Efflux Pump (QacA, QacB, and QacC) and β-Lactamase Inhibitors? An Evaluation of 1,8-Naphthyridines against Staphylococcus aureus Strains
Abstract
1. Introduction
2. Results and Discussion
2.1. Antibacterial Activity of 1,8-Naphthyridines
2.2. Confirmation of β-Lactamase Activity in the SA-K4414 and SA-4100 Strains of S. aureus
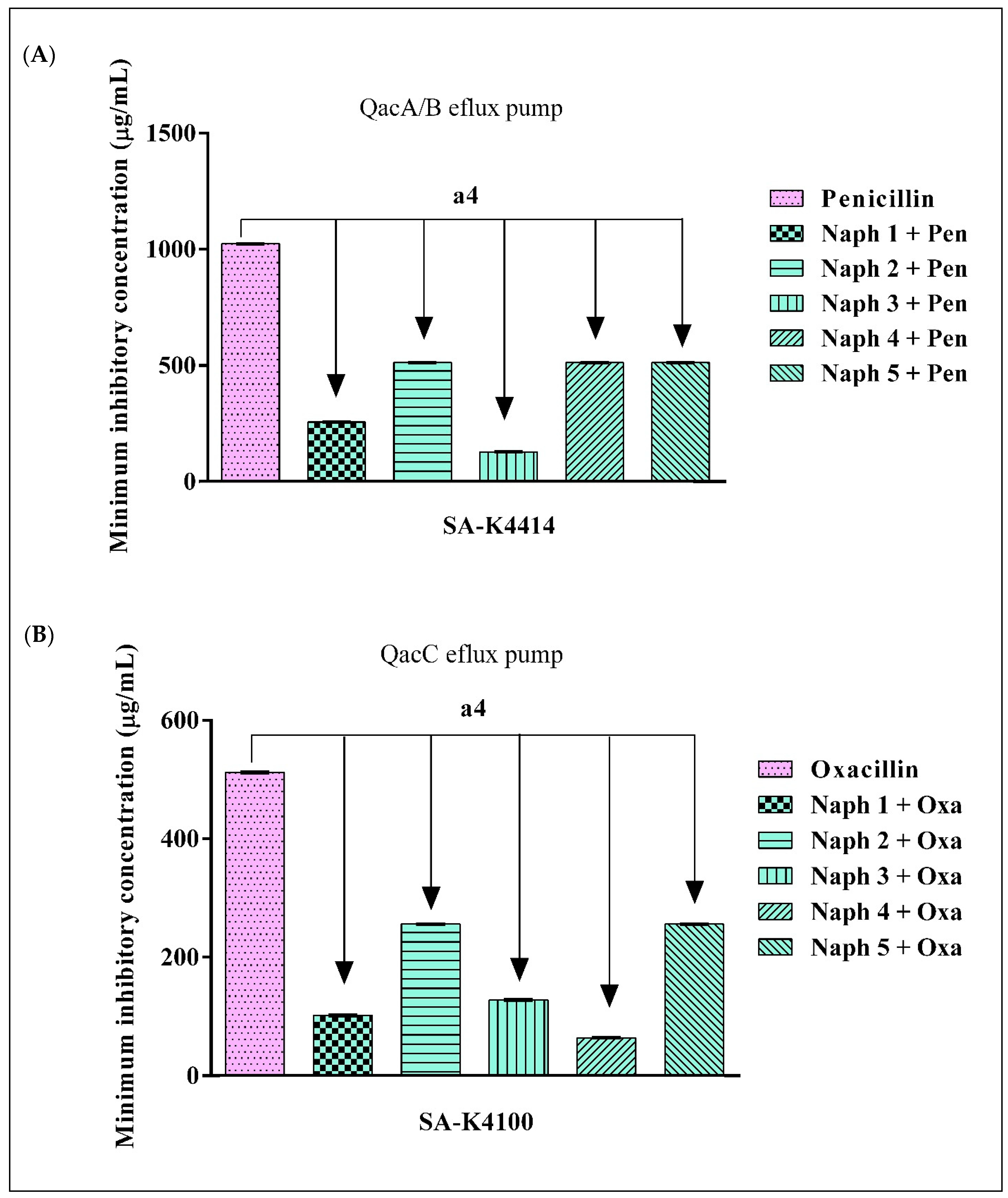
2.3. Evidence of β-Lactamase Inhibition by 1,8-Naphthyridines
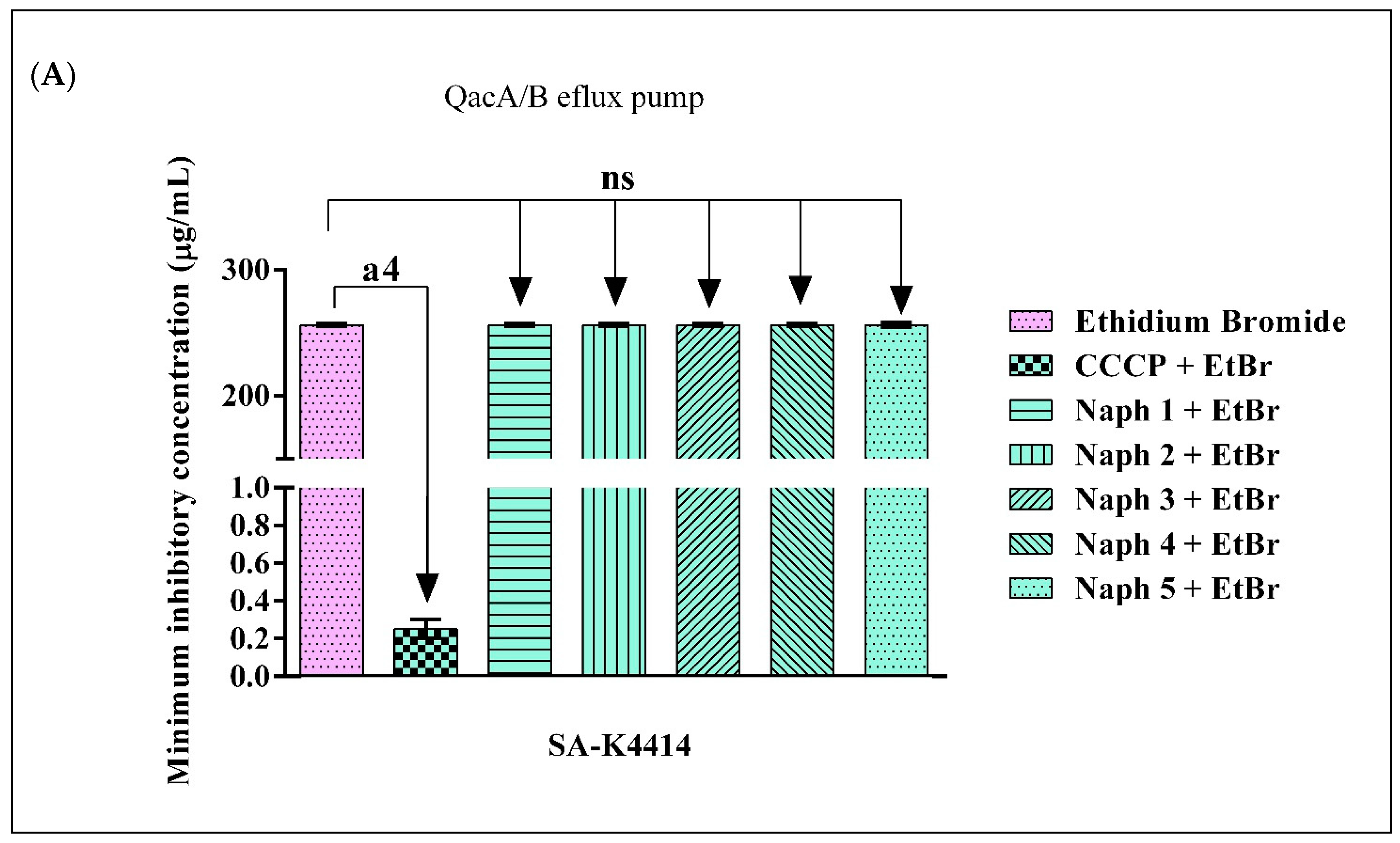
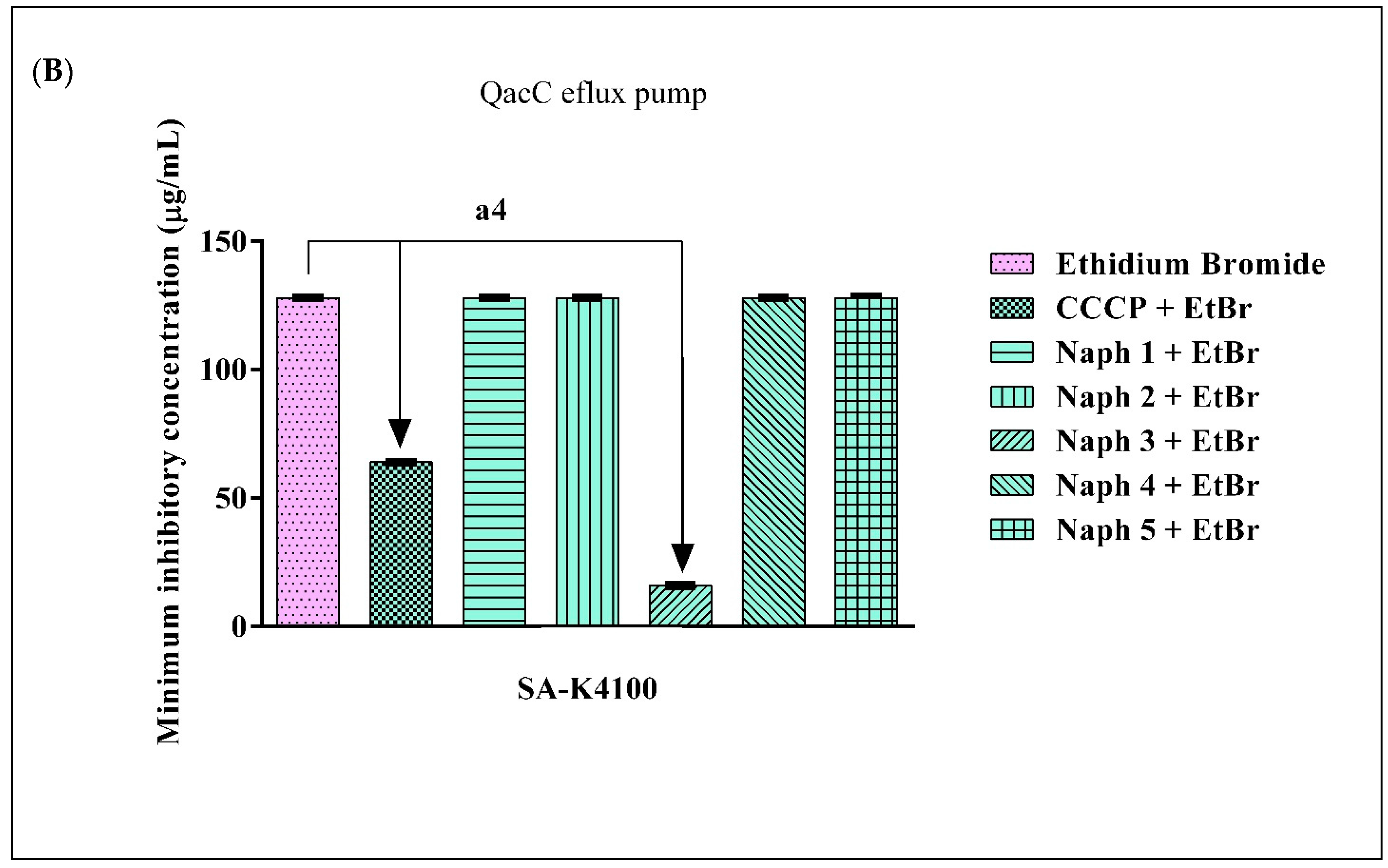
2.4. Evidence of Efflux Pump Inhibition by 1,8-Naphthyridines
3. Materials and Methods
3.1. Drugs and Reagents
3.2. Microorganisms
3.3. Minimum Inhibitory Concentration (MIC) Determination and Antibacterial Analysis
3.4. Analysis of β-Lactamase Activity
3.5. Verification of β-Lactamase and Efflux Pump Inhibition
3.5.1. Evaluation of β-Lactamase Inhibition through Reduction of Antibiotic MIC
3.5.2. Evaluation of Efflux Pump Inhibition through the Reduction of Ethidium Bromide MIC
3.6. Statistical Analysis of Microbiological Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Taylor, A.R. Methicillin-Resistant Staphylococcus aureus Infections. Prim. Care-Clin. Off. Pract. 2013, 40, 637–654. [Google Scholar] [CrossRef]
- Pollitt, E.J.G.; Szkuta, P.T.; Burns, N.; Foster, S.J. Staphylococcus aureus Infection Dynamics. PLoS Pathog 2018, 14, e1007112. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus Infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Roberts, S.; Chambers, S. Diagnosis and Management of Staphylococcus aureus Infections of the Skin and Soft Tissue. Intern. Med. J. 2005, 35, S97–S105. [Google Scholar] [CrossRef]
- Velázquez-Meza, M.E. Surgimiento y Diseminación de Staphylococcus aureus Meticilinorresistente. Salud Publica Mex. 2005, 47, 381–387. [Google Scholar] [CrossRef]
- Lutz, L.; Machado, A.; Kuplich, N.; Barth, A.L. Clinical Failure of Vancomycin Treatment of Staphylococcus aureus Infection in a Tertiary Care Hospital in Southern Brazil. Braz. J. Infect. Dis. 2003, 7, 224–228. [Google Scholar] [CrossRef]
- Knox, K.W.; Wicken, A.J. Immunological Properties of Teichoic Acids. Bacteriol. Rev. 1973, 37, 215–257. [Google Scholar] [CrossRef]
- J Jenul, C.; Horswill, A.R. Regulation of Staphylococcus aureus Virulence. Microbiol. Spectr. 2019, 7, 1–21. [Google Scholar] [CrossRef]
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed; WHO: Geneva, Switzerland, 2017.
- Baker, S.; Thomson, N.; Weill, F.X.; Holt, K.E. Genomic Insights into the Emergence and Spread of Antimicrobial-Resistant Bacterial Pathogens. Science 2018, 360, 733–738. [Google Scholar] [CrossRef]
- Gyles, C.; Boerlin, P. Horizontally Transferred Genetic Elements and Their Role in Pathogenesis of Bacterial Disease. Vet. Pathol. 2014, 51, 328–340. [Google Scholar] [CrossRef]
- Piddock, L.J. Understanding Drug Resistance Will Improve the Treatment of Bacterial Infections. Nat. Rev. Microbiol. 2017, 15, 639–640. [Google Scholar] [CrossRef] [PubMed]
- González-Bello, C. Antibiotic Adjuvants—A Strategy to Unlock Bacterial Resistance to Antibiotics. Bioorg. Med. Chem. Lett. 2017, 27, 4221–4228. [Google Scholar] [CrossRef]
- Abushaheen, M.A.; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; Vellappally, S. Antimicrobial Resistance, Mechanisms and Its Clinical Significance. Dis.-A-Mon. 2020, 66, 100971. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and Antagonism in Natural Product Extracts: When 1 + 1 Does Not Equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Wilke, M.S.; Lovering, A.L.; Strynadka, N.C.J. β-Lactam Antibiotic Resistance: A Current Structural Perspective. Curr. Opin. Microbiol. 2005, 8, 525–533. [Google Scholar] [CrossRef]
- Abraham, E.P.; Chain, E. An Enzyme from Bacteria Able to Destroy penicillin. Nature 1940, 146, 837. [Google Scholar] [CrossRef]
- Johnson, A.P.; Woodford, N. Global Spread of Antibiotic Resistance: The Example of New Delhi Metallo-β-Lactamase (NDM)-Mediated Carbapenem Resistance. J. Med. Microbiol. 2013, 62, 499–513. [Google Scholar] [CrossRef]
- Lynch, J.P.; Clark, N.M.; Zhanel, G.G. Evolution of Antimicrobial Resistance among Enterobacteriaceae (Focus on Extended Spectrum β-Lactamases and Carbapenemases). Expert Opin. Pharmacother. 2013, 14, 199–210. [Google Scholar] [CrossRef]
- Sidhu, M.S.; Heir, E.; Leegaard, T.; Wiger, K.; Holck, A. Frequency of Disinfectant Resistance Genes and Genetic Linkage with -Lactamase Transposon Tn552 among Clinical Staphylococci. Antimicrob. Agents Chemother. 2002, 46, 2797–2803. [Google Scholar] [CrossRef]
- Sidhu, M.S.; Heir, E.; Sørum, H.; Holck, A. Genetic Linkage between Resistance to Quaternary Ammonium Compounds and β-Lactam Antibiotics in Food-Related Staphylococcus spp. Microb. Drug Resist. 2001, 7, 363–371. [Google Scholar] [CrossRef]
- Costa, S.S.; Viveiros, M.; Amaral, L.; Couto, I. Multidrug Efflux Pumps in Staphylococcus aureus: An Update. Open Microbiol. J. 2013, 7, 59–71. [Google Scholar] [CrossRef]
- Kaatz, G.W.; DeMarco, C.E.; Seo, S.M. MepR, a Represser of the Staphylococcus aureus MATE Family Multidrug Efflux Pump MepA, Is a Substrate-Responsive Regulatory Protein. Antimicrob. Agents Chemother. 2006, 50, 1276–1281. [Google Scholar] [CrossRef]
- McAleese, F.; Petersen, P.; Ruzin, A.; Dunman, P.M.; Murphy, E.; Projan, S.J.; Bradford, P.A. A Novel MATE Family Efflux Pump Contributes to the Reduced Susceptibility of Laboratory-Derived Staphylococcus aureus Mutants to Tigecycline. Antimicrob. Agents Chemother. 2005, 49, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Van Hoek, A.H.A.M.; Mevius, D.; Guerra, B.; Mullany, P.; Roberts, A.P.; Aarts, H.J.M. Acquired Antibiotic Resistance Genes: An Overview. Front. Microbiol. 2011, 2, 203. [Google Scholar] [CrossRef] [PubMed]
- Vali, L.; Davies, S.E.; Lai, L.L.G.; Dave, J.; Amyes, S.G.B. Frequency of Biocide Resistance Genes, Antibiotic Resistance and the Effect of Chlorhexidine Exposure on Clinical Methicillin-Resistant Staphylococcus aureus Isolates. J. Antimicrob. Chemother. 2008, 61, 524–532. [Google Scholar] [CrossRef]
- Smith, K.; Gemmell, C.G.; Hunter, I.S. The Association between Biocide Tolerance and the Presence or Absence of Qac Genes among Hospital-Acquired and Community-Acquired MRSA Isolates. J. Antimicrob. Chemother. 2008, 61, 78–84. [Google Scholar] [CrossRef]
- Cervinkova, D.; Babak, V.; Marosevic, D.; Kubikova, I.; Jaglic, Z. The Role of the QacA Gene in Mediating Resistance to Quaternary Ammonium Compounds. Microb. Drug Resist. 2013, 19, 160–167. [Google Scholar] [CrossRef]
- Wassenaar, T.; Ussery, D.; Nielsen, L.; Ingmer, H. Review and Phylogenetic Analysis of Qac Genes That Reduce Susceptibility to Quaternary Ammonium Compounds in Staphylococcus Species. Eur. J. Microbiol. Immunol. 2015, 5, 44–61. [Google Scholar] [CrossRef]
- Kumar, S.; Varela, M.F. Biochemistry of Bacterial Multidrug Efflux Pumps. Int. J. Mol. Sci. 2012, 13, 4484. [Google Scholar] [CrossRef]
- Paulsen, I.T.; Brown, M.H.; Littlejohn, T.G.; Mitchell, B.A.; Skurray, R.A. Multidrug Resistance Proteins QacA and QacB from Staphylococcus aureus: Membrane Topology and Identification of Residues Involved in Substrate Specificity. Proc. Natl. Acad. Sci. USA 1996, 93, 3630–3635. [Google Scholar] [CrossRef]
- Lekshmi, M.; Ammini, P.; Adjei, J.; Sanford, L.M.; Shrestha, U.; Kumar, S.; Varela, M.F. Modulation of Antimicrobial Efflux Pumps of the Major Facilitator Superfamily in Staphylococcus aureus. AIMS Microbiol. 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- De Neto, L.J.L.; Ramos, A.G.B.; de Freitas, T.S.; Barbosa, C.R.D.S.; de Sousa Júnior, D.L.; Siyadatpanah, A.; Nejat, M.; Wilairatana, P.; Coutinho, H.D.M.; da Cunha, F.A.B. Evaluation of Benzaldehyde as an Antibiotic Modulator and Its Toxic Effect against Drosophila Melanogaster. Molecules 2021, 26, 5570. [Google Scholar] [CrossRef]
- Reddy, V.S.; Shlykov, M.A.; Castillo, R.; Sun, E.I.; Saier, M.H. The Major Facilitator Superfamily (MFS) Revisited. FEBS J. 2012, 279, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Antimicrobial Resistance: The Example of Staphylococcus Aureus. J Clin Invest 2003, 111, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.; Lomovskaya, O. Can Efflux Inhibitors Really Counter Resistance? Drug Discov. Today Ther. Strateg. 2006, 3, 145–152. [Google Scholar] [CrossRef]
- Mahamoud, A.; Chevalier, J.; Alibert-Franco, S.; Kern, W.V.; Pagès, J.M. Antibiotic Efflux Pumps in Gram-Negative Bacteria: The Inhibitor Response Strategy. J. Antimicrob. Chemother. 2007, 59, 1223–1229. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Bostian, K.A. Practical Applications and Feasibility of Efflux Pump Inhibitors in the Clinic—A Vision for Applied Use. Biochem. Pharmacol. 2006, 71, 910–918. [Google Scholar] [CrossRef]
- Wright, G.D. Resisting Resistance: New Chemical Strategies for Battling Superbugs. Chem. Biol. 2000, 7, R127–R132. [Google Scholar] [CrossRef]
- Gupta, V.; Datta, P. Next-Generation Strategy for Treating Drug Resistant Bacteria: Antibiotic Hybrids. Indian J. Med. Res. 2019, 149, 97. [Google Scholar] [CrossRef]
- Reissert, A. Ueber Di-(γ-Amidopropyl) Essigsäure (Diamino.1.7.Heptanmethylsäure.4) Und Ihr Inneres Condensationsproduct, Das Octohydro.1.8.Naphtyridin. Ber. Dtsch. Chem. Ges. 1893, 26, 2137–2144. [Google Scholar] [CrossRef]
- Bobrański, B.; Sucharda, E. Über Eine Synthese Des 1.5-Naphthyridins. Ber. Dtsch. Chem. Ges. (A B Ser.) 1927, 60, 1081–1084. [Google Scholar] [CrossRef]
- Koller, G. Über Eine Synthese von Derivaten Des 1.8-Naphthyridins. Ber. Dtsch. Chem. Ges. (A B Ser.) 1927, 60, 407–410. [Google Scholar] [CrossRef]
- Koller, G. Über Das 1.8-Naphthyridin Und Seine Derivate (Vorläufige Mitteilung). Ber. Dtsch. Chem. Ges. (A B Ser.) 1927, 60, 1572–1575. [Google Scholar] [CrossRef]
- Bachand, B. Antiviral Methods Using [1, 8] Naphthyridine Derivatives. U.S. Patent No. 6,340,690, 12 August 2002. [Google Scholar]
- Domagala, J.M.; Mich, T.F.; Nichols, J.B. Naphthyridine Antibacterial Agents. U.S. Patent No. 5,281,612, 25 January 1994. [Google Scholar]
- Brehmer, M.C. Síntese e Reatividade das Cloro 1,6-e 1,5-Naftiridinas e o Estudo de Suas Potencialidades. M.Sc. Dissertation, Federal University of Santa Catarina, Florianópolis, Brazil, 2002. [Google Scholar]
- Madaan, A.; Verma, R.; Kumar, V.; Singh, A.T.; Jain, S.K.; Jaggi, M. 1,8-Naphthyridine Derivatives: A Review of Multiple Biological Activities. Arch. Pharm. (Weinh.) 2015, 348, 837–860. [Google Scholar] [CrossRef] [PubMed]
- Blum, C.A.; Caldwell, T.; Zheng, X.; Bakthavatchalam, R.; Capitosti, S.; Brielmann, H.; De Lombaert, S.; Kershaw, M.T.; Matson, D.; Krause, J.E.; et al. Discovery of Novel 6,6-Heterocycles as Transient Receptor Potential Vanilloid (TRPV1) Antagonists. J. Med. Chem. 2010, 53, 3330–3348. [Google Scholar] [CrossRef]
- Di Braccio, M.; Grossi, G.; Alfei, S.; Ballabeni, V.; Tognolini, M.; Flammini, L.; Giorgio, C.; Bertoni, S.; Barocelli, E. 1,8-Naphthyridines IX. Potent Anti-Inflammatory and/or Analgesic Activity of a New Group of Substituted 5-Amino[1,2,4]Triazolo[4,3-a][1,8]Naphthyridine-6-Carboxamides, of Some Their Mannich Base Derivatives and of One Novel Substituted 5-Amino-10-Oxo-10H. Eur. J. Med. Chem. 2014, 6, 394–405. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Cheng, L.; Chen, L.; Wang, Y.; Qing, J.; Huang, S.; Wang, Y.; Lei, X.; Wu, Y.; et al. Discovery of Imidazo[1,2-α][1,8]Naphthyridine Derivatives as Potential HCV Entry Inhibitor. ACS Med. Chem. Lett. 2015, 6, 977–981. [Google Scholar] [CrossRef]
- Lesher, G.Y.; Froelich, E.J.; Gruett, M.D.; Bailey, J.H.; Brundage, R.P. 1,8-Naphthyridine Derivatives. A New Class of Chemotherapeutic Agents. J. Med. Pharm. Chem. 1962, 5, 1063–1065. [Google Scholar] [CrossRef]
- Thompson, R.E.M.; Rae, J. Negram (1-ethyI-7-methyl-1, 8-naphthyridine-4-one-3-carboxylic acid): A New Antibacterial Agent for the Treatment of Urinary Infection. Report of a Trial in General Practice. Br. J. Urol. 1964, 36, 42. [Google Scholar] [CrossRef]
- Guinea, J.; Gargallo-Viola, D.; Robert, M.; Tudela, E.; Xicota, M.A.; Garcia, J.; Esteve, M.; Coll, R.; Pares, M.; Roser, R. E-4695, a New C-7 Azetidinyl Fluoronaphthyridine with Enhanced Activity against Gram-Positive and Anaerobic Pathogens. Antimicrob. Agents Chemother. 1995, 39, 413–421. [Google Scholar] [CrossRef]
- Ashima, K. Bhardwaj; Priyabrata Mohanty Bacterial Efflux Pumps Involved in Multidrug Resistance and Their Inhibitors: Rejuvinating the Antimicrobial Chemotherapy. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Sundaramoorthy, N.S.; Mitra, K.; Ganesh, J.S.; Makala, H.; Lotha, R.; Bhanuvalli, S.R.; Ulaganathan, V.; Tiru, V.; Sivasubramanian, A.; Nagarajan, S. Ferulic Acid Derivative Inhibits NorA Efflux and in Combination with Ciprofloxacin Curtails Growth of MRSA in Vitro and in Vivo. Microb. Pathog. 2018, 124, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N. In Vitro Evaluation of E-4695, a New Fluoro-Naphthyridine. Eur. J. Clin. Microbiol. Infect. Dis. 1992, 11, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Parhi, A.K.; Zhang, Y.; Saionz, K.W.; Pradhan, P.; Kaul, M.; Trivedi, K.; Pilch, D.S.; Lavoie, E.J. Antibacterial Activity of Quinoxalines, Quinazolines, and 1,5-Naphthyridines. Bioorg. Med. Chem. Lett. 2013, 23, 4968–4974. [Google Scholar] [CrossRef]
- Laxminarayana, E.; Karunakar, T.; Shiva, S.; Thirumala Chary, M. A Study on Antibacterial Activity of Substituted 1, 8-Naphthyridines Containing Carbaldehydes, Methylene Hydrazines, Thiadiazol Amines and Triazole Thiols. J. Adv. Drug Res. 2012, 2, 6–11. [Google Scholar]
- Bryskier, A.; Chantot, J.F. Classification and Structure-Activity Relationships of Fluoroquinolones. Drugs 1995, 49 (Suppl. S2), 16–28. [Google Scholar] [CrossRef]
- Ball, P.; Tillotson, G. Tolerability of Fluoroquinolone Antibiotics: Past, Present and Future. Drug Saf. 1995, 13, 343–358. [Google Scholar] [CrossRef]
- Hong, C.Y.; Kim, Y.K.; Chang, J.H.; Kim, S.H.; Choi, H.; Nam, D.H.; Kim, Y.Z.; Kwak, J.H. Novel Fluoroquinolone Antibacterial Agents Containing Oxime-Substituted (Aminomethyl)Pyrrolidines: Synthesis and Antibacterial Activity of 7-(4- (Aminomethyl)-3-(Methoxyimino) Pyrrolidin-1-Yl)-1-Cyclopropyl-6-Fluoro-4-Oxo-1,4-Dihydro[1,8]Naphthyridine-3-carboxylic Acid (LB20304). J. Med. Chem. 1997, 40, 3584–3593. [Google Scholar] [CrossRef]
- Liliam, D.; Jackson, C. Quinolonas y Terapia Antimicrobiana. Acta Med. 1998, 8, 58–65. [Google Scholar]
- Tillotson, G.S. Quinolones: Structure-Activity Relationships and Future Predictions. J. Med. Microbiol. 1996, 44, 320–324. [Google Scholar] [CrossRef]
- Domagk, G. Chemotherapie Der Bakteriellen Infektionen. Angew. Chem. 1935, 61, 250–253. [Google Scholar] [CrossRef]
- Nasr, T.; Bondock, S.; Eid, S. Design, Synthesis, Antimicrobial Evaluation and Molecular Docking Studies of Some New 2,3-Dihydrothiazoles and 4-Thiazolidinones Containing Sulfisoxazole. J. Enzym. Inhib. Med. Chem. 2016, 31, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Holmes, N.E.; Lindsay Grayson, M. Sulfonamides. In Kucers the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs, 7th ed.; Taylor and Francis: London, UK, 2017; ISBN 9781498747967. [Google Scholar]
- Anand, N. Sulfonamides and Sulfones. In Mechanism of Action of Antimicrobial and Antitumor Agents. Antibiotics; Corcoran, J.W., Hahn, F.E., Snell, J.F., Arora, K.L., Eds.; Springer: Berlin/Heidelberg, Germany, 1975; Volume 3. [Google Scholar] [CrossRef]
- Trefouel, J.; Nitti, F.; Bovet, D. Action of P-Aminophenylsulfamide in Experimental Streptococcus Infections of Mice and Rabbits. Comptes Rendus Seances Soc. Biol. Ses Fil. 1935, 120, 756–758. [Google Scholar]
- Fouts, J.R.; Kamm, J.J.; Brodie, B.B. Enzymatic Reduction of Prontosil and Other Azo Dyes. J. Pharmacol. Exp. Ther. 1957, 120, 291–300. [Google Scholar]
- Paulsen, I.T.; Gillespie, M.T.; Littlejohn, T.G.; Hanvivatvong, O.; Rowland, S.J.; Dyke, K.G.H.; Skurray, R.A. Characterisation of Sin, a Potential Recombinase-Encoding Gene from Staphylococcus aureus. Gene 1994, 141, 109–114. [Google Scholar] [CrossRef]
- Berg, T.; Firth, N.; Apisiridej, S.; Hettiaratchi, A.; Leelaporn, A.; Skurray, R.A. Complete Nucleotide Sequence of PSK41: Evolution of Staphylococcal Conjugative Multiresistance Plasmids. J. Bacteriol. 1998, 180, 4350. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ahn, J. Associations between Resistance Phenotype and Gene Expression in Response to Serial Exposure to Oxacillin and Ciprofloxacin in Staphylococcus aureus. Lett. Appl. Microbiol. 2017, 65, 462–468. [Google Scholar] [CrossRef]
- Hadadi, F.; Ghaznavi Rad, E.; Almasi-Hashiani, A.; Abtahi, H. Detection of QacEΔ1, QacG, QacE, QacF Resistance Genes in Escherichia coli Producing Broad-Spectrum Beta-Lactamases to Benzalkonium Chloride. J. Babol Univ. Med. Sci. 2019, 21, 286–292. [Google Scholar] [CrossRef]
- De Sousa Júnior, D.L.; Cordeiro, P.P.M.; dos Santos Barbosa, C.R.; Muniz, D.F.; de Sousa Silveira, Z.; Macêdo, N.S.; de Lacerda Neto, L.J.; de Freitas, T.S.; dos Santos, J.F.S.; Coutinho, H.D.M.; et al. Evaluation of Isoeugenol in Inhibition of Staphylococcus aureus Efflux Pumps and Their Toxicity Using Drosophila Melanogaster Model. Life Sci. 2021, 285, 119940. [Google Scholar] [CrossRef]
- CLSI CLSI, M100ED29E. Performance Standards for Antimicrobial Susceptibility Testing: 29th Informational Supplement. 20; 29th ed. 2019. Available online: https://webstore.ansi.org/standards/clsi/clsim100ed29 (accessed on 13 January 2022).
- Drawz, S.M.; Bonomo, R.A. Three Decades of Beta-Lactamase Inhibitors. Clin. Microbiol. Rev. 2010, 23, 160–201. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Bethel, C.R.; Distler, A.M.; Kasuboski, C.; Taracila, M.; Bonomo, R.A. Inhibitor Resistance in the KPC-2 Beta-Lactamase, a Preeminent Property of This Class A Beta-Lactamase. Antimicrob. Agents Chemother. 2010, 54, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Coleman, K. Diazabicyclooctanes (DBOs): A Potent New Class of Non-β-Lactam β-Lactamase Inhibitors. Curr. Opin. Microbiol. 2011, 14, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Grigorenko, V.G.; Andreeva, I.P.; Rubtsova, M.Y.; Deygen, I.M.; Antipin, R.L.; Majouga, A.G.; Egorov, A.M.; Beshnova, D.A.; Kallio, J.; Hackenberg, C.; et al. Novel Non-β-Lactam Inhibitor of β-Lactamase TEM-171 Based on Acylated Phenoxyaniline. Biochimie 2017, 132, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Cahill, S.T.; Cain, R.; Wang, D.Y.; Lohans, C.T.; Wareham, D.W.; Oswin, H.P.; Mohammed, J.; Spencer, J.; Fishwick, C.W.G.; McDonough, M.A.; et al. Cyclic Boronates Inhibit All Classes of β-Lactamases. Antimicrob. Agents Chemother. 2017, 61, e02260-16. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, D.E.; Jahic, H.; Ross, P.L.; Gu, R.-F.; Hu, J.; Kern, G.; Walkup, G.K.; Fisher, S.L. Avibactam Is a Covalent, Reversible, Non-Lactam-Lactamase Inhibitor. Proc. Natl. Acad. Sci. USA 2012, 109, 11663–11668. [Google Scholar] [CrossRef] [PubMed]
- Drawz, S.M.; Papp-Wallace, K.M.; Bonomo, R.A. New β-Lactamase Inhibitors: A Therapeutic Renaissance in an MDR World. Antimicrob. Agents Chemother. 2014, 58, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Kaatz, G.W.; Moudgal, V.V.; Seo, S.M.; Kristiansen, J.E. Phenothiazines and Thioxanthenes Inhibit Multidrug Efflux Pump Activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Schindler, B.D.; Jacinto, P.; Kaatz, G.W. Inhibition of Drug Efflux Pumps in Staphylococcus aureus: Current Status of Potentiating Existing Antibiotics. Future Microbiol. 2013, 8, 491–507. [Google Scholar] [CrossRef]
- DeMarco, C.E.; Cushing, L.A.; Frempong-Manso, E.; Seo, S.M.; Jaravaza, T.A.A.; Kaatz, G.W. Efflux-Related Resistance to Norfloxacin, Dyes, and Biocides in Bloodstream Isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 3235–3239. [Google Scholar] [CrossRef]
- Kristiansen, J.E.; Thomsen, V.F.; Martins, A.; Viveiros, M.; Amaral, L. Non-Antibiotics Reverse Resistance of Bacteria to Antibiotics. In Vivo 2010, 24, 751–754. [Google Scholar]
- Patel, D.; Kosmidis, C.; Seo, S.M.; Kaatz, G.W. Ethidium Bromide MIC Screening for Enhanced Efflux Pump Gene Expression or Efflux Activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 5070–5073. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C. Emerging Mechanisms of Fluoroquinolone Resistance. Emerg. Infect. Dis. 2001, 7, 337–341. [Google Scholar]
- German, N.; Wei, P.; Kaatz, G.W.; Kerns, R.J. Synthesis and Evaluation of Fluoroquinolone Derivatives as Substrate-Based Inhibitors of Bacterial Efflux Pumps. Eur. J. Med. Chem. 2008, 43, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Tennent, J.M.; Lyon, B.R.; Gillespie, M.T.; May, J.W.; Skurray, R.A. Cloning and Expression of Staphylococcus aureus Plasmid-Mediated Quaternary Ammonium Resistance in Escherichia coli. Antimicrob. Agents Chemother. 1985, 27, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.A.; Brown, M.H.; Skurray, R.A. QacA Multidrug Efflux Pump from Staphylococcus aureus: Comparative Analysis of Resistance to Diamidines, Biguanidines, and Guanylhydrazones. Antimicrob. Agents Chemother. 1998, 42, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.H.; Skurray, R.A. Staphylococcal Multidrug Efflux Protein QacA. J. Mol. Microbiol. Biotechnol. 2001, 3, 163–170. [Google Scholar]
- Nakaminami, H.; Noguchi, N.; Nishijima, S.; Kurokawa, I.; Sasatsu, M. Characterization of the PTZ2162 Encoding Multidrug Efflux Gene QacB from Staphylococcus aureus. Plasmid 2008, 60, 108–117. [Google Scholar] [CrossRef]
- Leelaporn, A.; Paulsen, I.T.; Tennent, J.M.; Littlejohn, T.G.; Skurray, R.A. Multidrug Resistance to Antiseptics and Disinfectants in Coagulase-Negative Staphylococci. J. Med. Microbiol. 1994, 40, 214–220. [Google Scholar] [CrossRef]
- Jang, S. Multidrug Efflux Pumps in Staphylococcus aureus and Their Clinical Implications. J. Microbiol. 2016, 54, 1–8. [Google Scholar] [CrossRef]
- Sun, J.; Deng, Z.; Yan, A. Bacterial Multidrug Efflux Pumps: Mechanisms, Physiology and Pharmacological Exploitations. Biochem. Biophys. Res. Commun. 2014, 453, 254–267. [Google Scholar] [CrossRef]
- Schindler, B.D.; Kaatz, G.W. Multidrug Efflux Pumps of Gram-Positive Bacteria. Drug Resist. Updat. 2016, 27, 1–13. [Google Scholar] [CrossRef]
- Lolkema, J.S.; Poolman, B.; Konings, W.N. Bacterial Solute Uptake and Efflux Systems. Curr. Opin. Microbiol. 1998, 1, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Charushin, V.N.; Nosova, E.V.; Lipunova, G.N.; Chupakhin, O.N. Fluoroquinolones: Synthesis and Application. In Fluorine in Heterocyclic Chemistry: Volume 2: 6-Membered Heterocycles; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; pp. 111–179. ISBN 9783319044354. [Google Scholar]
- De Oliveira-Tintino, C.D.M.; Muniz, D.F.; dos Barbosa, C.R.S.; Pereira, R.L.S.; Begnini, I.M.; Rebelo, R.A.; da Silva, L.E.; Mireski, S.L.; Nasato, M.C.; Krautler, M.I.L.; et al. The 1,8-Naphthyridines Sulfonamides Are NorA Efflux Pump Inhibitors. J. Glob. Antimicrob. Resist. 2021, 24, 233–240. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira-Tintino, C.D.M.; Tintino, S.R.; Muniz, D.F.; dos Santos Barbosa, C.R.; Pereira, R.L.S.; Begnini, I.M.; Rebelo, R.A.; da Silva, L.E.; Mireski, S.L.; Nasato, M.C.; et al. Chemical Synthesis, Molecular Docking and MepA Efflux Pump Inhibitory Effect by 1,8-Naphthyridines Sulfonamides. Eur. J. Pharm. Sci. 2021, 160, 105753. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira-Tintino, C.D.M.; Tintino, S.R.; Muniz, D.F.; dos Barbosa, C.R.S.; Pereira, R.L.S.; Begnini, I.M.; Rebelo, R.A.; da Silva, L.E.; Mireski, S.L.; Nasato, M.C.; et al. Do 1,8-Naphthyridine Sulfonamides Possess an Inhibitory Action against Tet(K) and MsrA Efflux Pumps in Multiresistant Staphylococcus aureus Strains? Microb. Pathog. 2020, 147, 104268. [Google Scholar] [CrossRef] [PubMed]
- Cedraro, N.; Cannalire, R.; Astolfi, A.; Mangiaterra, G.; Felicetti, T.; Biavasco, F.; Sabatini, S. From Quinoline to Quinazoline-Based S. aureus NorA Efflux Pump Inhibitors by Coupling a Focused Scaffold Hopping Approach and a Pharmacophore Search. ChemMedChem 2021, 16, 3044–3059. [Google Scholar] [CrossRef]
- De Oliveira-Tintino, C.D.M.; Muniz, D.F.; dos Santos Barbosa, C.R.; Silva Pereira, R.L.; Begnini, I.M.; Rebelo, R.A.; da Silva, L.E.; Mireski, S.L.; Nasato, M.C.; Lacowicz Krautler, M.I.; et al. NorA, Tet(K), MepA, and MsrA Efflux Pumps in Staphylococcus aureus, Their Inhibitors and 1,8-Naphthyridine Sulfonamides. Curr. Pharm. Des. 2022, 29, 1–33. [Google Scholar] [CrossRef]
- Rodrigues, L.; Ramos, J.; Couto, I.; Amaral, L.; Viveiros, M. Ethidium Bromide Transport across Mycobacterium Smegmatis Cell-Wall: Correlation with Antibiotic Resistance. BMC Microbiol. 2011, 11, 35. [Google Scholar] [CrossRef]
- Lepecq, J.-B.; Paoletti, C. A Fluorescent Complex between Ethidium Bromide and Nucleic Acids. J. Mol. Biol. 1967, 27, 87–106. [Google Scholar] [CrossRef]
| Treatment | SA-K4414 | SA-K4100 |
|---|---|---|
| Ampicillin | ≥1024 μg/mL | 128 μg/mL |
| Ampicillin + Sulbactam | 128 μg/mL | 16 μg/mL |
| Ampicillin + CCCP (MIC/8) | ≥1024 μg/mL | 128 μg/mL |
| Ampicillin + Sulbactam + CCCP (MIC/8) | 128 μg/mL | 16 μg/mL |
| Compound | Structure | Compound Name | Yield % | Melting Point °C |
|---|---|---|---|---|
| Naph 1 | 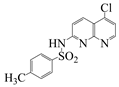 | 4-methyl-N-(5-chloro-1,8-naphthyridin-2-yl)- benzenesulfonamide | 84 | 249–250 |
| Naph 2 |  | 2.5-dichloro-N-(5-chloro-1,8-naphthyridin-2-yl)-benzenesulfonamide | 70.8 | 247.5–248 |
| Naph 3 | 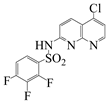 | 2,3,4-trifluoro-N-(5- chloro-1,8-naphthyridin-2-yl)-benzenesulfonamide | 56.5 | 198.6–199.6 |
| Naph 4 | 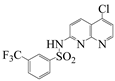 | 3-trifluoromethyl-N-(5-chloro-1,8-naphthyridin-2-yl)-benzenesulfonamide | 52.6 | 222.2–224 |
| 1,8-naphthyridinone |  | 7-acetamido-1,8-naphthyridin-4(1H)-one | - | 298–299 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira-Tintino, C.D.d.M.; Tintino, S.R.; Justino de Araújo, A.C.; dos Santos Barbosa, C.R.; Ramos Freitas, P.; Araújo Neto, J.B.d.; Begnini, I.M.; Rebelo, R.A.; Silva, L.E.d.; Mireski, S.L.; et al. Efflux Pump (QacA, QacB, and QacC) and β-Lactamase Inhibitors? An Evaluation of 1,8-Naphthyridines against Staphylococcus aureus Strains. Molecules 2023, 28, 1819. https://doi.org/10.3390/molecules28041819
Oliveira-Tintino CDdM, Tintino SR, Justino de Araújo AC, dos Santos Barbosa CR, Ramos Freitas P, Araújo Neto JBd, Begnini IM, Rebelo RA, Silva LEd, Mireski SL, et al. Efflux Pump (QacA, QacB, and QacC) and β-Lactamase Inhibitors? An Evaluation of 1,8-Naphthyridines against Staphylococcus aureus Strains. Molecules. 2023; 28(4):1819. https://doi.org/10.3390/molecules28041819
Chicago/Turabian StyleOliveira-Tintino, Cícera Datiane de Morais, Saulo Relison Tintino, Ana Carolina Justino de Araújo, Cristina Rodrigues dos Santos Barbosa, Priscilla Ramos Freitas, José Bezerra de Araújo Neto, Iêda Maria Begnini, Ricardo Andrade Rebelo, Luiz Everson da Silva, Sandro Lucio Mireski, and et al. 2023. "Efflux Pump (QacA, QacB, and QacC) and β-Lactamase Inhibitors? An Evaluation of 1,8-Naphthyridines against Staphylococcus aureus Strains" Molecules 28, no. 4: 1819. https://doi.org/10.3390/molecules28041819
APA StyleOliveira-Tintino, C. D. d. M., Tintino, S. R., Justino de Araújo, A. C., dos Santos Barbosa, C. R., Ramos Freitas, P., Araújo Neto, J. B. d., Begnini, I. M., Rebelo, R. A., Silva, L. E. d., Mireski, S. L., Nasato, M. C., Krautler, M. I. L., Barreto, H. M., Ribeiro-Filho, J., de Menezes, I. R. A., & Coutinho, H. D. M. (2023). Efflux Pump (QacA, QacB, and QacC) and β-Lactamase Inhibitors? An Evaluation of 1,8-Naphthyridines against Staphylococcus aureus Strains. Molecules, 28(4), 1819. https://doi.org/10.3390/molecules28041819










