Hesperidin, Hesperetin, Rutinose, and Rhamnose Act as Skin Anti-Aging Agents
Abstract
:1. Introduction
2. Results
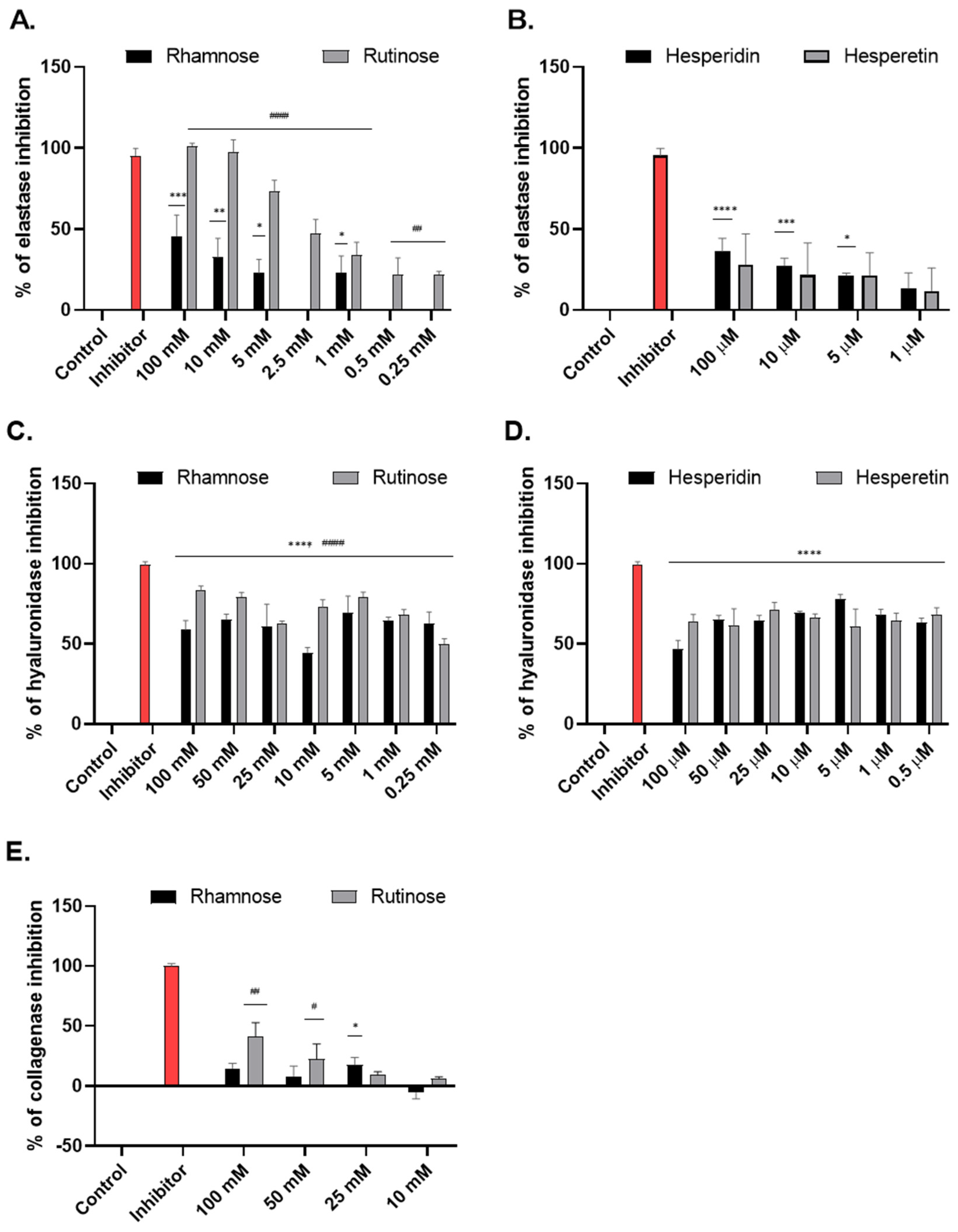
2.1. Effect of Studied Compounds on the Activity of Enzymes Associated with Skin Aging (Tube Tests)
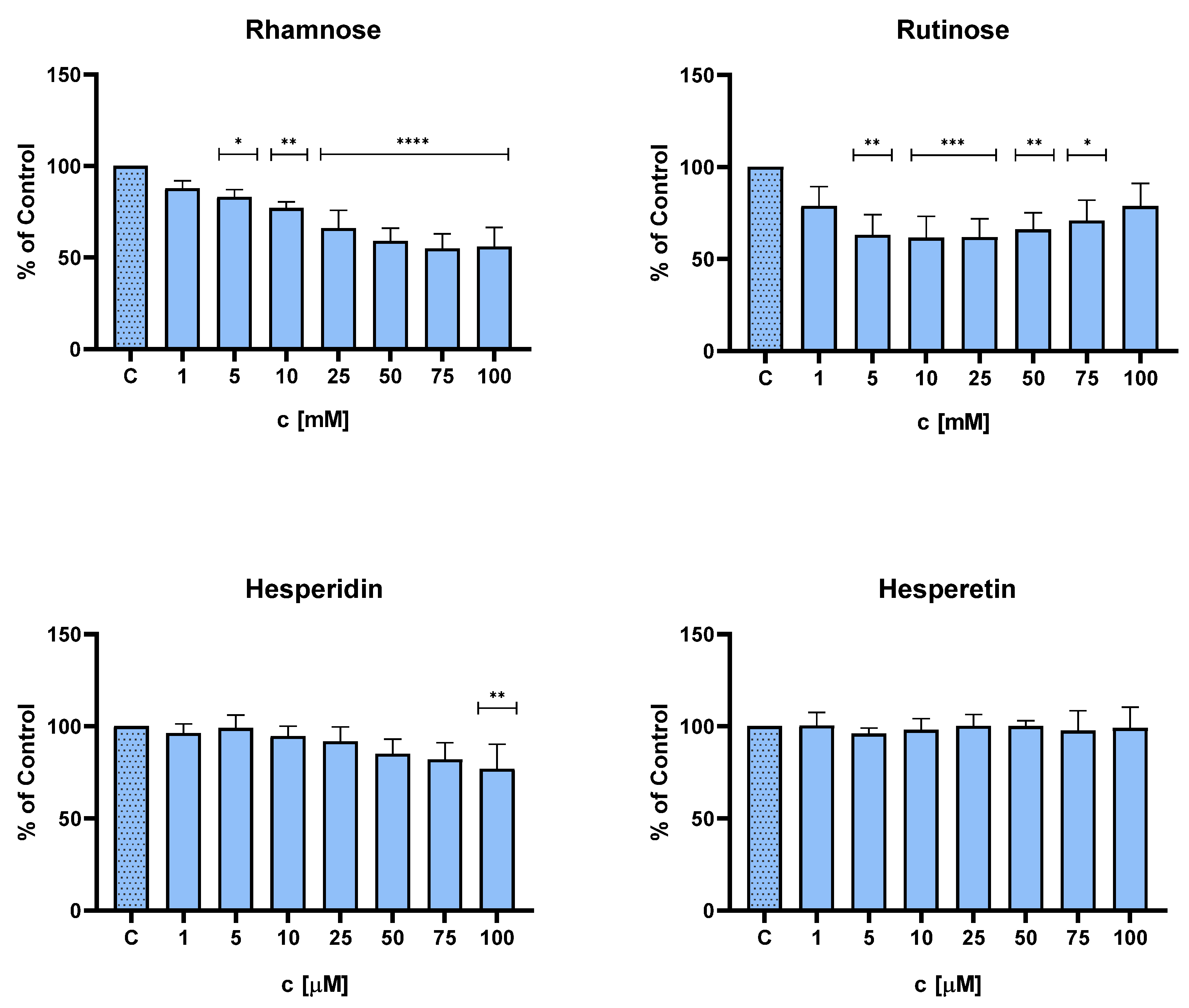
2.2. Effect of Hesperidin, Hesperetin, Rutinose, and Rhamnose on NHDFs Viability
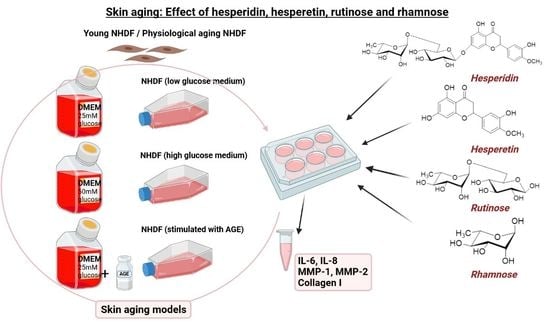
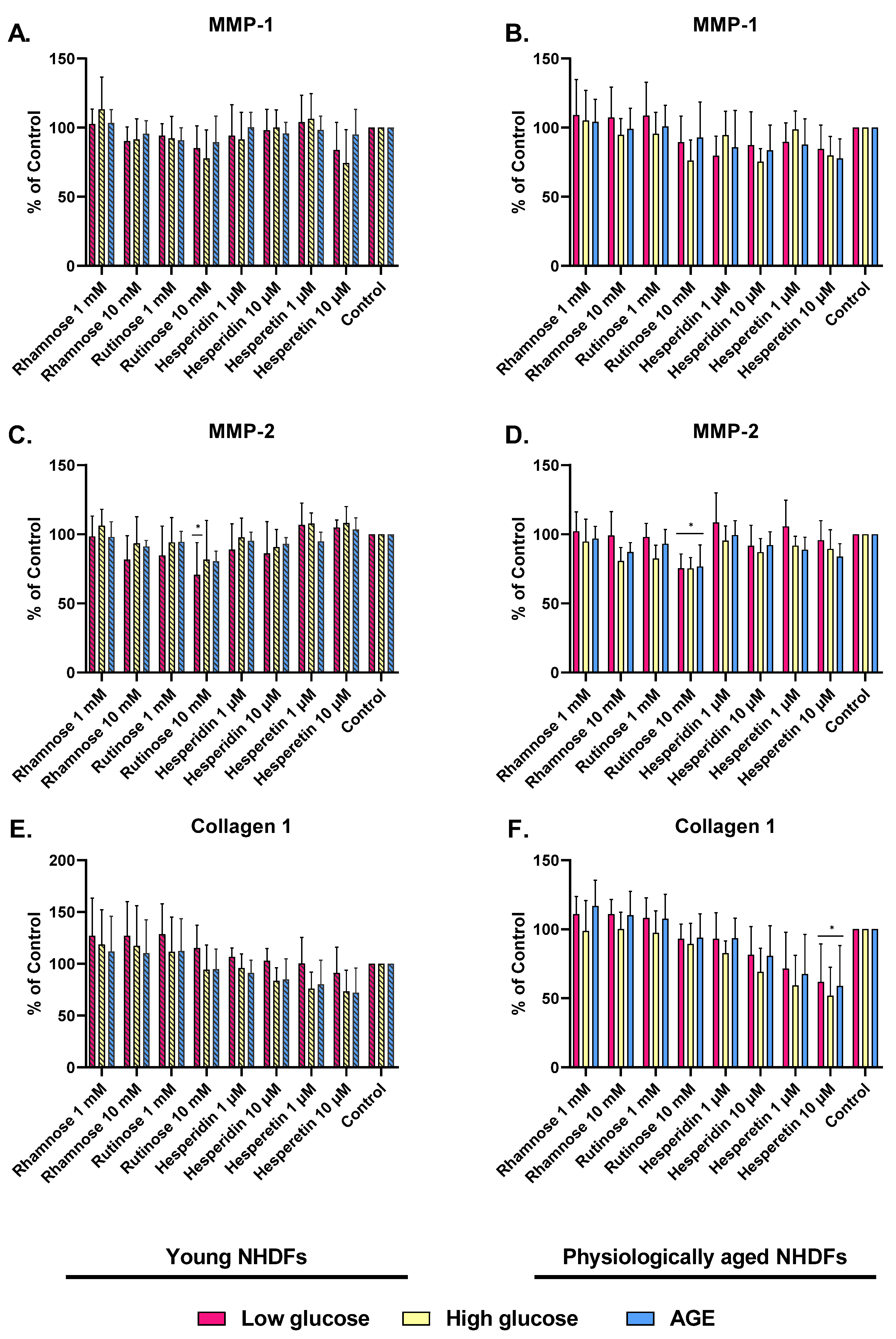
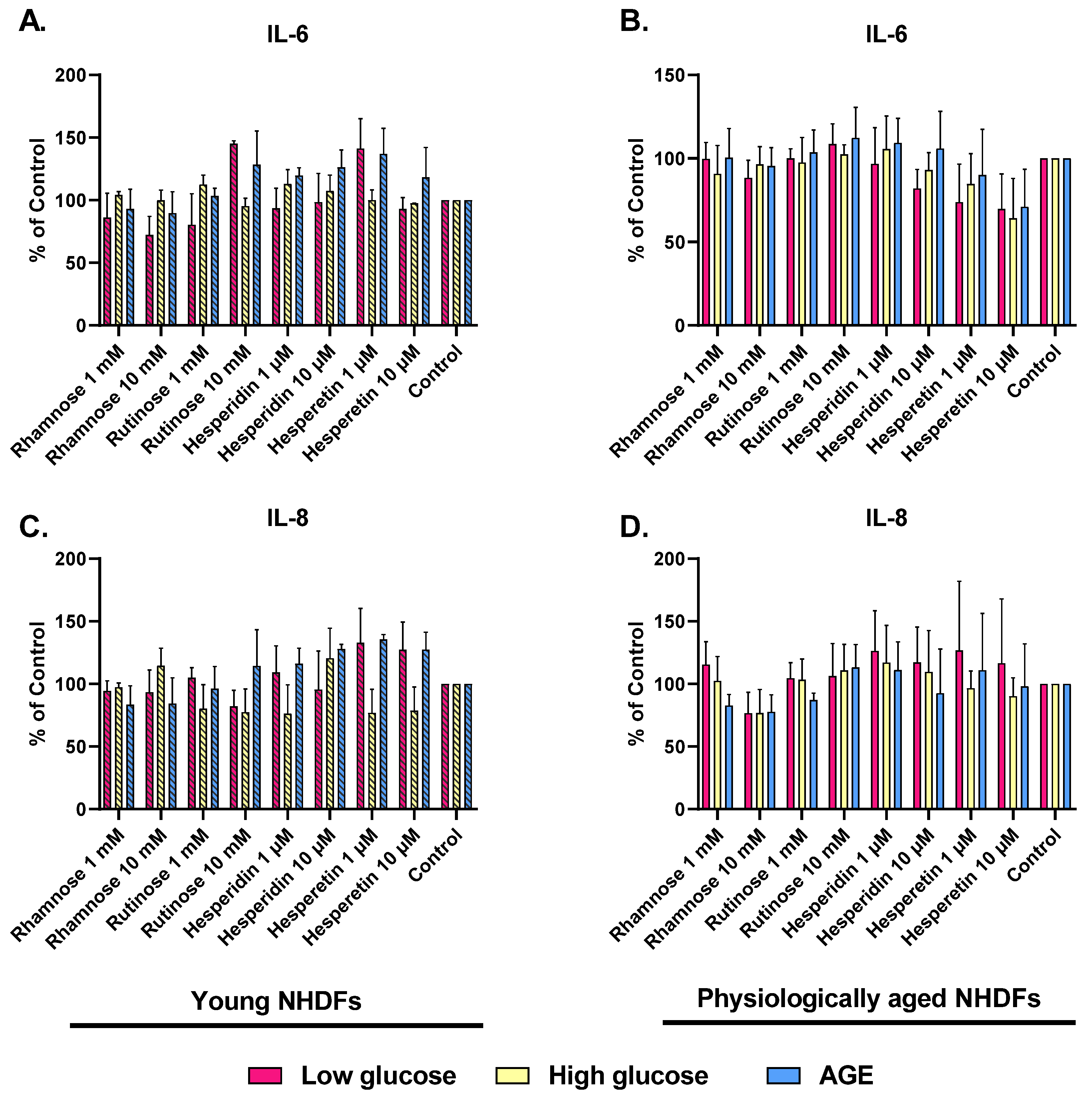
2.3. Effect of Hesperidin, Hesperetin, Rutinose, and Rhamnose on Skin-Aging Models
3. Discussion
4. Materials and Methods
4.1. Chemicals
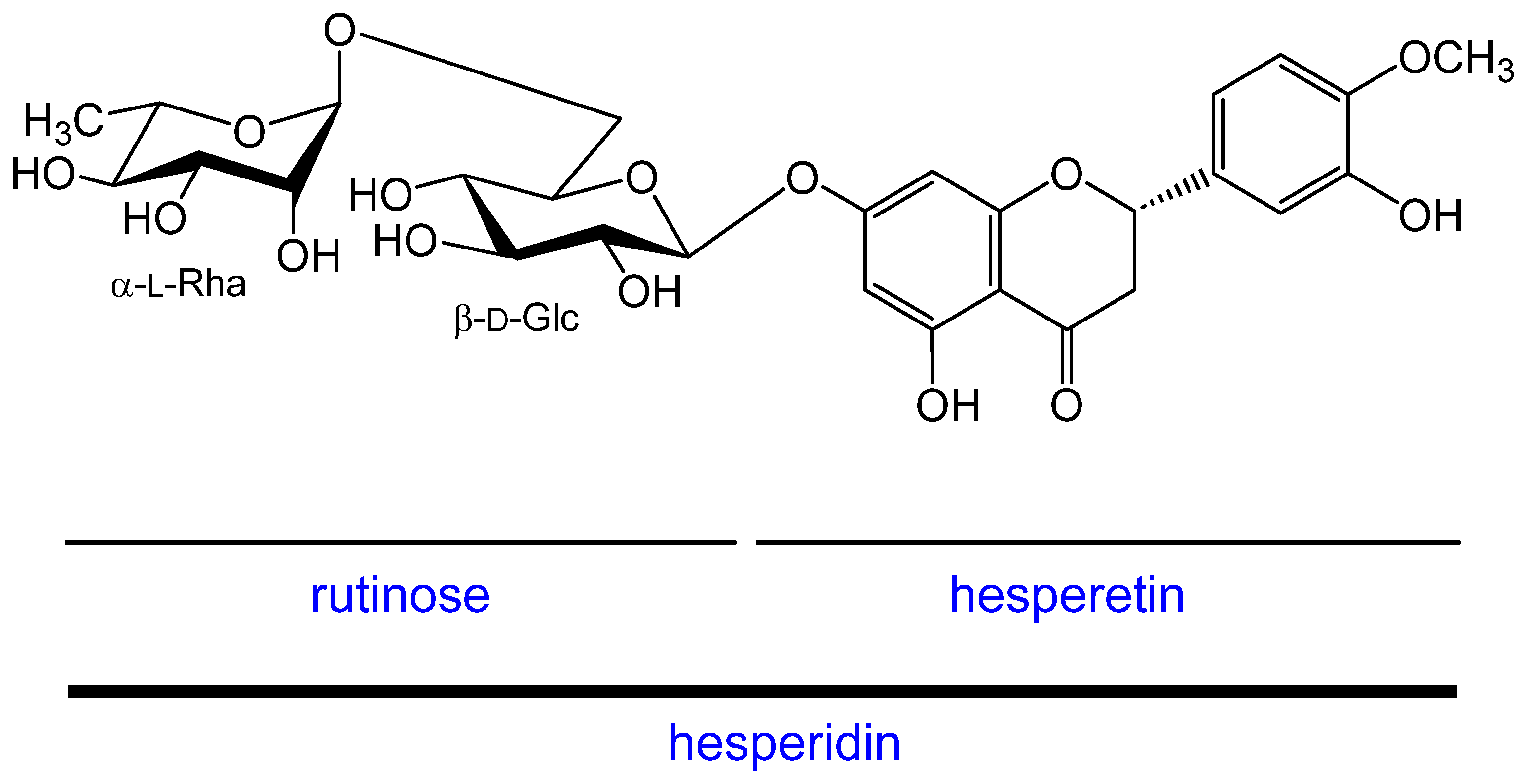
4.1.1. Rhamnose, Rutinose, Hesperidin, and Hesperetin
4.1.2. Other Chemicals
4.2. Determination of the Activity of Enzymes Associated with Skin Aging by Tube Tests
4.2.1. Elastase Activity
4.2.2. Hyaluronidase Activity
4.2.3. Collagenase Activity
4.3. Cell Culture and Preparation of Skin-Aging Models
4.4. Cell Viability Assay
4.5. Effect of Studied Compounds on Skin-Aging Models
4.6. Evaluation of the Effect of Hesperidin, Hesperetin, Rutinose, and Rhamnose on Skin Aging Models
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Vostálová, J.; Tinková, E.; Biedermann, D.; Kosina, P.; Ulrichová, J.; Rajnochová Svobodová, A. Skin protective activity of silymarin and its flavonolignans. Molecules 2019, 24, 1022. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.; Griffiths, T.; Watson, R.; Hay, R.; Griffiths, C. Age-associated skin conditions and diseases: Current perspectives and future options. Gerontologist 2016, 56, S230–S242. [Google Scholar] [CrossRef]
- Wang, A.; Dreesen, O. Biomarkers of cellular senescence and skin aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef]
- Csekes, E.; Račková, L. Skin aging, cellular senescence and natural polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Lu, C.; Wu, C.; Li, K.; Kuo, Y.; Hsieh, S.; Yu, C. The development of Maillard reaction, and advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling inhibitors as novel therapeutic strategies for patients with AGE-related diseases. Molecules 2020, 25, 5591. [Google Scholar] [CrossRef]
- Velichkova, S.; Foubert, K.; Pieters, L. Natural products as a source of inspiration for novel inhibitors of advanced glycation endproducts (AGEs) formation. Planta Med. 2021, 87, 780–801. [Google Scholar] [CrossRef] [PubMed]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products: Key players in skin aging? Derm.-Endocrinol. 2014, 4, 259–270. [Google Scholar] [CrossRef]
- Stanisic, D.; Liu, L.; dos Santos, R.; Costa, A.; Durán, N.; Tasic, L. New sustainable process for hesperidin isolation and anti-ageing effects of hesperidin nanocrystals. Molecules 2020, 25, 4534. [Google Scholar] [CrossRef]
- Lee, H.; Im, A.; Kim, S.; Kang, H.; Lee, J.; Chae, S. The flavonoid hesperidin exerts anti-photoaging effect by downregulating matrix metalloproteinase (MMP)-9 expression via mitogen activated protein kinase (MAPK)-dependent signaling pathways. BMC Complement. Altern. Med. 2018, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Sheen, Y.; Huang, H.; Liao, Y. The efficacy and safety of an antiaging topical serum containing hesperetin and sodium cyclic lysophosphatidic acid: A single-center clinical trial. J. Cosmet. Dermatol. 2021, 20, 3960–3967. [Google Scholar] [CrossRef]
- Hering, A.; Ochocka, J.R.; Baranska, H.; Cal, K.; Stefanowicz-Hajduk, J. Mangiferin and hesperidin transdermal distribution and permeability through the skin from solutions and honeybush extracts (Cyclopia sp.)-A comparison ex vivo study. Molecules 2021, 26, 6547. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Walkowiak, J.; Pietrzak, R.; Bazan-Woźniak, A.; Cielecka-Piontek, J. Bioavailability of hesperidin and its aglycone hesperetin-compounds found in citrus fruits as a parameter conditioning the pro-health potential (neuroprotective and antidiabetic activity)-mini-review. Nutrients 2022, 14, 2647. [Google Scholar] [CrossRef] [PubMed]
- Pageon, H.; Azouaoui, A.; Zucchi, H.; Ricois, S.; Tran, C.; Asselineau, D. Potentially beneficial effects of rhamnose on skin ageing: An in vitro and in vivo study. Int. J. Cosmet. Sci. 2019, 41, 213–220. [Google Scholar] [CrossRef]
- Ravelojaona, V.; Molinari, J.; Robert, L. Protection by rhamnose-rich polysaccharides against the cytotoxicity of Maillard reaction products. Biomed. Pharmacother. 2006, 60, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.; Molinari, J.; Ravelojaona, V.; Andrès, E.; Robert, A. Age- and passage-dependent upregulation of fibroblast elastase-type endopeptidase activity. Role of advanced glycation endproducts, inhibition by fucose- and rhamnose-rich oligosaccharides. Arch. Gerontol. Geriatr. 2010, 50, 327–331. [Google Scholar] [CrossRef]
- Ho, C.; Dreesen, O. Faces of cellular senescence in skin aging. Mech. Ageing Dev. 2021, 198, 111525. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Franco, A.; Aveleira, C.; Cavadas, C. Skin senescence: Mechanisms and impact on whole-body aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef]
- Nguyen, H.; Katta, R. Sugar sag: Glycation and the role of diet in aging skin. Skin Therapy Lett. 2015, 20, 1–5. [Google Scholar]
- Robert, L.; Labat-Robert, J.; Robert, A. Physiology of skin aging. Pathol. Biol. 2009, 57, 336–341. [Google Scholar] [CrossRef]
- Santhanam, R.; Fakurazi, S.; Ahmad, S.; Abas, F.; Ismail, I.; Rukayadi, Y.; Akhtar, M.; Shaari, K. Inhibition of UVB-induced pro-inflammatory cytokines and MMP expression by Zanthoxylum rhetsa bark extract and its active constituent hesperidin. Phytother. Res. 2018, 32, 1608–1616. [Google Scholar] [CrossRef]
- Lee, D.; Oh, J.; Chung, J. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 2016, 83, 174–181. [Google Scholar] [CrossRef]
- Andrès, E.; Molinari, J.; Péterszegi, G.; Mariko, B.; Ruszova, E.; Velebny, V.; Faury, G.; Robert, L. Pharmacological properties of rhamnose-rich polysaccharides, potential interest in age-dependent alterations of connectives tissues. Pathol. Biol. 2006, 54, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Faury, G.; Ruszova, E.; Molinari, J.; Mariko, B.; Raveaud, S.; Velebny, V.; Robert, L. The α-l-Rhamnose recognizing lectin site of human dermal fibroblasts functions as a signal transducer. Biochim. Biophys. Acta (BBA)—Gen. Subjects 2008, 1780, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Vidhya, R.; Anbumani, V.; Dinakara Rao, A.; Anuradha, C. Identification of novel human neutrophil elastase inhibitors from dietary phytochemicals using in silico and in vitro studies. J. Biomol. Struct. Dyn. 2022, 40, 3451–3461. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, R.; Cheng, Z.; Song, Z.; Wang, Z.; Duan, H.; Wu, X.; Ni, T. Comparative study on the interaction between flavonoids with different core structures and hyaluronidase. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120079. [Google Scholar] [CrossRef] [PubMed]
- Man, G.; Mauro, T.; Zhai, Y.; Kim, P.; Cheung, C.; Hupe, M.; Crumrine, D.; Elias, P.; Man, M. Topical hesperidin enhances epidermal function in an aged murine model. J. Investig. Dermatol. 2015, 135, 1184–1187. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.; Fisher, G. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef]
- Lu, Z.; Xia, Q.; Cheng, Y.; Lu, Q.; Li, Y.; Zeng, N.; Luan, X.; Li, Y.; Fan, L.; Luo, D. Hesperetin attenuates UVA-induced photodamage in human dermal fibroblast cells. J. Cosmet. Dermatol. 2022, 21, 6261–6269. [Google Scholar] [CrossRef]
- Hiraishi, N.; Maruno, T.; Tochio, N.; Sono, R.; Otsuki, M.; Takatsuka, T.; Tagami, J.; Kobayashi, Y. Hesperidin interaction to collagen detected by physico-chemical techniques. Dent. Mater. 2017, 33, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Péterszegi, G.; Andrès, E.; Molinari, J.; Ravelojaona, V.; Robert, L. Effect of cellular aging on collagen biosynthesis. Arch. Gerontol. Geriatr. 2008, 47, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lin, X.; Lu, J.; Zhou, B.; Luo, D. Hesperidin ameliorates UV radiation-induced skin damage by abrogation of oxidative stress and inflammatory in HaCaT cells. J. Photochem. Photobiol. B Biol. 2016, 165, 240–245. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Andrade, T.; Heimfarth, L.; dos Santos, D.; dos Santos, M.; de Albuquerque-Júnior, R.; dos Santos-Neto, A.; de Araujo, G.; Lira, A.; Matos, S.; Frank, L.; et al. Hesperetin-based hydrogels protect the skin against UV radiation-induced damage. AAPS PharmSciTech 2022, 23, 170. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Zhang, Y.; Ding, H.; Li, B.; Huang, C.; Meng, X.; Li, J. Design, synthesis and investigation of potential anti-inflammatory activity of O-alkyl and O-benzyl hesperetin derivatives. Int. Immunopharmacol. 2018, 61, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, X.; Zhang, L.; Meng, Y.; Li, N.; Wang, M.; Zhai, C.; Liu, Z.; Di, T.; Zhang, L.; et al. Hesperidin inhibits keratinocyte proliferation and imiquimod-induced psoriasis-like dermatitis via the IRS-1/ERK1/2 pathway. Life Sci. 2019, 219, 311–321. [Google Scholar] [CrossRef]
- Kapešová, J.; Petrásková, L.; Markošová, K.; Rebroš, M.; Kotik, M.; Bojarová, P.; Křen, V. Bioproduction of quercetin and rutinose catalyzed by rutinosidase: Novel concept of “solid state biocatalysis”. Int. J. Mol. Sci. 2019, 20, 1112. [Google Scholar] [CrossRef]
- Ndlovu, G.; Fouche, G.; Tselanyane, M.; Cordier, W.; Steenkamp, V. In vitro determination of the anti-aging potential of four southern African medicinal plants. BMC Complement. Altern. Med. 2013, 13, 304. [Google Scholar] [CrossRef]
- Ryšavá, A.; Čížková, K.; Franková, J.; Roubalová, L.; Ulrichová, J.; Vostálová, J.; Vrba, J.; Zálešák, B.; Rajnochová Svobodová, A. Effect of UVA radiation on the Nrf2 signalling pathway in human skin cells. J. Photochem. Photobiol. B Biol. 2020, 209, 111948. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P. Analysis of Cell Viability by the MTT Assay. Cold Spring Harbor Protocols 2018, 2018, 6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novotná, R.; Škařupová, D.; Hanyk, J.; Ulrichová, J.; Křen, V.; Bojarová, P.; Brodsky, K.; Vostálová, J.; Franková, J. Hesperidin, Hesperetin, Rutinose, and Rhamnose Act as Skin Anti-Aging Agents. Molecules 2023, 28, 1728. https://doi.org/10.3390/molecules28041728
Novotná R, Škařupová D, Hanyk J, Ulrichová J, Křen V, Bojarová P, Brodsky K, Vostálová J, Franková J. Hesperidin, Hesperetin, Rutinose, and Rhamnose Act as Skin Anti-Aging Agents. Molecules. 2023; 28(4):1728. https://doi.org/10.3390/molecules28041728
Chicago/Turabian StyleNovotná, Renáta, Denisa Škařupová, Jiří Hanyk, Jitka Ulrichová, Vladimír Křen, Pavla Bojarová, Katerina Brodsky, Jitka Vostálová, and Jana Franková. 2023. "Hesperidin, Hesperetin, Rutinose, and Rhamnose Act as Skin Anti-Aging Agents" Molecules 28, no. 4: 1728. https://doi.org/10.3390/molecules28041728
APA StyleNovotná, R., Škařupová, D., Hanyk, J., Ulrichová, J., Křen, V., Bojarová, P., Brodsky, K., Vostálová, J., & Franková, J. (2023). Hesperidin, Hesperetin, Rutinose, and Rhamnose Act as Skin Anti-Aging Agents. Molecules, 28(4), 1728. https://doi.org/10.3390/molecules28041728