Phytochemicals from Vanda bensonii and Their Bioactivities to Inhibit Growth and Metastasis of Non-Small Cell Lung Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Key Phytochemicals Isolated from V. bensonii
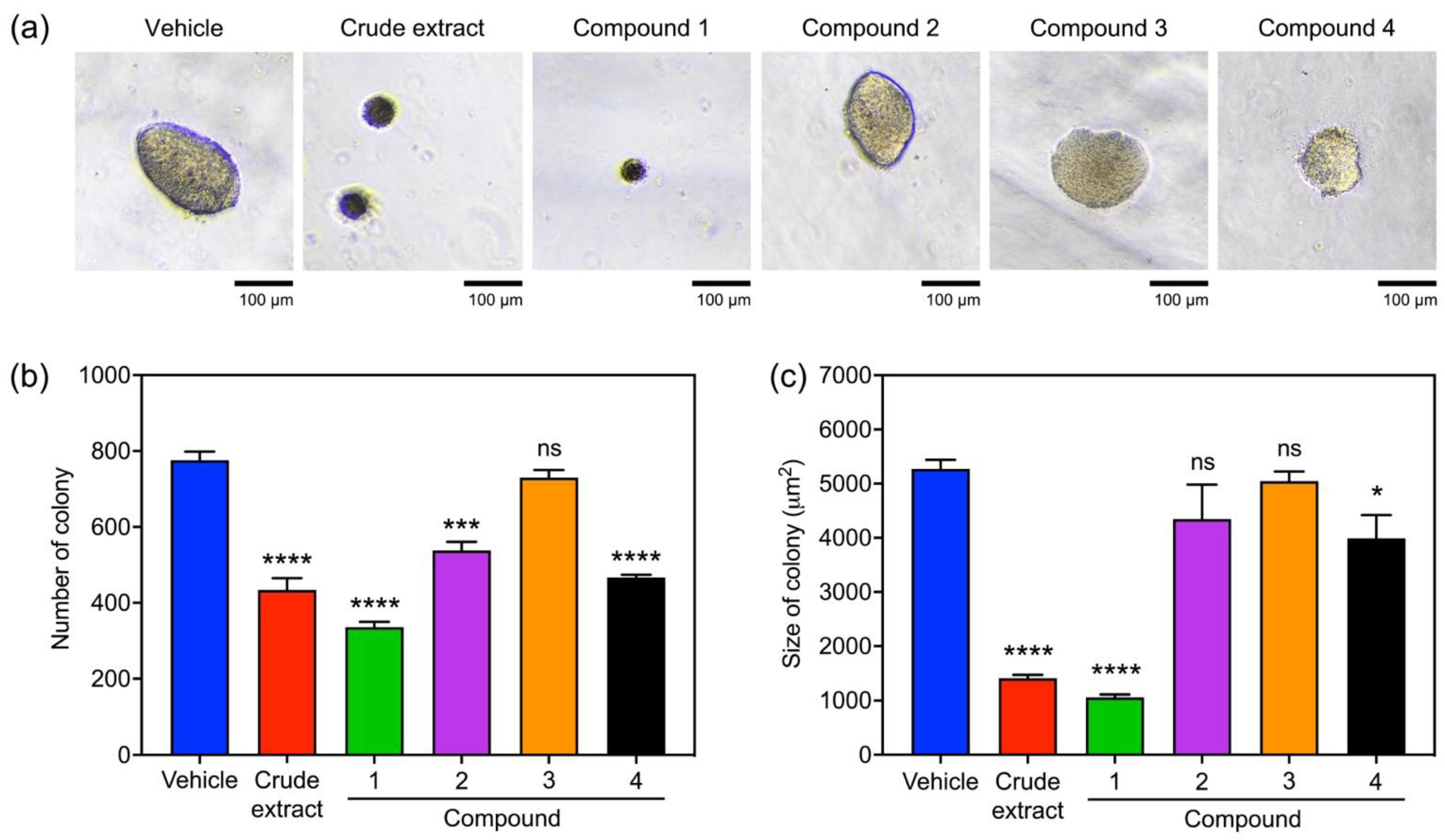
2.2. The Orchid Metabolites Exhibit a Broad Spectrum of Anticancer Activities
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chemicals and Reagents
4.3. Extraction, Fractionation, and Structural Elucidation of Key Phytochemicals
4.4. Cell Culture and Growth Conditions
4.5. Cell Viability Assay
4.6. Cell Proliferation Assay
4.7. Wound-Healing Assay
4.8. Soft Agar Colony Formation Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gardiner, L.M.; Kocyan, A.; Motes, M. Molecular phylogenetics of Vanda and related genera (Orchidaceae). Bot. J. Linn. Soc. 2013, 173, 549–572. [Google Scholar] [CrossRef]
- Hossain, M.M. Therapeutic orchids: Traditional uses and recent advances—An overview. Fitoterapia 2011, 82, 102–140. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Belwal, T.; Tariq, M.; Atanasov, A.G.; Devkota, H.P. Genus Vanda: A review on traditional uses, bioactive chemical constituents and pharmacological activities. J. Ethnopharmacol. 2019, 229, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.K.; Mandal, D. The folklore medicinal orchids of Sikkim. Anc. Sci. Life 2013, 33, 92. [Google Scholar] [CrossRef] [PubMed]
- Deb, C.R.; Deb, M.S.; Jamir, N.S.; Imchen, T. Orchids in indigenous system of medicine in Nagaland, India. Pleione 2009, 3, 209–211. [Google Scholar]
- Manandhar, N.P. Plants and People of Nepal; Timber Press: Portland, OR, USA, 2002; p. 599. [Google Scholar]
- Begum, Y.; Kumer Sen, P.; Bulbul, I.J.; Nasrin, F. Evaluation of analgesic and anti-inflammatory potentials of the leaf and root extracts of Vanda roxburghii (Roxb). J. Compl. Altern. Med. Res. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Joshi, P.R.; Paudel, M.R.; Chand, M.B.; Pradhan, S.; Pant, K.K.; Joshi, G.P.; Bohara, M.; Wagner, S.H.; Pant, B.; Pant, B. Cytotoxic effect of selected wild orchids on two different human cancer cell lines. Heliyon 2020, 6, e03991. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- What Is Lung Cancer? Available online: https://www.cancer.org/cancer/lung-cancer/about/what-is.html (accessed on 11 October 2022).
- Tamura, T.; Kurishima, K.; Nakazawa, K.; Kagohashi, K.; Ishikawa, H.; Satoh, H.; Hizawa, N. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol. Clin. Oncol. 2015, 3, 217–221. [Google Scholar] [CrossRef]
- Zimmermann, S.; Dziadziuszko, R.; Peters, S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat. Rev. 2014, 40, 716–722. [Google Scholar] [CrossRef]
- Tanee, T.; Chadmuk, P.; Sudmoon, R.; Chaveerach, A.; Noikotr, K. Genetic analysis for identification, genomic template stability in hybrids and barcodes of the Vanda species (Orchidaceae) of Thailand. Afr. J. Biotechnol. 2012, 11, 11772–11781. [Google Scholar]
- Ecoy, G.A.U.; Chamni, S.; Suwanborirux, K.; Chanvorachote, P.; Chaotham, C. Jorunnamycin A from Xestospongia sp. suppresses epithelial to mesenchymal transition and sensitizes anoikis in human lung cancer cells. J. Nat. Prod. 2019, 82, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Yang, X.W.; Zhang, Y.B.; Zhai, Y.Y.; Xu, W.; Zhao, B.; Liu, D.L.; Yu, H.J. Biotransformation of phlorizin by human intestinal flora and inhibition of biotransformation products on tyrosinase activity. Food Chem. 2012, 132, 936–942. [Google Scholar] [CrossRef]
- Metcalf, R.L.; Kogan, M. Plant volatiles as insect attractants. Crit. Rev. Plant Sci. 1987, 5, 251–301. [Google Scholar] [CrossRef]
- Barua, A.K.; Ghosh, B.B.; Ray, S.; Patra, A. Cymbinodin-A, a phenanthraquinone from Cymbidium aloifolium. Phytochemistry 1990, 29, 3046–3047. [Google Scholar] [CrossRef]
- Lertnitikul, N.; Pattamadilok, C.; Chansriniyom, C.; Suttisri, R. A new dihydrophenanthrene from Cymbidium finlaysonianum and structure revision of cymbinodin-A. J. Asian Nat. Prod. Res. 2020, 22, 83–90. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhang, L.; Chen, X.; Pan, Y.; Chen, S.S.; Zhang, S.; Wang, Z.; Xiao, W.; Yang, L.; et al. Systems pharmacology to decipher the combinational anti-migraine effects of Tianshu formula. J. Ethnopharmacol. 2015, 174, 45–56. [Google Scholar] [CrossRef]
- Axiotis, E.; Angelis, A.; Antoniadi, L.; Petrakis, E.; Skaltsounis, L.A. Phytochemical analysis and dermo-cosmetic evaluation of Cymbidium Sw. cultivation by-products. Circular economy in North Aegean Island of Samos. Planta Med. 2021, 87, 1288. [Google Scholar]
- Yoshikawa, K.; Ito, T.; Iseki, K.; Baba, C.; Imagawa, H.; Yagi, Y.; Morita, H.; Asakawa, Y.; Kawano, S.; Hashimoto, T. Phenanthrene derivatives from Cymbidium Great Flower Marie Laurencin and their biological activities. J. Nat. Prod. 2012, 75, 605–609. [Google Scholar] [CrossRef]
- Masuda, Y.; Suzuki, R.; Sakagami, H.; Umemura, N.; Shirataki, Y. Novel cytotoxic phenanthrenequinone from Odontioda Marie Noel ‘Velano’. Chem. Pharm. Bull. 2012, 60, 1216–1219. [Google Scholar] [CrossRef]
- Benahmed, M.; Akkal, S.; Elomri, A.; Laouer, H.; Vérité, P.; Seguin, E. Constituents from Bupleurum montanum (Coss. & Dur.) (Apiaceae). Arab. J. Chem. 2014, 7, 1065–1069. [Google Scholar]
- Gallage, N.J.; Møller, B.L. Vanillin–bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Mol. Plant 2015, 8, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.N.; Khang, D.T.; Tuyen, P.T.; Minh, L.T.; Anh, L.H.; Quan, N.V.; Ha, P.T.T.; Quan, N.T.; Toan, N.P.; Elzaawely, A.A.; et al. Phenolic compounds and antioxidant activity of Phalaenopsis orchid hybrids. Antioxidants 2016, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.; Kumar, V.; Grúz, J.; Doležal, K.; Van Staden, J. Deciphering the phenolic acid reserves and antioxidant activity within the protocorm like bodies of Ansellia africana: A vulnerable medicinal orchid. Ind. Crops Prod. 2019, 135, 21–29. [Google Scholar] [CrossRef]
- Kotiloğlu, D.; Acet, T.; Özcan, K. Phytochemical profile and biological activity of a therapeutic orchid from Anatolia: Dactylorhiza romana subsp. georgica. J. Food Meas. Charact. 2020, 14, 3310–3318. [Google Scholar] [CrossRef]
- Vaz, J.A.; Almeida, G.M.; Ferreira, I.C.; Martins, A.; Vasconcelos, M.H. Clitocybe alexandri extract induces cell cycle arrest and apoptosis in a lung cancer cell line: Identification of phenolic acids with cytotoxic potential. Food Chem. 2012, 132, 482–486. [Google Scholar] [CrossRef]
- Guadamillas, M.C.; Cerezo, A.; Del Pozo, M.A. Overcoming anoikis–pathways to anchorage-independent growth in cancer. J. Cell Sci. 2011, 124, 3189–3197. [Google Scholar] [CrossRef]
- Jimoh, T.O.; Costa, B.C.; Chansriniyom, C.; Chaotham, C.; Chanvorachote, P.; Rojsitthisak, P.; Likhitwitayawuid, K.; Sritularak, B. Three new dihydrophenanthrene derivatives from Cymbidium ensifolium and their cytotoxicity against cancer cells. Molecules 2022, 27, 2222. [Google Scholar] [CrossRef]
- Tsao, S.M.; Hsia, T.C.; Yin, M.C. Protocatechuic acid inhibits lung cancer cells by modulating FAK, MAPK, and NF-κ B pathways. Nutr. Cancer 2014, 66, 1331–1341. [Google Scholar] [CrossRef]
- Marks, D.C.; Belov, L.; Davey, M.W.; Davey, R.A.; Kidman, A.D. The MTT cell viability assay for cytotoxicity testing in multidrug-resistant human leukemic cells. Leuk. Res. 1992, 16, 1165–1173. [Google Scholar] [CrossRef]
- Khine, H.E.E.; Sungthong, R.; Sritularak, B.; Prompetchara, E.; Chaotham, C. Untapped pharmaceutical potential of 4, 5, 4′-trihydroxy-3, 3′-dimethoxybibenzyl for regulating obesity: A cell-based study with a focus on terminal differentiation in adipogenesis. J. Nat. Prod. 2022, 85, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.G.; Wu, X.; Guan, J.L. Wound-healing assay. In Cell Migration; Humana Press: Totowa, NJ, USA, 2005; pp. 23–29. [Google Scholar]
- Borowicz, S.; Van Scoyk, M.; Avasarala, S.; Rathinam, M.K.K.; Tauler, J.; Bikkavilli, R.K.; Winn, R.A. The soft agar colony formation assay. J. Vis. Exp. 2014, 92, e51998. [Google Scholar] [CrossRef] [PubMed]
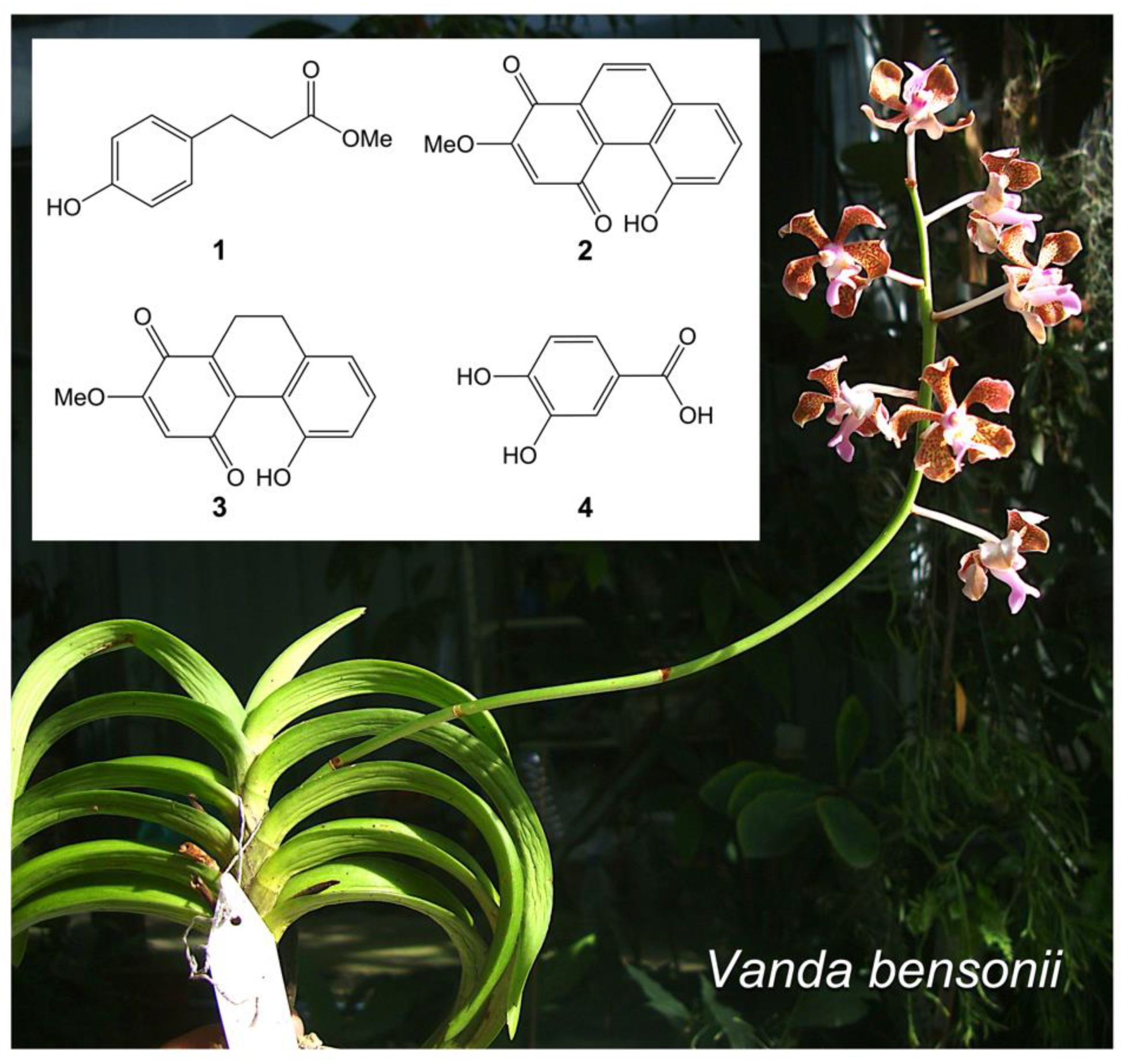
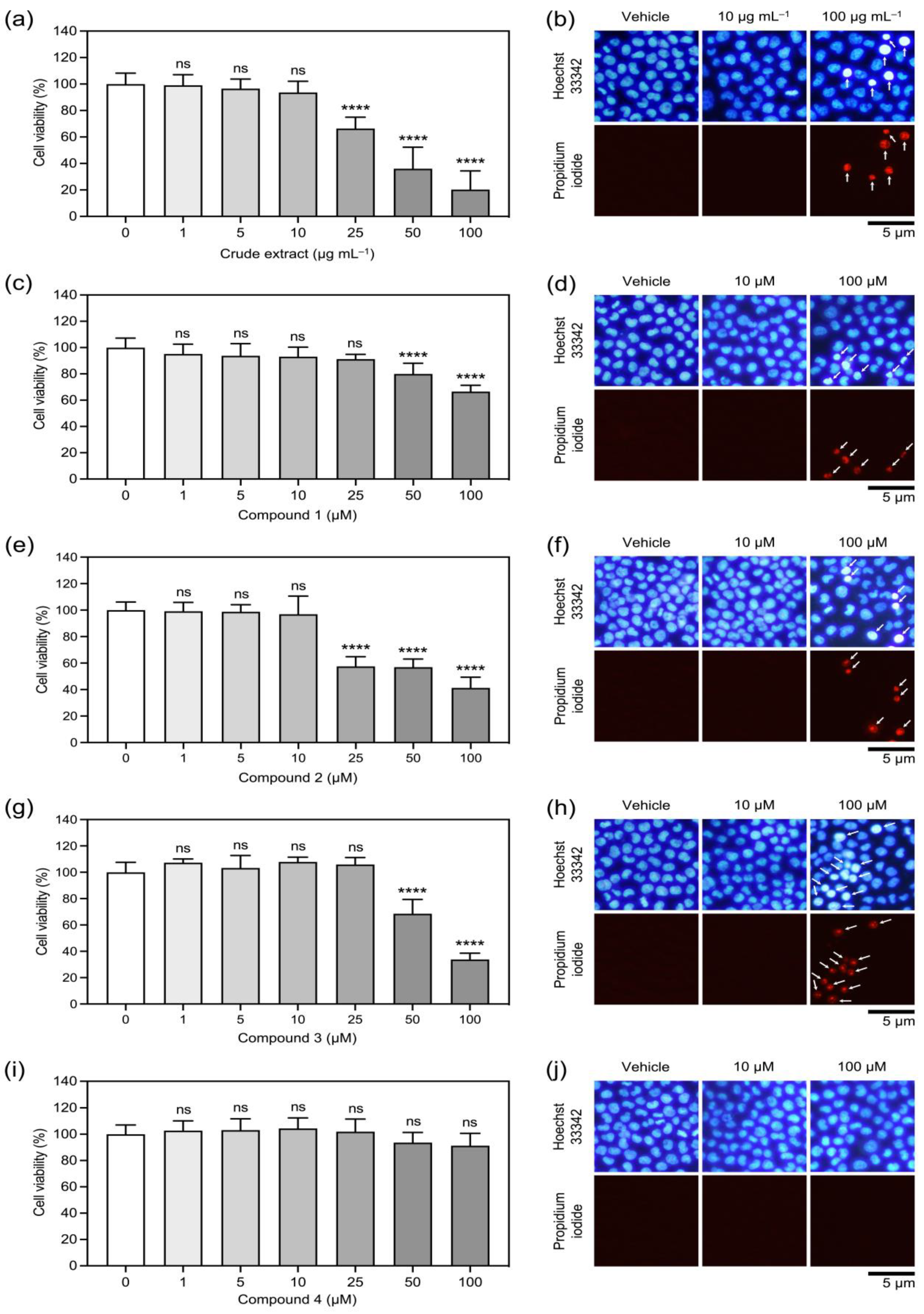
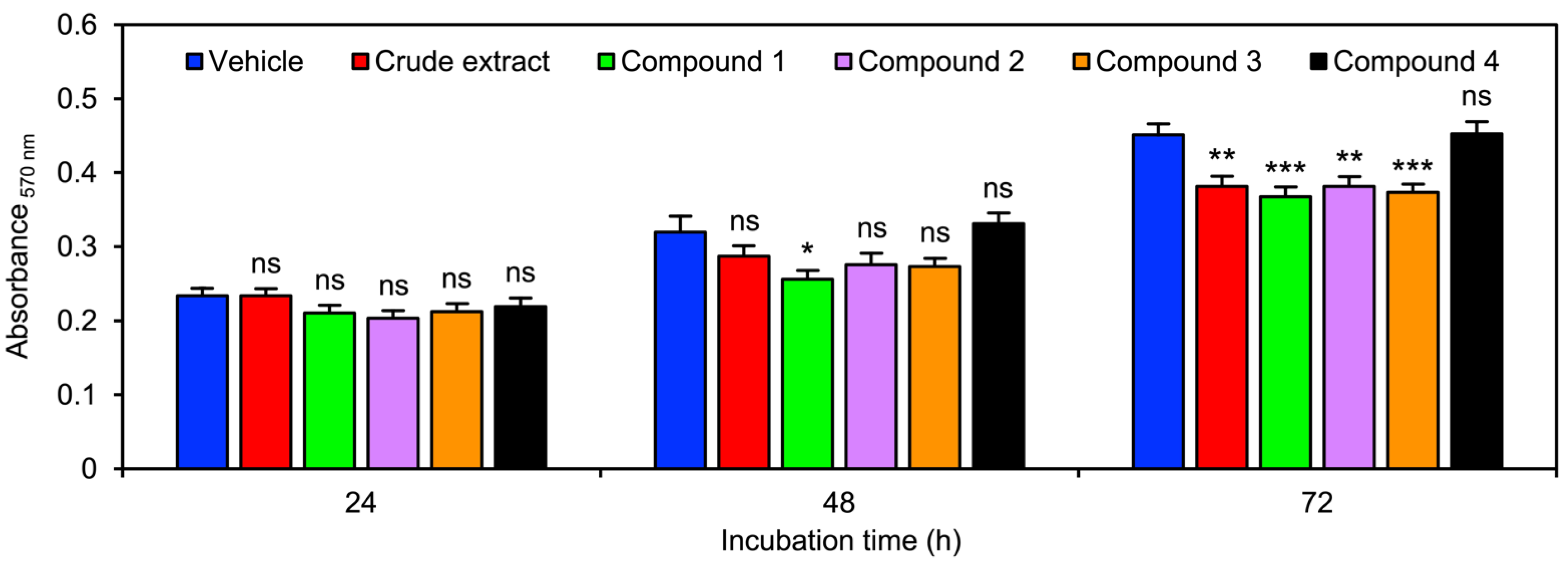
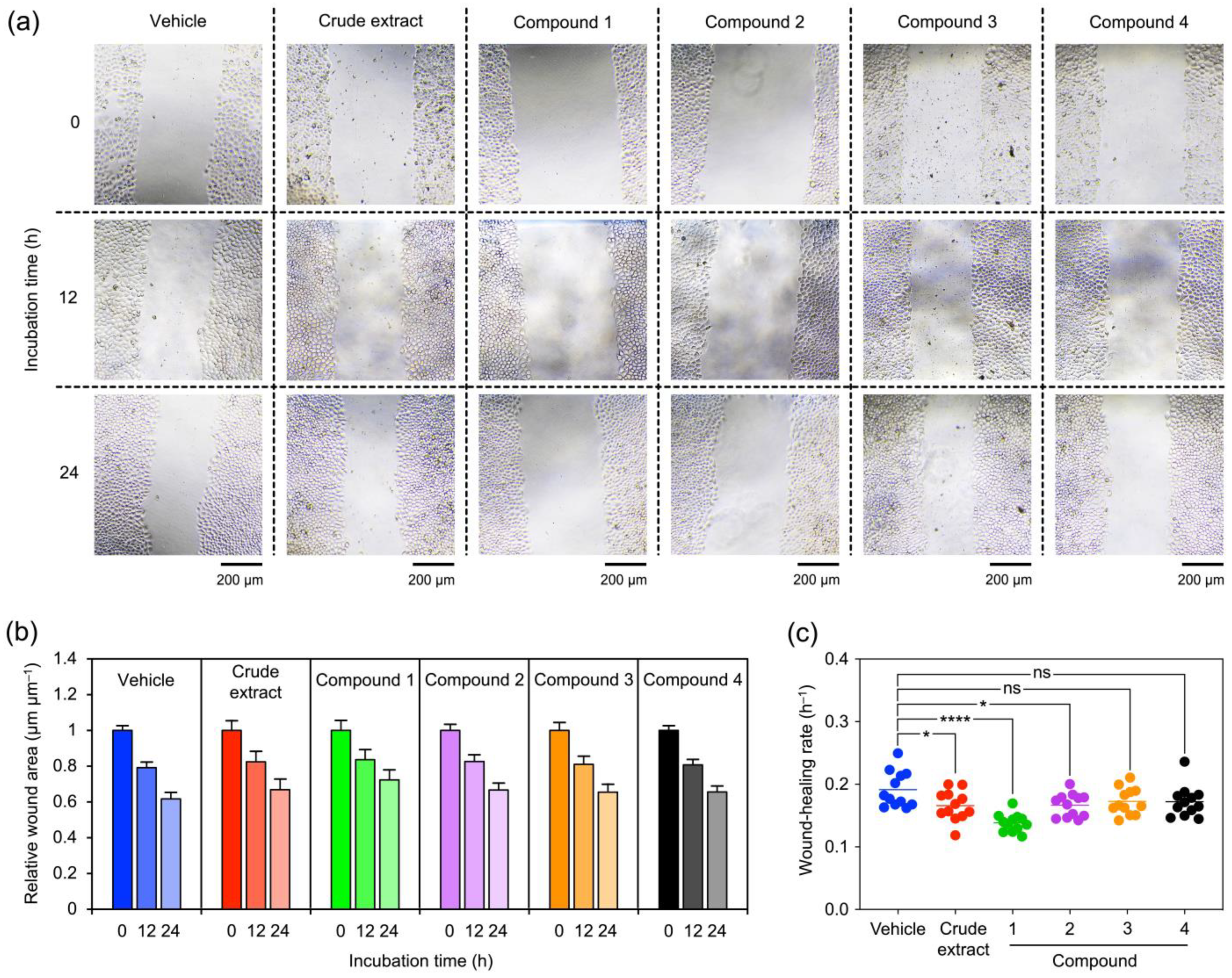
| Phytochemicals from V. bensonii | Absolute IC50 1 | Fold Change in Cell Proliferation 2 | Wound-Healing Rate (h−1) 3 | Anchorage Independence 4 | ||
|---|---|---|---|---|---|---|
| 48 h | 72 h | Fold Change in Survival | Fold Change in Growth | |||
| Crude methanolic extract | 40.39 μg mL−1 | 0.90 | 0.85 | 0.16 ± 0.02 | 0.56 | 0.27 |
| Phloretic acid methyl ester (1) | >100 μM | 0.80 | 0.81 | 0.14 ± 0.01 | 0.43 | 0.20 |
| Cymbinodin-A (2) | 50.82 μM | 0.86 | 0.85 | 0.17 ± 0.02 | 0.69 | 0.82 |
| Ephemeranthoquinone B (3) | 64.22 μM | 0.85 | 0.83 | 0.17 ± 0.02 | 0.94 | 0.96 |
| Protocatechuic acid (4) | >100 μM | 1.04 | 1.00 | 0.17 ± 0.02 | 0.60 | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimoh, T.O.; Nuamnaichati, N.; Sungthong, R.; Chansriniyom, C.; Chanvorachote, P.; Likhitwitayawuid, K.; Chaotham, C.; Sritularak, B. Phytochemicals from Vanda bensonii and Their Bioactivities to Inhibit Growth and Metastasis of Non-Small Cell Lung Cancer Cells. Molecules 2022, 27, 7902. https://doi.org/10.3390/molecules27227902
Jimoh TO, Nuamnaichati N, Sungthong R, Chansriniyom C, Chanvorachote P, Likhitwitayawuid K, Chaotham C, Sritularak B. Phytochemicals from Vanda bensonii and Their Bioactivities to Inhibit Growth and Metastasis of Non-Small Cell Lung Cancer Cells. Molecules. 2022; 27(22):7902. https://doi.org/10.3390/molecules27227902
Chicago/Turabian StyleJimoh, Tajudeen O., Narawat Nuamnaichati, Rungroch Sungthong, Chaisak Chansriniyom, Pithi Chanvorachote, Kittisak Likhitwitayawuid, Chatchai Chaotham, and Boonchoo Sritularak. 2022. "Phytochemicals from Vanda bensonii and Their Bioactivities to Inhibit Growth and Metastasis of Non-Small Cell Lung Cancer Cells" Molecules 27, no. 22: 7902. https://doi.org/10.3390/molecules27227902
APA StyleJimoh, T. O., Nuamnaichati, N., Sungthong, R., Chansriniyom, C., Chanvorachote, P., Likhitwitayawuid, K., Chaotham, C., & Sritularak, B. (2022). Phytochemicals from Vanda bensonii and Their Bioactivities to Inhibit Growth and Metastasis of Non-Small Cell Lung Cancer Cells. Molecules, 27(22), 7902. https://doi.org/10.3390/molecules27227902











