Abstract
Luteoloside has shown anti-inflammatory, antiviral, and antitumor properties. However, the effect and mechanism of luteoloside on neuroblastoma cells remain unknown. The proliferation of human neuroblastoma cells (SH-SY5Y and SK-N-AS) treated with different concentrations of luteoloside (0, 12.5, 25, and 50 μM) was detected by the MTT assay and colony formation assay. Cell apoptosis and cell cycle were examined by Hoechst staining and flow cytometry. A subcutaneous tumorigenesis model was established in nude mice to evaluate the effect of luteoloside on tumor growth in vivo. Bioinformatics, molecular docking techniques, and cellular thermal shift assays were utilized to predict the potential targets of luteoloside in neuroblastoma. The p38 MAPK inhibitor SB203580 was used to confirm the role of p38 MAPK. Luteoloside inhibited the proliferation of neuroblastoma cells in vitro and in vivo. Luteoloside slightly induced cellular G0/G1 phase arrest and reduced the expression levels of G0/G1 phase–related genes and the proteins cyclin D1, CDK4, and C-myc, which are downregulated by p38 MAPK pathways. Meanwhile, p38 was identified as the target of luteoloside, and inhibition of p38 MAPK reversed the inhibitory effect of luteoloside on neuroblastoma cells. Luteoloside is a potential anticancer drug for treating neuroblastoma by activating p38 MAPK.
1. Introduction
Neuroblastoma is one of the most malignant tumors in peripheral neuroma and often occurs in children under 5 years old, which makes neuroblastoma hard to diagnose and treat [1,2]. In spite of considerable progress in molecular mechanisms and therapeutic approach, survival rates remain low. Moreover, because of the high toxicity of radio-chemotherapy and the age of the patients, it is difficult to achieve therapeutic success [3,4]. Furthermore, oncology drugs have the lowest likelihood of approval in phase I (6.7%) compared with drugs treating other pediatric diseases [5]. Consequently, there is an urgent need to discover novel therapeutic agents that are well tolerated and have low toxicity to treat neuroblastoma. In recent years, researchers have found that traditional Chinese medicines, including flavonoids, exhibit significant antitumor effects and have begun clinical trials [6,7].
Luteoloside, a natural flavonoid substance also known as luteolin 7-O-glucoside, exhibits a variety of biological activities, such as anti-inflammatory [8], antiviral [9], cardiovascular protection [10], and anti-tumor activity [11,12,13,14]. Researchers found that luteoloside suppressed oral cancer cell migration by regulating matrix metalloproteinase-2 expression, and inhibited human nasopharyngeal carcinoma cell proliferation and promoted apoptosis [11,12]. Other researchers reported that luteoloside inhibited proliferation and modulated apoptosis by mitogen-activated protein kinase (MAPK) and mammalian target of rapamycin (mTOR) signaling pathways in human cervical cancer [13]. Our previous research suggests that luteoloside promoted the apoptosis in breast cancer cells [14]. In addition, some reports showed that luteolin, which has a similar structure to luteoloside, enhanced neuronal antioxidant defense capacity and suppressed glioma cell proliferation by inducing apoptosis and autophagy via MAPK activation [15,16].
The structure of a drug usually determines its function. This suggests that the molecular structure of luteoloside can be used to infer its mechanism of action. Network pharmacology and molecular docking are effective methods in drug design and screening, and are being used to explore the targets and mechanisms of traditional Chinese medicine [17,18]. Network pharmacology is a new discipline based on the theory of systems biology, which can predict the targets of drug action based on the target characteristics of drugs and diseases and their network relationships [19]. It is commonly used in research on multitarget and multiaction traditional Chinese medicine. Molecular docking allows a receptor–ligand complex to be docked according to an analysis of the specific receptor docking sites and the drug molecule [20]. Although studies have shown that luteoloside has anti-tumor effects, the specific targets are still unclear, and neuroblastoma has not yet been studied to determine the effects and mechanisms of luteoloside. Therefore, we may predict the possible molecules of action of luteoloside using network pharmacology and molecular docking techniques.
In this study, we attempted to elucidate the specific biological activity and crucial molecular mechanisms of luteoloside on neuroblastoma, possibly assisting in the treatment of neuroblastoma. We show that luteoloside targets to p38 MAPK and induces cell cycle arrest, which leads to the inhibition of neuroblastoma cell proliferation. This study shows that luteoloside exerts anti-neuroblastoma activity in a novel manner, and offers a promising approach for neuroblastoma treatment.
2. Results
2.1. Luteoloside Inhibits Proliferation of Neuroblastoma Cell
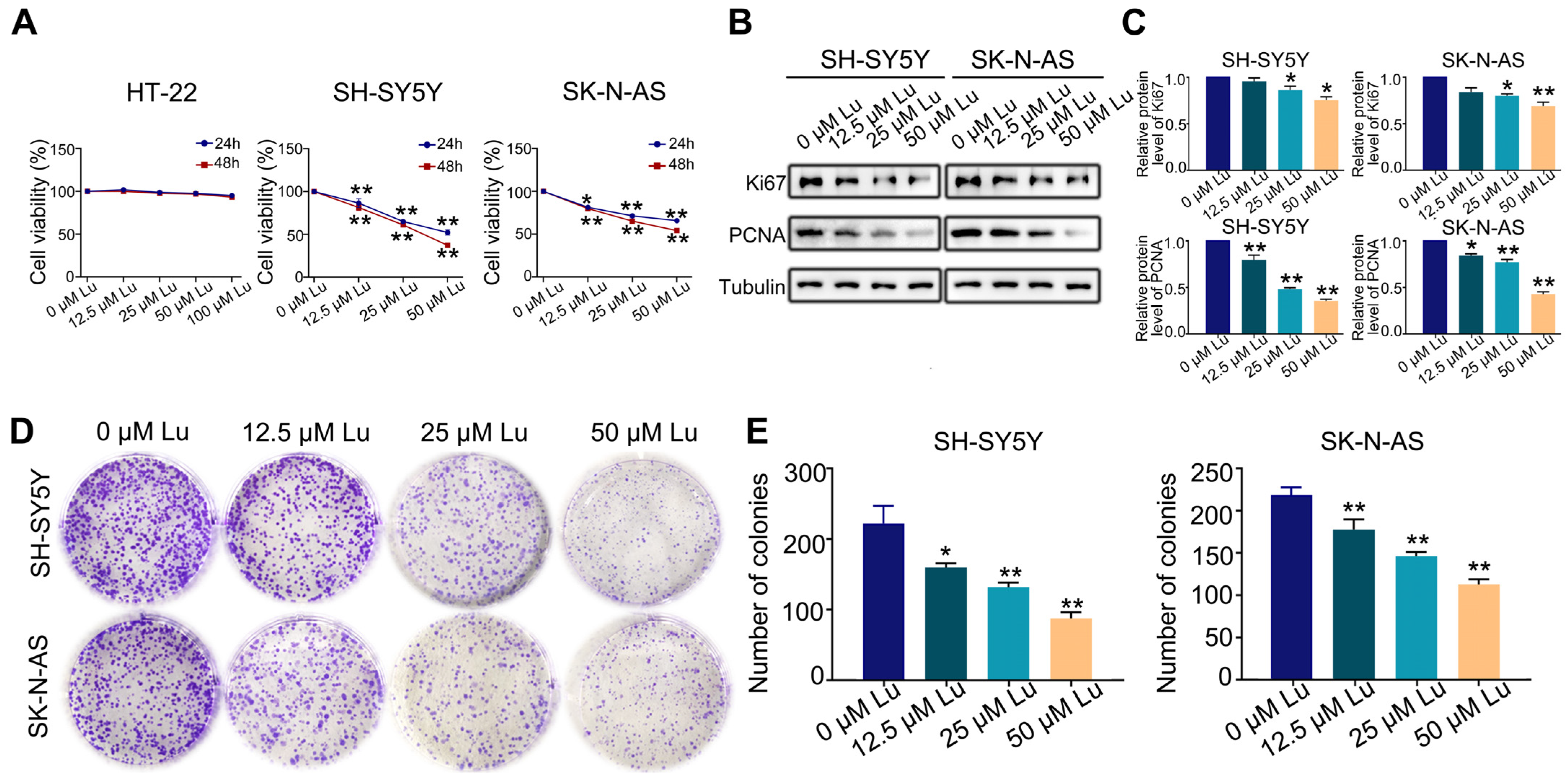
The first set of questions aimed to evaluate the proliferative effect of luteoloside on HT-22, SH-SY5Y, and SK-N-AS cells. SH-SY5Y and SK-N-AS cells were treated with varying concentrations (0, 12.5, 25, and 50 μM) of luteoloside for 24 and 48 h, while HT-22 cells were treated with different concentrations (0, 12.5, 25, 50, and 100 μM), also for 24 and 48 h. MTT assays revealed that luteoloside obviously decreased the proliferation rate of SH-SY5Y and SK-N-AS cells in a dose-dependent manner, but had no significant effect on normal cells of HT-22 (Figure 1A). The protein expressions of the cell proliferation markers Ki-67 and PCNA were both downregulated by luteoloside (Figure 1B,C). Additionally, colony formation assays demonstrated that SH-SY5Y and SK-N-AS exhibited low colony formation capacity after being treated with luteoloside (Figure 1D,E).
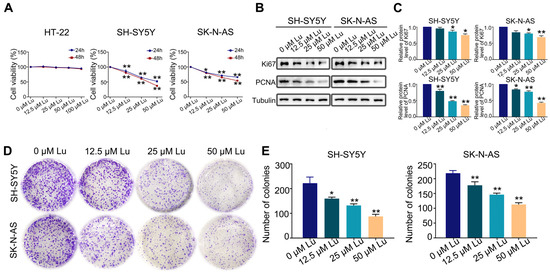
Figure 1.
Luteoloside inhibits the proliferation of neuroblastoma cells. SH-SY5Y and SK-N-AS cells were treated with varying concentrations (0, 12.5, 25, and 50 μM) of luteoloside for 24 or 48 h, while HT-22 was treated with different concentrations (0, 12.5, 25, 50, and 100 μM) of luteoloside for the same periods. (A) The viability of HT-22, SH-SY5Y, and SK-N-AS cells was detected in each group. (B) The expression of PCNA and Ki67 was tested by Western blot. (C) Statistical analysis of the expressions intensity of PCNA, Ki67 in SH-SY5Y and SK-N-AS cells. Tubulin is taken as internal reference. (D) The colony formation of SH-SY5Y and SK-N-AS cells was tested in each group. (E) Statistical analysis of the number of colonies in each group. *, p < 0.05; **, p < 0.01. n = 3. The control group is used for comparison. Data are shown as mean ± SD.
2.2. Luteoloside Slightly Improves Neuroblastoma Cell Apoptosis
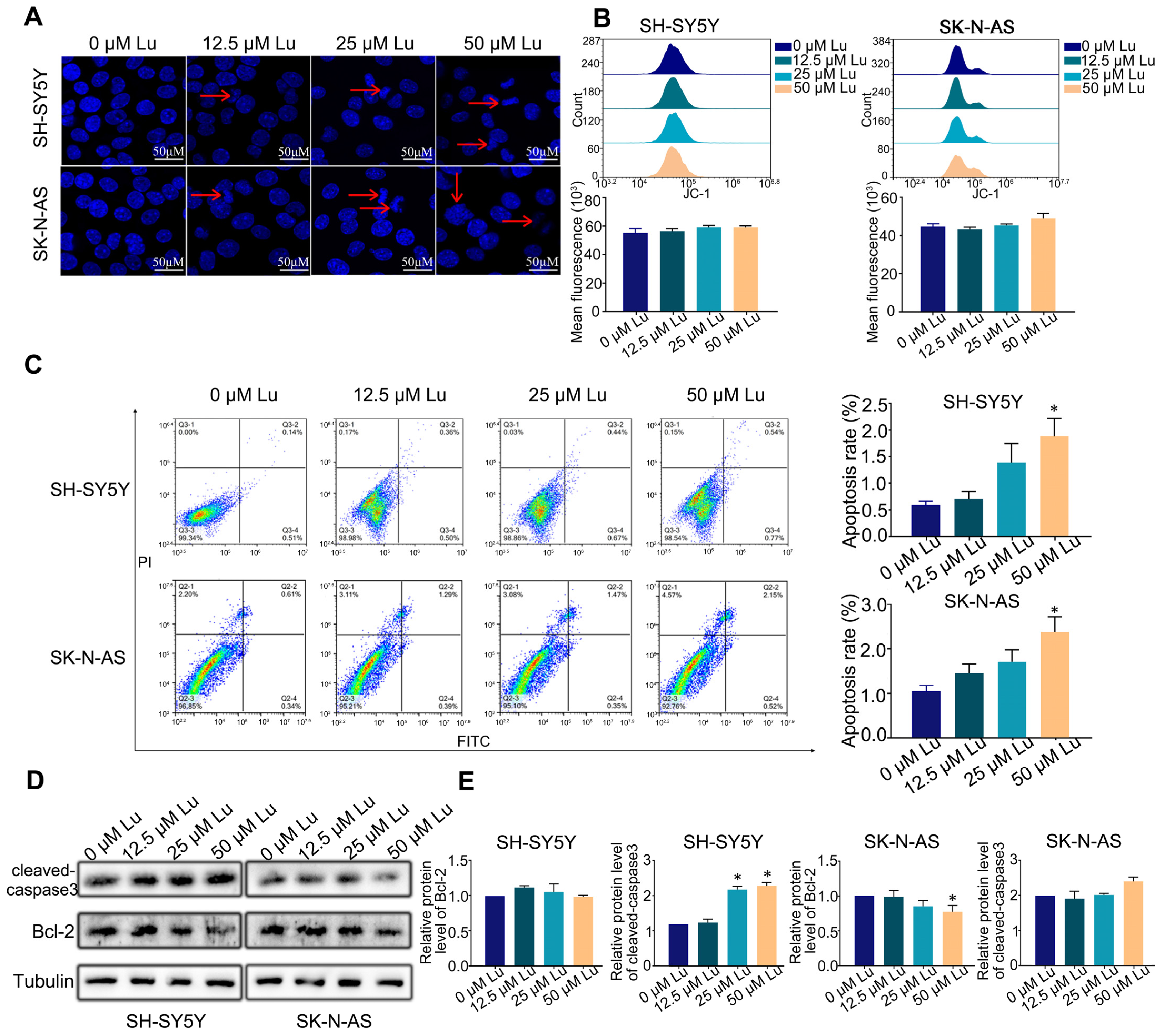
To assess the effect of luteoloside on the apoptosis of human neuroblastoma cells, cells were stained with Hoechst 33258 after being treated with different concentrations (0, 12.5, 25, and 50 μM) of luteoloside for 24 h. The cell nuclei of each group were uniformly stained, and the edge contours were clear; only a few cell nuclei of the 25 and 50 μM groups appeared with apoptotic bodies (Figure 2A). There were no significant changes of mitochondrial membrane potential (MMP) in either SH-SY5Y and SK-N-AS cells (Figure 2B). The apoptosis rates of SH-SY5Y and SK-N-AS cells were not significantly changed at in the 0, 12.5, or 25 μM luteoloside-treated groups, but were increased in the 50 μM luteoloside-treated groups (Figure 2C). Western blot results demonstrated that the expression levels of the Bcl-2 protein had no change in SH-SY5Y cells in each luteoloside-treated groups, and the levels of cleaved-caspase3 in the 25 and 50 μM groups were increased. As for SK-N-AS cells, the expression levels of Bcl-2 in the 25 and 50 μM luteoloside-treated groups were less than those of the control group (Figure 2D,E). These results indicate that luteoloside slightly induced apoptosis of neuroblastoma cells, but the inhibitory effect of luteoloside on human neuroblastoma cell proliferation is not primarily mediated by apoptosis.
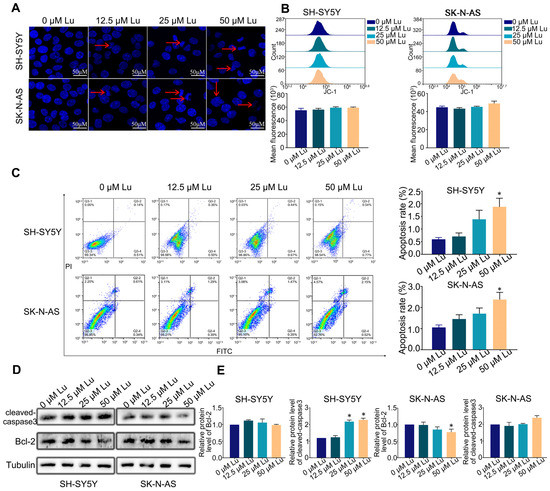
Figure 2.
Effect of luteoloside on the neuroblastoma cell apoptosis. SH-SY5Y and SK-N-AS cells were treated with varying concentrations (0, 12.5, 25, and 50 μM) of luteoloside for 24 h. (A) Cell morphology with Hoechst 33258 staining was observed under a fluorescence microscope. Red arrows show the apoptotic bodies. Bar = 50 μM. (B) Flow cytometry was used to detect the change of mitochondrial membrane potential (MMP) in each group. (C) Apoptosis distribution of SH-SY5Y and SK-N-AS cells was tested by flow cytometry. (D) The protein expressions of cleaved-caspase3 and Bcl-2 were tested by Western blot. (E) Statistical analysis of the expression intensity of cleaved caspase-3 and Bcl-2 in SH-SY5Y and SK-N-AS cells. Tubulin is taken as internal reference. *, p < 0.05. n = 3. The control group is used for comparison. Data are shown as mean ± SD.
2.3. Luteoloside Induces Neuroblastoma Cell Cycle Arrest
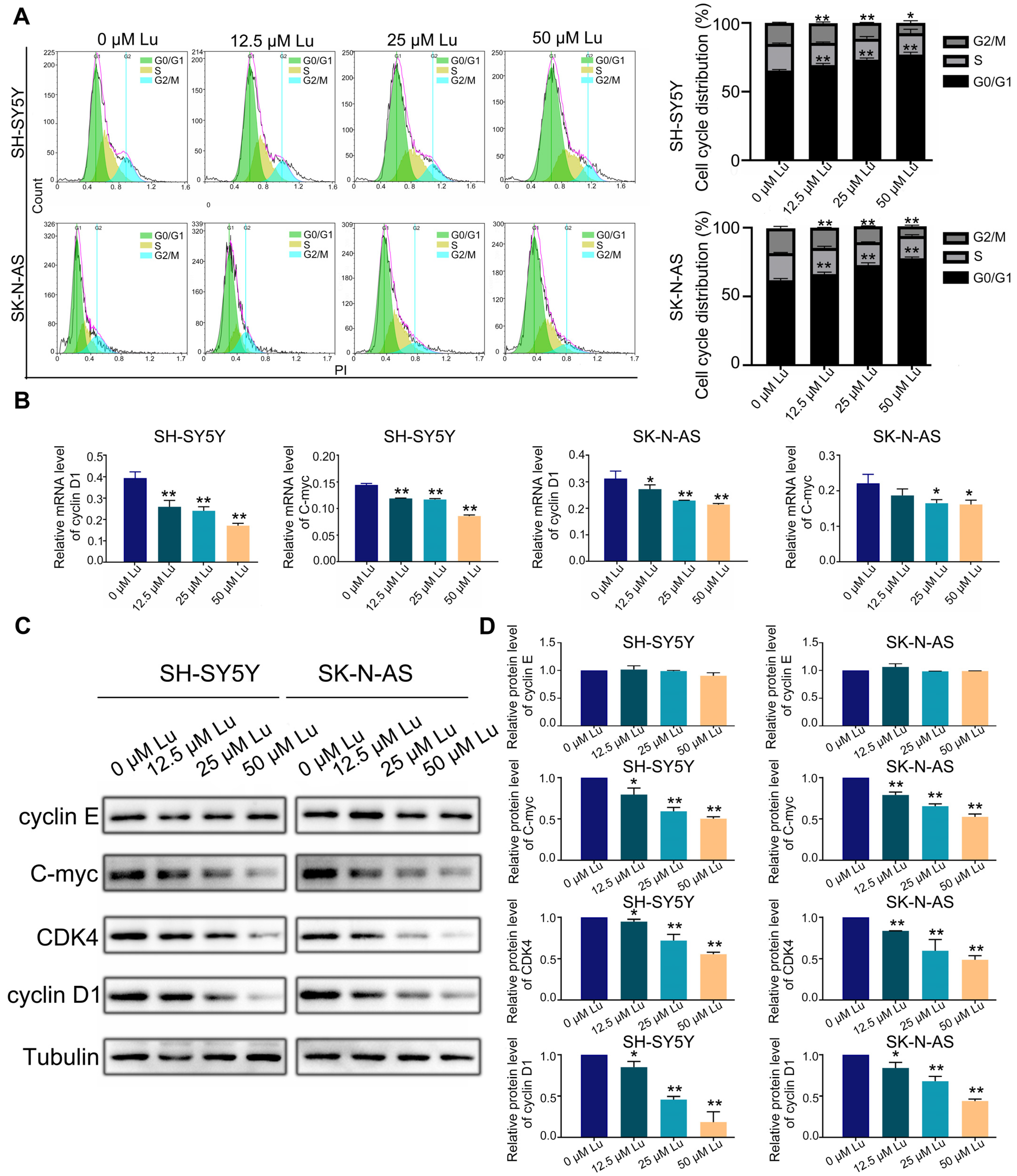
Further, we analyzed the cell cycle and related protein expression to explore the impact of luteoloside on DNA replication and cell cycle processes in neuroblastoma cells. The flow cytometry results revealed that luteoloside elevated the percentage of neuroblastoma cells in the G0/G1 phase, but significantly reduced the cell numbers in the G2 phase (Figure 3A). Western blot assay results demonstrated that the expression levels of the G1 phase–related proteins cyclin D1 and cyclin-dependent kinase 4 (CDK4) decreased after treatment with luteoloside. However, the expression of cyclin E proteins associated with the late G1 phase was not significantly changed. The expression level of C-myc was reduced in the luteoloside-treated group (Figure 3B,C). Meanwhile, the mRNA levels of cyclin D1 and C-myc were slightly decreased compared with the control groups (Figure 3D). These results show that luteoloside induces G0/G1 phase arrest in neuroblastoma cells by regulating the proteins cyclin D1 and C-myc.
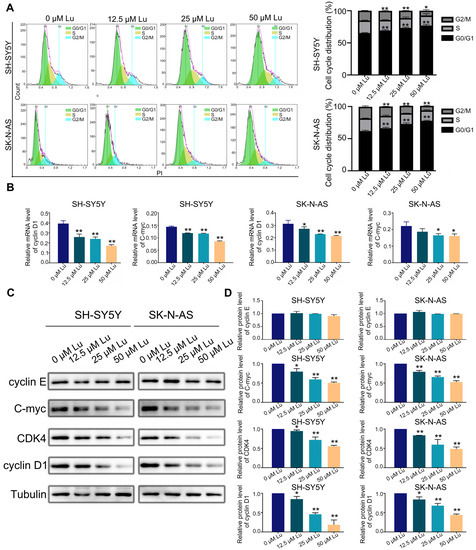
Figure 3.
Luteoloside induces neuroblastoma cell cycle arrest. SH-SY5Y and SK-N-AS cells were treated with varying concentrations (0, 12.5, 25, and 50 μM) of luteoloside for 24 h. (A) The SH-SY5Y and SK-N-AS cell cycle distributions were measured by flow cytometric analysis. (B) The protein expressions of cyclin D1, cyclin E, C-myc, and CDK4 were tested by Western blot. (C) Statistical analysis of the expression intensity of cyclin D1, cyclin E, C-myc, and CDK4 in SH-SY5Y and SK-N-AS cells. Tubulin is taken as internal reference. (D) The relative mRNA expression of cyclin D1 and C-myc in SH-SY5Y and SK-N-AS cells was analyzed using qRT-PCR. *, p < 0.05; **, p < 0.01. n = 3. The control group is used for comparison. Data are shown as mean ± SD.
2.4. Luteoloside Suppressed the Tumor Growth In Vivo
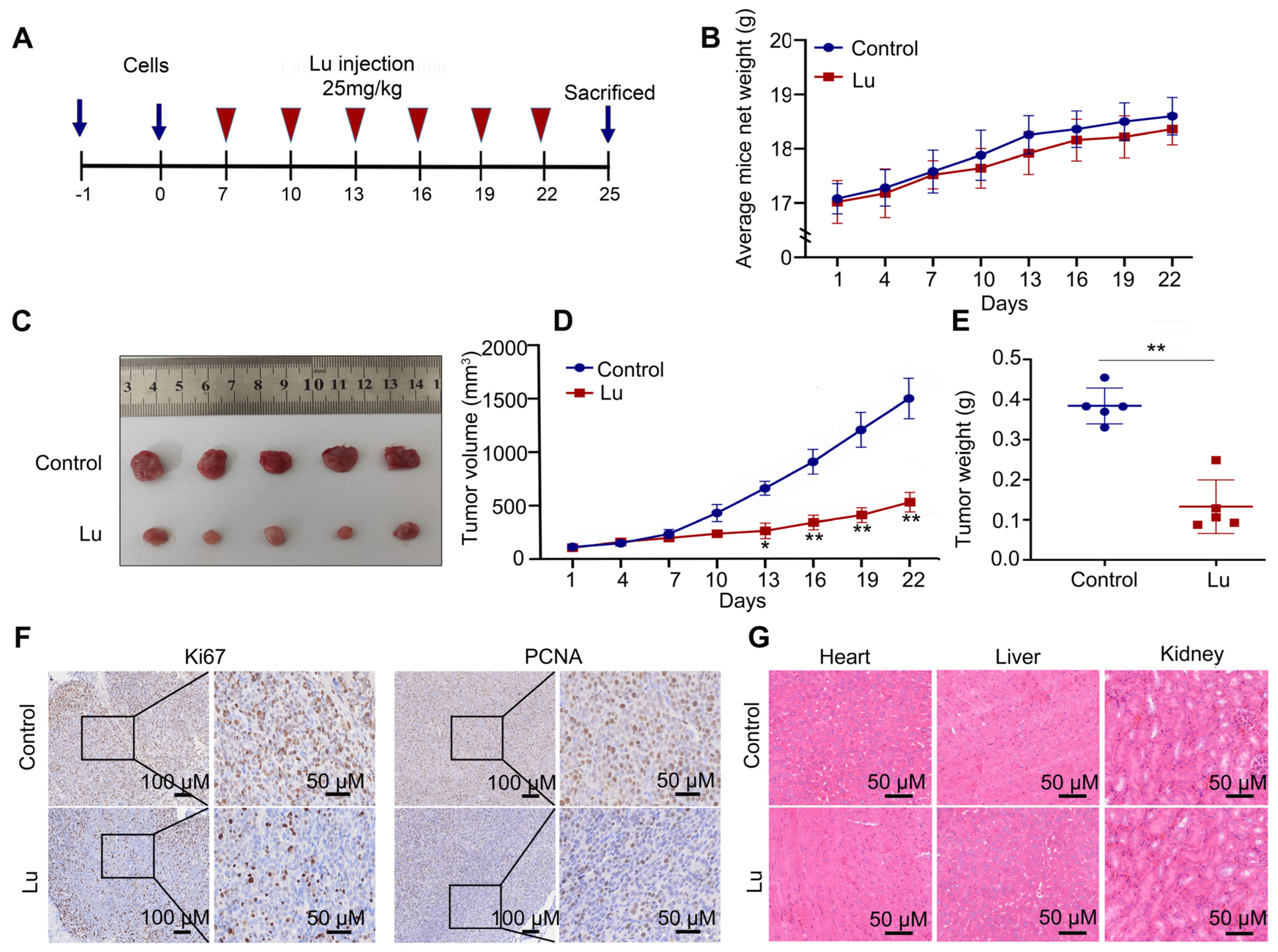
We also used female BALB/c nude mice as a subcutaneous tumorigenesis model to evaluate the antitumor potential of luteoloside in vivo (Figure 4A). Tumor volume and weight in the luteoloside-treated group were obviously decreased compared with the control group (Figure 4B–E). Immunohistochemical staining (IHC) results showed that neuroblastoma tissues treated with luteoloside had low expression of PCNA and Ki67 (Figure 4F). Luteoloside did not damage the heart, liver, or kidney (Figure 4G). These results suggest that luteoloside had the same inhibitory effect and no toxicity in vivo.
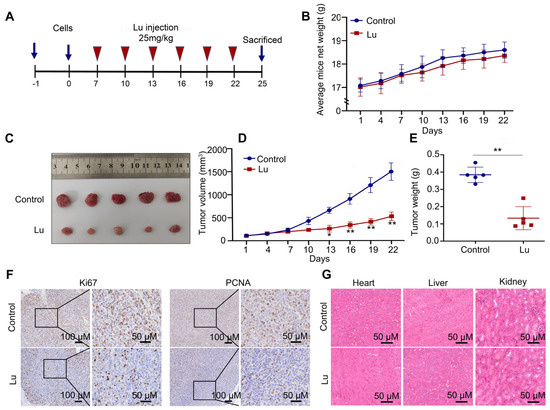
Figure 4.
Luteoloside suppressed tumor growth in vivo. (A) The process sketch diagram. (B) The average mouse weight treated with DMSO or luteoloside. (C–E) Tumor volume and weight of tissues treated with DMSO or luteoloside. (F) The expression of Ki67 and PCNA in neuroblastoma and adjacent tissues was analyzed using immunohistochemical staining (IHC). (G) Hematoxylin–eosin (HE) was used to evaluate toxicity to the heart, liver, and kidney after treatment with luteoloside. *, p < 0.05; **, p < 0.01. n = 5. The control group is used for comparison. Data are shown as mean ± SD.
2.5. Luteoloside Induces Neuroblastoma Cell Cycle Arrest through the p38 MAPK Signaling Pathway
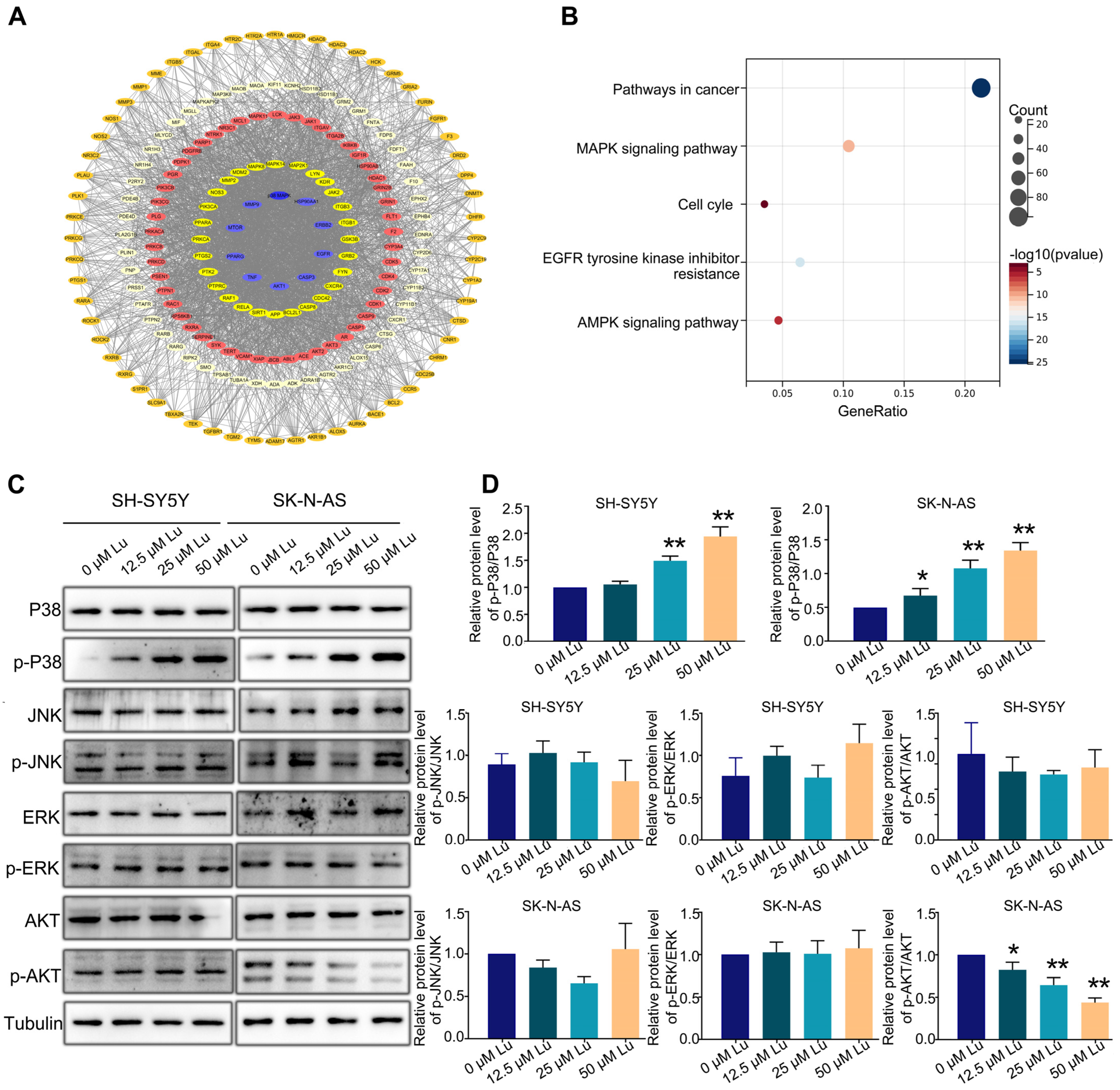
Next, we predicted the potential targets of luteoloside by using SwissTargetPrediction (http://swisstargetprediction.ch/) to identify the possible pathways involved in luteoloside-induced neuroblastoma cell cycle arrest; 495 potential targets were found (Figure 5A). Furthermore, using Kyoto Encyclopedia of Genes and Genomes (KEGG, https://david.ncifcrf.gov/tools.jsp) pathway analysis, we found that most of those targets were significantly enriched in cancer, MAPK signaling pathways and cell cycles (Figure 5B). We analyzed the expression levels of MAPK and Akt (protein kinase B) signaling pathway proteins, including AKT, p-AKT, c-Jun N-terminal kinase (JNK), p-JNK, extracellular regulated protein kinases (ERK), p-ERK, p38, and p-p38. The expression level of p-AKT was downregulated in SK-N-AS cells (Figure 5C). The protein level of p-p38 in was significantly upregulated in the luteoloside-treated groups compared with the control group (Figure 5C). Given these results, the activation of the p38 MAPK signaling pathway plays an essential role in the effect of luteoloside on neuroblastoma.
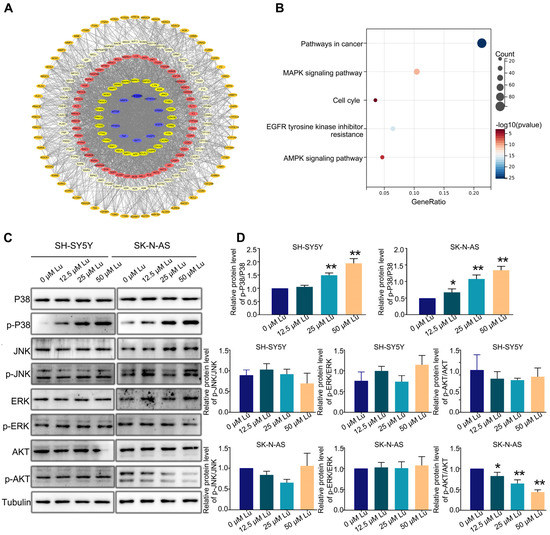
Figure 5.
Luteoloside induces neuroblastoma cell cycle arrest through the p38 MAPK signaling pathway. SH-SY5Y and SK-N-AS cells were treated with different concentrations (0, 12.5, 25, and 50 μM) of luteoloside for 24 h. (A) The protein–protein interaction (PPI) network of potential luteoloside targets. (B) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. (C) The protein expressions of JNK, p-JNK, ERK, p-ERK, p38, p-P38, AKT, and p-AKT were tested by Western blot. (D) Statistical analysis of the expression intensity of p-AKT/AKT, p-JNK/JNK, p-ERK/ERK, p-P38/P38 in SH-SY5Y and SK-N-AS cells. *, p < 0.05; **, p < 0.01. n = 3. The control group is used for comparison. Data are shown as mean ± SD.
2.6. P38 MAPK May Be a Key Target of Luteoloside
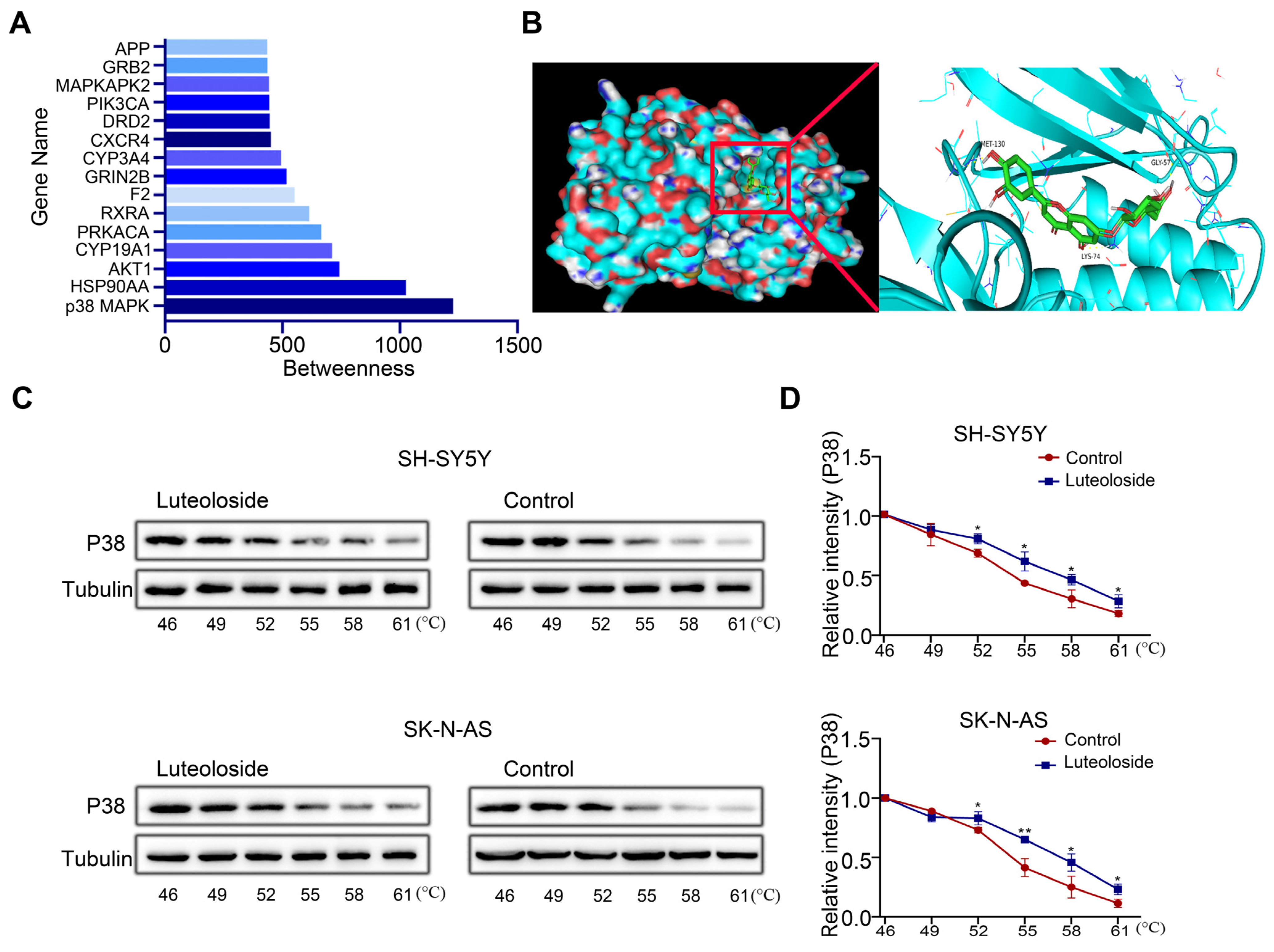
Finding a drug’s target is key to drug research. To further explore the target of luteoloside, we evaluated the betweenness centrality of these targets, and found that p38 MAPK had the highest betweenness centrality (Figure 6A). Then, we analyzed the binding model of luteoloside and the p38 MAPK protein by using molecular docking technology. The 3D drawing showed that luteoloside can bind to the residues Gly57, Lys74, and Met-130 of the p38 MAPK protein, exhibiting a stabilizing binding conformation (Figure 6B). The correlation between luteoloside and p38 MAPK was tested using a CETSA experiment, and it was found that after treatment with luteoloside, the thermal stability of p38 MAPK was increased compared with the control group (Figure 6C,D). These results suggest that p38 MAPK may be a key target of luteoloside.
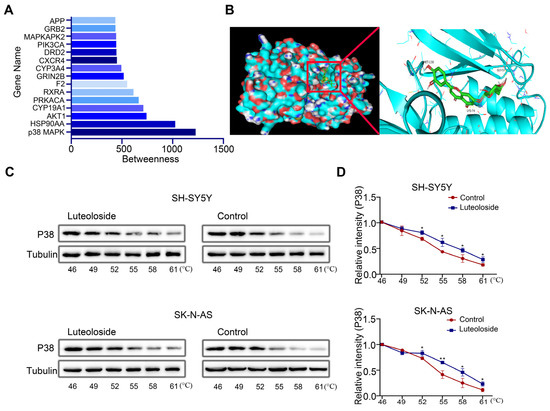
Figure 6.
P38 MAPK was identified as a key target of luteoloside. (A) The top 15 luteoloside targets were visualized using Cytoscape. (B) The binding mode of luteoloside with p38 MAPK and the 3D illustration of the details of the interaction. Green shows luteoloside, aquamarine shows the protein of p38 MAPK. (C) The cellular thermal shift assay was used to evaluate luteoloside–p38 MAPK interactions in SH-SY5Y and SK-N-AS cells. (D) Quantification of the relative intensity of the p38 MAPK protein versus increased temperature. Tubulin is taken as internal reference. *, p < 0.05; **, p < 0.01. n = 3. The control group is used for comparison. Data are shown as mean ± SD.
2.7. P38 MAPK Inhibitor Reversed Proliferate Inhibition and Cell Cycle Arrest Induced by Luteoloside in Neuroblastoma Cells
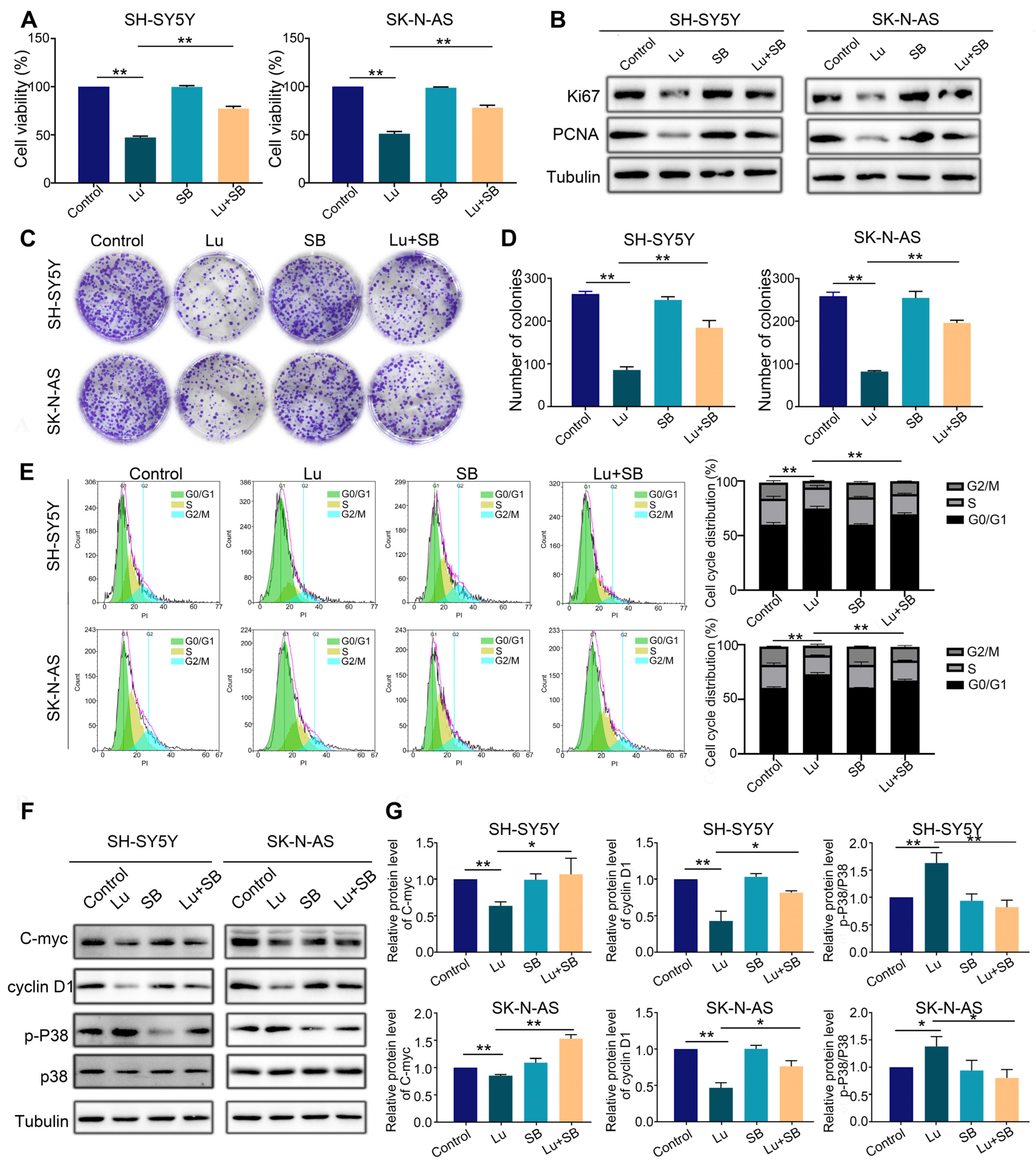
Finally, to confirm that p38 MAPK is the target of luteoloside, we utilized a specific p38 MAPK inhibitor, SB203580 (20 μM), to pretreat SH-SY5Y and SK-N-AS cells for 2 h, followed by combined treatment with luteoloside (50 μM) for 24 h. MTT assays revealed that the SB203580 pretreated group could partially recover from proliferation inhibition compared with the group treated with luteoloside alone (Figure 7A). The protein expression of the cell proliferation markers Ki-67 and PCNA was upregulated by luteoloside in the SB203580 pretreated group (Figure 7B). Additionally, colony formation assays demonstrated that SH-SY5Y and SK-N-AS partially recovered colony formation capacity when pretreated with SB203580 (Figure 7C,D). The results showed that SB203580 pretreatment could partially reverse G1 phase arrest from luteoloside (Figure 7E). Additionally, the p-P38 level of the SB203580 pretreated groups was clearly downregulated, while the levels of cyclin D1 and C-myc were clearly upregulated (Figure 7F,G). These data further reveal that luteoloside induces neuroblastoma cell cycle arrest via the p38 MAPK signaling pathway.
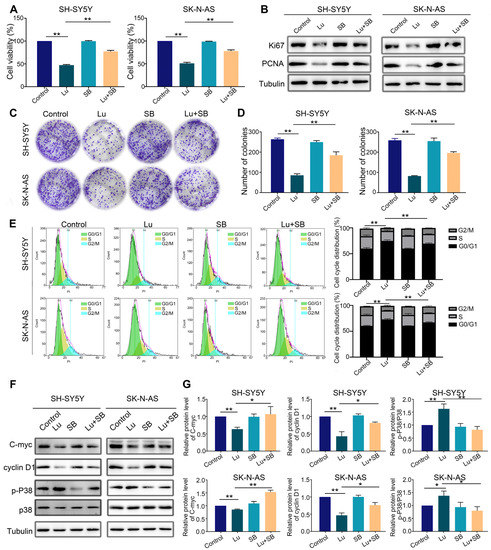
Figure 7.
Inhibition of the p38 MAPK signaling pathway reversed cell cycle arrest caused by luteoloside on neuroblastoma cells. SH-SY5Y and SK-N-AS cells were treated with 0 μM luteoloside (control group), 50 μM luteoloside (Lu group), 20 μM SB203580 (SB group), and luteoloside + SB203580 (Lu + SB group) for 24 h. (A) The viability of SH-SY5Y and SK-N-AS cells was detected in each group. (B) The protein expressions of PCNA and Ki67 were tested by Western blot. (C) The colony formation of SH-SY5Y and SK-N-AS cells was tested in each group. (D) Statistical analysis of the number of colonies in each group. (E) SH-SY5Y and SK-N-AS cell cycle distributions were measured by flow cytometry in each group. (F) The expression of cyclin D1, C-myc, p38, and p-P38 was tested by Western blot. (G) Statistical analysis of the expression intensity of cyclin D1, C-myc, and p-P38/p38 in SH-SY5Y and SK-N-AS cells. Tubulin is taken as internal reference. *, p < 0.05; **, p < 0.01. n = 3. The control group is used for comparison. Data are shown as mean ± SD.
3. Discussion
Traditional therapeutic strategies for neuroblastoma that utilize radio-chemotherapy, bone marrow transplantation, and immunotherapy still result in many side effects [21,22]. Safer and better-tolerated therapies and new drugs are urgently needed. This study found that luteoloside inhibited the proliferation of neuroblastoma cells through G0/G1 phase arrest. We revealed that luteoloside targets p38 MAPK by increasing the phosphorylation level, thereby downregulating the expression of key downstream proteins of the G1 phase, such as C-myc, cyclin D1, and CDK4, eventually causing G0/G1 phase arrest.
A disruption of cell cycle progression is one of the basic mechanisms of tumorigenesis. Cells increase in number through repeated cycles of division, which is called the cell cycle. The cycle includes two parts: the interphase consisting of the G1, S, and G2 phases and the mitosis phase. Disturbances of the cell cycle and its regulatory systems commonly lead to uncontrolled cell proliferation and, eventually, carcinogenesis [23,24]. Therefore, controlling the cell cycle is key to suppressing tumor cell proliferation. Cyclins and cyclin-dependent kinases (CDKs) are responsible for the progression of cells past various checkpoints. Cyclin D1 is a regulatory subunit of CDK4 or CDK6 that binds with CDK4/6 to form a complex. This complex triggers the activation of genes related to DNA replication to ensure that cells transition from the G1 phase checkpoint to the S phase [25]. Some studies have indicated that the overexpression of cyclin D1 or CDK4 caused tumor in mice [26,27,28], while knockout cyclin D1 or CDK4 caused resistance to the development of murine breast cancer [29]. Deletion of CDK4 caused cell cycle arrest and promoted cell senescence in lung cancer cells with the endogenous K-RAS oncogene [30]. Several clinical studies on inhibitors targeting CDK4 and CDK6 in pRb-positive tumors (breast cancer, melanoma, liposarcoma, and so on) have shown that the cyclin D–CDK4/6–pRb axis has a significant impact on tumor proliferation and transformation [31]. These studies suggest that regulators of the expressions of cell cycle proteins could be developed for neuroblastoma treatment. Many researchers have reported that natural products, including artemisinin, olive leaves extract, and oleuropein, can inhibit the proliferation of neuroblastoma cells by mediating cell cycle [32,33,34,35]. Similar mechanisms for luteoloside were also found by Zhou et al. They found that luteoloside caused G0/G1 arrest and autophagy through the AKT/mTOR signaling pathway in human non–small cell lung cancer, but this process does not effectively lead to apoptotic cell death [36]. In this study, we observed a small amount of apoptosis and increased the expression of cleaved-caspase3 induced by luteoloside. This may be due to luteoloside regulation of other pathways or proteins, which requires further study. In addition, we revealed that SH-SY5Y and SK-N-AS cells were arrested in the G0/G1 phase, and the expression of the cyclin D1 and CDK4 proteins was significantly decreased after treatment with luteoloside.
In recent years, the emerging field of network pharmacology has had a significant impact on traditional Chinese medicine [37]. An important part of network pharmacology is the forecasting of the molecular mechanisms of drugs based on complex biological network models and interactions between targets and diseases [38,39]. We predicted the potential targets of luteoloside using SwissTargetPrediction, and KEGG pathway analysis showed that the 495 targets were significantly enriched in the MAPK signaling pathway. The MAPK signaling pathway is an essential pathway in cell growth. It includes the ERK, p38 MAPK, and JNK subfamilies [40]. ERK exists widely in tissues and participates in the regulation of cell proliferation and differentiation; JNK is a vital signal molecule in cellular signal transduction; and p38 MAPK consists of five members (p38α, p38β1, p38β2, p38γ, and p38δ) that mediate apoptosis and inflammation [41], and can either inhibit tumor cell homeostasis, proliferation, differentiation, and apoptosis, or enhance tumor development and metastasis. Our results show that luteoloside could bind to the residues Gly57, Lys74, and Met 130 of p38 MAPK, and this interaction was confirmed by the CETSA experiment. Furthermore, an upregulating p-p38 MAPK was observed in the luteoloside-treated group. We speculate that this may be due to the binding of luteoloside and p38 MAPK, inducing increased activity of p38, but the specific structural mechanism needs further investigation. Some studies have clarified the role of the p38 MAPK signaling pathway in various tumors, providing theoretical guidance for p38 MAPK as a potential target of cancer therapy [42,43,44]. A report demonstrated that the flavonoid isoliquiritigenin exhibited antiproliferative effects in neuroblastoma cells by upregulating p-p38 MAPK [45]. Other research showed that notoginsenoside induced G2/M phase arrest and apoptosis by the activation of p38 MAPK [46].
The activation of p38 MAPK regulates cell cycle progression at both the G1/S and G2/M transitions through multiple mechanisms, including the downregulation of cyclins [47]. Cyclin D1, as the main regulator of the G1 phase of the cell cycle, promotes progression to the S phase and is downregulated by the activation of p38 MAPK [48]. In our study, the gene and protein expression levels of cyclin D1 were downregulated by luteoloside, and p38 MAPK phosphorylation was remarkably increased. This effect was reversed after by treatment with p38 MAPK inhibitor SB203580. Interestingly, we found that C-myc was decreased by luteoloside in neuroblastoma, which was reversed by the inhibition of p38 MAPK. C-myc is an important transcription factor involved in cell cycle control and tumorigenesis that can promote G1–S progression of the cell cycle and is regulated by p38 MAPK [49]. C-myc has been shown to collaborate with cyclin D1, N-myc, or L-myc to induce lymphomas in transgenic mice [50]. Although it is clear that cyclin D1 and C-myc were downregulated by luteoloside in this study, the relationship between these two needs to be further investigated.
In this study, p38 MAPK was identified as a potential target of luteoloside, and p38 MAPK phosphorylation was remarkably increased by luteoloside. Furthermore, cell cycle arrest caused by luteoloside in neuroblastoma cells could be reversed by the inhibition of p38 MAPK. These data reveal that luteoloside induces neuroblastoma cell cycle arrest via the p38 MAPK signaling pathway. In summary, our study shows that luteoloside has anti- neuroblastoma activity and potential applications against human neuroblastoma cells.
4. Materials and Methods
4.1. Cell Culture
SH-SY5Y and HT-22 cell lines were purchased from DSMZ (Braunschweig, Germany); SK-N-AS cell line was purchased from Procell Life Science&Technology Co. (Wuhan, China). SH-SY5Y cells were cultured in DMEM/F12 (1:1), while HT-22 and SK-N-AS cells were cultured in DMEM (Gibco, Grand Island, NY, USA) with 10% FBS (BI, Kibbutz Beit Haemek, Israel) and 1% penicillin/streptomycin (Invitrogen; Carlsbad, CA, USA) at 37 °C with 5% CO2. Luteoloside (HY-N0540) and SB203580 (HY-10256) were purchased from MedChemExpress (MCE, Wuhan, China). Cells were treated with different concentrations of luteoloside (0, 12.5, 25, and 50 μM) for 24 h unless otherwise specified.
4.2. MTT Assay
SH-SY5Y, SK-N-AS, and HT-22 cells were cultured in 96-well plates with a density of 1 × 104/well. Different concentrations (0, 12.5, 25, and 50 μM) of luteoloside were used to treat the SH-SY5Y and SK-N-AS cells for 24 or 48 h, while HT-22 was treated with 0, 12.5, 25, 50, and 100 μM of luteoloside. An amount of 20 µL of MTT solution (M1020, Solarbio, Beijing, China) was added to incubate cells in each well for 4 h; then DMSO (D8370, Solarbio, Beijing, China) was used to stop the reaction. Cell proliferation rates were measured using a spectrophotometer (BioTek, Winooski, VT, USA) set at 490 nm.
4.3. Colony Formation Assay
SH-SY5Y and SK-N-AS cells were cultured in 6-well plates (1000 cells/well). Different concentrations (0, 12.5, 25, and 50 μM) of luteoloside were used to treat the cells. After 10 days, a 4% paraformaldehyde solution (BL539A, Biosharp, Hefei, China) was used to immobilize the cells, and a 0.5% crystal violet solution (G1064, Solarbio, Beijing, China) was used to stain. Three replicates were used for each treatment group.
4.4. Flow Cytometry Analysis
SH-SY5Y cells and SK-N-AS cells were cultured with different concentrations (0, 12.5, 25, and 50 μM) of luteoloside in 6-well plates. Cells were subjected to serum starvation for about 24 h for cell cycle synchronization. Subsequently, cells were resuspended in DMEM with 10% FBS and fixed in 70% ethanol overnight at −20 °C. After washing the cells three times with PBS and collecting them, the cells were stained using the Annexin V-FITC Apoptosis Detection Kit (40302ES60, Yeason, Shanghai, China) and Cell Cycle Detection Kit (KGA512, KeyGEN, Nanjing, China). Lastly, the cell samples were determined by flow cytometry (Beckman, Brea, CA, USA). Data were analyzed by NovoExpress (Agilent, Beijing, China).
4.5. Measurement of Mitochondrial Membrane Potential (MMP)
SH-SY5Y and SK-N-AS cells were seeded in 6-well plates and treated with different concentrations of luteoloside (0, 12.5, 25, and 50 μM) for 24 h. After washing the cells three times with PBS, they were stained with JC-1 staining working solution (M8650, Solarbio, Beijing, China) for 20 min at 37 °C in the dark and then detected through flow cytometry (Beckman, Brea, CA, USA). NovoExpress was used to analyze data.
4.6. Hoechst 33258 Staining Assay
SH-SY5Y and SK-N-AS cells were cultured in 12-well plates and treated with luteoloside (0, 12.5, 25, and 50 μM) for 24 h. After washing the cells three times with PBS, the cells were fixed with 4% paraformaldehyde solution for 30 min. Next, they were stained with 1 μg/mL Hoechst 33258 (C0021, Solarbio, Beijing, China) for 15 min in the dark, and morphological changes of the nuclei were observed with a fluorescence microscope (Leica, Frankfurt, Germany). Nuclear condensation and fragmentation of cells were considered to indicate apoptotic cells.
4.7. qRT-PCR Analysis
RNA extraction reagent was used to extract RNA from SH-SY5Y and SK-N-AS cells. RNA purity and concentration were measured with a UV spectrophotometer according to the instructions. Reverse transcription of RNA to cDNA was performed by a gDNA digester plus (11141ES60, YEASEN, Shanghai, China). Finally, 1 μL of cDNA template, 0.5 μL of primers, and 5 μL of TB Green (RR820A, TaKaRa, Kyoto, Japan) were used for PCR amplification. Each sample was detected using a real-time PCR system (Thermo Fisher Scientific, Billerica, MA, USA). Tubulin served as endogenous control. The primer sequences are listed in Supplementary Table S1.
4.8. Western Blot Assay
Radio immunoprecipitation assay buffer (R0010, Solarbio, Beijing, China) was used to lyse the cells for 20 min to extract total protein. The protein concentrations were detected using the Bio-Rad Protein Assay Kit (PC0020, Servicebio, Wuhan, China). Then the proteins were isolated with 12% SDS-PAGE and retransferred onto PVDF membranes (Meilunbio, Dalian, China). The membranes were blocked with 5% skimmed milk for 2 h and incubated with primary antibodies, including cyclin D1 (26939-1-AP), cyclin E (11554-1-AP), C-myc (10828-1-AP), CDK4 (11026-1-AP), p38 (14064-1-AP), p-P38 (Thr180/Tyr182)-(28796-1-AP), ERK (16443-1-AP), p-ERK (Thr202/Tyr204)-(28733-1-AP), AKT (10176-2-AP), p-AKT (Ser473)-(66444-1-Ig), Bcl-2 (60178-1-lg), and tubulin (11224-1-AP), which were purchased from Proteintech Company (Wuhan, China). JNK (9252) and p-JNK (Thr183/Tyr185)-(9255) were obtained from Cell Signaling Technology (Danvers, MA, USA). Cleaved-caspase3 (A11021) was obtained from ABclonal (Wuhan, China). After washing three times with tris-buffered saline, the secondary antibodies were incubated for 2 h. Band intensity was measured using a chemiluminescence Ultra High Sensitivity ECL Kit (AR1190, Boster, Wuhan, China) and analyzed by the ImageJ software (ImageJ 6.0, Bethesda, MD, USA).
4.9. Subcutaneous Tumorigenesis Model
Female BALB/c nude mice (4–6 weeks old, body weight of 16–20 g) were received from the Experimental Animal Center of Guizhou Medical University. SH-SY5Y cells (2 × 106 cells/200 µL) were injected subcutaneously into the right upper flank of the mice. After 1 week, when tumors reached 40–60 mm3, the mice were randomly subdivided into the luteoloside and DMSO treatment groups (n = 5 per group) and injected with DMSO or luteoloside (25 mg/kg/d), respectively, every 3 days. Meanwhile, 3-day measurements of tumor volume and body weight were taken using vernier calipers. Tumor volume was determined according to the formula: (mm3) = (Long × Width2)/2.21 days. Later, the mice were killed, and kidney, liver, heart, and tumor tissues were taken from the mouse bodies for further study.
4.10. Immunohistochemical Staining (IHC)
To dewax and rehydrate paraffin-embedded tumor tissues from mice, different grades of xylene and ethanol solutions were used. After utilizing sodium citrate to retrieve antigens and blocking endogenous peroxidase activity using H2O2, the tumor samples were incubated with PCNA (GB11010, 1:400, Servicebio, Wuhan, China) and Ki67 (27309-1-AP, 1:300, Proteintech, Wuhan, China) overnight at 4 °C. According to DAB and hematoxylin staining, the secondary antibody was incubated with the uncombined antibody after washing with PBS. Lastly, observations were made by a microscope (Olympus, Kyoto, Japan).
4.11. Hematoxylin–Eosin (HE) Staining Assay
After deparaffinization and rehydration, 1% acid ethanol was applied to 5 m longitudinal sections of the kidney, liver, and heart for 5 min, followed by 5 dips in hematoxylin solution (G1120, Solarbio, Beijing, China) and rinsing in distilled water. After staining with eosin solution for 3 min, the sections were dehydrated with graded alcohol and cleared with xylene. HE staining intensity was determined by a microscope (Olympus, Kyoto, Japan).
4.12. Network Pharmacology Analysis
The 2D structure (ID: 126832) of luteoloside was downloaded from the PubChem compound database. To predict all potential luteoloside targets, we imported the 2D structure of luteoloside into SwissTargetPrediction (http://swisstargetprediction.ch/), all luteoloside targets are listed in Supplementary Table S2. The potential luteoloside targets were introduced into Cytoscape (https://cytoscape.org/) to construct the compound target diagram. “Betweenness centrality” indicates the target’s importance in transferring information. We introduced the potential target into DAVID (https://david.ncifcrf.gov/) to obtain KEGG enrichment analysis. A bubble map of enrichment analysis was produced using the R programming language.
4.13. Molecular Modelling
The crystal structure of the p38 MAPK protein was obtained from the PDB database. The 2D structure (ID: 126832) of luteoloside was downloaded from the PubChem Compound database and transformed to a 3D structure using the Chem3D software (Chem3D 17.0, Waltham, MA, USA). The crystal structure of the p38 MAPK protein and the 3D structure of luteoloside were input the AutoDock software (AutoDock 4.2.6, Beijing, China). Protein activity pockets were identified by automatic pattern recognition after hydrogenation, dehydration, calculation, and atom type addition. According to the binding score, the binding degree of molecule to protein was judged. The results were visualized by the PyMOL software (PyMOL 2.5, Schrödinger, New York, NY, USA).
4.14. Cellular Thermal Shift Assay (CETSA)
SH-SY5Y and SK-N-AS cells were cultured in 10 cm culture dishes for 24 h and then centrifuged at 12,000 rpm for 2 min at 37 °C. Then cells were resuspended with 1 mL of PBS containing 20 mM Tris-HCl, 100 mM NaCl, 5 mM EDTA, 2 mM phenylmethylsulfonyl fluoride (PMSF), 10 ng/mL leupeptin, and 10 μg/mL aprotinin. Three freeze–thaw cycles were performed on the cells. Cells were exposed to liquid nitrogen for 3 min each cycle, then heated at 25 °C for 3 min. After centrifuging at 12,000 rpm at 4 °C for half hour, the supernatants from each group were collected. For the luteoloside-treated groups, luteoloside was treated with a final concentration of 50 μM luteoloside. For the control groups, the same quantity of DMSO was added as before. All groups were heated at 25 °C for an hour. Following this, pairs of control and treatment groups were heated for 3 min at 46, 49, 52, 55, 58, and 61 °C. Finally, the samples were analyzed by Western blot.
4.15. Statistical Analysis
The GraphPad Prism software (GraphPad Prism 6.01, San Diego, CA, USA) was used for processing. An unpaired t-test was used to measure the differences between the two groups with a cut-off of p < 0.05; a one-way analysis of variance combined with a least significant difference t-test was performed to compare differences between multiple groups with a cut-off of p < 0.05.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28041748/s1, Table S1: Primer sequences for qRT-PCR Detection; Table S2: All the potential targets of luteoloside.
Author Contributions
Conceptualization, Y.H. and L.W. (Lu Wang); data curation, L.W. (Lu Wang); formal analysis, Y.H. and L.W. (Lu Wang); investigation, Y.H., M.L., S.L., Z.Z., T.C., Y.W., D.W. and L.W. (Long Wang); methodology, Y.H. and L.W. (Lu Wang); resources, L.W. (Lu Wang); software, M.L.; validation, Y.H., M.L., S.L., Z.Z., T.C., Y.W., D.W., L.W. (Long Wang) and L.W. (Lu Wang); writing—original draft, Y.H., M.L. and L.W. (Lu Wang); writing—review and editing, Y.H. and L.W. (Lu Wang). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Office of Science and Technology of Guizhou (QKHLH [2016]7351) and Guizhou Provincial Natural Science Foundation (No. [2021]4029 and [2022]4017).
Institutional Review Board Statement
Animal experiments were approved by the Ethics Committee of Guizhou Medical University and were in accordance with the Chinese Guidelines for Ethical Review of Laboratory Animal Welfare (approval number: 2200350).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Office of Science and Technology and Provincial Natural Science Foundation of Guizhou.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Abbreviations
| Lu | luteoloside |
| SB | SB203580 |
| IHC | immunohistochemical staining |
| MAPK | mitogen-activated protein kinase |
| HE | hematoxylin–eosin |
| MMP | mitochondrial membrane potential. |
References
- Tan, W.Q.; Chen, G. Artemether Regulates Chemosensitivity to Doxorubicin via Regulation of B7-H3 in Human Neuroblastoma Cells. Med. Sci. Monit. 2017, 23, 4252–4259. [Google Scholar] [CrossRef]
- Wang, L.L.; Teshiba, R. Augmented expression of MYC and/or MYCN protein defines highly aggressive MYC-driven neuroblastoma: A Children’s Oncology Group study. Br. J. Cancer 2015, 113, 57–63. [Google Scholar] [CrossRef]
- Jurkowska, H.; Wróbel, M. A possible mechanism of inhibition of U87MG and SH-SY5Y cancer cell proliferation by diallyl trisulfide and other aspects of its activity. Amino Acids 2017, 49, 1855–1866. [Google Scholar] [CrossRef]
- Liu, W.; Fan, Z.; Gao, F.; Ou, L.; Li, M.; Zhou, X.; Luo, W.; Wei, P.; Miao, F. Emodin inhibits zinc-induced neurotoxicity in neuroblastoma SH-SY5Y cells. Biosci. Rep. 2019, 39, bsr20182378. [Google Scholar] [CrossRef]
- London, W.B.; Bagatell, R. Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children’s Oncology Group early-phase trials. Cancer 2017, 123, 4914–4923. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Ponte, L.G.S.; Pavan, I.C.B. The Hallmarks of Flavonoids in Cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tian, Z. Luteoloside attenuates neuroinflammation in focal cerebral ischemia in rats via regulation of the PPARγ/Nrf2/NF-κB signaling pathway. Int. Immunopharmacol. 2019, 66, 309–316. [Google Scholar] [CrossRef]
- Cao, Z.; Ding, Y.; Ke, Z.; Cao, L.; Li, N.; Ding, G.; Wang, Z.; Xiao, W. Luteoloside Acts as 3C Protease Inhibitor of Enterovirus 71 In Vitro. PLoS ONE 2016, 11, e0148693. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Shang, Z.; Wang, P.; Li, S.; Zhang, Q.; Tian, H.; Ren, D.; Han, X. Protection of Luteolin-7-O-Glucoside Against Doxorubicin-Induced Injury Through PTEN/Akt and ERK Pathway in H9c2 Cells. Cardiovasc. Toxicol. 2016, 16, 101–110. [Google Scholar] [CrossRef]
- Velmurugan, B.K.; Lin, J.T. Luteolin-7-O-Glucoside Inhibits Oral Cancer Cell Migration and Invasion by Regulating Matrix Metalloproteinase-2 Expression and Extracellular Signal-Regulated Kinase Pathway. Biomolecules 2020, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.Y.; Chen, P.J. Luteolin-7-O-glucoside inhibits cell proliferation and modulates apoptosis through the AKT signaling pathway in human nasopharyngeal carcinoma. Environ. Toxicol. 2021, 36, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wang, C.; Li, L.; Liang, H.; Dai, J.; Ling, X.; Tang, H. Luteoloside Inhibits Proliferation and Promotes Intrinsic and Extrinsic Pathway-Mediated Apoptosis Involving MAPK and mTOR Signaling Pathways in Human Cervical Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1664. [Google Scholar] [CrossRef]
- Jian, C.; Lu, W. Effects of luteoloside on proliferation, migration and invasion of MCF-7 cells and its mechanism. Propr. Chin. Med. 2022, 44, 587–592. [Google Scholar]
- Lin, C.W.; Wu, M.J. Neurotrophic and cytoprotective action of luteolin in PC12 cells through ERK-dependent induction of Nrf2-driven HO-1 wxpression. J. Agric. Food Chem. 2010, 58, 4477–4486. [Google Scholar] [CrossRef]
- You, Y.; Wang, R.; Shao, N.; Zhi, F.; Yang, Y. Luteolin suppresses tumor proliferation through inducing apoptosis and autophagy via MAPK activation in glioma. OncoTargets Ther. 2019, 12, 2383–2396. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Ma, Z. Multi-target mechanism of Tripteryguim wilfordii Hook for treatment of ankylosing spondylitis based on network pharmacology and molecular docking. Ann. Med. 2021, 53, 1091–1099. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, Q.; Wang, Y. Network pharmacology-based investigation of potential targets of astragalus membranaceous-angelica sinensis compound acting on diabetic nephropathy. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology. Nat. Biotechnol. 2007, 25, 1110–1111. [Google Scholar] [CrossRef]
- Diachkov, M.V.; Ferrer, K.; Oklestkova, J.; Rarova, L.; Bazgier, V.; Kvasnica, M. Synthesis and Biological Activity of Brassinosteroid Analogues with a Nitrogen-Containing Side Chain. Int. J. Mol. Sci. 2020, 22, 155. [Google Scholar] [CrossRef]
- Le Grand, M.; Mukha, A. Interplay between MycN and c-Myc regulates radioresistance and cancer stem cell phenotype in neuroblastoma upon glutamine deprivation. Theranostics 2020, 10, 6411–6429. [Google Scholar] [CrossRef] [PubMed]
- Peet, A.C.; McConville, C. 1H MRS identifies specific metabolite profiles associated with MYCN-amplified and non-amplified tumour subtypes of neuroblastoma cell lines. NMR Biomed. 2007, 20, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. Cancer cell cycles. Science 1996, 274, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Peng, Y.; Wei, W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022, 32, 30–44. [Google Scholar] [CrossRef]
- Suski, J.M.; Braun, M. Targeting cell-cycle machinery in cancer. Cancer Cell 2021, 39, 759–778. [Google Scholar] [CrossRef] [PubMed]
- Costa-Guda, J.; Corrado, K. CDK4/6 Dependence of Cyclin D1-Driven Parathyroid Neoplasia in Transgenic Mice. Endocrinology 2020, 161, bqaa159. [Google Scholar] [CrossRef]
- Miliani de Marval, P.L.; Macias, E. Enhanced malignant tumorigenesis in Cdk4 transgenic mice. Oncogene 2004, 23, 1863–1873. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nature reviews. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef]
- Álvarez-Fernández, M.; Malumbres, M. Mechanisms of Sensitivity and Resistance to CDK4/6 Inhibition. Cancer Cell 2020, 37, 514–529. [Google Scholar] [CrossRef]
- Puyol, M.; Martín, A.; Dubus, P.; Mulero, F.; Pizcueta, P.; Khan, G.; Guerra, C.; Santamaría, D.; Barbacid, M. A Synthetic Lethal Interaction between K-Ras Oncogenes and Cdk4 Unveils a Therapeutic Strategy for Non-small Cell Lung Carcinoma. Cancer Cell 2010, 18, 63–73. [Google Scholar] [CrossRef]
- Van Arsdale, T.; Boshoff, C. Molecular Pathways: Targeting the Cyclin D-CDK4/6 Axis for Cancer Treatment. Clin. Cancer Res. 2015, 21, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Ferlazzo, N. Moringin from Moringa Oleifera Seeds Inhibits Growth, Arrests Cell-Cycle, and Induces Apoptosis of SH-SY5Y Human Neuroblastoma Cells through the Modulation of NF-κB and Apoptotic Related Factors. Int. J. Mol. Sci. 2019, 20, 1930. [Google Scholar] [CrossRef]
- Morandi, F.; Bensa, V. The Olive Leaves Extract Has Anti-Tumor Effects against Neuroblastoma through Inhibition of Cell Proliferation and Induction of Apoptosis. Nutrients 2021, 13, 2178. [Google Scholar] [CrossRef] [PubMed]
- Seçme, M.; Eroğlu, C.; Dodurga, Y.; Bağcı, G. Investigation of anticancer mechanism of oleuropein via cell cycle and apoptotic pathways in SH-SY5Y neuroblastoma cells. Gene 2016, 585, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liu, W.; Ke, X.; Li, J.; Hu, R.; Cui, H.; Song, G. Artemisinin reduces cell proliferation and induces apoptosis in neuroblastoma. Oncol. Rep. 2014, 32, 1094–1100. [Google Scholar] [CrossRef]
- Zhou, M.; Shen, S. Luteoloside induces G(0)/G(1) arrest and pro-death autophagy through the ROS-mediated AKT/mTOR/p70S6K signalling pathway in human non-small cell lung cancer cell lines. Biochem. Biophys. Res. Commun. 2017, 494, 263–269. [Google Scholar] [CrossRef]
- Yuan, Z.; Pan, Y.; Leng, T.; Chu, Y.; Zhang, H.; Ma, J.; Ma, X. Progress and Prospects of Research Ideas and Methods in the Network Pharmacology of Traditional Chinese Medicine. J. Pharm. Pharm. Sci. 2022, 25, 218–226. [Google Scholar] [CrossRef]
- Li, H.; Zhao, L.; Zhang, B.; Jiang, Y.; Wang, X.; Guo, Y.; Liu, H.; Li, S.; Tong, X. A Network Pharmacology Approach to Determine Active Compounds and Action Mechanisms of Ge-Gen-Qin-Lian Decoction for Treatment of Type 2 Diabetes. Evid.-Based Complement. Altern. Med. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Lu, X.; Zheng, Y.; Wen, F.; Huang, W.; Chen, X.; Ruan, S.; Gu, S.; Hu, Y.; Teng, Y.; Shu, P. Study of the active ingredients and mechanism of Sparganii rhizoma in gastric cancer based on HPLC-Q-TOF–MS/MS and network pharmacology. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-Activated Protein (MAP) Kinase Pathways: Regulation and Physiological Functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Igea, A.; Nebreda, A.R. The Stress Kinase p38α as a Target for Cancer Therapy. Cancer Res. 2015, 75, 3997–4002. [Google Scholar] [CrossRef]
- Ono, K.; Han, J. The p38 signal transduction pathway Activation and function. Cell. Signal. 2000, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Canals, D.; Adada, M.; Coant, N.; Salama, M.F.; Helke, K.L.; Arthur, J.S.; Shroyer, K.R.; Kitatani, K.; Obeid, L.M.; et al. P38 delta MAPK promotes breast cancer progression and lung metastasis by enhancing cell proliferation and cell detachment. Oncogene 2017, 36, 6649–6657. [Google Scholar] [CrossRef]
- Escobar, S.J.M.; Fong, G.M. Anti-proliferative and cytotoxic activities of the flavonoid isoliquiritigenin in the human neuroblastoma cell line SH-SY5Y. Chem.-Biol. Interact. 2019, 299, 77–87. [Google Scholar] [CrossRef]
- Gao, B.; Shi, H.L. p38 MAPK and ERK1/2 pathways are involved in the pro-apoptotic effect of notoginsenoside Ft1 on human neuroblastoma SH-SY5Y cells. Life Sci. 2014, 108, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, C.; Nebreda, A.R. Cell cycle regulation by p38 MAP kinases. Biol. Cell 2001, 93, 47–51. [Google Scholar] [CrossRef]
- Zhao, X.-M.; Xiang, Z.-L.; Chen, Y.-X.; Yang, P.; Hu, Y.; Zeng, Z.-C. A sequence polymorphism on 8q24 is associated with survival in hepatocellular carcinoma patients who received radiation therapy. Sci. Rep. 2018, 8, 2264. [Google Scholar] [CrossRef]
- Berger, A.; Roberts, M.A.; Hoff, B. How dietary arachidonic- and docosahexaenoic- acid rich oils differentially affect the murine hepatic transcriptome. Lipids Health Dis. 2006, 5, 10. [Google Scholar] [CrossRef]
- Bodrug, S.; Warner, B.; Bath, M.; Lindeman, G.; Harris, A.; Adams, J. Cyclin D1 transgene impedes lymphocyte maturation and collaborates in lymphomagenesis with the myc gene. EMBO J. 1994, 13, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).