Melamine Foam-Derived Carbon Scaffold for Dendrite-Free and Stable Zinc Metal Anode
Abstract
:1. Introduction
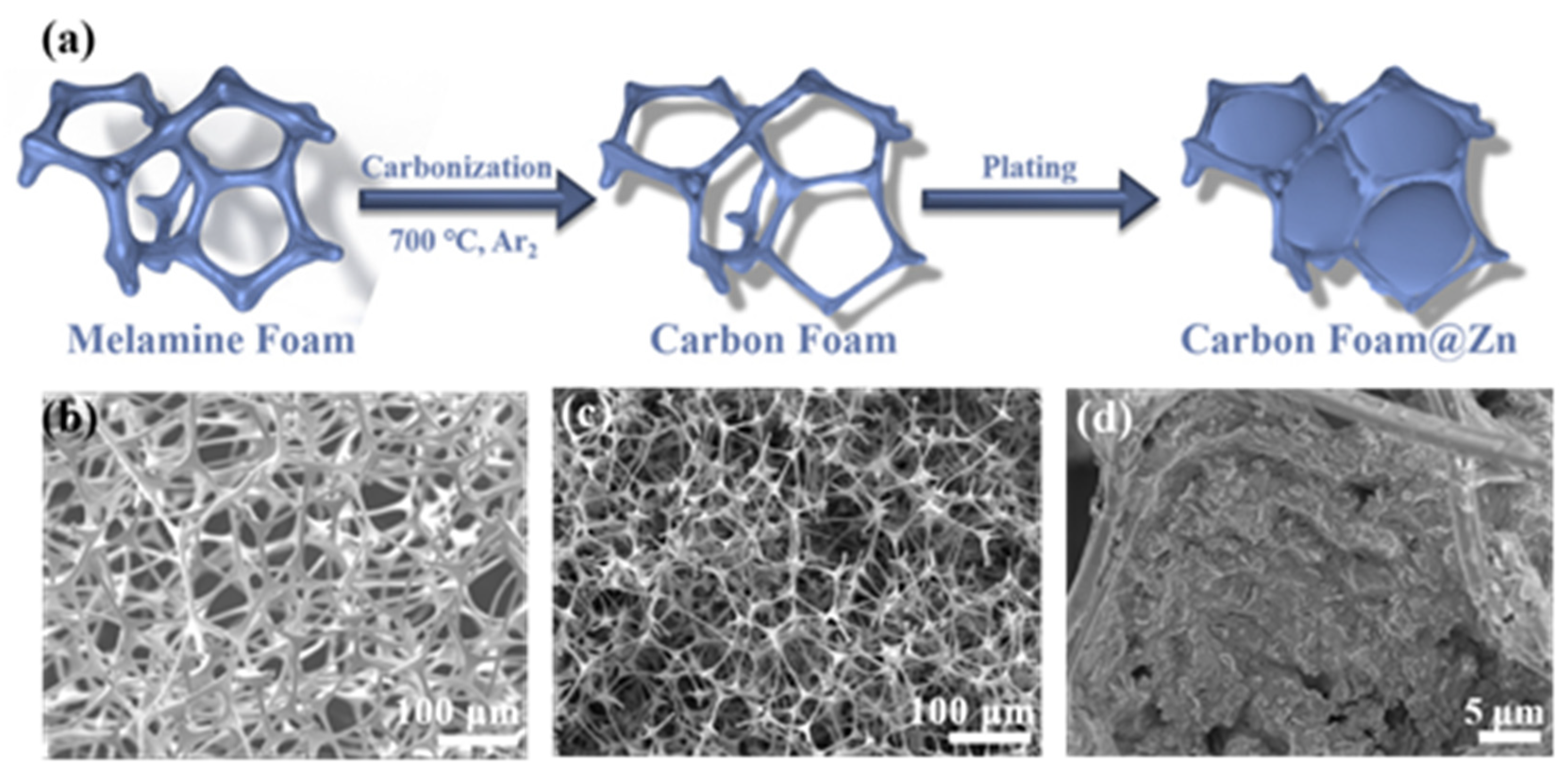
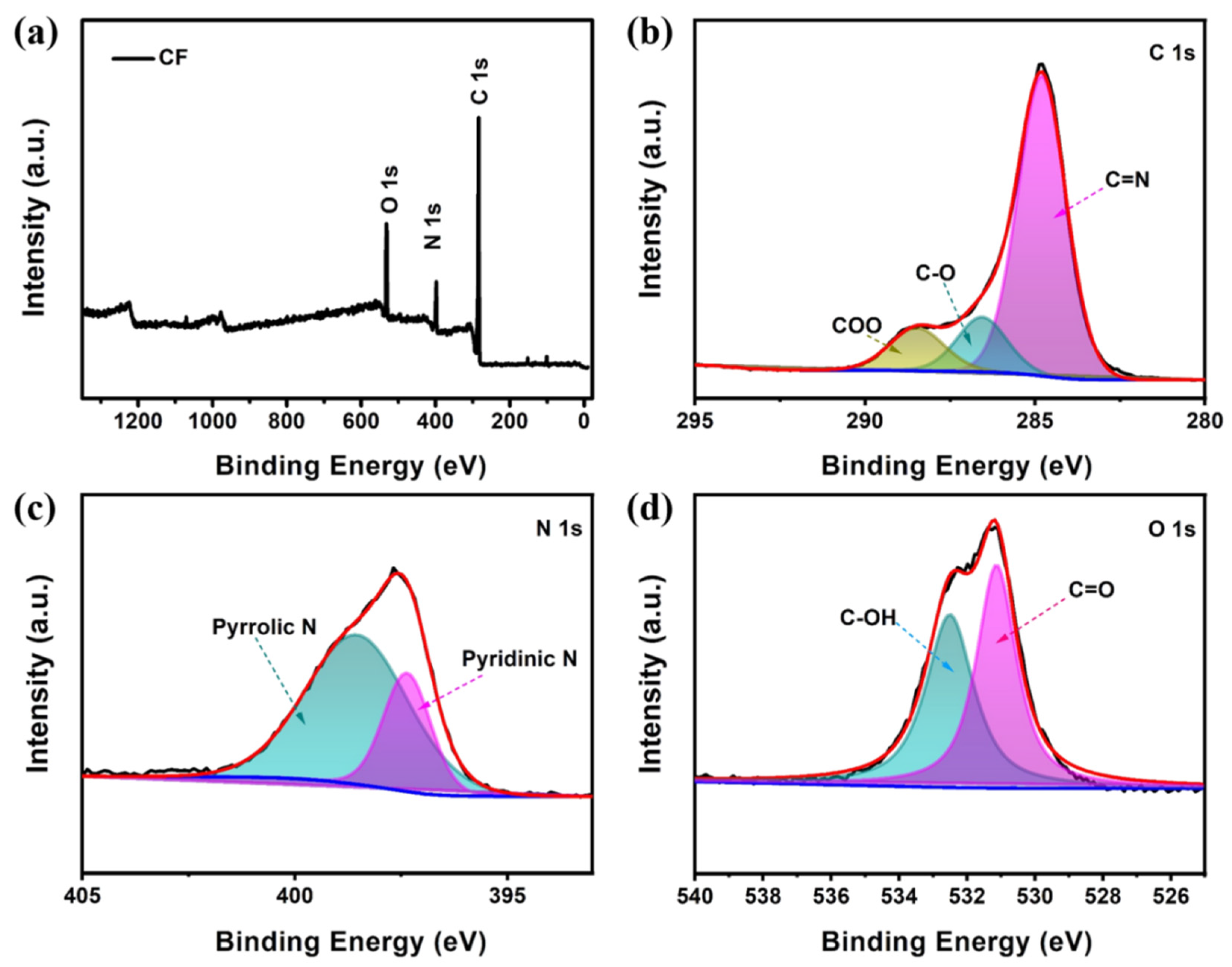
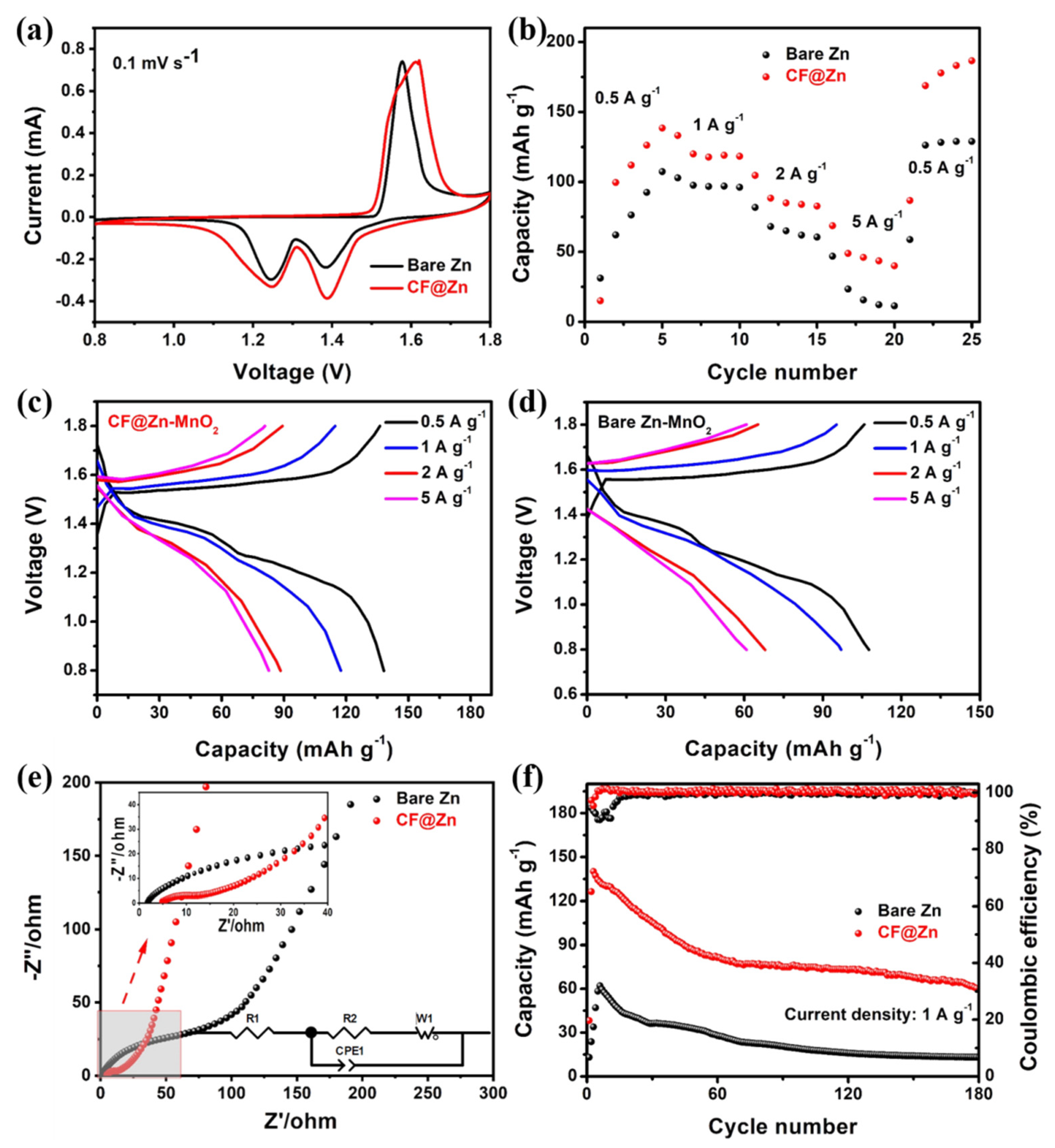
2. Results
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Carbon Foam
3.3. Preparation of the α-MnO2 Cathode
3.4. Electrochemical Measurements
3.5. Materials Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Du, W.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2020, 13, 3330–3360. [Google Scholar] [CrossRef]
- Han, C.; Li, W.; Liu, H.K.; Dou, S.; Wang, J. Principals and strategies for constructing a highly reversible zinc metal anode in aqueous batteries. Nano Energy 2020, 74, 104880. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Z.; Tawiah, B.; Wang, Y.; Chan, C.-Y.; Fei, B.; Pan, F. Recent advances in zinc anodes for high-performance aqueous Zn-ion batteries. Nano Energy 2020, 70, 104523. [Google Scholar] [CrossRef]
- Li, C.; Xie, X.; Liang, S.; Zhou, J. Issues and Future Perspective on Zinc Metal Anode for Rechargeable Aqueous Zinc-Ion Batteries. Energy Environ. Mater. 2020, 3, 146–159. [Google Scholar] [CrossRef]
- Ma, L.; Zhi, C. Zn electrode/electrolyte interfaces of Zn batteries: A mini review. Electrochem. Commun. 2021, 122, 106898. [Google Scholar] [CrossRef]
- Pu, X.; Jiang, B.; Wang, X.; Liu, W.; Dong, L.; Kang, F.; Xu, C. High-Performance Aqueous Zinc-Ion Batteries Realized by MOF Materials. Nanomicro Lett. 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Xiong, T.; Chen, T.; Yu, C.; Zhang, M.; Yang, Y.; Deng, Z.; Xiao, H.; Lee, W.S.V.; Xue, J. Dendrite-Free Anodes Enabled by a Composite of a ZnAl Alloy with a Copper Mesh for High-Performing Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 28129–28139. [Google Scholar] [CrossRef]
- Ye, J.; Xia, G.; Yang, X.; Li, X.; Wang, J.; Zheng, Z.; Fu, Z.; Zhang, Q.; Hu, C. Ultrafine Ni5P4 nanoparticles embedded in 3D porous carbon foams for high-performance lithium-ion and potassium-ion batteries. J. Alloys Compd. 2022, 891, 161951. [Google Scholar] [CrossRef]
- Cao, P.; Zhou, X.; Wei, A.; Meng, Q.; Ye, H.; Liu, W.; Tang, J.; Yang, J. Fast-Charging and Ultrahigh-Capacity Zinc Metal Anode for High-Performance Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2100398. [Google Scholar] [CrossRef]
- Chen, P.; Yuan, X.; Xia, Y.; Zhang, Y.; Fu, L.; Liu, L.; Yu, N.; Huang, Q.; Wang, B.; Hu, X.; et al. An Artificial Polyacrylonitrile Coating Layer Confining Zinc Dendrite Growth for Highly Reversible Aqueous Zinc-Based Batteries. Adv. Sci. 2021, 8, 2100309. [Google Scholar] [CrossRef]
- Tao, F.; Feng, K.; Liu, Y.; Ren, J.; Xiong, Y.; Li, C.; Ren, F. Suppressing interfacial side reactions of zinc metal anode via isolation effect toward high-performance aqueous zinc-ion batteries. Nano Res. 2022. [Google Scholar] [CrossRef]
- Zhai, P.; Zhai, X.; Jia, Z.; Zhang, W.; Pan, K.; Liu, Y. Inhibiting Corrosion and Side Reactions of Zinc Metal Anode by Nano-CaSiO3 Coating towards High-performance Aqueous Zinc-Ion Batteries. Nanotechnology 2022, 34, 085402. [Google Scholar] [CrossRef]
- Zhu, D.; Zheng, Y.; Xiong, Y.; Cui, C.; Ren, F.; Liu, Y. In situ growth of S-doped ZnO thin film enabling dendrite-free zinc anode for high-performance aqueous zinc-ion batteries. J. Alloys Compd. 2022, 918, 165486. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, Y.; Yan, B.; Wang, C.; Liu, Z.; Huang, Z.; Wang, Y.; Li, Y.; Li, H.; Song, L.; et al. Hydrogen-Substituted Graphdiyne Ion Tunnels Directing Concentration Redistribution for Commercial-Grade Dendrite-Free Zinc Anodes. Adv. Mater. 2020, 32, 2001755. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, W.; Chen, M.; Huang, A.; Tian, Q.; Xu, X.; Chen, J. Integrated design of aqueous zinc-ion batteries based on dendrite-free zinc microspheres/carbon nanotubes/nanocellulose composite film anode. J. Colloid Interface Sci. 2021, 594, 389–397. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, R.; Wang, Y.; Bai, Y.; Wu, C. Regulating Uniform Zn Deposition via Hybrid Artificial Layer for Stable Aqueous Zn-Ion Batteries. Energy Mater. Adv. 2022, 2022, 9809626. [Google Scholar] [CrossRef]
- Zhao, J.W.; Zhang, J.; Yang, W.H.; Chen, B.B.; Zhao, Z.M.; Qiu, H.Y.; Dong, S.M.; Zhou, X.H.; Cui, G.L.; Chen, L.Q. “Water-in-deep eutectic solvent” electrolytes enable zinc metal anodes for rechargeable aqueous batteries. Nano Energy 2019, 57, 625–634. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.L.; Sun, W.; Han, F.D.; Faraone, A.; Dura, J.A.; Xu, K.; Wang, C.S. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef]
- Han, M.; Huang, J.; Wu, X.; Liang, S.; Zhou, J. Electrolyte Modulation Strategies for Rechargeable Zn Batteries. Chin. J. Inorg. Chem. 2022, 38, 1451–1469. [Google Scholar] [CrossRef]
- Song, Y.; Ruan, P.; Mao, C.; Chang, Y.; Wang, L.; Dai, L.; Zhou, P.; Lu, B.; Zhou, J.; He, Z. Metal–Organic Frameworks Functionalized Separators for Robust Aqueous Zinc-Ion Batteries. Nanomicro Lett. 2022, 14, 218. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Huang, X.; Zhu, L.; Tang, Y.; Ji, X.; Wang, H. Simultaneously Regulating the Ion Distribution and Electric Field to Achieve Dendrite-Free Zn Anode. Small 2020, 16, e2000929. [Google Scholar] [CrossRef]
- Yang, J.; Yang, P.; Yan, W.; Zhao, J.; Fan, H. 3D zincophilic micro-scaffold enables stable Zn deposition. Energy Storage Mater. 2022, 51, 259–265. [Google Scholar] [CrossRef]
- Li, D.; Lv, T.; Chen, Z.; Yang, Y.; Liu, Y.; Wan, J.; Qi, Y.; Cao, S.; Chen, T. High-Performance Compressible Zinc Ion Battery Based on Melamine Foam-Derived Electrodes. Small Struct. 2022, 3, 2200027. [Google Scholar] [CrossRef]
- Li, C.; Xie, X.; Liu, H.; Wang, P.; Deng, C.; Lu, B.; Zhou, J.; Liang, S. Integrated ‘all-in-one’ strategy to stabilize zinc anodes for high-performance zinc-ion batteries. Natl. Sci. Rev. 2022, 9, nwab177. [Google Scholar] [CrossRef]
- He, H.; Tong, H.; Song, X.; Song, X.; Liu, J. Highly stable Zn metal anodes enabled by atomic layer deposited Al2O3 coating for aqueous zinc-ion batteries. J. Mater. Chem. A 2020, 8, 7836–7846. [Google Scholar] [CrossRef]
- Li, C.; Shi, X.; Liang, S.; Ma, X.; Han, M.; Wu, X.; Zhou, J. Spatially homogeneous copper foam as surface dendrite-free host for zinc metal anode. Chem. Eng. J. 2020, 379, 122248. [Google Scholar] [CrossRef]
- Tao, F.; Liu, Y.; Ren, X.; Wang, J.; Zhou, Y.; Miao, Y.; Ren, F.; Wei, S.; Ma, J. Different surface modification methods and coating materials of zinc metal anode. J. Energy Chem. 2022, 66, 397–412. [Google Scholar] [CrossRef]
- Ma, X.; Xiong, X.; Zou, P.; Liu, W.; Wang, F.; Liang, L.; Liu, Y.; Yuan, C.; Lin, Z. General and Scalable Fabrication of Core-Shell Metal Sulfides@C Anchored on 3D N-Doped Foam toward Flexible Sodium Ion Batteries. Small 2019, 15, e1903259. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yue, M.; Zhang, C.J.; Dong, Y.; Lu, P.; Zheng, S.; Huang, H.; Chen, J.; Wen, P.; Xu, Z.; et al. 3D Flexible, Conductive, and Recyclable Ti3C2Tx MXene-Melamine Foam for High-Areal-Capacity and Long-Lifetime Alkali-Metal Anode. ACS Nano 2020, 14, 8678–8688. [Google Scholar] [CrossRef]
- Tao, F.; Liu, Y.; Ren, X.; Jiang, A.; Wei, H.; Zhai, X.; Wang, F.; Stock, H.R.; Wen, S.; Ren, F. Carbon Nanotube-based Nanomaterials for High-performance Sodium-ion batteries: Recent advances and perspectives. J. Alloys Compd. 2021, 873, 159742. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, Y.; Zhang, S.; Xu, Z.; Feng, T.; Zhou, H.; Wu, M. Binder-Free Freestanding 3D Zn-Graphene Anode Induced from Commercial Zinc Powders and Graphene Oxide for Zinc Ion Battery with High Utilization Rate. ACS Appl. Energy Mater. 2022, 5, 15222–15232. [Google Scholar] [CrossRef]
- Xie, R.; Fang, Z.; Yan, J.; Wang, W.; Cao, X.; Qiu, X. Fabrication of diverse carbon forms and their reversed applications in hexane/water separation. Water Sci. Technol. 2020, 82, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, J.; Liu, Y.; Ren, F. An amorphous ZnO and oxygen vacancy modified nitrogen-doped carbon skeleton with lithiophilicity and ionic conductivity for stable lithium metal anodes. J. Mater. Chem. A 2022, 10, 17395–17405. [Google Scholar] [CrossRef]
- Zhou, J.; Xie, M.; Wu, F.; Mei, Y.; Hao, Y.; Huang, R.; Wei, G.; Liu, A.; Li, L.; Chen, R. Ultrathin Surface Coating of Nitrogen-Doped Graphene Enables Stable Zinc Anodes for Aqueous Zinc-Ion Batteries. Adv. Mater. 2021, 33, e2101649. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Y.; Wei, H.J.; Li, T.F.; Volinsky, A.A. Recent advances and perspective in metal coordination materials-based electrode materials for potassium-ion batteries. Rare Met. 2021, 40, 448–470. [Google Scholar] [CrossRef]
- Wu, C.; Xie, K.; Ren, K.; Yang, S.; Wang, Q. Dendrite-free Zn anodes enabled by functional nitrogen-doped carbon protective layers for aqueous zinc-ion batteries. Dalton Trans. 2020, 49, 17629–17634. [Google Scholar] [CrossRef]
- Shen, C.; Li, X.; Li, N.; Xie, K.; Wang, J.-g.; Liu, X.; Wei, B. Graphene-Boosted, High-Performance Aqueous Zn-Ion Battery. Acs Appl. Mater. Inter. 2018, 10, 25446–25453. [Google Scholar] [CrossRef]
- Li, Z.; Wu, L.; Dong, S.; Xu, T.; Li, S.; An, Y.; Jiang, J.; Zhang, X. Pencil Drawing Stable Interface for Reversible and Durable Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2006495. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Z.; Hao, R.; Huang, Y.; Liu, W.; Tan, Y.; Li, P.; Yan, J.; Liu, K. Ultra-highly stable zinc metal anode via 3D-printed g-C3N4 modulating interface for long life energy storage systems. Chem. Eng. J. 2021, 403, 126425. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, W.; Huang, A.; Chen, M.; Chen, J.; Tian, Q.; Xu, J. Modifying the Zn anode with carbon black coating and nanofibrillated cellulose binder: A strategy to realize dendrite-free Zn-MnO2 batteries. J. Colloid Interface Sci. 2020, 577, 256–264. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Wei, C.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Flexible and Free-Standing Ti3C2Tx MXene@Zn Paper for Dendrite-Free Aqueous Zinc Metal Batteries and Nonaqueous Lithium Metal Batteries. ACS Nano 2019, 13, 11676–11685. [Google Scholar] [CrossRef]
- Wang, L.-P.; Li, N.-W.; Wang, T.-S.; Yin, Y.-X.; Guo, Y.-G.; Wang, C.-R. Conductive graphite fiber as a stable host for zinc metal anodes. Electrochim. Acta 2017, 244, 172–177. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Tao, F.; Xing, Y.; Pei, Y.; Ren, F. Melamine Foam-Derived Carbon Scaffold for Dendrite-Free and Stable Zinc Metal Anode. Molecules 2023, 28, 1742. https://doi.org/10.3390/molecules28041742
Liu Y, Tao F, Xing Y, Pei Y, Ren F. Melamine Foam-Derived Carbon Scaffold for Dendrite-Free and Stable Zinc Metal Anode. Molecules. 2023; 28(4):1742. https://doi.org/10.3390/molecules28041742
Chicago/Turabian StyleLiu, Yong, Feng Tao, Yibo Xing, Yifei Pei, and Fengzhang Ren. 2023. "Melamine Foam-Derived Carbon Scaffold for Dendrite-Free and Stable Zinc Metal Anode" Molecules 28, no. 4: 1742. https://doi.org/10.3390/molecules28041742
APA StyleLiu, Y., Tao, F., Xing, Y., Pei, Y., & Ren, F. (2023). Melamine Foam-Derived Carbon Scaffold for Dendrite-Free and Stable Zinc Metal Anode. Molecules, 28(4), 1742. https://doi.org/10.3390/molecules28041742





