Tin(II) and Tin(IV) Complexes Incorporating the Oxygen Tripodal Ligands [(η5-C5R5)Co{P(OEt)2O}3]−, (R = H, Me; Et = -C2H5) as Potent Inflammatory Mediator Inhibitors: Cytotoxic Properties and Biological Activities against the Platelet-Activating Factor (PAF) and Thrombin
Abstract
1. Introduction
2. Results and Discussion
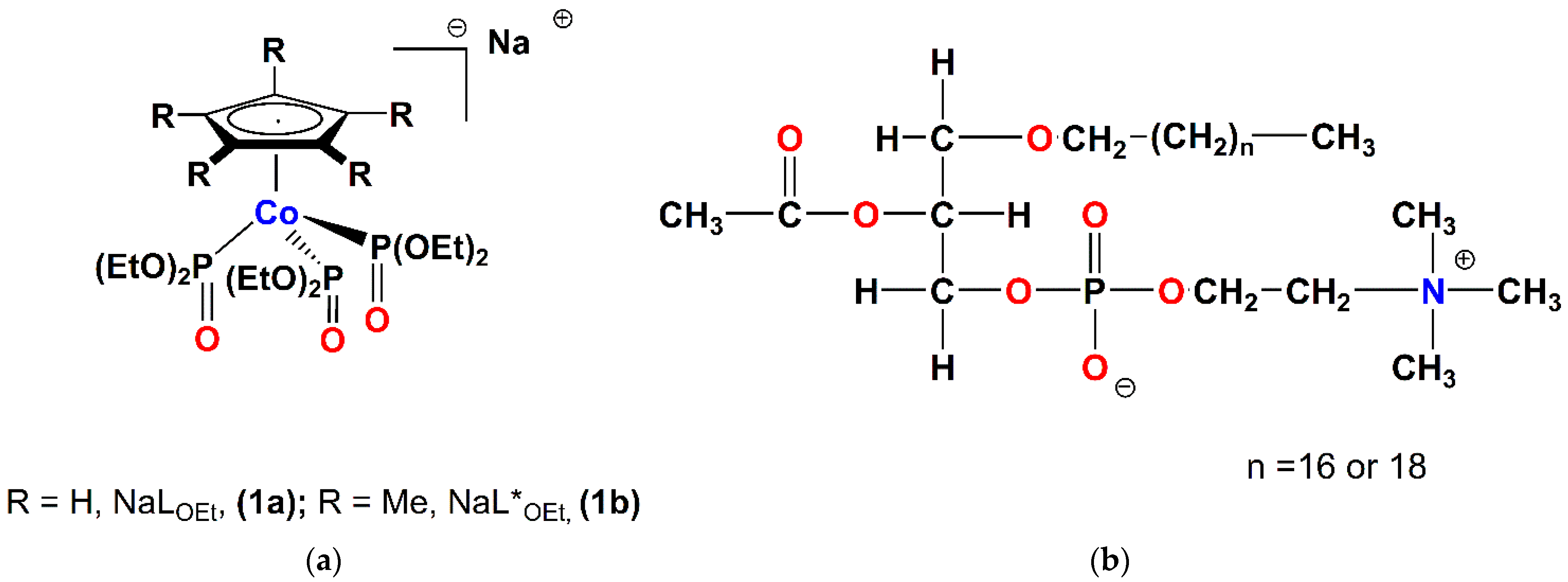
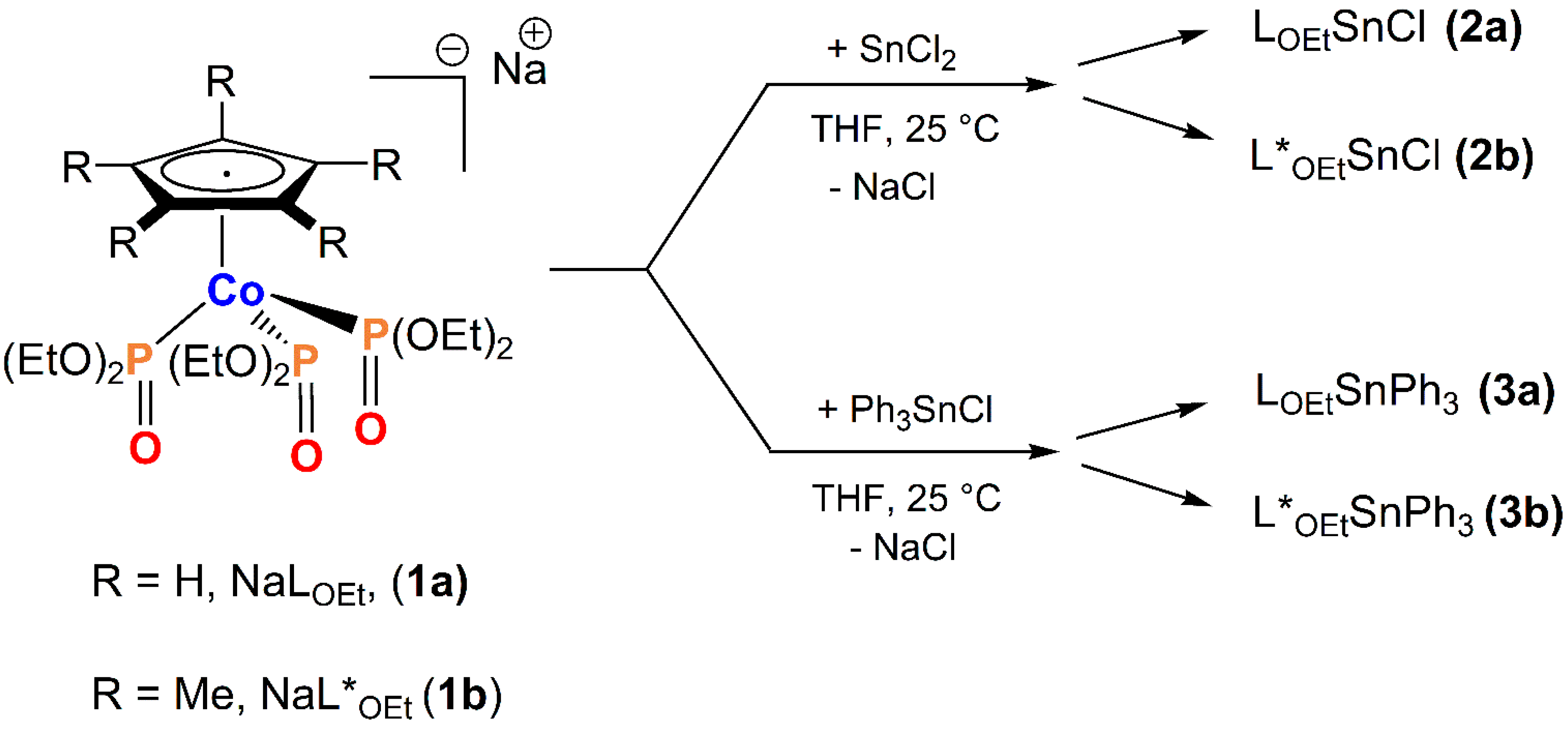
2.1. Chemistry
2.2. Evaluation of Biological Activity
2.2.1. Inhibitory Effects against the Biological Activities of PAF in Washed Rabbit Platelets (WRPs) and in Rabbit PRPs (rPRPs)
Inhibitory Effects against the Biological Activities of PAF in Washed Rabbit Platelets (WRPs)
Inhibitory Effect towards the PAF-Induced rPRPs Aggregation
2.2.2. Inhibitory Effect against Thrombin
2.2.3. Desensitization Tests
2.2.4. Cell Viability Assay
3. Materials and Methods
3.1. General
Synthesis of the Organotin Complexes 2–3
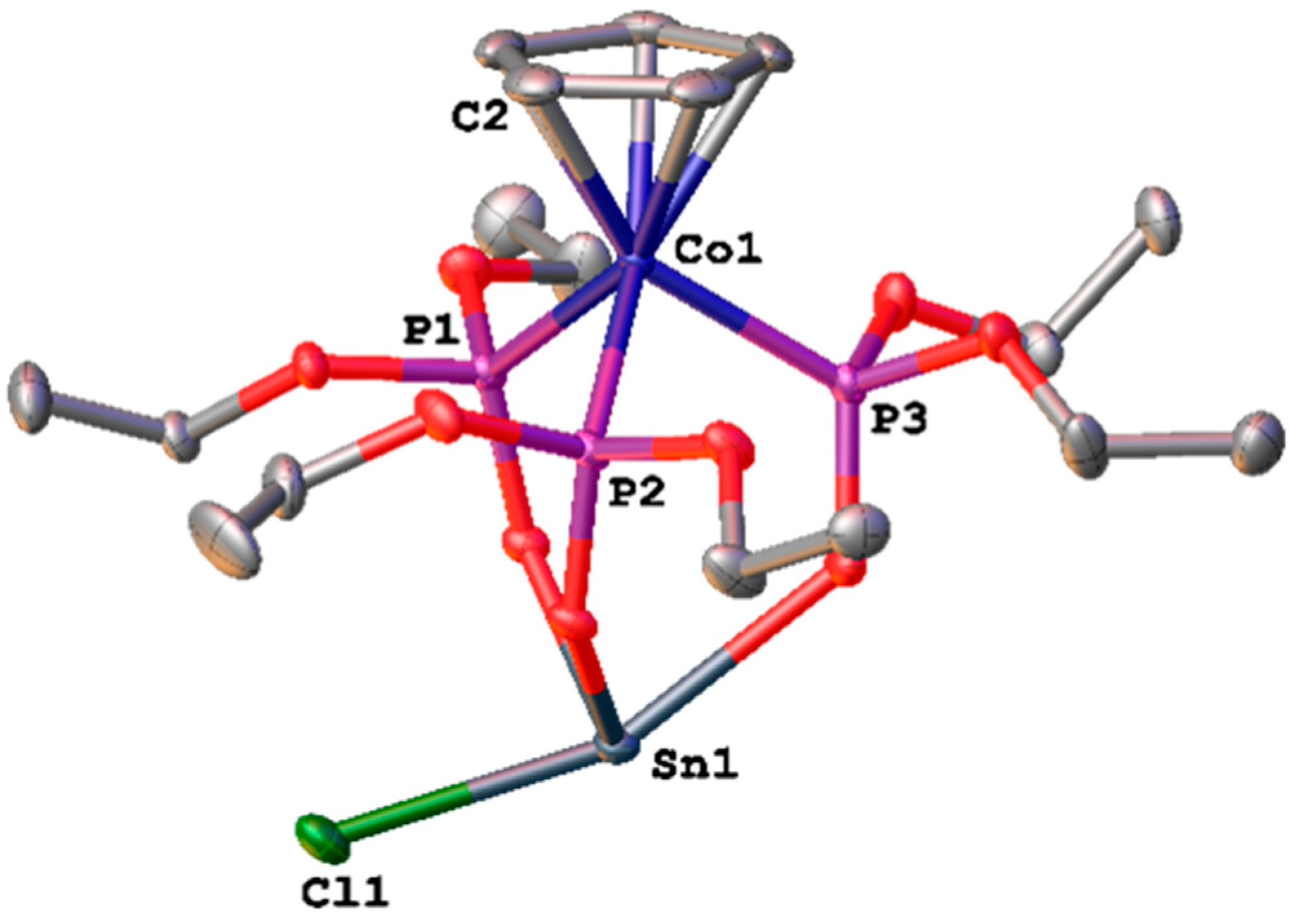
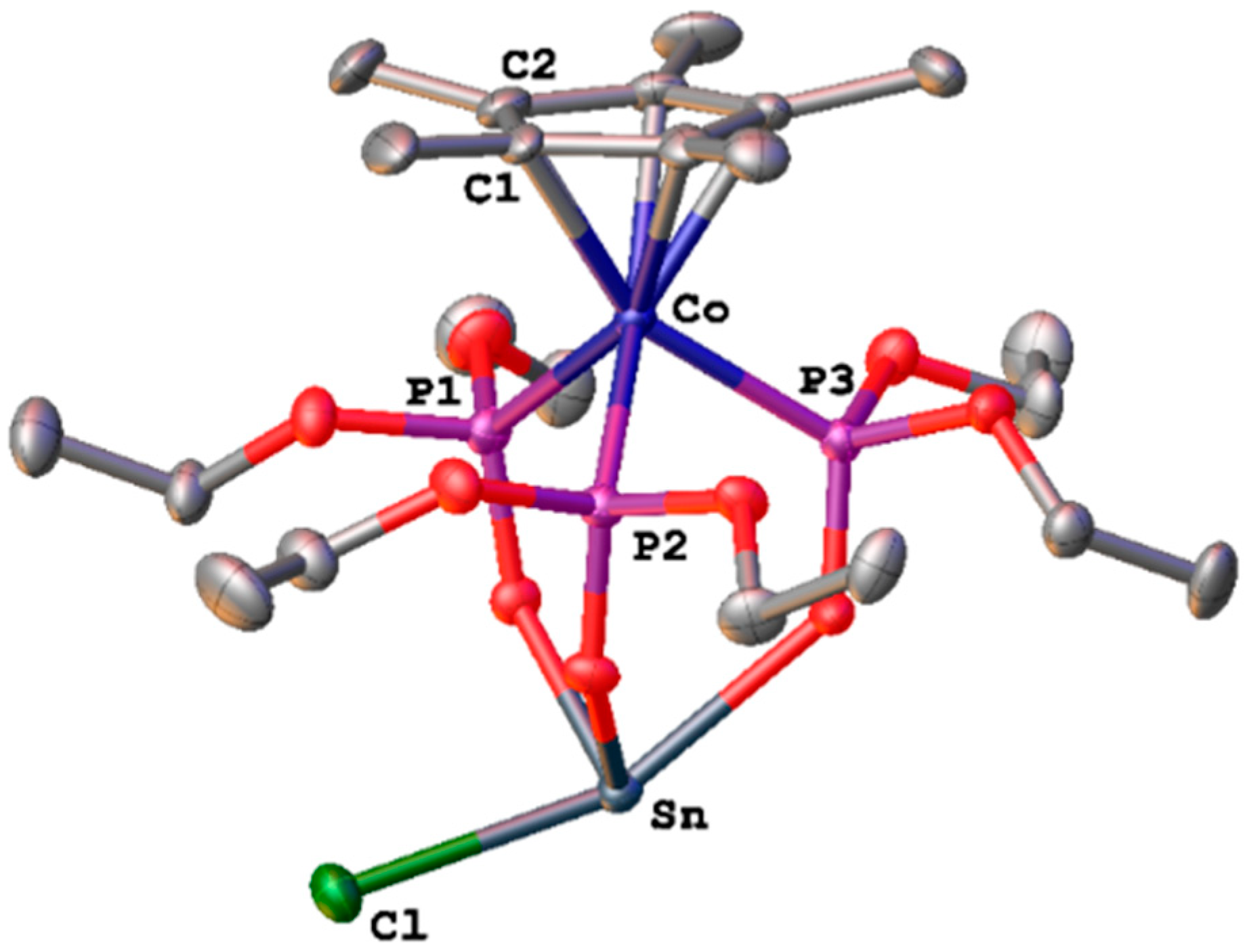
3.2. Single-Crystal X-ray Structural Determination
3.3. Biological Evaluation
3.3.1. Materials and Methods for Biological Experiments in Washed Rabbit Platelets (WRPs)
3.3.2. Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Duaa, G.; Zahraa, R.; Emad, Y. A Review of Organotin Compounds: Chemistry and Applications. Arc. Org. Inorg. Chem. Sci. 2018, 3, 349–352. [Google Scholar] [CrossRef]
- Martínez, A.R.; Morales, L.P.; Ojeda, E.D.; Rodríguez, M.C.; Rodríguez-García, I. The Proven Versatility of Cp2TiCl. J. Org. Chem. 2021, 86, 1311–1329. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.G. Introduction and Overview. In Tin Chemistry Fundamentals, Frontiers and Applications; Davies, A.G., Gielen, M., Pannell, K.H., Eds.; Tiekink, John Wiley & Sons: Chichester, UK, 2008; pp. 1–15. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, X.; Ma, X.; Liu, Y.; Zhong, M.; Li, W.; Yang, Z.; Yang, Y. Synthesis, characterization, and catalytic performance of Aluminum and Tin Compounds with β-diketiminato ligand. J. Organomet. Chem. 2018, 868, 55–60. [Google Scholar] [CrossRef]
- Saxena, A.K. Organotin compounds: Toxicology and biomedicinal applications. Appl. Organomet. Chem. 1987, 1, 39–56. [Google Scholar] [CrossRef]
- Basu Baul, T.S. Antimicrobial activity of organotin(IV) compounds: A review. Appl. Organomet. Chem. 2008, 22, 195–204. [Google Scholar] [CrossRef]
- Omae, I. Organotin antifouling paints and their alternatives. Appl. Organomet. Chem. 2003, 17, 81–105. [Google Scholar] [CrossRef]
- Arjmand, F.; Parveen, S.; Tabassum, S.; Pettinari, C. Organo-tin antitumor compounds: Their present status in drug development and future perspectives. Inorg. Chim. Acta 2014, 423 Pt B, 26–37. [Google Scholar] [CrossRef]
- Yusof, E.N.M.; Ravoof, T.B.S.A.; Page, A.J. Cytotoxicity of Tin(IV)-based compounds: A review. Polyhedron 2021, 198, 115069. [Google Scholar] [CrossRef]
- Niu, L.; Li, Y.; Li, Q. Medicinal properties of organotin compounds and their limitations caused by toxicity. Inorg. Chim. Acta 2014, 423 Pt B, 2–13. [Google Scholar] [CrossRef]
- Banti, C.N.; Hadjikakou, S.K.; Sismanoglu, T.; Hadjiliadis, N. Anti-proliferative and antitumor activity of organotin(IV) compounds. An overview of the last decade and future perspectives. J. Inorg. Biochem. 2019, 194, 114–152. [Google Scholar] [CrossRef]
- Ayers, A.E.; Klapötke, T.M.; Dias, H.V.R. Azido Derivatives of Germanium(II) and Tin(II): Syntheses and Characterization of [(Mes)2DAP]GeN3, [(Mes)2DAP]SnN3, and the Corresponding Chloro Analogues Featuring Heterocyclic Six-π-Electron Ring Systems (where [(Mes)2DAP] = {N(Mes)C(Me)}2CH). Inorg. Chem. 2001, 40, 1000–1005. [Google Scholar] [CrossRef]
- Ding, Y.; Roesky, H.W.; Noltemeyer, M.; Schmidt, H.-G.; Power, P.P. Synthesis and Structures of Monomeric Divalent Germanium and Tin Compounds Containing a Bulky Diketiminato Ligand. Organometallics 2001, 20, 1190–1194. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.-J.; Kuo, T.-S.; Shen, L.-C.; Liu, H.-J. Ambiphilic Nature of Dipyrrolylpyridine-Supported Divalent Germanium and Tin Compounds. Organometallics 2021, 40, 3659–3667. [Google Scholar] [CrossRef]
- Kläui, W. [(C5H5)CO{P(O)(OC2H5)2}3]-: Eine einfache Synthese eines metallorganischen Tripodliganden und seine Reaktivität gegenüber den Kationen der III. Hauptgruppe/[(C5H5)CO{P(O)(OC2H5)2}3]-: A Convenient Synthesis of an Organometallic Tripod Ligand and its Reactivity towards the Main Group III Cations. Z. Naturforsch. B. 1979, 34, 1403–1407. [Google Scholar] [CrossRef]
- Román, E.E.; Tapia, C.F.; Sergio, H.M. A novel access to the anionic permethylated fac-tripod ligand [C5Me5Co&P(O)(OEt)2&3]−, and its derived trinuclear and binuclear complexes. Polyhedron 1986, 5, 917–920. [Google Scholar] [CrossRef]
- Filippou, A.C.; Portius, P.; Kociok-Köhn, G.; Volker, A. Oxidation of germanium(II) azides with HN3: A convenient route to six-co-ordinate triazidogermanium(IV) compounds. J. Chem. Soc. Dalton Trans. 2000, 11, 1759–1768. [Google Scholar] [CrossRef]
- Leung, W.-H.; Zhang, Q.-F.; Yi, X.-Y. Recent developments in the coordination and organometallic chemistry of Kläui oxygen tripodal ligands. Coord. Chem. Rev. 2007, 251, 2266–2279. [Google Scholar] [CrossRef]
- Grunwald, A.C.; Scholtysik, C.; Hagenbach, A.; Abram, U. One Ligand, One Metal, Seven Oxidation States: Stable Technetium omplexes with the Kläui Ligand. Inorg. Chem. 2020, 59, 9396–9405. [Google Scholar] [CrossRef]
- Kläui, W.; Müller, A.; Eberspach, W.; Boese, R.; Goldberg, I. Crystal structure and coordination chemistry of the pentane-soluble sodium salt of an oxygen tripod ligand. J. Am. Chem. Soc. 1987, 109, 164–169. [Google Scholar] [CrossRef]
- Shinar, H.; Navon, G.; Kläui, W. Novel organometallic ionophore with specificity toward lithium ion. J. Am. Chem. Soc. 1986, 108, 5005–5006. [Google Scholar] [CrossRef]
- Demopoulos, C.A.; Pinckard, R.N.; Hanahan, D.J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J. Biol. Chem. 1979, 254, 9355–9358. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Chini, M.; Tsogas, N.; Lioni, A.; Tsekes, G.; Demopoulos, C.A.; Lazanas, M.C. In vitro anti-inflammatory and anti-coagulant effects of antibiotics towards Platelet Activating Factor and thrombin. J. Inflamm. 2011, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I.; Demopoulos, C.A. Forty Years Since the Structural Elucidation of Platelet-Activating Factor (PAF): Historical, Current, and Future Research Perspectives. Molecules 2019, 24, 4414. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.B.; Papakyriakou, A.; Demopoulos, A.C.; Philippopoulos, A.I. Synthesis, biochemical evaluation and molecular modeling studies of novel rhodium complexes with nanomolar activity against Platelet Activating Factor. J. Inorg. Biochem. 2013, 120, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Margariti, A.; Papakonstantinou, V.D.; Stamatakis, G.M.; Demopoulos, C.A.; Schnakenburg, G.; Andreopoulou, A.K.; Giannopoulos, P.; Kallitsis, J.K.; Philippopoulos, A.I. Substituted pyridine-quinoline ligands as building blocks for neutral rhodium(III) complexes. Synthesis, structural characterization studies and anti-platelet activity towards the Platelet-Activating Factor (PAF). Polyhedron 2020, 178, 114336. [Google Scholar] [CrossRef]
- Kalampalidis, A.; Peppas, A.; Schnakenburg, G.; Papakyriakoy, A.; Tsoupras, A.; Zabetakis, I.; Philippopoulos, A.I. Antithrombotic and antiplatelet activity of an organometallic rhodium(I) complex incorporating a substituted thieno-[2,3-d]-pyrimidine ligand: Synthesis, structural characterization, and molecular docking calculations. Appl. Organomet. Chem. 2021, 35, e6210. [Google Scholar] [CrossRef]
- Falaras, P.; Philippopoulos, A.I. Platelet Activating Factor (PAF) Inhibitors with Possible Antitumor Activity. Industrial Property Organization of Greece Patent Number 1006959, 2009. [Google Scholar]
- Papakonstantinou, V.D.; Lagopati, N.; Tsilibary, E.C.; Demopoulos, C.A.; Philippopoulos, A.I. A Review on Platelet Activating Factor Inhibitors: Could a New Class of Potent Metal-Based Anti-Inflammatory Drugs Induce Anticancer Properties? Bioinorg. Chem. Appl. 2017, 2017, 6947034. [Google Scholar] [CrossRef]
- Peppas, A.; Kalabalidis, A.; Papakonstantinou, V.D.; Demopoulos, C.A.; Schnakenburg, G.; Philippopoulos, A.I. Rhodium-based Inhibitors of the Platelet Activating Factor (PAF). A new class of potent anti-inflammatory drugs. In Chemical Elements (Fluorine, Rhodium and Rubidium). Properties, Synthesis and Applications; Huff, A., Ed.; Nova Science Publishers: New York, NY, USA, 2018; pp. 127–169. [Google Scholar]
- Heuer, H.O. Current status of PAF antagonists. Clin. Exp. Allergy 1992, 22, 980–983. [Google Scholar] [CrossRef]
- Terashita, Z.; Tsushima, S.; Yoshioka, Y.; Nomura, H.; Inada, Y.; Nishikawa, K. CV-3988—A specific antagonist of platelet activating factor (PAF). Life Sci. 1983, 32, 1975–1982. [Google Scholar] [CrossRef]
- Ferentinos, E.; Tsoupras, A.B.; Roulia, M.; Chatziefthimiou, S.D.; Demopoulos, C.A.; Kyritsis, P. Inhibitory activity of the novel Zn[(OPPh 2)(SePPh 2)N]2 complex towards the Platelet Activating Factor (PAF) and thrombin: Comparison with its isomorphous Co(II) and Ni(II) analogues. Inorg. Chim. Acta 2011, 378, 102–108. [Google Scholar] [CrossRef]
- Sioriki, E.; Lordan, R.; Nahra, F.; Van Hecke, K.; Zabetakis, I.; Nolan, S.P. In vitro Anti-atherogenic Properties of N-Heterocyclic Carbene Aurate(I) Compounds. ChemMedChem 2018, 13, 2484–2487. [Google Scholar] [CrossRef]
- Hyland, I.K.; O’Toole, R.F.; Smith, J.A.; Bissember, A.C. Progress in the Development of Platelet-Activating Factor Receptor (PAFr) Antagonists and Applications in the Treatment of Inflammatory Diseases. ChemMedChem 2018, 13, 1873–1884. [Google Scholar] [CrossRef]
- Sohrabi, M.; Saeedi, M.; Larijani, B.; Mahdavi, M. Recent advances in biological activities of rhodium complexes: Their applications in drug discovery research. Eur. J. Med. Chem. 2021, 216, 113308. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Kläui, W.; Dehnicke, K. Dreikernige Sandwichkomplexe mit Dialkylphosphonat-Brückenliganden: Synthese, NMR-, IR- und Raman-spektroskopische Untersuchungen. Chem. Ber. 1978, 111, 451–468. [Google Scholar] [CrossRef]
- Fritz, H.P. Infrared and Raman Spectral Studies of π Complexes Formed Between Metals and CnHnRings. In Advances in Organometallic Chemistry; Stone, F.G.A., West, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1964; Volume 1, pp. 239–316. [Google Scholar] [CrossRef]
- Dubler, E.; Linowsky, L.; Kläui, W. Molecular structures and solid-solid phase transitions of trinuclear {CpCo[P(O)(OR)2]3}2M complexes. Transit. Metal Chem. 1979, 4, 191–198. [Google Scholar] [CrossRef]
- Kläui, W.; Peters, W.; Liedtke, N.; Trofimenko, S.; Rheingold, A.; Sommer, R. Nitrogen Tripod Ligand/Oxygen Tripod Ligand Indium(III) Complexes: The First Structurally Characterized Trofimenko−Kläui Complex [η5-(C5H5)Co{P(OCH3)2(O)}3InHB(pz)3][InCl4] (pz = pyrazol-1-yl). Eur. J. Inorg. Chem. 2001, 2001, 693–699. [Google Scholar] [CrossRef]
- Bos, K.D.; Bulten, E.J.; Noltes, J.G.; Spek, A.L. The crystal and molecular structure of cyclopentadienyltin(II) chloride. The bonding of the cyclopentadienyl group in tin(II) compounds. J. Organomet. Chem. 1975, 99, 71–77. [Google Scholar] [CrossRef]
- Constantine, S.P.; De Lima, G.M.; Hitchcock, P.B.; Keates, J.M.; Lawless, G.A.; Marziano, I. Synthesis and Characterization of Sn(η5-C5Me5)Cl and Sn{η5-C5Me4(SiMe2But)}Cl. Organometallics 1997, 16, 793–795. [Google Scholar] [CrossRef]
- Cowley, A.H.; Geerts, R.L.; Numn, C.M.; Carrano, C.J. Synthesis and structure of two tris(pyrazolyl)boratotin(II) compounds. J. Organomet. Chem. 1988, 341, C27–C30. [Google Scholar] [CrossRef]
- Lloyd, N.C.; Nicholson, B.K.; Wilkins, A.L. Six-coordinate organotin(IV) complexes formed using the Kläui ligands; [CpCo{P(OR’)2O}3]SnR3−nCln. J. Organomet. Chem. 2006, 691, 2757–2766. [Google Scholar] [CrossRef]
- Mehring, M.; Schürmann, M.; Jurkschat, K. The First Rigid O,C,O-Pincer Ligand and Its Application for the Synthesis of Penta- and Hexacoordinate Organotin(IV) Compounds. Organometallics 1998, 17, 1227–1236. [Google Scholar] [CrossRef]
- Ronconi, L.; Marzano, C.; Russo, U.; Sitran, S.; Graziani, R.; Fregona, D. Synthesis, characterization and in vitro cytotoxicity of new organotin(IV) derivatives of N-methylglycine. J. Inorg. Biochem. 2002, 91, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Prokopi, M. Ir(I) and Ir(III) Complexes and Study of Their Biological Activity against the Platelet Acivating Factor. Master’s Thesis, National and Kapodistrian University of Athens, Athens, Greece, 2015. [Google Scholar]
- Tsoupras, A.-B.; Papakonstantinou, V.; Stamatakis, G.M.; Demopoulos, C.A.; Falaras, P.; Philippopoulos, A.I. Biochemical evaluation of ruthenium-based complexes towards PAF (Platelet Activating Factor) and Thrombin. Potent anti-inflammatory agents. Sci. Lett. J. 2015, 4, 208. [Google Scholar]
- Ravishankar, D.; Salamah, M.; Attina, M.A.; Pothi, R.; Vallance, T.M.; Javed, M.; Williams, H.F.; Alzahranil, E.M.S.; Kabove, E.; Vaiyapuri, R.; et al. Ruthenium-conjugated chrysin analogues modulate platelet activity, thrombus formation and haemostasis with enhanced efficacy. Sci. Rep. 2017, 7, 5738. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Harrington, J.; Pienaar, R.; Devaney, K.; Heaney, S.; Koidis, A.; Zabetakis, I. The Effects of Oxidation on the Antithrombotic Properties of Tea Lipids against PAF, Thrombin, Collagen, and ADP. Foods 2020, 9, 385. [Google Scholar] [CrossRef]
- Khamrang, T.; Hung, K.-C.; Hsia, C.-H.; Hsieh, C.-Y.; Velusamy, M.; Jayakumar, T.; Sheu, J.-R. Antiplatelet Activity of a Newly Synthesized Novel Ruthenium (II): A Potential Role for Akt/JNK Signaling. Int. J. Mol. Sci. 2017, 18, 916. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Demopoulos, C.A.; Pappas, K.M. Platelet-activating factor detection, metabolism, and inhibitors in the ethanologenic bacterium Zymomonas mobilis. Eur. J. Lipid Sci. Technol. 2012, 114, 123–133. [Google Scholar] [CrossRef]
- Koukouraki, P.; Tsoupras, A.; Sotiroudis, G.; Demopoulos, C.A.; Sotiroudis, T.G. Antithrombotic properties of Spirulina extracts against platelet-activating factor and thrombin. Food Biosc. 2020, 37, 100686. [Google Scholar] [CrossRef]
- Damati, A.; Vlastos, D.; Philippopoulos, A.I.; Matthopoulos, D.P. Genotoxic evaluation of newly synthesized organometallic compounds of tin. Glob. Nest J. 2015, 17, 574–582. [Google Scholar] [CrossRef]
- Collins, A.R.; Oscoz, A.A.; Brunborg, G.; Gaivão, I.; Giovannelli, L.; Kruszewski, M.; Smith, C.C.; Stetina, R. The comet assay: Topical issues. Mutagenesis 2008, 23, 143–151. [Google Scholar] [CrossRef]
- Damati, A.; Koutsoumplias, D.; Vlastos, D.; Philippopoulos, A.Ι.; Matthopoulos, D.P. Evaluation of Cytostatic Action of New Organotin Compounds on the Lymphoid Jurkat Cell Line. 2023; manuscript in preparation. [Google Scholar]
- Khandani, M.; Sedaghat, T.; Erfani, N.; Haghshenas, M.R.; Khavasi, H.R. Synthesis, spectroscopic characterization, structural studies and antibacterial and antitumor activities of diorganotin complexes with 3-methoxysalicylaldehyde thiosemicarbazone. J. Mol. Struct. 2013, 1037, 136–143. [Google Scholar] [CrossRef]
- Khan, N.; Farina, Y.; Mun, L.K.; Rajab, N.F.; Awang, N. Triorganotin(IV) complexes with o-substituted arylhydroxamates: Synthesis, spectroscopic characterization, X-ray structures and in vitro cytotoxic activities. J. Organomet. Chem. 2014, 763–764, 26–33. [Google Scholar] [CrossRef]
- Khan, N.; Farina, Y.; Mun, L.K.; Rajab, N.F.; Awang, N. Syntheses, characterization, X-ray diffraction studies and in vitro antitumor activities of diorganotin(IV) derivatives of bis(p-substituted-N-methylbenzylaminedithiocarbamates). Polyhedron 2015, 85, 754–760. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Iatrou, C.; Frangia, C.; Demopoulos, A.C. The implication of platelet activating factor in cancer growth and metastasis: Potent beneficial role of PAF-inhibitors and antioxidants. Infect. Disord. Drug Targets 2009, 9, 390–399. [Google Scholar] [CrossRef]
- Onuchic, A.C.; Machado, C.M.; Saito, R.F.; Rios, F.J.; Jancar, S.; Chammas, R. Expression of PAFR as Part of a Prosurvival Response to Chemotherapy: A Novel Target for Combination Therapy in Melanoma. Mediators Inflamm. 2012, 2012, 175408. [Google Scholar] [CrossRef]
- Bussolati, B.; Biancone, L.; Cassoni, P.; Russo, S.; Rola-Pleszczynski, M.; Montrucchio, G.; Camussi, G. PAF produced by human breast cancer cells promotes migration and proliferation of tumor cells and neo-angiogenesis. Am. J. Pathol. 2000, 157, 1713–1725. [Google Scholar] [CrossRef]
- Chatzovoulos, P.; Tsoupras, A.B.; Samiotaki, M.; Panayotou, G.; Demopoulos, C.A.; Dotsika, E. Paf-Metabolic Enzymes and Paf-like Activity in L. Infantum and L. Major Promastigotes. Eur. J. Inflamm. 2011, 9, 231–239. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Antonopoulou, S.; Baltas, G.; Samiotaki, M.; Panayotou, G.; Kotsifaki, H.; Mantzavinos, Z.; Demopoulos, C.A. Isolation and identification of hydroxyl–platelet-activating factor from natural sources. Life Sci. 2006, 79, 1796–1803. [Google Scholar] [CrossRef]
- Stivaktakis, P.; Vlastos, D.; Giannakopoulos, E.; Matthopoulos, D.P. Differential micronuclei induction in human lymphocyte cultures by imidacloprid in the presence of potassium nitrate. Sci. World J. 2010, 10, 80–89. [Google Scholar] [CrossRef]
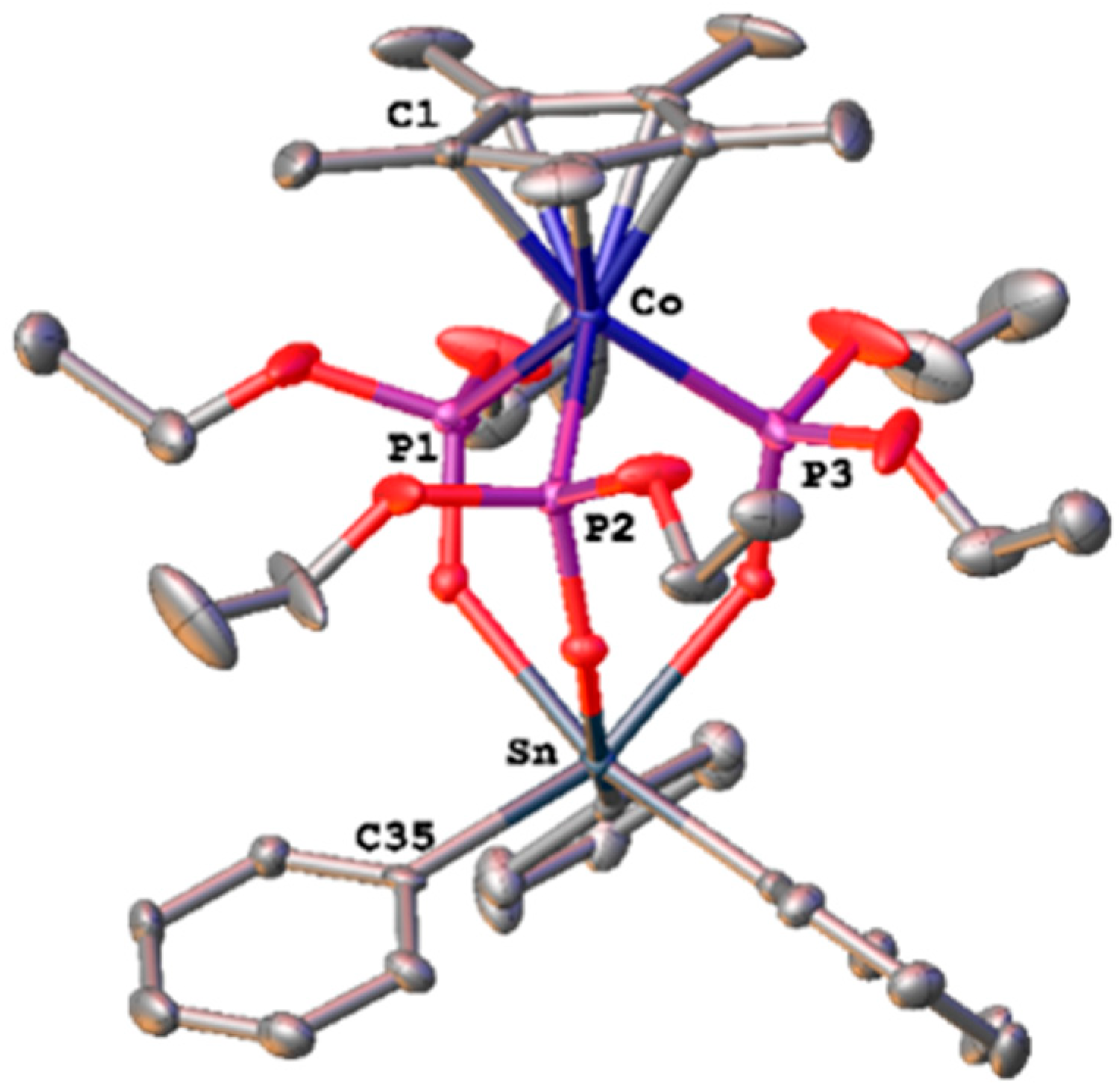
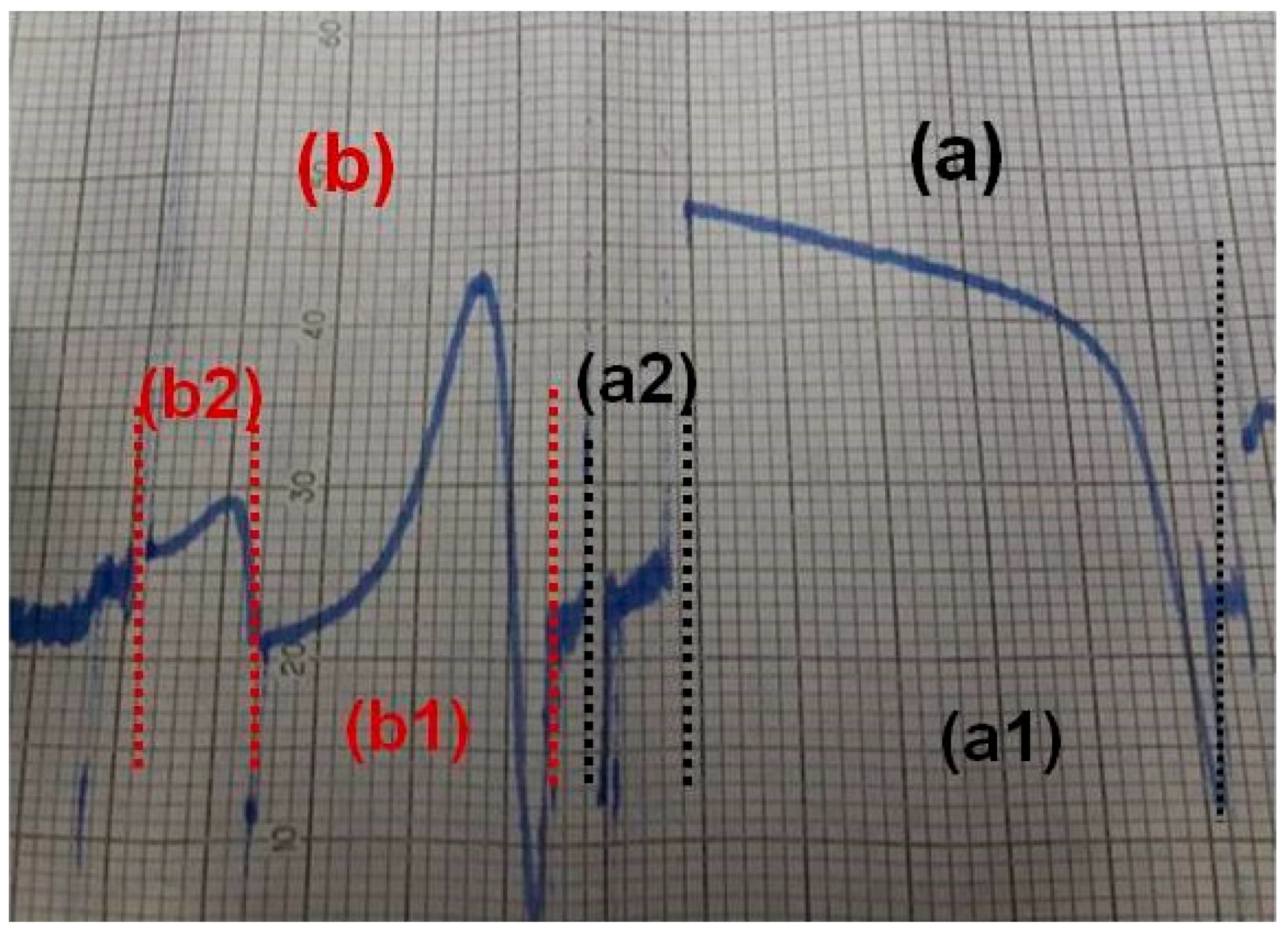
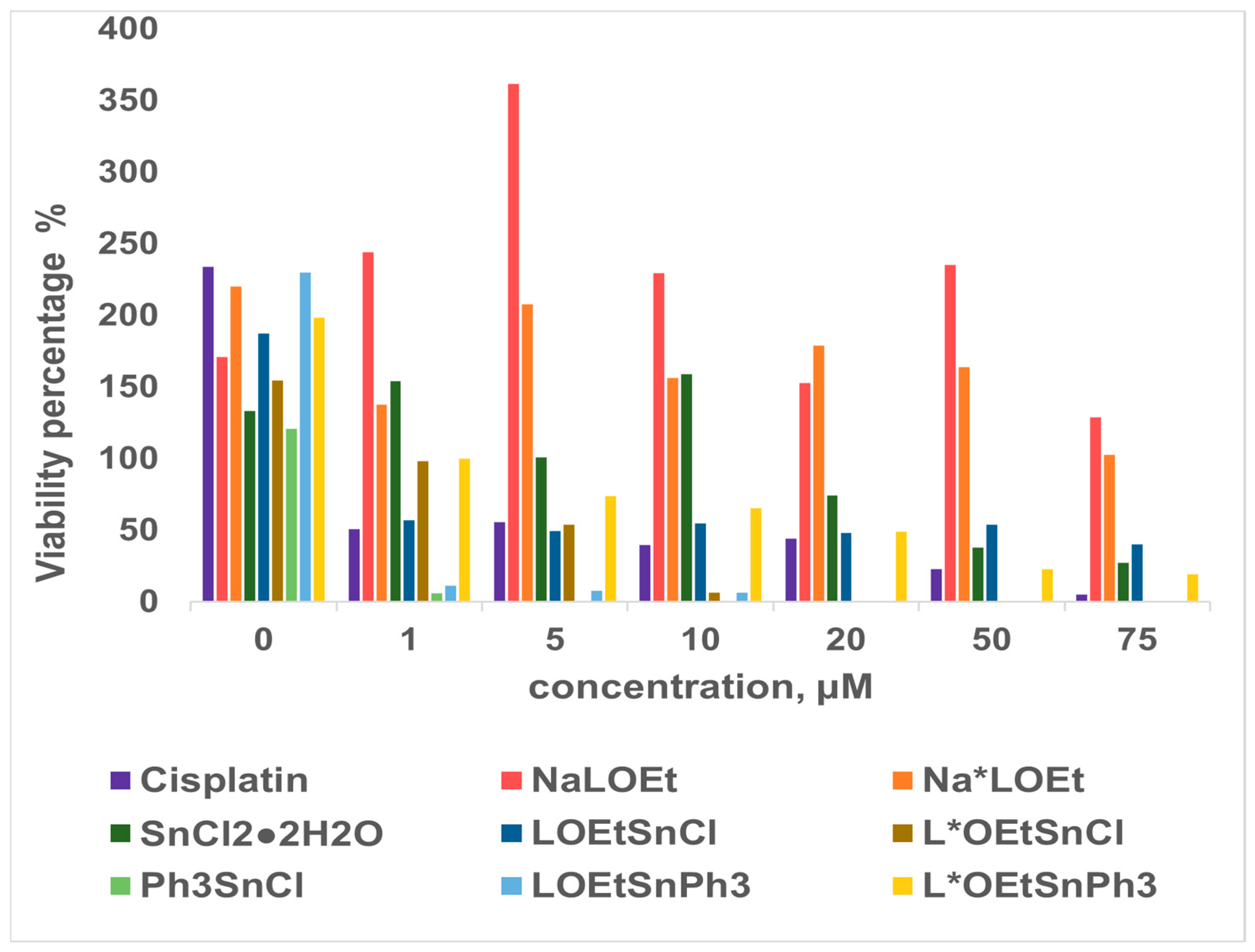
| Precursors and Sn Complexes | IC50 (μM) towards PAF in WRPs | IC50 (μM) towards PAF in rPRPs | IC50 (μM) towards Thrombin in WRPs |
|---|---|---|---|
| Ligands | |||
| 1a | 0.88 ± 0.15 | ND | 1.0 ± 0.1 |
| 1b | 0.95 ± 0.21 | 91 ± 9 | 13.6 ± 1.3 |
| Metal precursors | |||
| SnCl2 × 2H2O | 63 ± 8 | 224 ± 24 | 23.6 ± 5.3 |
| Ph3SnCl | 0.88 ± 0.15 | 105 ± 10 | 2.7 ± 0.8 |
| Complexes | |||
| 2a | 10.3 ± 1.1 | 323 ± 22 | 10.3 ± 1.3 |
| 2b | 0.5 ± 0.1 | 4.5 ± 0.8 | 1.8 ± 0.7 |
| 3a | 0.5 ± 0.1 | 18.8 ± 1.7 | 0.6 ± 0.1 |
| 3b | 4.4 ± 0.6 | 15.7 ± 1.3 | 0.23 ± 0.02 |
| cis-platin | 0.55 ± 0.22 | 728 ± 121 | 56 ± 16 |
| 2a | 2b | 3b | |
|---|---|---|---|
| Empirical formula | C17H35ClO9P3Sn | C22H45ClCoO9P3Sn | C40H60CoO9P3Sn |
| Molecular weight | 689.43 | 759.56 | 955.41 |
| Crystal color | Yellow | Orange | Yellow |
| Crystal size (mm3) | 0.60 × 0.36 × 0.24 | 0.48 × 0.48 × 0.28 | 1.04 × 0.60 × 0.52 |
| Temperature (K) | 180(2) | 180(2) | 180(2) |
| Crystal system | Triclinic | Monoclinic | Monoclinic |
| Space group | P − 1 | P21 | C2/c |
| Unit cell dimensions | |||
| α (Å) | 12.425(2) | 9.504(3) | 17.927(3) |
| β (Å) | 11.690(2) | 15.456(4) | 14.341(2) |
| γ (Å) | 18.852(3) | 11.843(3) | 33.527(4) |
| α (°) | 90.49(2) | 90 | 90 |
| β (°) | 112.98(3) | 112.98(3) | 95.12(2) |
| γ (°) | 90.01(2) | 90 | 90 |
| V (Å3) | 2720.8(8) | 1601.6(7) | 8585(2) |
| Z | 4 | 2 | 8 |
| ρcalc (g cm−3) | 1.683 | 1.575 | 1.478 |
| μ mm−1) | 1.843 | 1.574 | 1.131 |
| max θ (°) | 25.25 | 25.25 | 22.38 |
| hkl range | −14, 14/−14, 13/−22, 22 | −11, 11/−18, 18/−13, 13 | −19, 19/−15, 14/−35, 35 |
| Total data | 17,594 | 10,316 | 17,938 |
| Data unique [I > 2σ(I)] | 9195 | 5468 | 5414 |
| Rint | 0.0373 | 0.1481 | 0.0869 |
| Parameters refined | 589 | 345 | 498 |
| refinement method | Full matrix on F2 | Full matrix on F2 | Full matrix on F2 |
| R1, wR2 | 0.0286, 0.0723 | 0.0687, 0.1022 | 0.0542, 0.1178 |
| R1, wR2 (all data) | 0.0339, 0.0743 | 0.0738, 0.1054 | 0.0731, 0.1378 |
| Min./max. density (e Å−3) | 0.787 and −0.733 | 1.010 and −1.397 | 0.843 and −0.754 |
| Goodness-of-fit | 1.039 | 1.029 | 1.136 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalampalidis, A.; Damati, A.; Matthopoulos, D.; Tsoupras, A.B.; Demopoulos, C.A.; Schnakenburg, G.; Philippopoulos, A.I. Tin(II) and Tin(IV) Complexes Incorporating the Oxygen Tripodal Ligands [(η5-C5R5)Co{P(OEt)2O}3]−, (R = H, Me; Et = -C2H5) as Potent Inflammatory Mediator Inhibitors: Cytotoxic Properties and Biological Activities against the Platelet-Activating Factor (PAF) and Thrombin. Molecules 2023, 28, 1859. https://doi.org/10.3390/molecules28041859
Kalampalidis A, Damati A, Matthopoulos D, Tsoupras AB, Demopoulos CA, Schnakenburg G, Philippopoulos AI. Tin(II) and Tin(IV) Complexes Incorporating the Oxygen Tripodal Ligands [(η5-C5R5)Co{P(OEt)2O}3]−, (R = H, Me; Et = -C2H5) as Potent Inflammatory Mediator Inhibitors: Cytotoxic Properties and Biological Activities against the Platelet-Activating Factor (PAF) and Thrombin. Molecules. 2023; 28(4):1859. https://doi.org/10.3390/molecules28041859
Chicago/Turabian StyleKalampalidis, Alexandros, Artemis Damati, Demetrios Matthopoulos, Alexandros B. Tsoupras, Constantinos A. Demopoulos, Gregor Schnakenburg, and Athanassios I. Philippopoulos. 2023. "Tin(II) and Tin(IV) Complexes Incorporating the Oxygen Tripodal Ligands [(η5-C5R5)Co{P(OEt)2O}3]−, (R = H, Me; Et = -C2H5) as Potent Inflammatory Mediator Inhibitors: Cytotoxic Properties and Biological Activities against the Platelet-Activating Factor (PAF) and Thrombin" Molecules 28, no. 4: 1859. https://doi.org/10.3390/molecules28041859
APA StyleKalampalidis, A., Damati, A., Matthopoulos, D., Tsoupras, A. B., Demopoulos, C. A., Schnakenburg, G., & Philippopoulos, A. I. (2023). Tin(II) and Tin(IV) Complexes Incorporating the Oxygen Tripodal Ligands [(η5-C5R5)Co{P(OEt)2O}3]−, (R = H, Me; Et = -C2H5) as Potent Inflammatory Mediator Inhibitors: Cytotoxic Properties and Biological Activities against the Platelet-Activating Factor (PAF) and Thrombin. Molecules, 28(4), 1859. https://doi.org/10.3390/molecules28041859









