One-Pot Synthesis of Melamine Formaldehyde Resin-Derived N-Doped Porous Carbon for CO2 Capture Application
Abstract
:1. Introduction
2. Results and Discussion
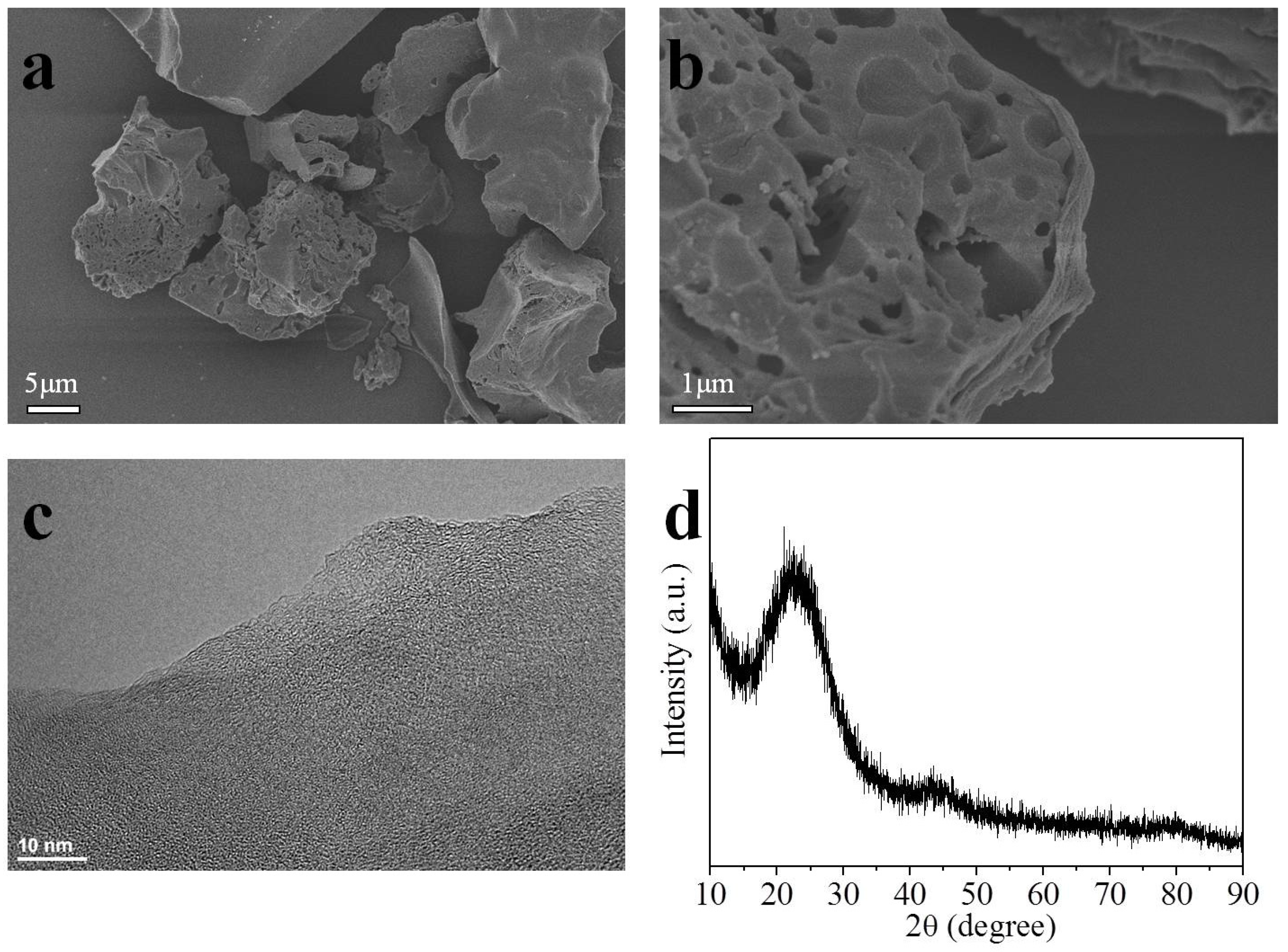
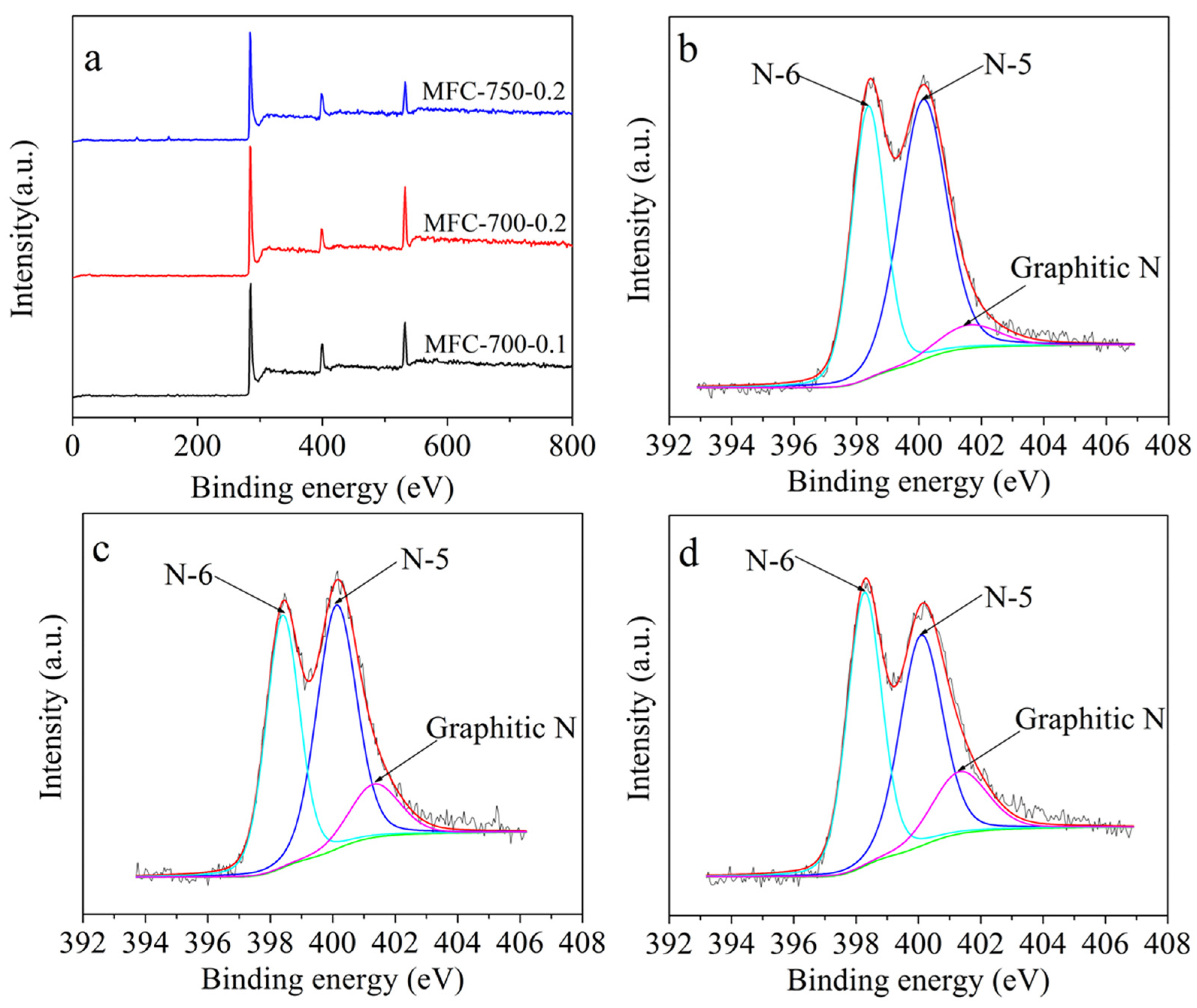
2.1. Morphological, Phase Structural, and Surface Chemical Properties
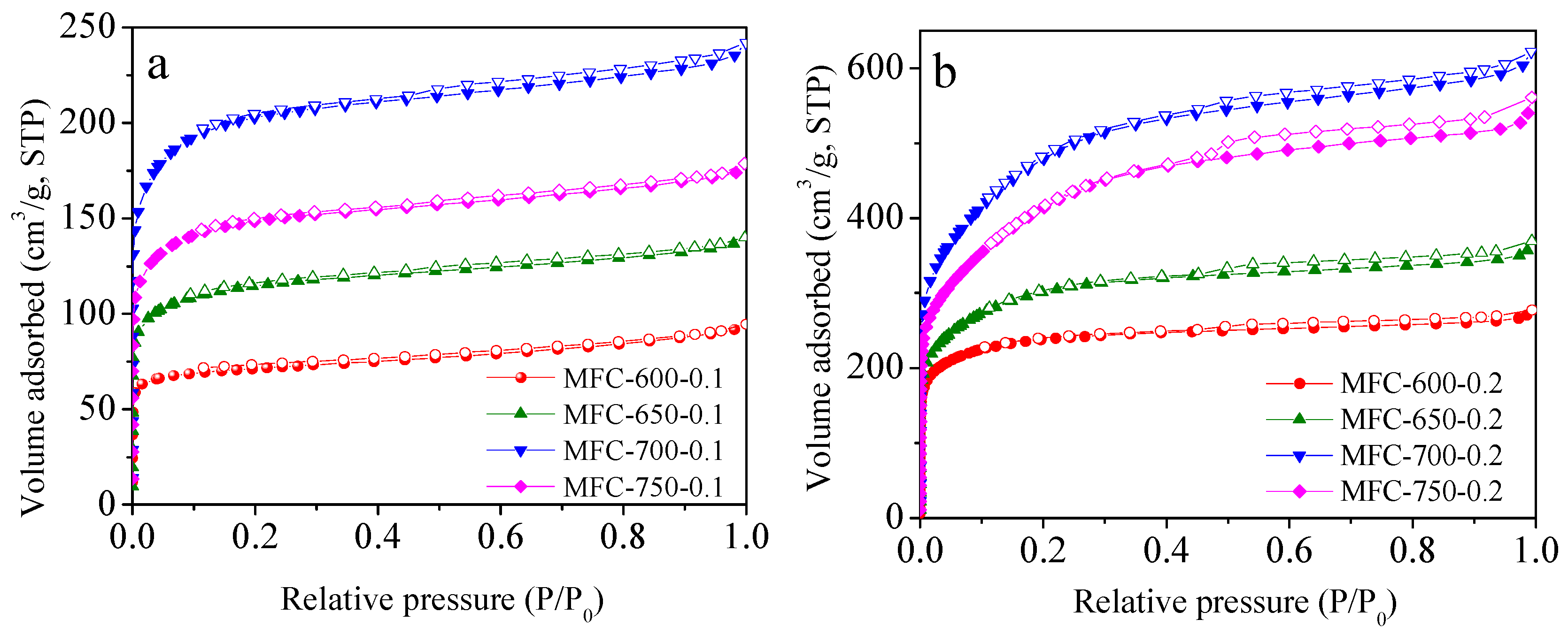
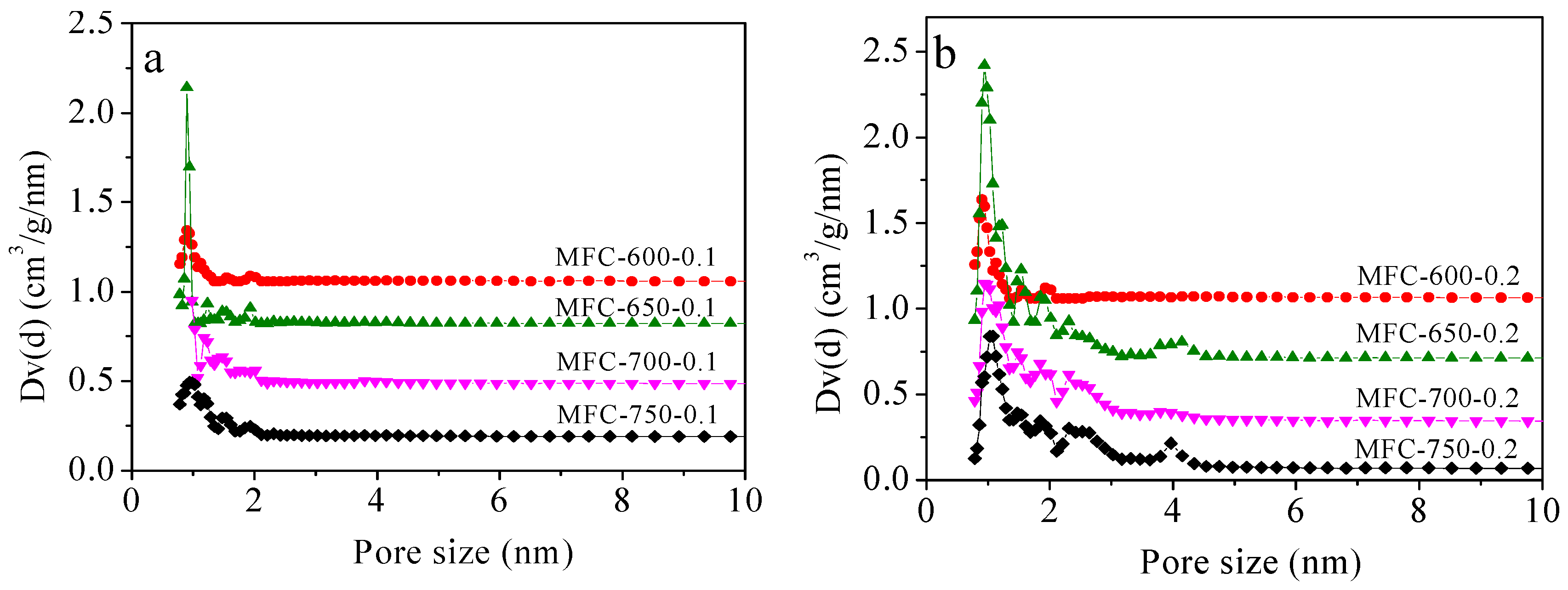
2.2. Porous Textual Properties
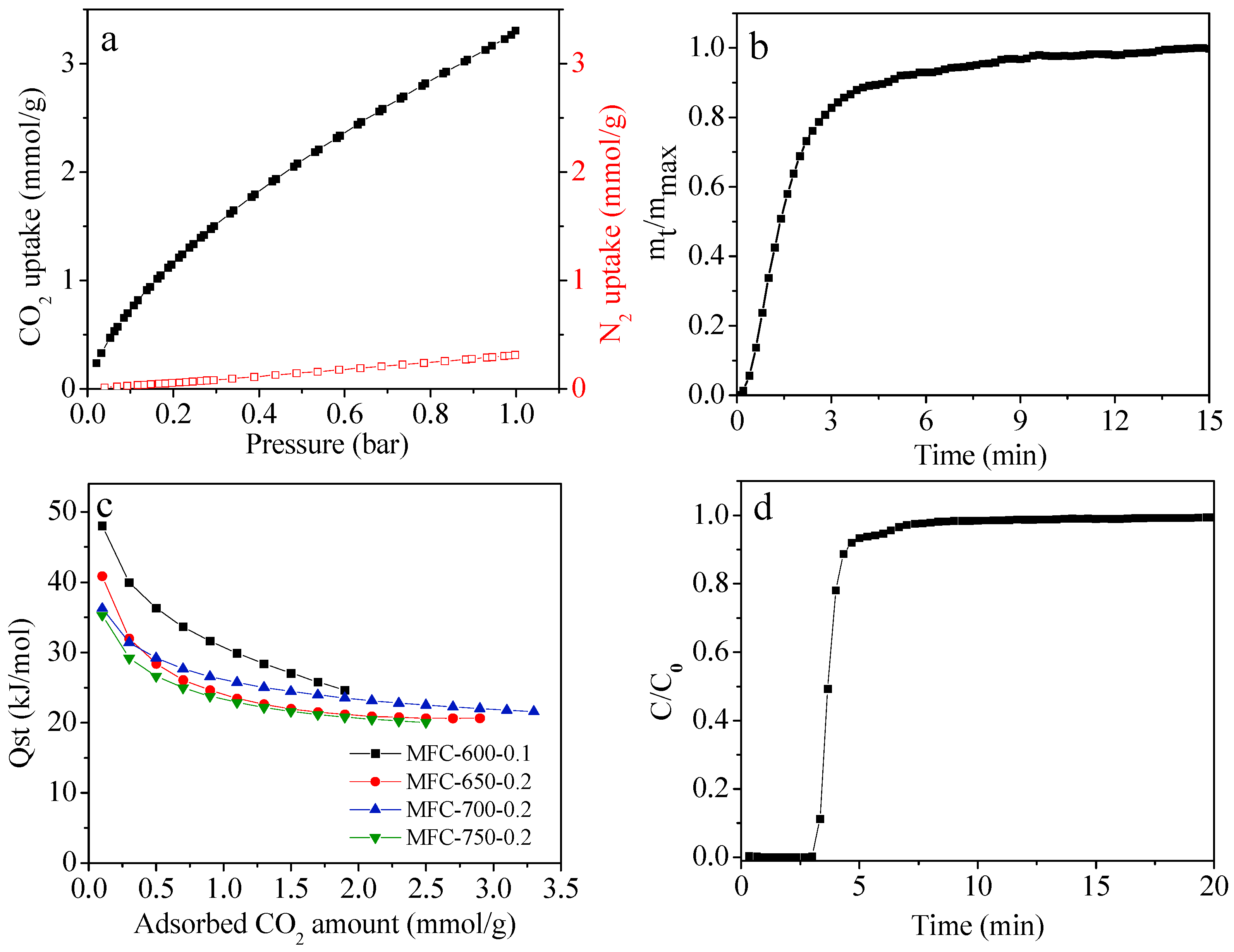
2.3. CO2 Adsorption Performance of the N-Doped Porous Carbons
3. Synthesis and Characterization
3.1. KOH Activation
3.2. Characterization
3.3. Measurement of Dynamic CO2 Uptake of the Sorbents
3.4. Measurement of CO2 Adsorption Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Available online: https://gml.noaa.gov/ccgg/trends/global.html (accessed on 2 January 2023).
- Wu, Z.; Huang, X.; Chen, R.; Mao, X.; Qi, X. The United States and China on the paths and policies to carbon neutrality. J. Environ. Manag. 2022, 320, 115785. [Google Scholar] [CrossRef] [PubMed]
- To, J.W.; He, J.; Mei, J.; Haghpanah, R.; Chen, Z.; Kurosawa, T.; Chen, S.; Bae, W.-G.; Pan, L.; Tok, J.B.-H. Hierarchical N-doped carbon as CO2 adsorbent with high CO2 selectivity from rationally designed polypyrrole precursor. J. Am. Chem. Soc. 2016, 138, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, J.; Zhang, Q.; Teng, F.; McLellan, B.C. A critical review on deployment planning and risk analysis of carbon capture, utilization, and storage (CCUS) toward carbon neutrality. Renew. Sustain. Energy Rev. 2022, 167, 112537. [Google Scholar] [CrossRef]
- Zhao, B.; Tao, W.; Zhong, M.; Su, Y.; Cui, G. Process, performance and modeling of CO2 capture by chemical absorption using high gravity: A review. Renew. Sustain. Energy Rev. 2016, 65, 44–56. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Aghel, B.; Janati, S.; Wongwises, S.; Shadloo, M.S. Review on CO2 capture by blended amine solutions. Int. J. Greenh. Gas Control 2022, 119, 103715. [Google Scholar] [CrossRef]
- Liu, F.; Huang, K.; Jiang, L. Promoted adsorption of CO2 on amine-impregnated adsorbents by functionalized ionic liquids. AIChE J. 2018, 64, 3671–3680. [Google Scholar] [CrossRef]
- Lou, Y.-C.; Qi, S.-C.; Xue, D.-M.; Gu, C.; Zhou, R.; Liu, X.-Q.; Sun, L.-B. Solvent-free synthesis of N-containing polymers with high cross-linking degree to generate N-doped porous carbons for high-efficiency CO2 capture. Chem. Eng. J. 2020, 399, 125845. [Google Scholar] [CrossRef]
- Shi, J.; Cui, H.; Xu, J.; Yan, N.; Liu, Y. Design and fabrication of hierarchically porous carbon frameworks with Fe2O3 cubes as hard template for CO2 adsorption. Chem. Eng. J. 2020, 389, 124459. [Google Scholar] [CrossRef]
- Shi, S.; Liu, Y. Nitrogen-doped activated carbons derived from microalgae pyrolysis by-products by microwave/KOH activation for CO2 adsorption. Fuel 2021, 306, 121762. [Google Scholar] [CrossRef]
- Comroe, M.L.; Kolasinski, K.W.; Saha, D. Direct Ink 3D Printing of Porous Carbon Monoliths for Gas Separations. Molecules 2022, 27, 5653. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Liu, P.; Wang, Y.; Yang, J.; Li, J.; Li, L. CO2 Capture from High-Humidity Flue Gas Using a Stable Metal-Organic Framework. Molecules 2022, 27, 5608. [Google Scholar] [CrossRef]
- Chatterjee, S.; Jeevanandham, S.; Mukherjee, M.; Vo, D.-V.N.; Mishra, V. Significance of re-engineered zeolites in climate mitigation–A review for carbon capture and separation. J. Environ. Chem. Eng. 2021, 9, 105957. [Google Scholar] [CrossRef]
- Sun, L.-B.; Kang, Y.-H.; Shi, Y.-Q.; Jiang, Y.; Liu, X.-Q. Highly Selective Capture of the Greenhouse Gas CO2 in Polymers. ACS Sustain. Chem. Eng. 2015, 3, 3077–3085. [Google Scholar] [CrossRef]
- Shao, L.; Li, Y.; Huang, J.; Liu, Y.-N. Synthesis of Triazine-Based Porous Organic Polymers Derived N-Enriched Porous Carbons for CO2 Capture. Ind. Eng. Chem. Res. 2018, 57, 2856–2865. [Google Scholar] [CrossRef]
- Sang, Y.; Cao, Y.; Wang, L.; Yan, W.; Chen, T.; Huang, J.; Liu, Y.-N. N-rich porous organic polymers based on Schiff base reaction for CO2 capture and mercury(II) adsorption. J. Colloid Interface Sci. 2021, 587, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.Y.; Zhang, G.J.; Xu, Y.; Zhang, Q.Q.; Liu, J.; Li, G.Q.; Zhao, Y.Q.; Wang, Y.; Zhang, Y.F. High CO2 adsorption on amine-functionalized improved macro-/mesoporous multimodal pore silica. Fuel 2022, 315, 123195. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, P.; Hao, L.; Xu, Y.; Cheng, H. A novel amine double functionalized adsorbent for carbon dioxide capture using original mesoporous silica molecular sieves as support. Sep. Purif. Technol. 2019, 209, 516–527. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, C.; Zhang, Z.; Usadi, A.K.; Calabro, D.C.; Baugh, L.S.; Di Yuan, Y.; Zhao, D. Evaluation of Schif-Base Covalent Organic Frameworks for CO2 Capture: Structure-Performance Relationships, Stability, and Performance under Wet Conditions. ACS Sustain. Chem. Eng. 2021, 10, 332–341. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Liu, L.; Zhang, Y.; Yang, J.; Zeng, Z.; Deng, S. Controllable synthesis of bifunctional porous carbon for efficient gas-mixture separation and high-performance supercapacitor. Chem. Eng. J. 2018, 348, 57–66. [Google Scholar] [CrossRef]
- Peng, H.-L.; Zhang, J.-B.; Zhang, J.-Y.; Zhong, F.-Y.; Wu, P.-K.; Huang, K.; Fan, J.-P.; Liu, F. Chitosan-derived mesoporous carbon with ultrahigh pore volume for amine impregnation and highly efficient CO2 capture. Chem. Eng. J. 2019, 359, 1159–1165. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.F.; Tan, C.; Sun, J.; Li, W.L.; Zhang, J.B.; Zhao, C.W. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Shao, J.; Ma, C.; Zhao, J.; Wang, L.; Hu, X. Effective nitrogen and sulfur co-doped porous carbonaceous CO2 adsorbents derived from amino acid. Colloids Surf. Physicochem. Eng. Asp. 2022, 632, 127750. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Park, S.-J. Role of heteroatoms (nitrogen and sulfur)-dual doped corn-starch based porous carbons for selective CO2 adsorption and separation. J. CO2 Util. 2021, 51, 101641. [Google Scholar] [CrossRef]
- Yu, Q.Y.; Bai, J.L.; Huang, J.M.; Demir, M.; Altay, B.N.; Hu, X.; Wang, L.L. One-Pot Synthesis of N-Rich Porous Carbon for Efficient CO2 Adsorption Performance. Molecules 2022, 27, 6816. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Park, S.J. Valorization of shrimp shell biowaste for environmental remediation: Efficient contender for CO2 adsorption and separation. J. Environ. Manag. 2021, 299, 113661. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Park, S.J. Self-activated, urea modified microporous carbon cryogels for high-performance CO2 capture and separation. Carbon 2022, 192, 14–29. [Google Scholar] [CrossRef]
- Rehman, A.; Nazir, G.; Rhee, K.Y.; Park, S.-J. Valorization of orange peel waste to tunable heteroatom-doped hydrochar-derived microporous carbons for selective CO2 adsorption and separation. Sci. Total Environ. 2022, 849, 157805. [Google Scholar] [CrossRef]
- Choudhury, F.A.; Norouzi, N.; Amir, K.; Demir, M.; El-Kaderi, H.M. Iron-based sulfur and nitrogen dual doped porous carbon as durable electrocatalysts for oxygen reduction reaction. Int. J. Hydrog. Energy 2022, 47, 6078–6088. [Google Scholar] [CrossRef]
- Guo, L.P.; Li, W.C.; Qiu, B.; Ren, Z.X.; Du, J.; Lu, A.H. Interfacial assembled preparation of porous carbon composites for selective CO2 capture at elevated temperatures. J. Mater. Chem. 2019, 7, 5402–5408. [Google Scholar] [CrossRef]
- Liu, Z.; Du, Z.; Song, H.; Wang, C.; Subhan, F.; Xing, W.; Yan, Z. The fabrication of porous N-doped carbon from widely available urea formaldehyde resin for carbon dioxide adsorption. J. Colloid Interface Sci. 2014, 416, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.F.; Chen, G.; Huang, J.H. Oxygen-rich porous carbons from carbonyl modified hyper-cross-linked polymers for efficient CO2 capture. J. Polymer Res. 2020, 27, 36. [Google Scholar] [CrossRef]
- Ma, C.; Bai, J.; Demir, M.; Hu, X.; Liu, S.; Wang, L. Water chestnut shell-derived N/S-doped porous carbons and their applications in CO2 adsorption and supercapacitor. Fuel 2022, 326, 125119. [Google Scholar] [CrossRef]
- Ma, C.; Lu, T.; Shao, J.; Huang, J.; Hu, X.; Wang, L. Biomass derived nitrogen and sulfur co-doped porous carbons for efficient CO2 adsorption. Sep. Purif. Technol. 2022, 281, 119899. [Google Scholar] [CrossRef]
- Ma, C.D.; Lu, T.Y.; Demir, M.; Yu, Q.Y.; Hu, X.; Jiang, W.H.; Wang, L.L. Polyacrylonitrile-Derived N-Doped Nanoporous Carbon Fibers for CO2 Adsorption. ACS Appl. Nano Mater. 2022, 5, 13473–13481. [Google Scholar] [CrossRef]
- Hao, G.-P.; Li, W.-C.; Qian, D.; Lu, A.-H. Rapid Synthesis of Nitrogen-Doped Porous Carbon Monolith for CO2 Capture. Adv. Mater. 2010, 22, 853–857. [Google Scholar] [CrossRef]
- Xing, W.; Liu, C.; Zhou, Z.; Zhang, L.; Zhou, J.; Zhuo, S.; Yan, Z.; Gao, H.; Wang, G.; Qiao, S.Z. Superior CO2 uptake of N-doped activated carbon through hydrogen-bonding interaction. Energy Environ. Sci. 2012, 5, 7323–7327. [Google Scholar] [CrossRef]
- Rehman, A.; Park, S.-J. From chitosan to urea-modified carbons: Tailoring the ultra-microporosity for enhanced CO2 adsorption. Carbon 2020, 159, 625–637. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Bud, J.; Byambajav, E.; Tsubouchi, N. Influence of ammonia treatment on the CO2 adsorption of activated carbon. J. Environ. Chem. Eng. 2022, 10, 107273. [Google Scholar] [CrossRef]
- Li, Q.; Liu, S.; Wang, L.; Chen, F.; Shao, J.; Hu, X. Efficient nitrogen doped porous carbonaceous CO2 adsorbents based on lotus leaf. J. Environ. Sci. 2021, 103, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Bai, J.; Demir, M.; Hu, X.; Jiang, Z.; Wang, L. Efficient N-Doped Porous Carbonaceous CO2 Adsorbents Derived from Commercial Urea-Formaldehyde Resin. Energy Fuels 2022, 36, 5825–5832. [Google Scholar] [CrossRef]
- Ma, C.; Bai, J.; Hu, X.; Jiang, Z.; Wang, L. Nitrogen-doped porous carbons from polyacrylonitrile fiber as effective CO2 adsorbents. J. Environ. Sci. 2023, 125, 533–543. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wang, H.; Li, C.C.; Wang, S.H.; Li, L.; Song, C.W.; Wang, T.H. Hierarchical flaky porous carbon derived from waste polyimide film for high-performance aqueous supercapacitor electrodes. Int. J. Energy Res. 2021, 46, 370–382. [Google Scholar] [CrossRef]
- Prasankumar, T.; Salpekar, D.; Bhattacharyya, S.; Manoharan, K.; Yadav, R.M.; Campos Mata, M.A.; Miller, K.A.; Vajtai, R.; Jose, S.; Roy, S.; et al. Biomass derived hierarchical porous carbon for supercapacitor application and dilute stream CO2 capture. Carbon 2022, 199, 249–257. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, Y.; Zhang, H.N.; Yang, H.; Yin, X.T.; Li, Z.G.; Ma, X.G. Synergistic effect of porous structure and heteroatoms in carbon materials to boost high-performance supercapacitor. Int. J. Energy Res. 2021, 45, 10963–10973. [Google Scholar] [CrossRef]
- Rehman, A.; Nazir, G.; Rhee, K.Y.; Park, S.-J. A rational design of cellulose-based heteroatom-doped porous carbons: Promising contenders for CO2 adsorption and separation. Chem. Eng. J. 2021, 420, 130421. [Google Scholar] [CrossRef]
- Yadav, R.M.; Li, Z.; Zhang, T.; Sahin, O.; Roy, S.; Gao, G.; Guo, H.; Vajtai, R.; Wang, L.; Ajayan, P.M.; et al. Amine-Functionalized Carbon Nanodot Electrocatalysts Converting Carbon Dioxide to Methane. Adv. Mater. 2022, 34, 2105690. [Google Scholar] [CrossRef]
- Li, H.M.; Li, J.H.; Thomas, A.; Liao, Y.Z. Ultra-High Surface Area Nitrogen-Doped Carbon Aerogels Derived From a Schiff-Base Porous Organic Polymer Aerogel for CO2 Storage and Supercapacitors. Adv. Funct. Mater. 2019, 29, 1904785. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, Á.; Suárez-García, F.; Martínez-Alonso, A.; Tascón, J.M.D. Influence of Porous Texture and Surface Chemistry on the CO2 Adsorption Capacity of Porous Carbons: Acidic and Basic Site Interactions. ACS Appl. Mater. Interface 2014, 6, 21237–21247. [Google Scholar] [CrossRef]
- Ma, X.; Cao, M.; Hu, C. Bifunctional HNO3 catalytic synthesis of N-doped porous carbons for CO2 capture. J. Mater. Chem. 2013, 1, 913–918. [Google Scholar] [CrossRef]
- Huang, G.G.; Wu, X.X.; Hou, Y.R.; Cai, J.J. Sustainable porous carbons from garlic peel biowaste and KOH activation with an excellent CO2 adsorption performance. Biomass Convers. Biorefin. 2020, 10, 267–276. [Google Scholar] [CrossRef]
- Lu, T.Y.; Bai, J.L.; Demir, M.; Hu, X.; Huang, J.M.; Wang, L.L. Synthesis of potassium Bitartrate-derived porous carbon via a facile and Self-Activating strategy for CO2 adsorption application. Sep. Purif. Technol. 2022, 296, 121368. [Google Scholar] [CrossRef]
- Lu, T.; Ma, C.; Demir, M.; Yu, Q.; Aghamohammadi, P.; Wang, L.; Hu, X. One-pot synthesis of potassium benzoate-derived porous carbon for CO2 capture and supercapacitor application. Sep. Purif. Technol. 2022, 301, 122053. [Google Scholar] [CrossRef]
- Li, H.Q.; Xiao, N.; Hao, M.Y.; Song, X.D.; Wang, Y.W.; Ji, Y.Q.; Liu, C.; Li, C.; Guo, Z.; Zhang, F.; et al. Efficient CO(2) electroreduction over pyridinic-N active sites highly exposed on wrinkled porous carbon nanosheets. Chem. Eng. J. 2018, 351, 613–621. [Google Scholar] [CrossRef]
- Kou, J.H.; Sun, L.B. Nitrogen-Doped Porous Carbons Derived from Carbonization of a Nitrogen-Containing Polymer: Efficient Adsorbents for Selective CO2 Capture. Ind. Eng. Chem. Res. 2016, 55, 10916–10925. [Google Scholar] [CrossRef]
- Presser, V.; McDonough, J.; Yeon, S.-H.; Gogotsi, Y. Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ. Sci. 2011, 4, 3059–3066. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 2011, 4, 1765–1771. [Google Scholar] [CrossRef]
- Xie, W.H.; Yao, X.Y.; Li, H.; Li, H.R.; He, L.N.A. Biomass-Based N-Rich Porous Carbon Materials for CO2 Capture and in-situ Conversion. ChemSusChem 2022, 15, e202201004. [Google Scholar] [CrossRef]
- Balahmar, N.; Mitchell, A.C.; Mokaya, R. Generalized Mechanochemical Synthesis of Biomass-Derived Sustainable Carbons for High Performance CO2 Storage. Adv. Energy Mater. 2015, 5, 1500867. [Google Scholar] [CrossRef]
- Sui, Z.-Y.; Cui, Y.; Zhu, J.-H.; Han, B.-H. Preparation of Three-Dimensional Graphene Oxide–Polyethylenimine Porous Materials as Dye and Gas Adsorbents. ACS Appl. Mater. Interfaces 2013, 5, 9172–9179. [Google Scholar] [CrossRef]
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef] [PubMed]
- Ben, T.; Li, Y.; Zhu, L.; Zhang, D.; Cao, D.; Xiang, Z.; Yao, X.; Qiu, S. Selective adsorption of carbon dioxide by carbonized porous aromatic framework (PAF). Energy Environ. Sci. 2012, 5, 8370–8376. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Shi, W.W.; Wang, R.Z.; Liu, H.L.; Chang, B.B.; Yang, B.C.; Zhang, Z.L. Biowaste-derived 3D honeycomb-like N and S dual-doped hierarchically porous carbons for high-efficient CO2 capture. RSC Adv. 2019, 9, 23241–23253. [Google Scholar] [CrossRef]

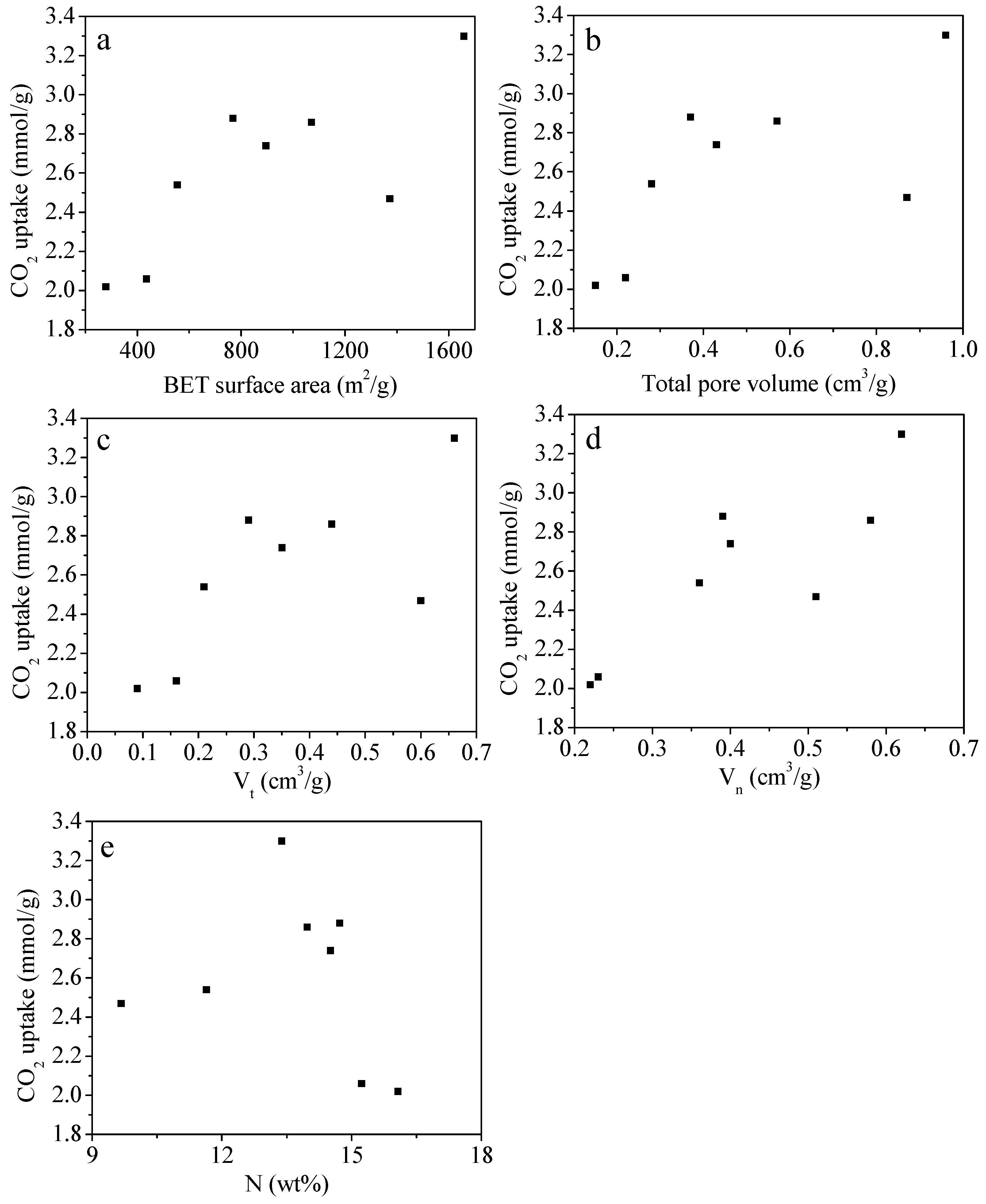

| Sample | SBET a (m2/g) | V0 b (cm3/g) | Vt c (cm3/g) | Vn d (cm3/g) | N (wt%) | C (wt%) | H (wt%) | CO2 Uptake (mmol/g) | |
|---|---|---|---|---|---|---|---|---|---|
| 25 °C | 0 °C | ||||||||
| MFC-600-0.1 | 278 | 0.15 | 0.09 | 0.22 | 16.07 | 58.18 | 3.86 | 2.02 | 2.55 |
| MFC-600-0.2 | 895 | 0.43 | 0.35 | 0.40 | 14.51 | 57.94 | 3.59 | 2.74 | 3.76 |
| MFC-650-0.1 | 435 | 0.22 | 0.16 | 0.23 | 15.23 | 59.64 | 3.12 | 2.06 | 2.72 |
| MFC-650-0.2 | 1070 | 0.57 | 0.44 | 0.58 | 13.97 | 59.11 | 3.82 | 2.86 | 4.28 |
| MFC-700-0.1 | 768 | 0.37 | 0.29 | 0.39 | 14.72 | 60.65 | 3.41 | 2.88 | 4.25 |
| MFC-700-0.2 | 1658 | 0.96 | 0.66 | 0.62 | 13.38 | 64.13 | 4.04 | 3.30 | 4.95 |
| MFC-750-0.1 | 554 | 0.28 | 0.21 | 0.36 | 11.64 | 58.26 | 2.60 | 2.54 | 3.26 |
| MFC-750-0.2 | 1373 | 0.87 | 0.60 | 0.51 | 9.67 | 60.34 | 2.98 | 2.47 | 3.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Bai, J.; Huang, J.; Demir, M.; Farghaly, A.A.; Aghamohammadi, P.; Hu, X.; Wang, L. One-Pot Synthesis of Melamine Formaldehyde Resin-Derived N-Doped Porous Carbon for CO2 Capture Application. Molecules 2023, 28, 1772. https://doi.org/10.3390/molecules28041772
Yu Q, Bai J, Huang J, Demir M, Farghaly AA, Aghamohammadi P, Hu X, Wang L. One-Pot Synthesis of Melamine Formaldehyde Resin-Derived N-Doped Porous Carbon for CO2 Capture Application. Molecules. 2023; 28(4):1772. https://doi.org/10.3390/molecules28041772
Chicago/Turabian StyleYu, Qiyun, Jiali Bai, Jiamei Huang, Muslum Demir, Ahmed A. Farghaly, Parya Aghamohammadi, Xin Hu, and Linlin Wang. 2023. "One-Pot Synthesis of Melamine Formaldehyde Resin-Derived N-Doped Porous Carbon for CO2 Capture Application" Molecules 28, no. 4: 1772. https://doi.org/10.3390/molecules28041772
APA StyleYu, Q., Bai, J., Huang, J., Demir, M., Farghaly, A. A., Aghamohammadi, P., Hu, X., & Wang, L. (2023). One-Pot Synthesis of Melamine Formaldehyde Resin-Derived N-Doped Porous Carbon for CO2 Capture Application. Molecules, 28(4), 1772. https://doi.org/10.3390/molecules28041772







