Orbital Polarization-Dependent Fragment Twist-Induced Intramolecular Electric-Field-Driven Charge Transfer
Abstract
1. Introduction
2. Results and Discussion
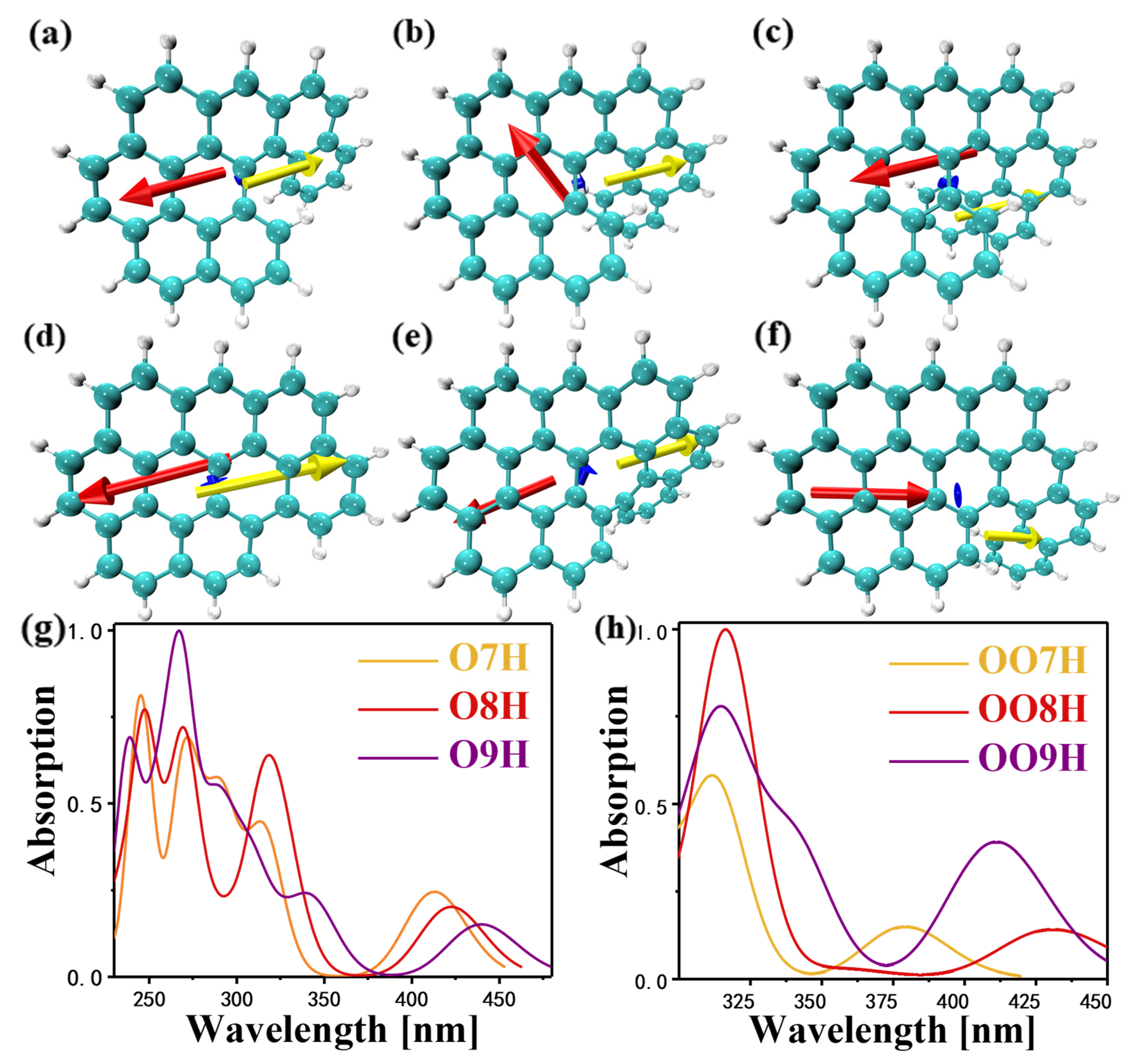
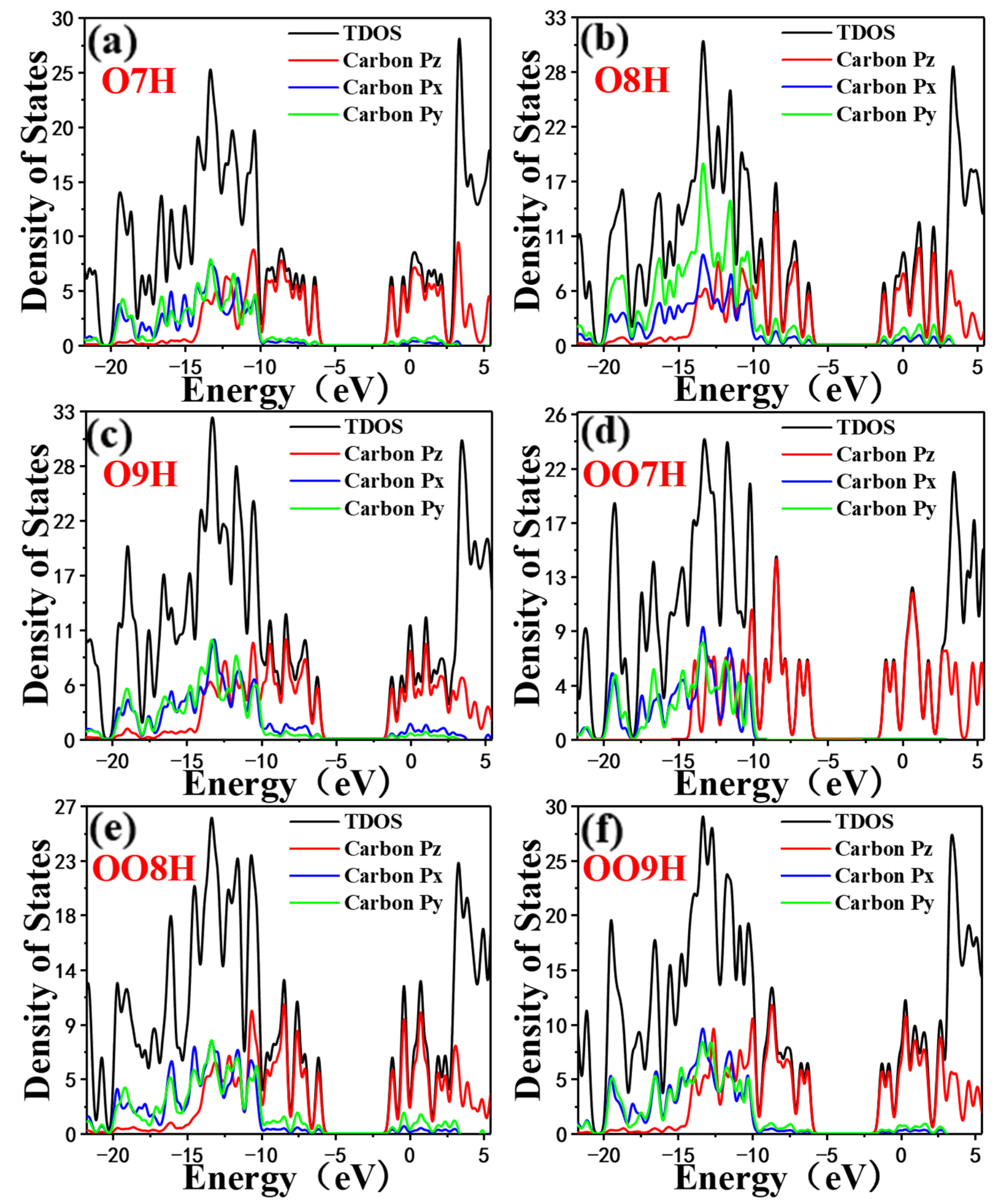
2.1. Inner Electric Field of Nanographene
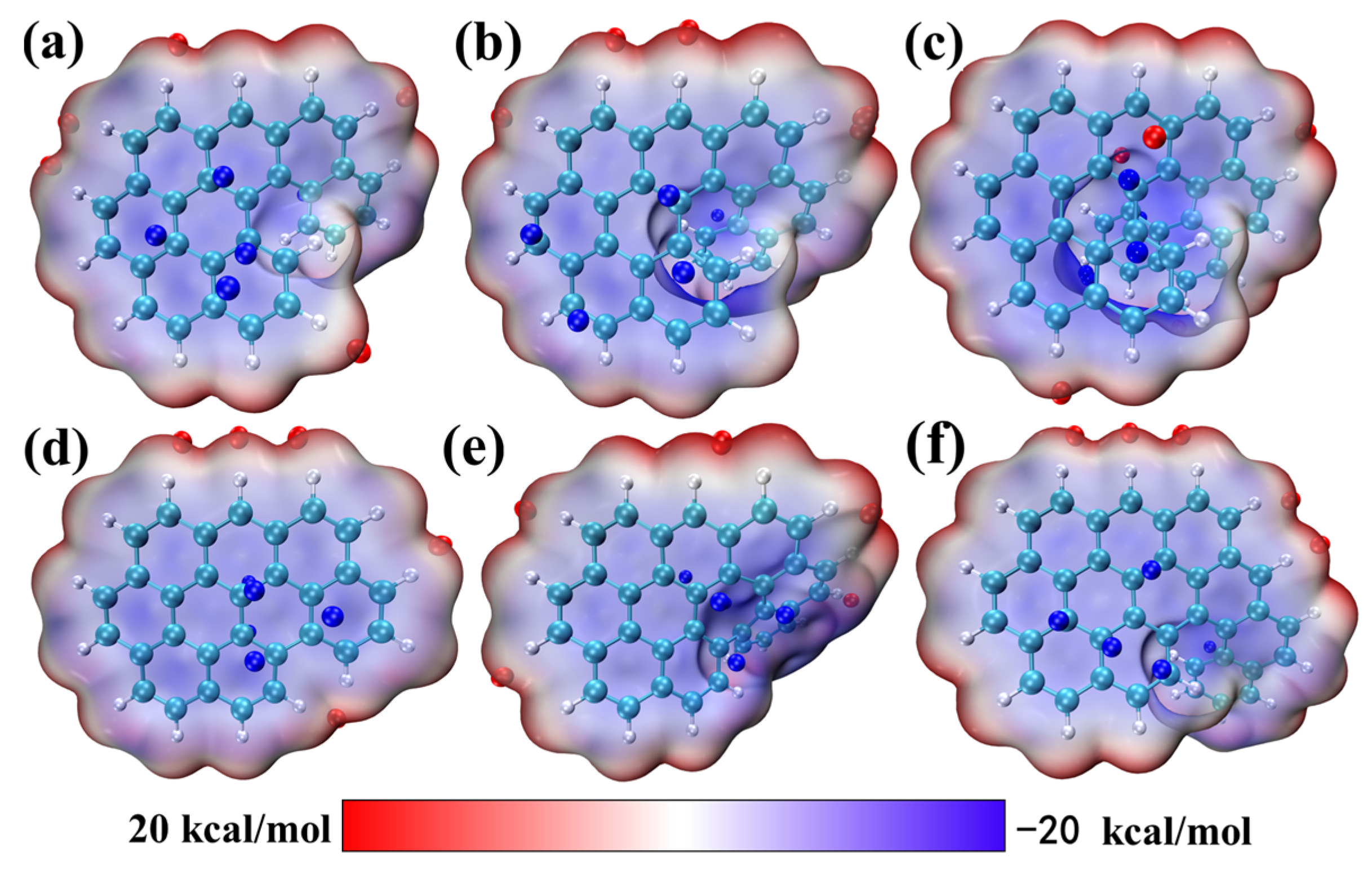
2.2. Electrostatic Potential
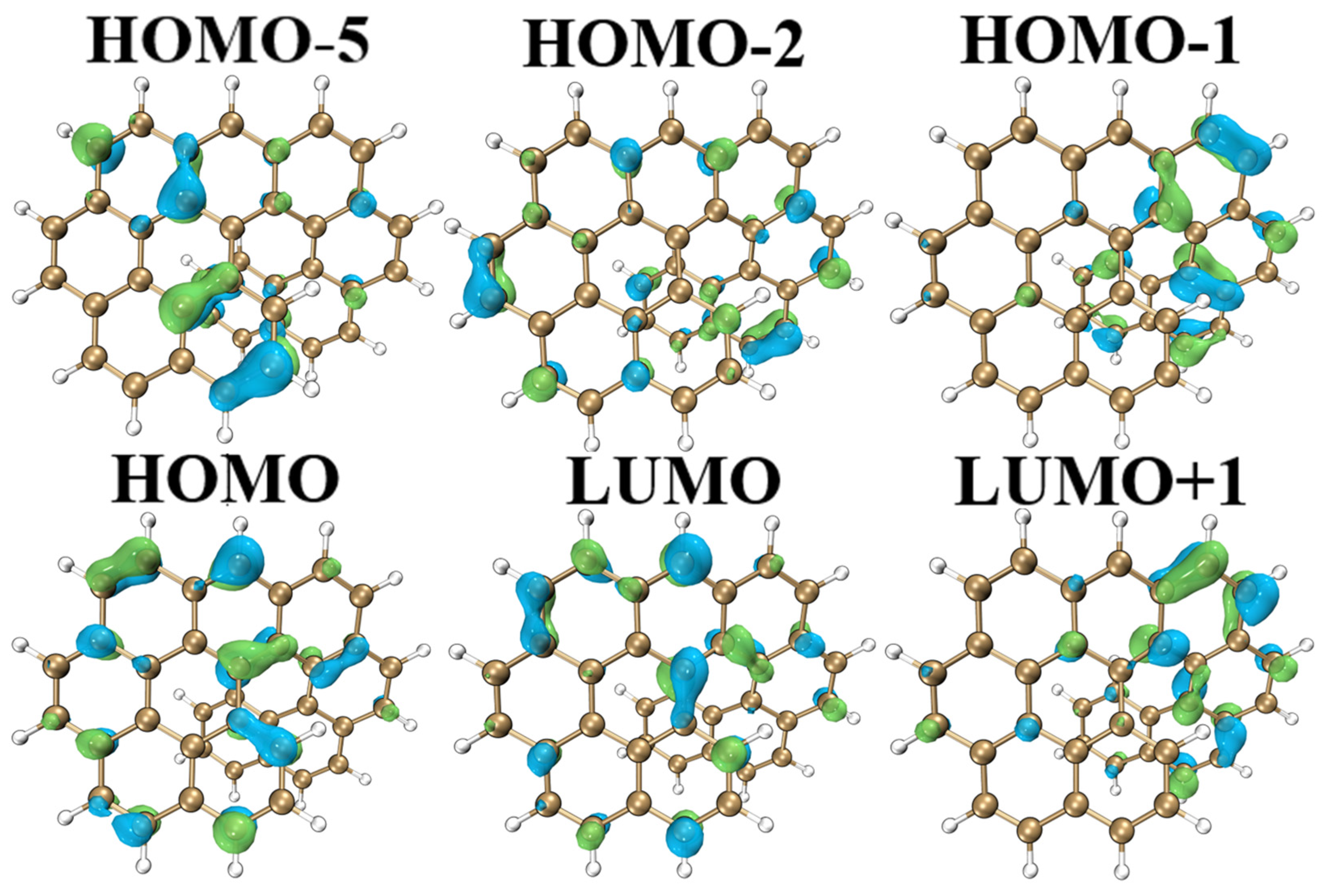
2.3. Molecular Orbital Analysis
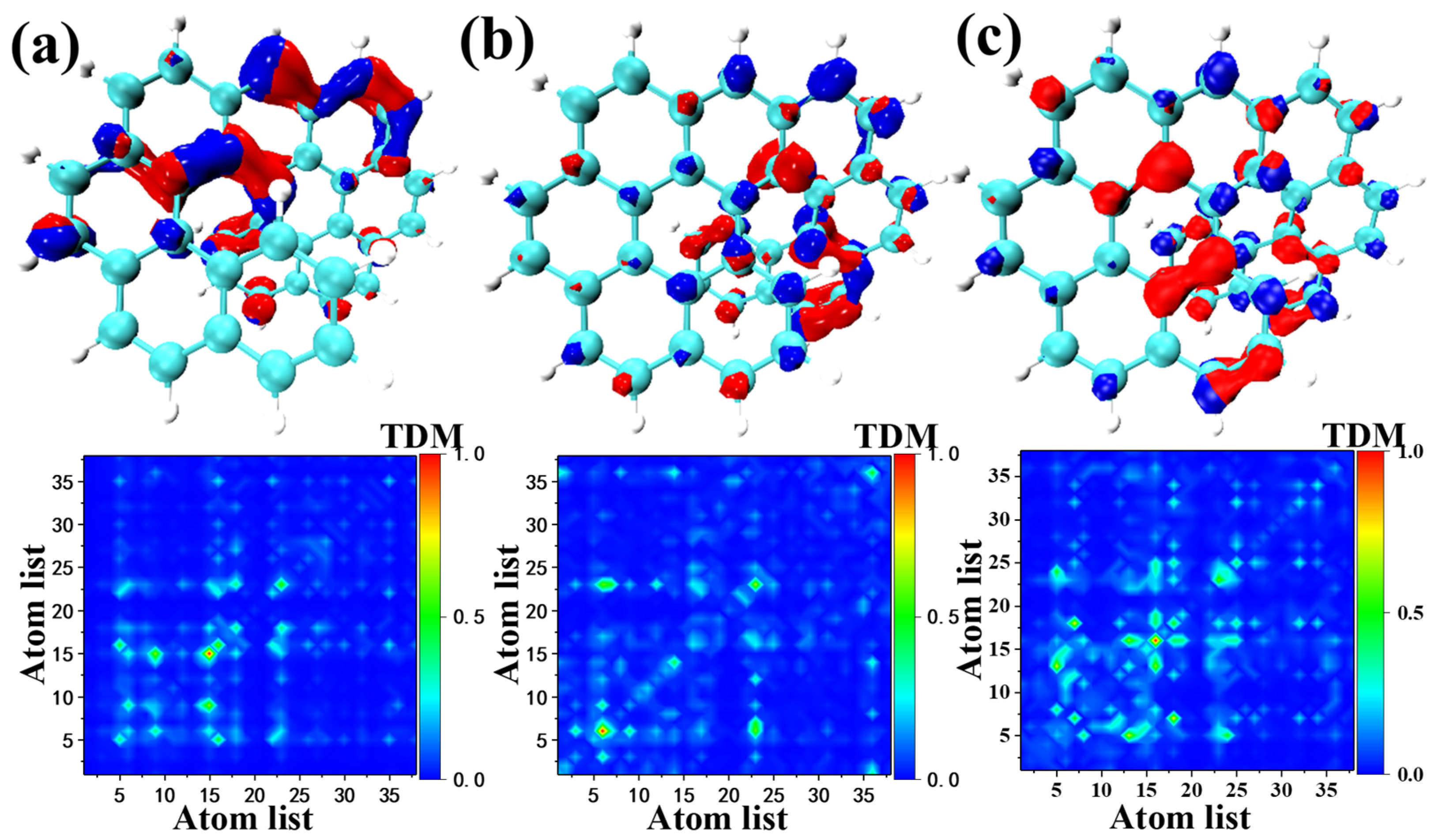
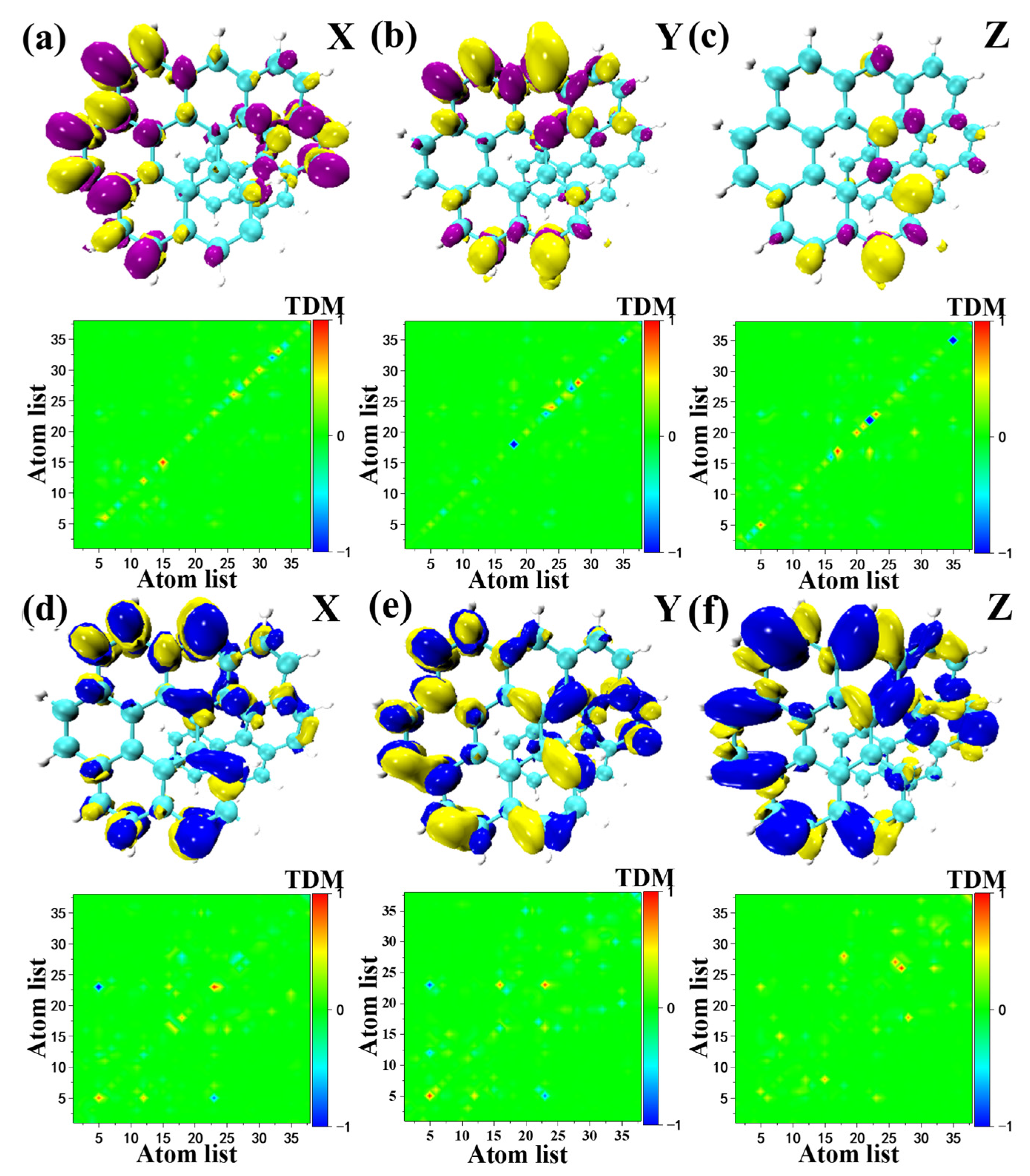
2.4. One Photon Electron-Hole Map (OPA) and Transition Density Matrix (TDM)
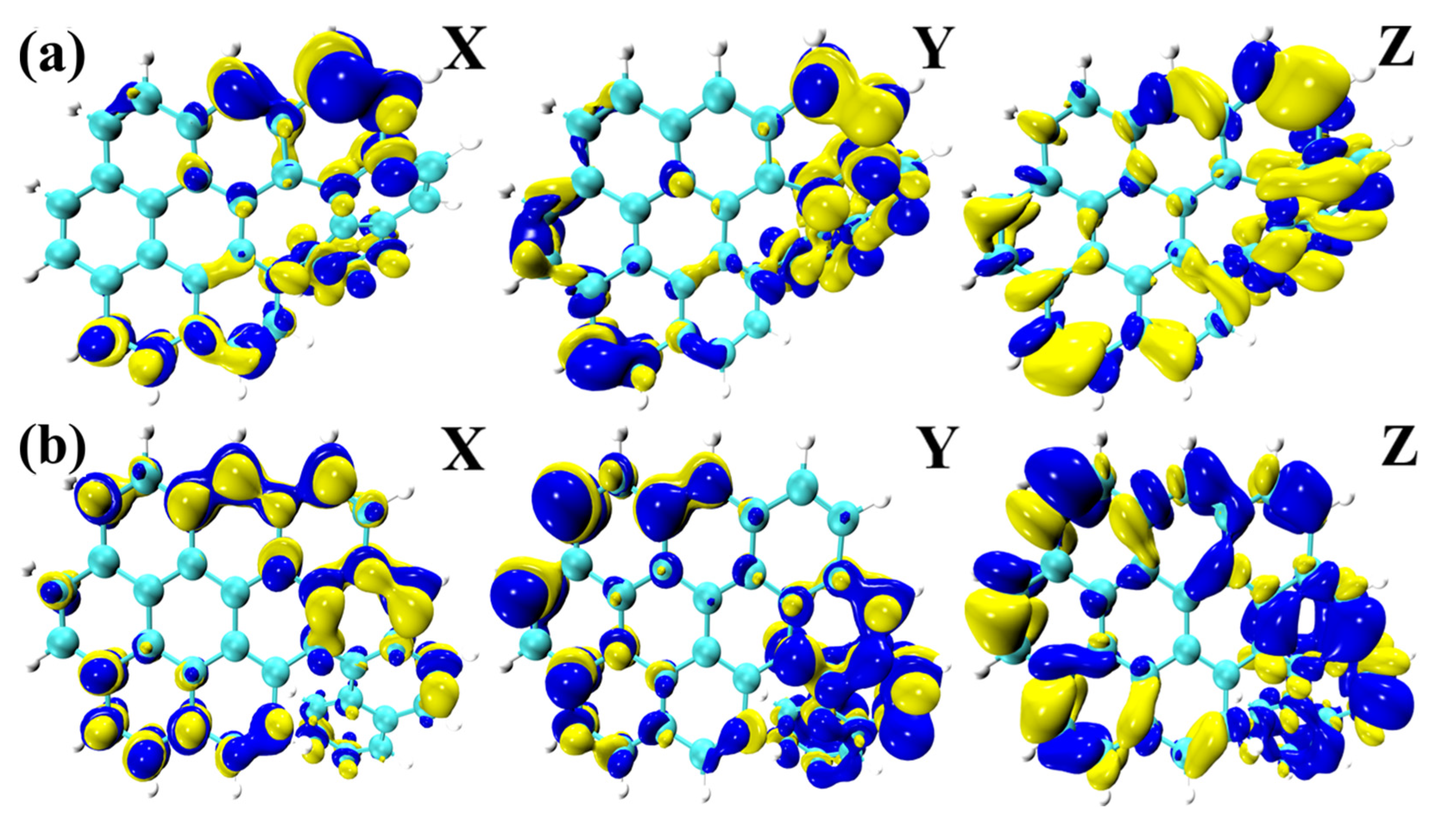
2.5. Transition Electric Dipole Moment (TEDM) and Transition Magnetic Dipole Moment (TMDM)
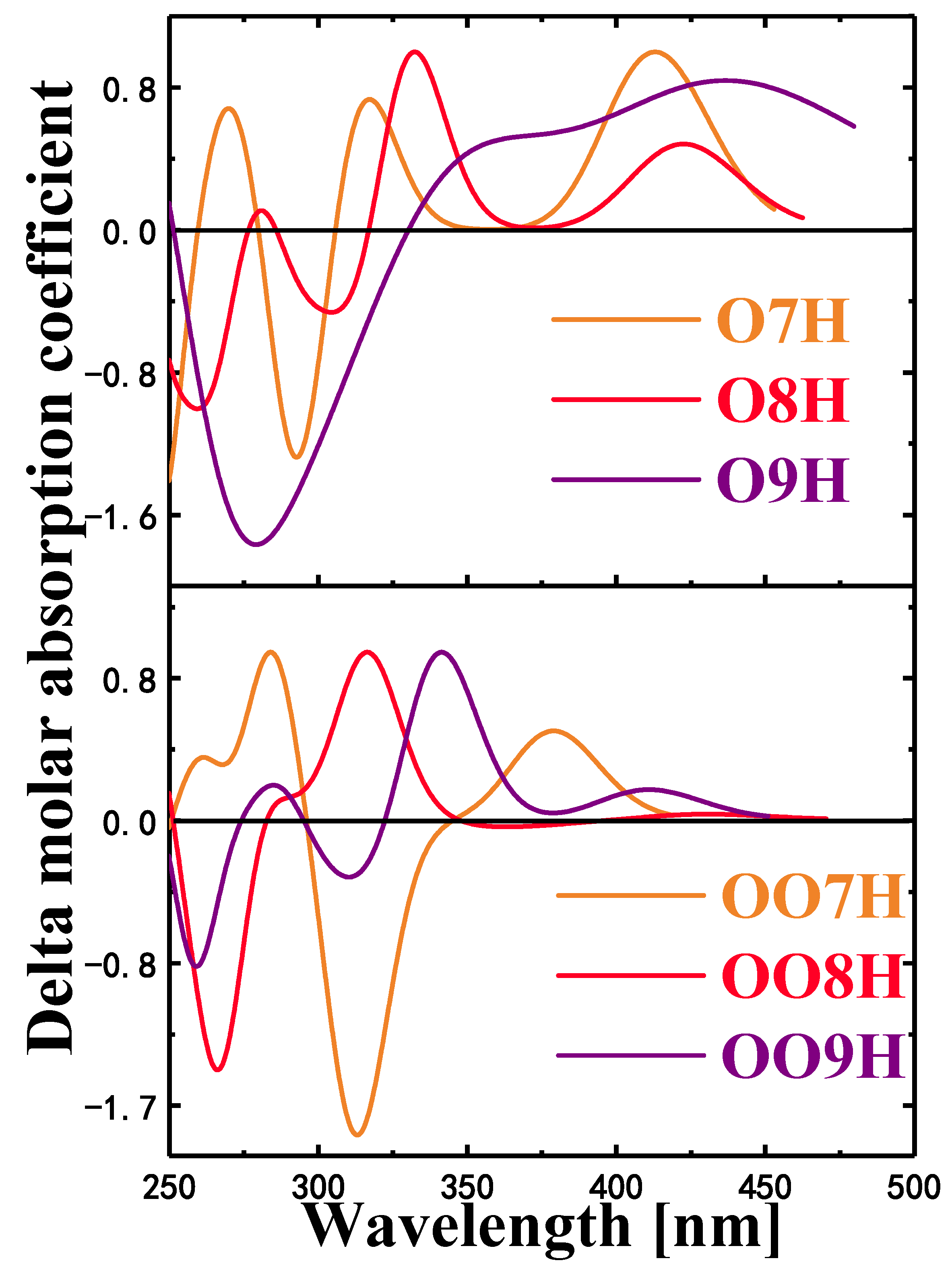
2.6. Circular Dichroism
3. Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jing, J.; Yang, J.; Zhang, Z.; Zhu, Y. Supramolecular Zinc Porphyrin Photocatalyst with Strong Reduction Ability and Robust Built-In Electric Field for Highly Efficient Hydrogen Production. Adv. Energy Mater. 2021, 11, 2101392. [Google Scholar] [CrossRef]
- Yang, J.; Jing, J.; Li, W.; Zhu, Y. Electron Donor–Acceptor Interface of TPPS/PDI Boosting Charge Transfer for Efficient Photocatalytic Hydrogen Evolution. Adv. Sci. 2022, 9, 2201134. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Yang, J.; Li, W.; Wu, Z.; Zhu, Y. Construction of Interfacial Electric Field via Dual-Porphyrin Heterostructure Boosting Photocatalytic Hydrogen Evolution. Adv. Mater. 2022, 34, 2106807. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xue, J.; Meng, L. Caffeine improves the performance and thermal stability of perovskite solar cells. Joule 2019, 3, 1464–1477. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-B.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- Chen, H.Y.; Hou, J.; Zhang, S.; Liang, Y.; Yang, G.; Yang, Y.; Yu, L.; Wu, Y.; Li, G. Polymer solar cells with enhanced open-circuit voltage and efficiency. Nat. Photonics 2009, 3, 649–653. [Google Scholar] [CrossRef]
- You, J.; Chen, C.-C.; Dou, L.; Murase, S.; Duan, H.-S.; Hawks, S.A.; Xu, T.; Son, H.J.; Yu, L.; Li, G.; et al. Metal oxide nanoparticles as an electron-transport layer in high-performance and stable inverted polymer solar cells. Adv. Mater. 2012, 24, 5267–5272. [Google Scholar] [CrossRef]
- Mu, X.; Mai, S.; Li, C.; Cao, J. Molecular Electric Field Regulation of Porphyrin/Phthalocyanine Optoelectronic Materials. J. Sci. Sin. Chim. 2022, 8, 1341–1356. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, L.; Mu, X.; Chen, C.C.; Wu, Y.; Cao, J.; Tang, Y. Intramolecular Electric Field Construction in Metal Phthalocyanine as Dopant-Free Hole Transporting Material for Stable Perovskite Solar Cells with >21% Efficiency. Angew. Chem. Int. Ed. 2021, 60, 6294–6299. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, B.; Li, Q. Confusion Approach Modulated Syntheses of Corrorin Parasitized Hexaphyrins (1.1. 1.1. 1.0) and the Oxidative Ring Cleavage Behavior. Org. Lett. 2021, 23, 8307–8311. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Kim, J.; Ishida, M.; Li, X.; Gu, T.; Liang, X.; Zhu, W.; Gren, H.; Kim, D.; et al. Regioselectively halogenated expanded porphyrinoids as building blocks for constructing porphyrin–porphyrinoid heterodyads with tunable energy transfer. J. Am. Chem. Soc. 2019, 141, 5294–5302. [Google Scholar] [CrossRef]
- Li, C.; Zhang, K.; Ishida, M.; Li, Q.; Shimomura, K.; Baryshnikov, G.; Li, X.; Savage, M.; Wu, X.; Yang, S.; et al. Tripyrrin-armed isosmaragdyrins: Synthesis, heterodinuclear coordination, and protonation-triggered helical inversion. Chem. Sci. 2020, 11, 2790–2795. [Google Scholar] [CrossRef]
- Cao, J.; Li, C.; Lv, X.; Feng, X.; Meng, R.; Wu, Y.; Tang, Y. Efficient Grain Boundary Suture by Low-Cost Tetra-ammonium Zinc Phthalocyanine for Stable Perovskite Solar Cells with Expanded Photoresponse. J. Am. Chem. Soc. 2018, 140, 11577–11580. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, B.; Zhang, X.; Jin, X.; Guo, K.; Zhou, D.; Bian, H.; Zhang, W.; Apfel, U.; Cao, R. Introducing Water-Network-Assisted Proton Transfer for Boosted Electrocatalytic Hydrogen Evolution with Cobalt Corrole. Angew. Chem. Int. Ed. 2022, 61, e202114310. [Google Scholar]
- Li, X.; Lv, B.; Zhang, X.; Jin, X.; Guo, K.; Zhou, D.; Bian, H.; Zhang, W.; Apfel, U.; Cao, R. Rücktitelbild: Introducing Water-Network-Assisted Proton Transfer for Boosted Electrocatalytic Hydrogen Evolution with Cobalt Corrole (Angew. Chem. 9/2022). Angew. Chem. 2022, 134, e202200909. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Han, T.; Cao, B.; Qiu, Z.; Li, Y.; Li, Q.; Hu, Y.; Liu, Z.; Lam, J.W.Y.; et al. In situ generation of azonia-containing polyelectrolytes for luminescent photopatterning and superbug killing. J. Am. Chem. Soc. 2019, 141, 11259–11268. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, L.; Lu, C.; Sun, Y.; Wang, J. Physical Mechanism of Spectra in Carbon Nanobelts under Quantum Size Effect. Nanomaterials 2022, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Feng, X.; Yang, Y.; Lv, X.; Cao, J.; Tang, Y. Cerium-oxide-modified anodes for efficient and UV-stable ZnO-based perovskite solar cells. ACS Appl. Mater. Interfaces 2019, 11, 13273–13278. [Google Scholar] [CrossRef]
- Xiao, G.B.; Wang, L.Y.; Mu, X.J.; Zou, X.X.; Wu, Y.Y.; Cao, J. Lead and iodide fixation by thiol copper (II) porphyrin for stable and environmental-friendly perovskite solar cells. CCS Chem. 2021, 3, 25–36. [Google Scholar] [CrossRef]
- Cao, J.; Lv, X.; Zhang, P.; Chuong, T.T.; Wu, B.; Feng, X.; Shan, C.; Liu, J.; Tang, Y. Plant Sunscreen and Co (II)/(III) Porphyrins for UV-Resistant and Thermally Stable Perovskite Solar Cells: From Natural to Artificial. Adv. Mater. 2018, 30, 1800568. [Google Scholar] [CrossRef]
- Wang, J.; Song, J.; Mu, X.; Sun, M. Optoelectronic and photoelectric properties and applications of graphene-based nanostructures. Mater. Today Phys. 2020, 13, 100196. [Google Scholar] [CrossRef]
- Lu, C.; Yu, J.; Sheng, H.; Jiang, Y.; Zhao, F.; Wang, J. Linear and Nonlinear Photon-Induced Cross Bridge/Space Charge Transfer in STC Molecular Crystals. Nanomaterials 2022, 12, 535. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, C.; Wang, L.; Wang, J. Angle-resolved One and Two-Photon Absorption spectrum in Twisted Bilayer Graphene Quantum Dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 271, 120894. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Wang, L.; Mu, X.; Wang, J. The magical photoelectric and optoelectronic properties of graphene nanoribbons and their applications. J. Mater. Chem. C 2021, 9, 13600–13616. [Google Scholar] [CrossRef]
- Bo, W.; Zou, Y.; Wang, J. Novel electrical properties and applications in kaleidoscopic graphene nanoribbons. RSC Adv. 2021, 11, 33675–33691. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Chang, K.; Peeters, F.M. Tuning of energy levels and optical properties of graphene quantum dots. Phys. Rev. B 2008, 77, 235411. [Google Scholar] [CrossRef]
- Zhou, G.D.; Duan, L.Y. Chemical Structure Basis.Beijing; Peking University Press: Beijing, China, 2008; pp. 133–137. [Google Scholar]
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. Molecular electrostatic potentials and noncovalent interactions. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 7, e1326. [Google Scholar] [CrossRef]
- Lu, T.; Manzetti, S. Wavefunction and reactivity study of benzo [a] pyrene diol epoxide and its enantiomeric forms. Struct. Chem. 2014, 25, 1521–1533. [Google Scholar] [CrossRef]
- Manzetti, S.; Lu, T. The geometry and electronic structure of Aristolochic acid: Possible implications for a frozen resonance. J. Phys. Org. Chem. 2013, 26, 473–483. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, T. Efficient evaluation of electrostatic potential with computerized optimized code. Phys. Chem. Chem. Phys. 2021, 23, 20323–20328. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. Molecular electrostatic potentials and chemical reactivity. Rev. Comput. Chem. 1991, 2, 273–312. [Google Scholar]
- Liu, Z.; Lu, T.; Chen, Q. Intermolecular interaction characteristics of the all-carboatomic ring, cyclo [18] carbon: Focusing on molecular adsorption and stacking. Carbon 2021, 171, 514–523. [Google Scholar] [CrossRef]
- Tian, L. Multiwfn Manual, version 3.7, Section 3.15.1. Available online: http://sobereva.com/multiwfn (accessed on 29 September 2020).
- Liu, Y.; Xue, Y.; Hui, L.; Yu, H.; Fang, Y.; He, F.; Li, Y. Porous graphdiyne loading CoOx quantum dots for fixation nitrogen reaction. Nano Energy 2021, 89, 106333. [Google Scholar] [CrossRef]
- Long, M.; Tang, L.; Wang, D.; Li, Y.; Shuai, Z. Electronic structure and carrier mobility in graphdiyne sheet and nanoribbons: Theoretical predictions. ACS Nano 2011, 5, 2593–2600. [Google Scholar] [CrossRef]
- Xue, Y.; Hui, L.; Yu, H.; Liu, Y.; Fang, Y.; Huang, B.; Zhao, Y.; Li, Z.; Li, Y. Rationally engineered active sites for efficient and durable hydrogen generation. Nat. Commun. 2019, 10, 2281. [Google Scholar] [CrossRef]
- Lin, Z.; Lu, T.; Ding, X.-L. A theoretical investigation on doping superalkali for triggering considerable nonlinear optical properties of Si12C12 nanostructure. J. Comput. Chem. 2017, 38, 1574–1582. [Google Scholar] [CrossRef]
- Chakraborty, D.; Das, R.; Chattaraj, P.K. Change in optoelectronic properties of ExBox+4 on functionalization and guest encapsulation. Phys. Chem. Chem. Phys. 2017, 19, 23373–23385. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Chen, Q. An sp-hybridized all-carboatomic ring, cyclo [18] carbon: Electronic structure, electronic spectrum, and optical nonlinearity. Carbon 2020, 165, 461–467. [Google Scholar] [CrossRef]
- Ortega Lorenzo, M.; Baddeley, C.J.; Muryn, C.; Raval, R. Extended surface chirality from supramolecular assemblies of adsorbed chiral molecules. Nature 2000, 404, 376. [Google Scholar] [CrossRef]
- Bentley, R. Role of sulfur chirality in the chemical processes of biology. Chem. Soc. Rev. 2005, 34, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G. Nakatsuji, Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Weigend, F.; Furche, F.; Ahlrichs, R. Gaussian basis sets of quadruple zeta valence quality for atoms H–Kr. J. Chem. Phys. 2003, 119, 12753–12762. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Furche, F.; Ahlrichs, R. Adiabatic time-dependent density functional methods for excited state properties. J. Chem. Phys. 2002, 117, 7433–7447. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange−correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bo, W.; Sheng, H.; Wang, J. Orbital Polarization-Dependent Fragment Twist-Induced Intramolecular Electric-Field-Driven Charge Transfer. Molecules 2023, 28, 1801. https://doi.org/10.3390/molecules28041801
Bo W, Sheng H, Wang J. Orbital Polarization-Dependent Fragment Twist-Induced Intramolecular Electric-Field-Driven Charge Transfer. Molecules. 2023; 28(4):1801. https://doi.org/10.3390/molecules28041801
Chicago/Turabian StyleBo, Wenjing, Hao Sheng, and Jingang Wang. 2023. "Orbital Polarization-Dependent Fragment Twist-Induced Intramolecular Electric-Field-Driven Charge Transfer" Molecules 28, no. 4: 1801. https://doi.org/10.3390/molecules28041801
APA StyleBo, W., Sheng, H., & Wang, J. (2023). Orbital Polarization-Dependent Fragment Twist-Induced Intramolecular Electric-Field-Driven Charge Transfer. Molecules, 28(4), 1801. https://doi.org/10.3390/molecules28041801





