Chemical Composition, Antimicrobial and Antioxidant Bioautography Activity of Essential Oil from Leaves of Amazon Plant Clinopodium brownei (Sw.)
Abstract
1. Introduction
2. Results
2.1. Clinopodiun Brownei Essential Oil Extraction
2.2. Chemical Composition
2.3. Antioxidant Activity
2.4. Bioautography Antioxidant
2.5. Antimicrobial Activity
2.6. Bioautography Antimicrobial
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Extraction
4.3. GC/FID Analysis
4.4. GC/MS Analysis
4.5. Identification of Compounds
4.6. Antioxidant Activity, DPPH Assay
4.7. Antioxidant Activity, ABTS Assay
4.8. Antioxidant Bioautography
4.9. Antimicrobial Activity
4.10. Antimicrobial Bioautography
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cuesta, F.; Peralvo, M.; Merino-Viteri, A.; Bustamante, M.; Baquero, F.; Freile, J.; Muriel, P.; Torres-Carvajal, O. Priority areas for biodiversity conservation in mainland Ecuador. Neotrop. Biodivers. 2017, 3, 93–106. [Google Scholar] [CrossRef]
- Sierra, R.; Campos, F.; Chamberlin, J. Assessing biodiversity conservation priorities: Ecosystem risk and representativeness in continental Ecuador. Landsc. Urban Plan. 2002, 59, 95–110. [Google Scholar] [CrossRef]
- Mestanza-Ramón, C.; Henkanaththegedara, S.M.; Vásconez Duchicela, P.; Vargas Tierras, Y.; Sánchez Capa, M.; Constante Mejía, D.; Mestanza Ramón, P. In-situ and ex-situ biodiversity conservation in Ecuador: A review of policies, actions and challenges. Diversity 2020, 12, 315. [Google Scholar] [CrossRef]
- De la Torre, L.; Navarrete, H.; Macía, M.; Balslev, H.; Muriel, P. Enciclopedia de las Plantas Útiles del Ecuador (con Extracto de Datos), 1st ed.; Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia Universidad Católica del Ecuador & Herbario AAU del Departamento de Ciencias Biológicas de la Universidad de Aarhus: Quito, Ecuador, 2008. [Google Scholar]
- Giovannini, P. Medicinal plants of the Achuar (Jivaro) of Amazonian Ecuador: Ethnobotanical survey and comparison with other Amazonian pharmacopoeias. J. Ethnopharmacol. 2015, 164, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, J.L.; Bracco, F.; Cerna, M.; Vita Finzi, P.; Vidari, G. Ethnobotanical research at the Kutukú Scientific Station, Morona-Santiago, Ecuador. BioMed Rev. Int. 2016, 2016, 9105746. [Google Scholar] [CrossRef]
- Cerón, C.; Montalvo, C.; Calazacón, A. Etnobotánica Tsáchila, Pichincha-Ecuador. Cinchonia 2004, 5, 109–194. [Google Scholar]
- Velásquez, G.; Moreira, W.; Caicedo, C.; Preciado, O.; Jaramillo, T.; Iturralde, G.; Aizaga, G. El uso de la etnobotánica como cura de eruptivas de la infancia por la etnia chachi. Esmeraldas. Ecuador. Rev. Investig. Talentos 2016, 3, 1–10. [Google Scholar]
- Cerón, C.; Reyes, C. Parches de bosque y etnobotánica Shuar en Palora, Morona Santiago-Ecuador. Cinchonia 2007, 8, 66–83. [Google Scholar]
- Zhiñin, H.R.; Poma, B.V.; González, L.P.; Quito, G.B. Etnobotánica y derechos de la naturaleza en el aja shuar: Caso de estudio parroquia Nankais, cantón Nangaritza, provincia Zamora Chinchipe, Ecuador. Siembra 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Cerón, C. Etnobotánica Huaorani de Tivacuno-Tiputini Parque Nacional Yasuní. Cinchonia 2002, 3, 64–94. [Google Scholar]
- Noriega, P. Extracción, química, actividad biológica, control de calidad y potencial económico de los aceites esenciales. La Granja 2009, 10, 3–13. [Google Scholar] [CrossRef]
- Marriott, P.J.; Shellie, R.; Cornwell, C. Gas chromatographic technologies for the analysis of essential oils. J. Chromatogr. A 2001, 936, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Baptista, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Aziz, Z.A.; Ahmad, A.; Setapar, S.H.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Ashraf, G.M. Essential oils: Extraction techniques, pharmaceutical and therapeutic potential-a review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; Moraes, A.A.; Costa, K.S.; Pereira, J.M.; Taube, P.S.; Costa, C.M.; Faria, L.J. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Maccioni, A.M.; Anchisi, C.; Sanna, A.; Sardu, C.; Dessi, S. Preservative systems containing essential oils in cosmetic products. Int. J. Cosmet. Sci. 2002, 24, 53–59. [Google Scholar] [CrossRef]
- Vankar, P.S. Essential oils and fragrances from natural sources. Resonance 2004, 9, 30–41. [Google Scholar] [CrossRef]
- Bruni, R.; Medici, A.; Andreotti, E.; Fantin, C.; Muzzoli, M.; Dehesa, M.; Sacchetti, G. Chemical composition and biological activities of Ishpingo essential oil, a traditional Ecuadorian spice from Ocotea quixos (Lam.) Kosterm. (Lauraceae) flower calices. Food Chem. 2004, 85, 415–421. [Google Scholar] [CrossRef]
- Sacchetti, G.; Guerrini, A.; Noriega, P.; Bianchi, A.; Bruni, R. Essential oil of wild ocotea quixos (lam.) kosterm. (lauraceae) leaves from amazonian Ecuador. Flavour Fragr. J. 2006, 21, 674–676. [Google Scholar] [CrossRef]
- Noriega, P.; Mosquera, T.; Paredes, E.; Parra, M.; Zappia, M.; Herrera, M.; Osorio, E. Antimicrobial and antioxidant bioautography activity of bark essential oil from Ocotea quixos (Lam.) kosterm. J. Planar Chromatogr.-Mod. TLC 2018, 31, 163–168. [Google Scholar] [CrossRef]
- Guerrini, A.; Sacchetti, G.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Andreotti, E.; Bruni, R. Bioactivities of Piper aduncum L. and Piper obliquum Ruiz & Pavon (Piperaceae) essential oils from eastern Ecuador. Environ. Toxicol. Pharmacol. 2009, 27, 39–48. [Google Scholar] [PubMed]
- Scalvenzi, L.; Radice, M.; Toma, L.; Severini, F.; Boccolini, D.; Bella, A.; Di Luca, M. Larvicidal activity of Ocimum campechianum, Ocotea quixos and Piper aduncum essential oils against Aedes aegypti. Parasite 2019, 26, 23. [Google Scholar] [CrossRef]
- Ballesteros, J.L.; Tacchini, M.; Spagnoletti, A.; Grandini, A.; Paganetto, G.; Neri, L.M.; Sacchetti, G. Rediscovering medicinal Amazonian aromatic plants: Piper carpunya (Piperaceae) essential oil as paradigmatic study. Evid. Based Complement. Altern. Med. 2019, 2019, 6194640. [Google Scholar] [CrossRef] [PubMed]
- Valarezo, E.; Rivera, J.X.; Coronel, E.; Barzallo, M.A.; Calva, J.; Cartuche, L.; Meneses, M.A. Study of volatile secondary metabolites present in Piper carpunya leaves and in the traditional ecuadorian beverage Guaviduca. Plants 2021, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Noriega, P.; Guerrini, A.; Sacchetti, G.; Grandini, A.; Ankuash, E.; Manfredini, S. Chemical composition and biological activity of five essential oils from the Ecuadorian Amazon rain forest. Molecules 2019, 24, 1637. [Google Scholar] [CrossRef]
- Noriega, P.; Mosquera, T.; Osorio, E.; Guerra, P.; Fonseca, A. Clinopodium nubigenum (Kunth) Kuntze essential oil: Chemical composition, antioxidant activity, and antimicrobial test against respiratory pathogens. J. Pharmacogn. Phytother. 2018, 10, 149–157. [Google Scholar]
- Salto, M.; Puente, B.; Malafronte, N.; Braca, A. Phenolic compounds from Clinopodium tomentosum (Kunth) govaerts (Lamiaceae). J. Braz. Chem. Soc. 2014, 25, 2121–2124. [Google Scholar]
- Matailo, A.; Bec, N.; Calva, J.; Ramírez, J.; Andrade, J.M.; Larroque, C.; Vidari, G.; Armijos, C. Selective BuChE inhibitory activity, chemical composition, and enantiomer content of the volatile oil from the Ecuadorian plant Clinopodium brownei. Rev. Bras. Farmacogn. 2019, 29, 749–754. [Google Scholar] [CrossRef]
- Armijos, C.; Ramírez, J.; Salinas, M.; Vidari, G.; Suárez, A.I. Pharmacology and Phytochemistry of Ecuadorian Medicinal Plants: An Update and Perspectives. Pharmaceuticals 2021, 14, 1145. [Google Scholar] [CrossRef]
- Vandebroek, I.; Picking, D. Clinopodium brownei (Sw.) Kuntze (Lamiaceae). In Popular Medicinal Plants in Portland and Kingston, Jamaica, 1st ed.; Springer: Cham, Switzerland; Kingston: Fantinwali, CA, USA, 2020; pp. 89–94. [Google Scholar]
- Ansaloni, R.; Wilches, I.; León, F.; Peñaherrera, E.; Orellana, A.; Tobar, V.; De Witte, P. Estudio preliminar sobre plantas medicinales utilizadas en algunas comunidades de las provincias de Azuay, Cañar y Loja, para afecciones del aparato gastrointestinal. Rev. Tecnológica-ESPOL 2010, 23, 89–97. [Google Scholar]
- Rojas, L.B.; Usubillaga, A. Composition of the essential oil of Satureja brownei (SW.) Briq. from Venezuela. Flavour Fragr. J. 2000, 15, 21–22. [Google Scholar] [CrossRef]
- Jaramillo, B.E.; Stashenko, E.; Martínez, J.R. Composición química volátil de Satureja brownei (Sw.) Briq. colombiana y determinación de su actividad antioxidante. Rev. Cuba. Plantas Med. 2010, 15, 52–63. [Google Scholar]
- Adams, R.P. Identification of Essential Oils by Ion Trap Mass Spectroscopy, 1st ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101–043102. [Google Scholar] [CrossRef]
- Suryanti, V.; Wibowo, F.R.; Khotijah, S.; Andalucki, N. Antioxidant activities of cinnamaldehyde derivatives. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Surakarta, Indonesia, 2018. [Google Scholar]
- Oladimeji, O.H.; Essien, A.E.; Sheriff, A.J.; Silaev, T.E. Evaluation of antioxidant activity of cinnamic acid and some of its derivatives. Eur. Chem. Bull. 2019, 8, 224–226. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Calleja, M.A.; Vieites, J.M.; Montero-Meterdez, T.; Torres, M.I.; Faus, M.J.; Gil, A.; Suárez, A. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 2013, 109, 394–401. [Google Scholar] [CrossRef]
- Gushiken, L.F.; Beserra, F.P.; Hussni, M.F.; Gonzaga, M.T.; Ribeiro, V.P.; De Souza, P.F.; Pellizzon, C.H. Beta-caryophyllene as an antioxidant, anti-inflammatory and re-epithelialization activities in a rat skin wound excision model. Oxid. Med. Cell. Longevity. 2022, 2022, 9004014. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, I.E.; Galviz, R.A. Determinación de la Composición Química y Actividad Antibacteriana del Aceite Esencial extraído de la planta Satureja brownei (Briq.) cultivada en el Jardín de plantas Medicinales “Dr. Luis Ruiz Terán” de la Facultad de Farmacia y Bioanálisis, Universidad de los Andes. Bachelor’s Thesis, Universidad de los Andes, Mérida, Venezuela, 2015. [Google Scholar]
- Yoo, H.J.; Jwa, S.K. Efficacy of β-caryophyllene for periodontal disease related factors. Arch. Oral. Biol. 2019, 100, 113–118. [Google Scholar] [CrossRef]
- Moo, C.L.; Yang, S.K.; Osman, M.A.; Yuswan, M.H.; Loh, J.Y.; Lim, W.M.; Lai, K.S. Antibacterial Activity and Mode of Action of β-caryophyllene on. Pol. J. Microbiol. 2020, 69, 49–54. [Google Scholar] [CrossRef]
- Mahdavi, B.; Ghorat, F.; Nasrollahzadeh, M.S.; Hosseyni-Tabar, M.; Rezaei-Seresht, H. Chemical composition, antioxidant, antibacterial, cytotoxicity, and hemolyses activity of essential oils from flower of Matricaria chamomilla var. chamomilla. Anti-Infect. Agents 2020, 18, 224–232. [Google Scholar] [CrossRef]
- El-Baroty, G.S.; Abd El-Baky, H.H.; Farag, R.S.; Saleh, M.A. Characterization of antioxidant and antimicrobial compounds of cinnamon and ginger essential oils. Afr. J. Biochem. Res. 2010, 4, 167–174. [Google Scholar]
- Garzoli, S.; Laghezza Masci, V.; Franceschi, S.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Headspace/GC–MS analysis and investigation of antibacterial, antioxidant and cytotoxic activity of essential oils and hydrolates from Rosmarinus officinalis L. and Lavandula angustifolia Miller. Foods 2021, 10, 1768. [Google Scholar] [CrossRef]
- Sabulal, B.; Dan, M.; Kurup, R.; Pradeep, N.S.; Valsamma, R.K.; George, V. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry 2006, 67, 2469–2473. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Zitterl-Eglseer, K.; Deans, S.G.; Franz, C.M. Essential oils of different cultivars of Cannabis sativa L. and their antimicrobial activity. Flavour Fragr. J. 2001, 16, 259–262. [Google Scholar] [CrossRef]
- Montanari, R.M.; Barbosa, L.C.; Demuner, A.J.; Silva, C.J.; Carvalho, L.S.; Andrade, N.J. Chemical composition and antibacterial activity of essential oils from Verbenaceae species: Alternative sources of (E)-caryophyllene and germacrene-D. Quim. Nova 2011, 34, 1550–1555. [Google Scholar] [CrossRef]
- Balcerzak, L.; Gibka, J.; Sikora, M.; Kula, J.; Strub, D.J. Minor constituents of essential oils and aromatic extracts. Oximes derived from natural flavor and fragrance raw materials–Sensory evaluation, spectral and gas chromatographic characteristics. Food Chem. 2019, 301, 125283. [Google Scholar] [CrossRef]
- NIST/02, Mass Spectral Library; United States Government: Washington, DC, USA, 2001. Available online: https://chemdata.nist.gov (accessed on 22 December 2022).
- Sirivibulkovit, K.; Nouanthavong, S.; Sameenoi, Y. Based DPPH assay for antioxidant activity analysis. Anal. Sci. 2018, 34, 795–800. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Noriega, P.; Ballesteros, J.; De la Cruz, A.; Veloz, T. Chemical composition and preliminary antimicrobial activity of the hydroxylated sesquiterpenes in the essential oil from Piper barbatum Kunth leaves. Plants 2020, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, D.; Abril-Novillo, A.; Khachatryan, A.; Jerves-Andrade, L.; Peñaherrera, E.; Cuzco, N.; Wilches, I.; Calle, J.; León, F. Validation of a method of broth microdilution for the determination of antibacterial activity of essential oils. BMC Res. Notes 2021, 14, 1–7. [Google Scholar] [CrossRef]
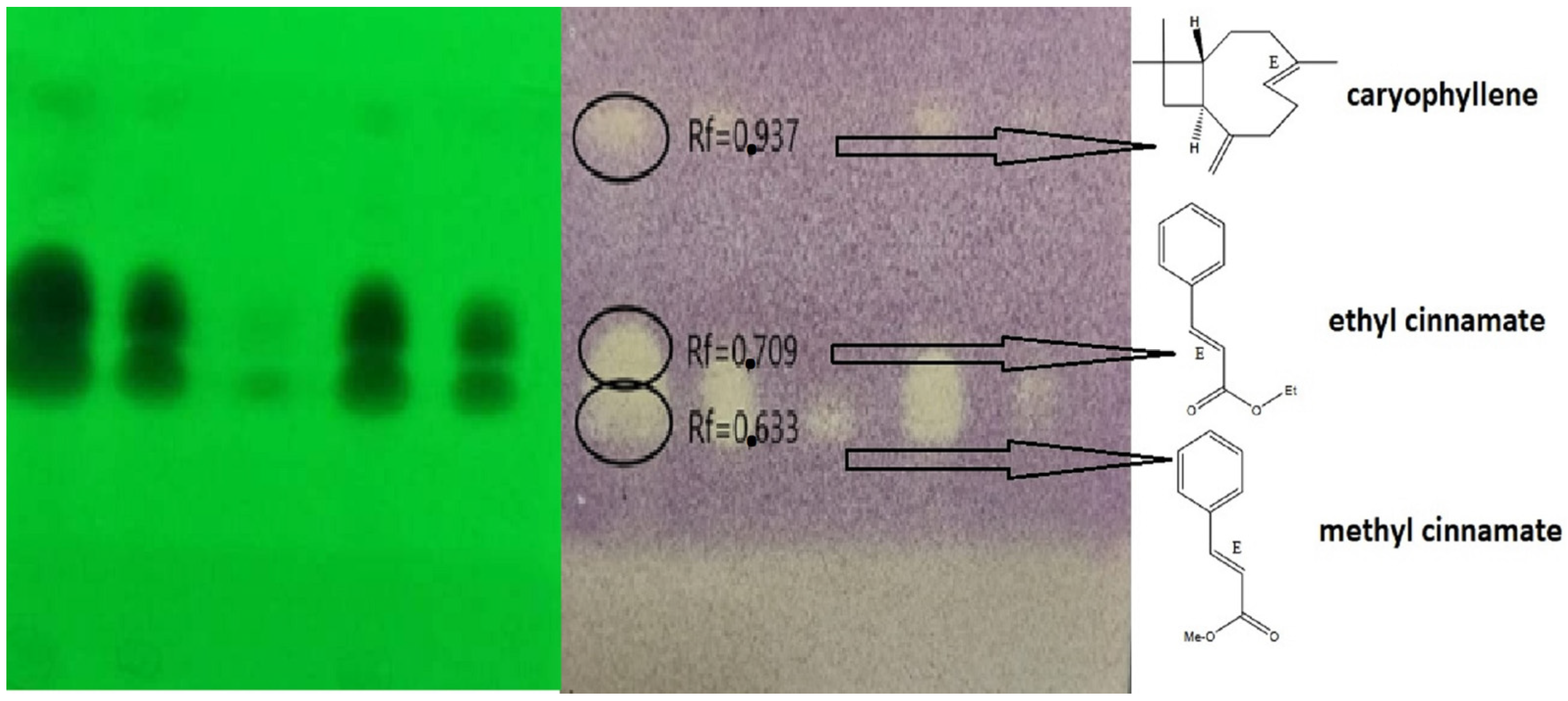

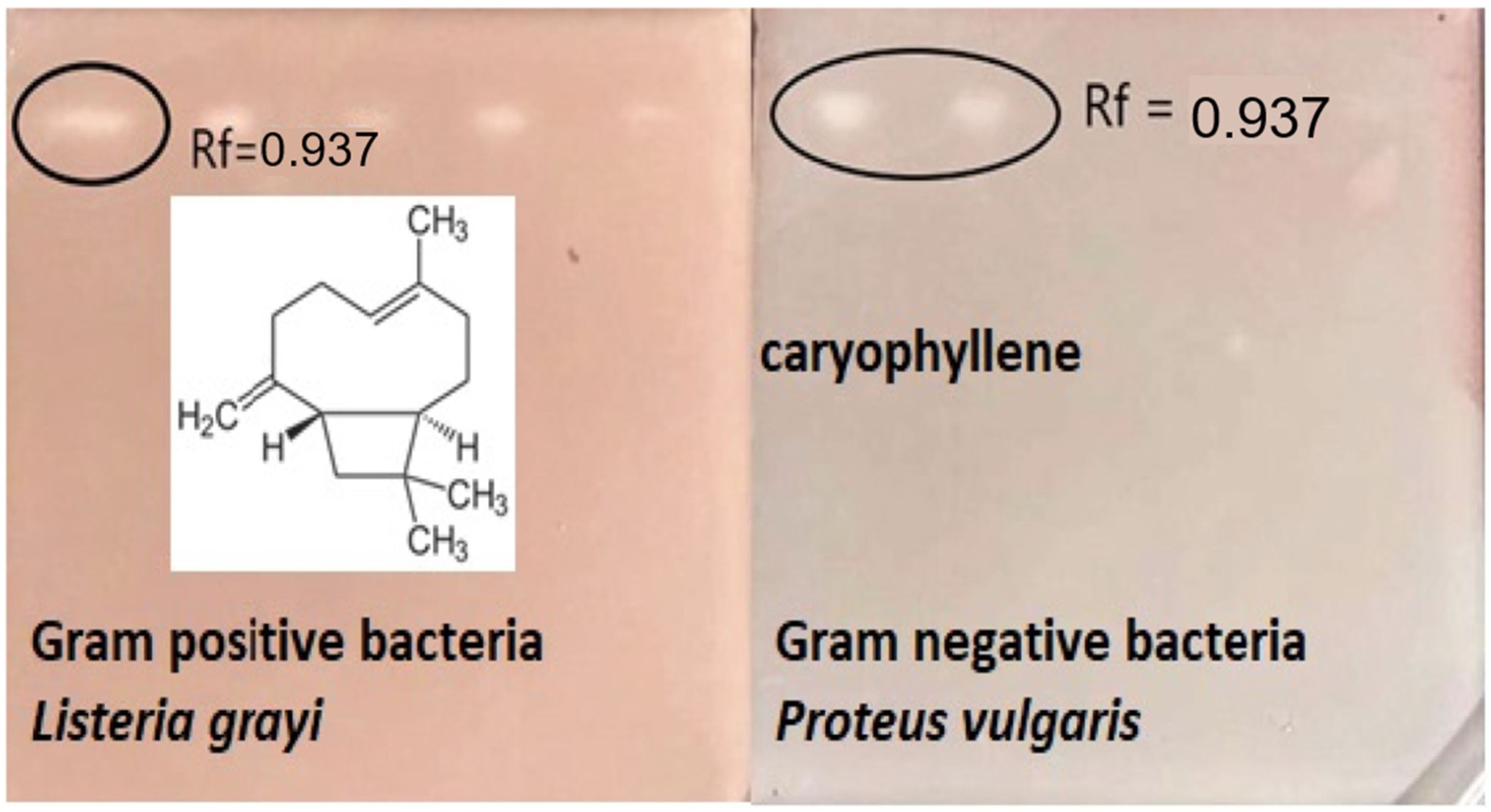
| Components (Non-Polar Column) | TR5MS RI cal a | RI Lit b | %RDA | Components (Polar Column) | DB5-Wax RI cal c | RT Lit d | % RDA |
|---|---|---|---|---|---|---|---|
| 3-octanone | 992 | 979 | 0.24 | 3-octanone | 1263 | 1255 | 0.22 |
| menthone | 1164 | 1148 | 7.51 | 2-pentanone, 4-hydroxy-4-methyl | 1371 | - | 1.42 |
| iso-isopulegol | 1172 | 1159 | 1.18 | menthone | 1471 | 1465 | 8.04 |
| neoiso.pulegol | 1176 | 1167 | 0.49 | α-copaene | 1496 | 1491 | 3.07 |
| pulegone | 1252 | 1233 | 20.76 | β-elemene | 1594 | 1591 | 0.64 |
| α-copaene | 1376 | 1374 | 2.87 | (E)-caryophyllene | 1602 | 1599 | 10.0 |
| (E)-methyl cinnamate | 1401 | 1376 | 16.68 | pulegone | 1653 | 1655 | 29.9 |
| (E)-caryophyllene | 1421 | 1417 | 8.17 | β-humulene | 1672 | 1667 | 1.96 |
| (E)-ethyl cinnamate | 1465 | 1443 | 21.4 | γ-gurjunene | 1692 | 1668 | 0.49 |
| β-humulene | 1458 | 1436 | 2.17 | α-terpinyl acetate | 1697 | 1695 | 0.31 |
| γ-gurjunene | 1487 | 1475 | 0.81 | β-selinene | 1722 | 1717 | 6.46 |
| β-selinene | 1493 | 1489 | 7.92 | α-selinene | 1726 | 1725 | 1.46 |
| α-selinene | 1499 | 1498 | 2.12 | α-panasinsene | 1764 | - | 0.34 |
| δ-selinene | 1495 | 1492 | 0.31 | Caryophyllene oxide | 1986 | 1986 | - |
| spathulenol | 1585 | 1577 | 0.33 | (E)-cinnamaldehyde | 2031 | 2033 | 0.93 |
| Caryophyllene oxide | 1589 | 1582 | 2.33 | (E)-mehtyl cinnamate | 2056 | 2075 | 13.82 |
| benzyl benzoate | 1772 | 1759 | 0.17 | (E)-ethyl cinnamate | 2110 | - | 18.75 |
| Total amount of identified compounds | 98.52 | 98.35 |
| Essential Oils/Antioxidant Molecule | DPPH IC50 (mg/mL) | ABTS IC50 (mg/mL) |
|---|---|---|
| Clinopodium brownei | 1.771 ± 0.260 | 0.060 ± 0.002 |
| Thymus vulgaris | 0.010 ± 0.005 | 0.008 ± 0.002 |
| butylated hydroxyanisole (BHA) | 0.004 ± 0.000 | 0.002 ± 0.000 |
| Microorganism | C. brownie MIC (mg/mL) | T. vulgaris MIC (mg/mL) |
|---|---|---|
| Gram Positive Bacteria | ||
| Staphylococcus aureus ATCC 6328 | 7.92 ± 0.21 | 3.02 ± 0.16 |
| Enterococcus faecalis ATCC 29212 | 5.54 ± 0.25 | 2.89 ± 0.14 |
| Listeria grayi ATCC 1912 | 3.84 ± 0.16 | 2.28 ± 0.11 |
| Staphylococcus epidermidis ATCC 14990 | 13.57 ± 0.21 | 7.98 ± 0.37 |
| Gram negative Bacteria | ||
| Escherichia coli ATCC 25922 | 6.22 ± 0.16 | 2.13 ± 0.09 |
| Proteus vulgaris ATCC 6380 | 4.62 ± 0.18 | 2.77 ± 0.08 |
| Klebsiella oxytoca ATCC 8724 | 7.19 ± 0.35 | 1.30 ± 0.05 |
| Pseudomonas aeruginosa ATCC 9027 | 8.38 ± 0.41 | 3.10 ± 0.12 |
| Yeast | ||
| Candida albicans ATCC 10231 | 3.11 ± 0.10 | 1.45 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noriega, P.; Calderón, L.; Ojeda, A.; Paredes, E. Chemical Composition, Antimicrobial and Antioxidant Bioautography Activity of Essential Oil from Leaves of Amazon Plant Clinopodium brownei (Sw.). Molecules 2023, 28, 1741. https://doi.org/10.3390/molecules28041741
Noriega P, Calderón L, Ojeda A, Paredes E. Chemical Composition, Antimicrobial and Antioxidant Bioautography Activity of Essential Oil from Leaves of Amazon Plant Clinopodium brownei (Sw.). Molecules. 2023; 28(4):1741. https://doi.org/10.3390/molecules28041741
Chicago/Turabian StyleNoriega, Paco, Lissette Calderón, Andrea Ojeda, and Erika Paredes. 2023. "Chemical Composition, Antimicrobial and Antioxidant Bioautography Activity of Essential Oil from Leaves of Amazon Plant Clinopodium brownei (Sw.)" Molecules 28, no. 4: 1741. https://doi.org/10.3390/molecules28041741
APA StyleNoriega, P., Calderón, L., Ojeda, A., & Paredes, E. (2023). Chemical Composition, Antimicrobial and Antioxidant Bioautography Activity of Essential Oil from Leaves of Amazon Plant Clinopodium brownei (Sw.). Molecules, 28(4), 1741. https://doi.org/10.3390/molecules28041741








