composition of fresh peach palm (Bactris gasipaes Kunth) fruits in Costa Rica. Braz. J. Food Technol. 2016, 19, e2015097. [Google Scholar] [CrossRef]
- Fernandes, F.H.A.; de Batista, R.S.; De Medeiros, F.D.; Santos, F.S.; Medeiros, A.C.D. Development of a rapid and simple HPLC-UV method for determination of gallic acid in Schinopsis brasiliensis. Rev. Bras. Farmacogn. 2015, 25, 208–211. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.; Nishika, J.; Suprakash, R. Spectrophotometric Analysis of Chlorophylls and Carotenoids from Commonly Grown Fern Species by Using Various Extracting Solvents. Res. J. Chem. Sci. 2014, 4, 63–69. Available online: http://www.isca.in/rjcs/Archives/v4/i9/12.ISCA-RJCS-2014-146.php (accessed on 8 November 2021).
- Wang, J.; Wang, J.; Ye, J.; Vanga, S.K.; Raghavan, V. Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: Profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control. 2019, 96, 128–136. [Google Scholar] [CrossRef]
- Zamora, G.; Beukelman, K.; Berg, B.; Arias, M.; Umana, E.; Aguilar, I.; Ufford, L.; Worm, E.; Fallas, N.; Solorzano, R. The antioxidant capacity and immunomodulatory activity of stingless bee honeys proceeding from Costa Rica. Oxid. Antioxidants Med. Sci. 2015, 4, 49–55. [Google Scholar] [CrossRef]
- Fratianni, F.; Cardinale, F.; Cozzolino, A.; Granese, T.; Albanese, D.; Di Matteo, M.; Zaccardelli, M.; Coppola, R.; Nazzaro, F. Polyphenol composition and antioxidant activity of different grass pea (Lathyrus sativus), lentils (Lens culinaris), and chickpea (Cicer arietinum) ecotypes of the Campania region (Southern Italy). J. Funct. Foods 2014, 7, 551–557. [Google Scholar] [CrossRef]
- Neveu, V.; Pérez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Suwa, K. Search extension transforms Wiki into a relational system: A case for flavonoid metabolite database. BioData Min. 2008, 1, 7. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Calvo-Castro, L.; Syed, D.N.; Chamcheu, J.C.; Vilela, F.M.P.; Pérez, A.M.; Vaillant, F.; Rojas, M.; Mukhtar, H. Protective Effect of Tropical Highland Blackberry Juice (Rubus adenotrichos Schltdl.) Against UVB-Mediated Damage in Human Epidermal Keratinocytes and in a Reconstituted Skin Equivalent Model. Photochem. Photobiol. 2013, 89, 1199–1207. [Google Scholar] [CrossRef]
- Aaby, K.; Mazur, S.; Arnfinn Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef]
- Hernanz, D.; Recamales, Á.F.; Meléndez-Martínez, A.J.; González-Miret, M.L.; Heredia, F.J. Assessment of the Differences in the Phenolic Composition of Five Strawberry Cultivars (Fragaria × ananassa Duch.) Grown in Two Different Soilless Systems. J. Agric. Food Chem. 2007, 55, 1846–1852. [Google Scholar] [CrossRef]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; de Vos, C.H.R.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, Phenolic Compounds, and Nutritional Quality of Different Strawberry Genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, D.S.; Kim, D.Y.; Chun, C. Variation of bioactive compounds content of 14 oriental strawberry cultivars. Food Chem. 2015, 184, 196–202. [Google Scholar] [CrossRef]
- Skupień, K.; Oszmiański, J. Comparison of six cultivars of strawberries (Fragaria x ananassa Duch.) grown in northwest Poland. Eur. Food Res. Technol. 2004, 219, 66–70. [Google Scholar] [CrossRef]
- Wang, M.; Yao, C.; Li, J.; Wei, X.; Xu, M.; Huang, Y.; Mei, Q.; Guo, D.-A. Software Assisted Multi-Tiered Mass Spectrometry Identification of Compounds in Traditional Chinese Medicine: Dalbergia odorifera as an Example. Molecules 2022, 27, 2333. [Google Scholar] [CrossRef]
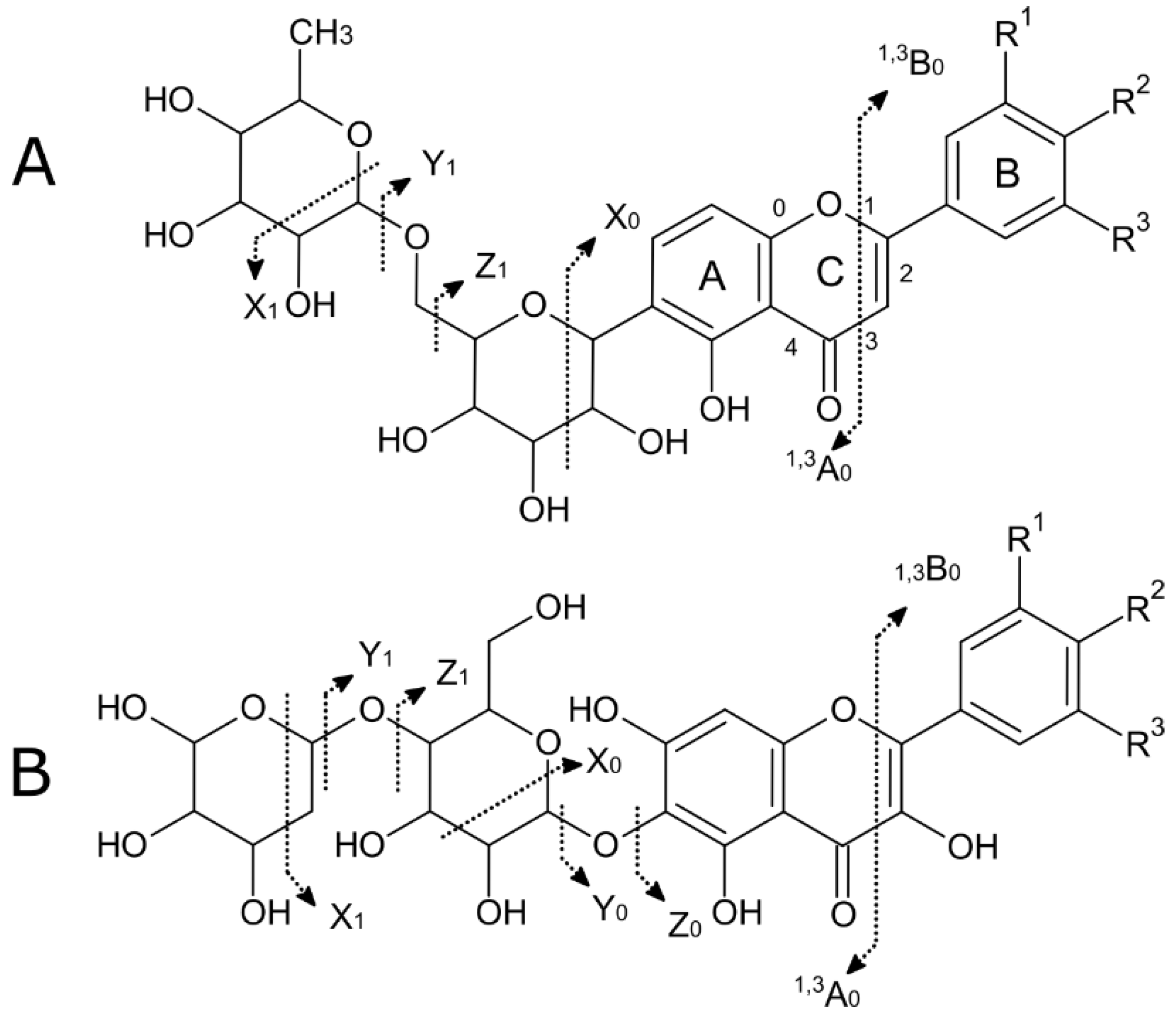
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Zagórska-Dziok, M.; Bujak, T.; Wójciak, M.; Sowa, I. Evaluation of Cosmetic and Dermatological Properties of Kombucha-Fermented Berry Leaf Extracts Considered to Be By-Products. Molecules 2022, 27, 2345. [Google Scholar] [CrossRef]
- Markiewicz, A.; Zasada, M.; Erkiert-Polguj, A.; Wieckowska-Szakiel, M.; Budzisz, E. An evaluation of the antiaging properties of strawberry hydrolysate treatment enriched with L-ascorbic acid applied with microneedle mesotherapy. J. Cosmet. Dermatol. 2018, 18, 129–135. [Google Scholar] [CrossRef]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Alvarez-Suarez, J.M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Giampieri, F. A Pilot Study of the Photoprotective Effects of Strawberry-Based Cosmetic Formulations on Human Dermal Fibroblasts. Int. J. Mol. Sci. 2015, 16, 17870–17884. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Reboredo-Rodriguez, P.; Cianciosi, D.; Mezzetti, B.; Quiles, J.L.; Bompadre, S.; Battino, M.; Giampieri, F. Strawberry-Based Cosmetic Formulations Protect Human Dermal Fibroblasts against UVA-Induced Damage. Nutrients 2017, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Forbes-Hernandez, T.; Giampieri, F.; Battino, M.; Gasparrini, M. The photoprotective effects of strawberry-based cosmetic formulations on human dermal fibroblasts. Acta Hortic. 2017, 1156, 397–404. [Google Scholar] [CrossRef]
- Nordin, M.L.; Kadir, A.A.; Zakaria, Z.A.; Abdullah, R.; Abdullah, M.N.H. In vitro investigation of cytotoxic and antioxidative activities of Ardisia crispa against breast cancer cell lines, MCF-7 and MDA-MB-231. BMC Complement. Altern. Med. 2018, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Gado, F.; Digiacomo, M.; Salsano, J.E.; Macchia, M.; Manera, C. Phenolic Compounds in Prevention and Treatment of Skin Cancers: A Review. Curr. Med. Chem. 2021, 28, 6730–6752. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Potapovich, A.I.; Kostyuk, V.A.; Kostyuk, T.V.; de Luca, C.; Korkina, L.G. Effects of pre- and post-treatment with plant polyphenols on human keratinocyte responses to solar UV. Inflamm. Res. 2013, 62, 773–780. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Fruit Extract | Leaf Extract |
|---|---|---|
| Protein (mg g−1 DW) | 28.63 ± 2.09 | 80.63 ± 1.13 |
| Soluble solids (° Brix) | 1% | 0.40% |
| Saponins (foam test) | Negative | Positive |
| Steroids and terpenoids (Liebermann–Buchard test) | Positive | Negative (no color) |
| Alkaloids (Dragendorf test) | Negative (no color) | Negative (no color) |
| Flavonoids (Shinoda test) | Positive (light red) | Positive (red color) |
| Total polyphenols (mg GAE g−1 DW) | 0.89 ± 0.05 | 108.83 ± 4.65 |
| Total flavonoids (mg QE g−1 DW) | 0.32 ± 0.01 | 10.25 ± 0.50 |
| Total chlorophyll (mg g−1 DM) | 0.2832 ± 0.0224 | 1.9470 ± 0.6399 |
| Total carotenoids (mg g−1 DW) | 0.0013 ± 0.0020 | 0.0074 ± 0.0933 |
| ORAC (µmol TE g−1 DW) | 221 ± 70 | 2918 ± 281 |
| Peak | Compound | Retention Time (min) | UV/Vis Wavelength (nm) | Polyphenol Concentration in [µg mg−1 DW] in the Extract | ||||
|---|---|---|---|---|---|---|---|---|
| Insoluble Extract | Soluble Extract | Total Extract | ||||||
| Fruit | Leaf | Fruit | Leaf | Leaf | ||||
| 1 | Gallic acid | 2.06 | 201/220/270 | 2.10 ± 1.40 | 57.53 ± 36.84 | 0.04 ± 0.01 | n.d. | 0.0010 ± 0.0001 |
| 2 | Caffeic acid | 8.51 | 216/240/322 | n.d. | 3.62 ± 4.97 | 0.14 ± 0.12 | n.d. | 0.0028 ± 0.0002 |
| 3 | Chlorogenic acid | 8.69 | 215/320 | n.d. | n.d. | 0.17 ± 0.18 | 0.66 ± 0.31 | 0.20 ± 0.02 |
| 4 | p-coumaric acid | 10.51 | 209/310 | 1.50 ± 0.56 | 3.70 ± 2.68 | 0.21 ± 0.06 | 1.08 ± 0.09 | 1.14 ± 0.23 |
| 5 | Rutin | 10.63 | 201/256/355 | n.d. | 7.14 ± 3.40 | 0.58 ± 0.07 | 4.67 ± 0.70 | 5.59 ± 2.69 |
| 6 | Ellagic acid | 11.15 | 196/254/366 | 0.11 ± 0.21 | 21.90 ± 39.72 | 0.26 ± 0.09 | 1.35 ± 0.08 | 0.88 ± 0.57 |
| 7 | Quercetin | 13.91 | 201/255/370 | n.d. | 19.44 ± 3.98 | 0.05 ± 0.09 | n.d. | 0.098 ± 0.003 |
| Peak | Compound Subclass | Tentative Identification | Formula | m/z | Adducts | Rt (min) | Assigned Fragments (m/z) |
|---|---|---|---|---|---|---|---|
| 1 | Flavan 3-ol derivatives | Catechin | C15H14O6 | 291.0887 | [M+H]+ | 2.91 | 273, 165, 147,139, 123 |
| 2 | Pelargonidin 3-glucoside | Pelargonidin 3-glucoside | C21H21O10+ | 433.1130 | M+ | 3.32 | 433, 271 |
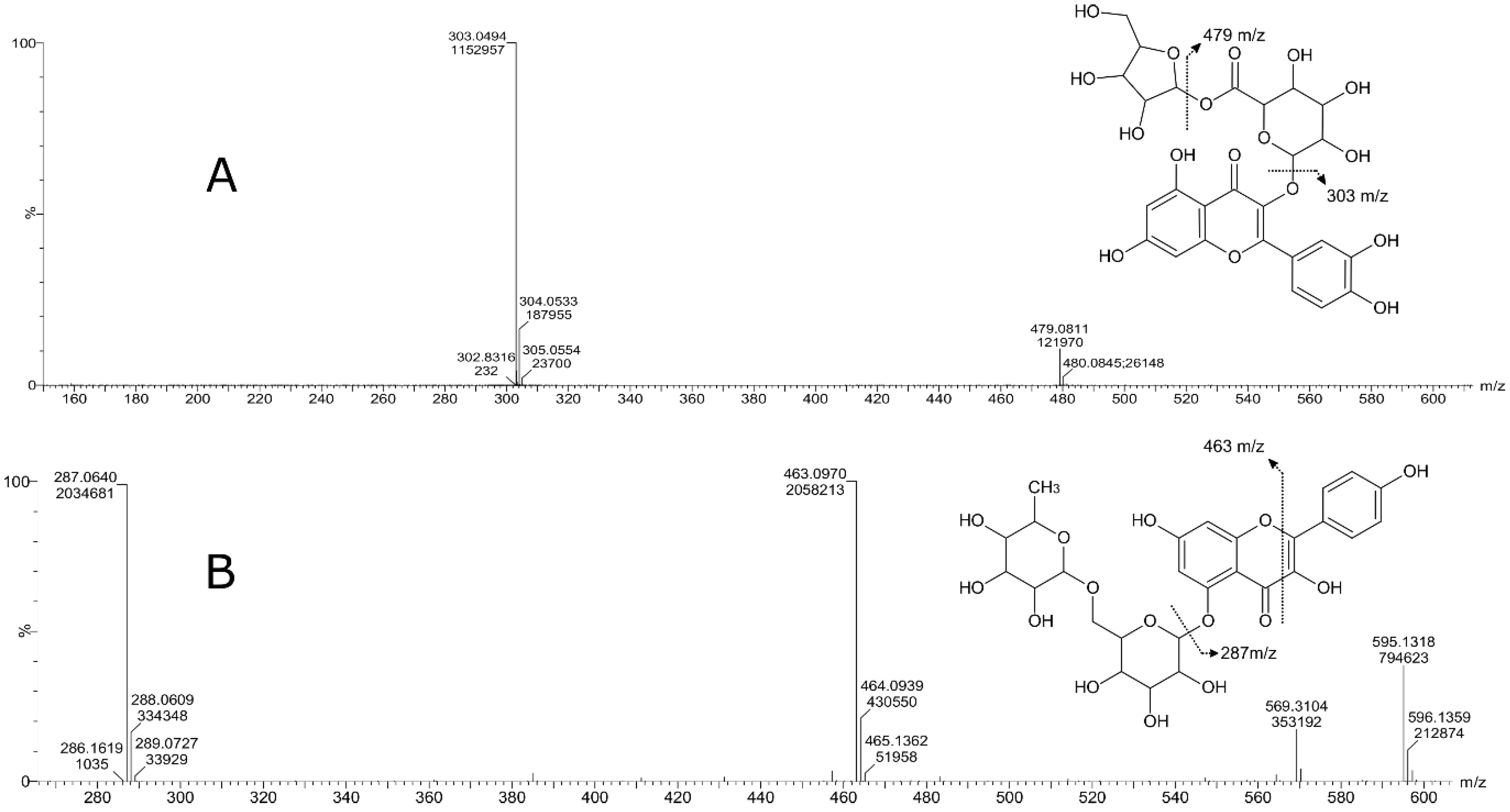
| 3 | Flavonoid-3-o-glucuronides | Quercetin 3-glucosyl-(1->2)-glucuronide | C27H28O18 | 641.1355 | M+H, M+Na | 6.8 | 641, 465, 303 |
| 4 | Flavonoid-3-o-glucuronides | Quercetin 3-o-xylosyl-glucuronide | C26H26O17 | 611.1324 | [M+H]+ | 7.4 | 611, 479, 303 |
| 5 | Anthocyanin glycoside | Delphinidin 3-galactoside | C21H21O12+ | 465.1051 | M+ | 7.51 | 465, 303 |
| 6 | Flavonoid o-rutinoside | Kaempferol 7-o-rutinoside | C30H26O13 | 595.1318 | M+H | 7.55 | 595, 463, 287, 153, 113 |
| 7 | Flavone o-glucoside | Kaempferol 3′-glucuronide | C21H18O12 | 463.0876 | M+H, M+Na | 7.76 | 436, 287, 165, 153 |
| 8 | Flavonoid-3-o-glucuronides | Quercetin 3-glucuronide | C21H18O13 | 479.0827 | M+H, M+Na | 7.61 | 303, 301, 179, 151 |
| 9 | Flavonoid 3-o-p-coumaroyl glycoside | Kaempferol 3-(6′′-p-coumarylgalactoside) | C30H26O13 | 595.1461 | M+H | 8.24 | 287, 147, 119 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas-Arias, K.; Irías-Mata, A.; Sánchez-Kopper, A.; Hernández-Moncada, R.; Salas-Morgan, B.; Villalta-Romero, F.; Calvo-Castro, L.A. Strawberry Fragaria x ananassa cv. Festival: A Polyphenol-Based Phytochemical Characterization in Fruit and Leaf Extracts. Molecules 2023, 28, 1865. https://doi.org/10.3390/molecules28041865
Salas-Arias K, Irías-Mata A, Sánchez-Kopper A, Hernández-Moncada R, Salas-Morgan B, Villalta-Romero F, Calvo-Castro LA. Strawberry Fragaria x ananassa cv. Festival: A Polyphenol-Based Phytochemical Characterization in Fruit and Leaf Extracts. Molecules. 2023; 28(4):1865. https://doi.org/10.3390/molecules28041865
Chicago/Turabian StyleSalas-Arias, Karla, Andrea Irías-Mata, Andrés Sánchez-Kopper, Ricardo Hernández-Moncada, Bridget Salas-Morgan, Fabián Villalta-Romero, and Laura A. Calvo-Castro. 2023. "Strawberry Fragaria x ananassa cv. Festival: A Polyphenol-Based Phytochemical Characterization in Fruit and Leaf Extracts" Molecules 28, no. 4: 1865. https://doi.org/10.3390/molecules28041865
APA StyleSalas-Arias, K., Irías-Mata, A., Sánchez-Kopper, A., Hernández-Moncada, R., Salas-Morgan, B., Villalta-Romero, F., & Calvo-Castro, L. A. (2023). Strawberry Fragaria x ananassa cv. Festival: A Polyphenol-Based Phytochemical Characterization in Fruit and Leaf Extracts. Molecules, 28(4), 1865. https://doi.org/10.3390/molecules28041865







