Mechanisms of Arachidonic Acid In Vitro Tumoricidal Impact
Abstract
1. Introduction
2. Results
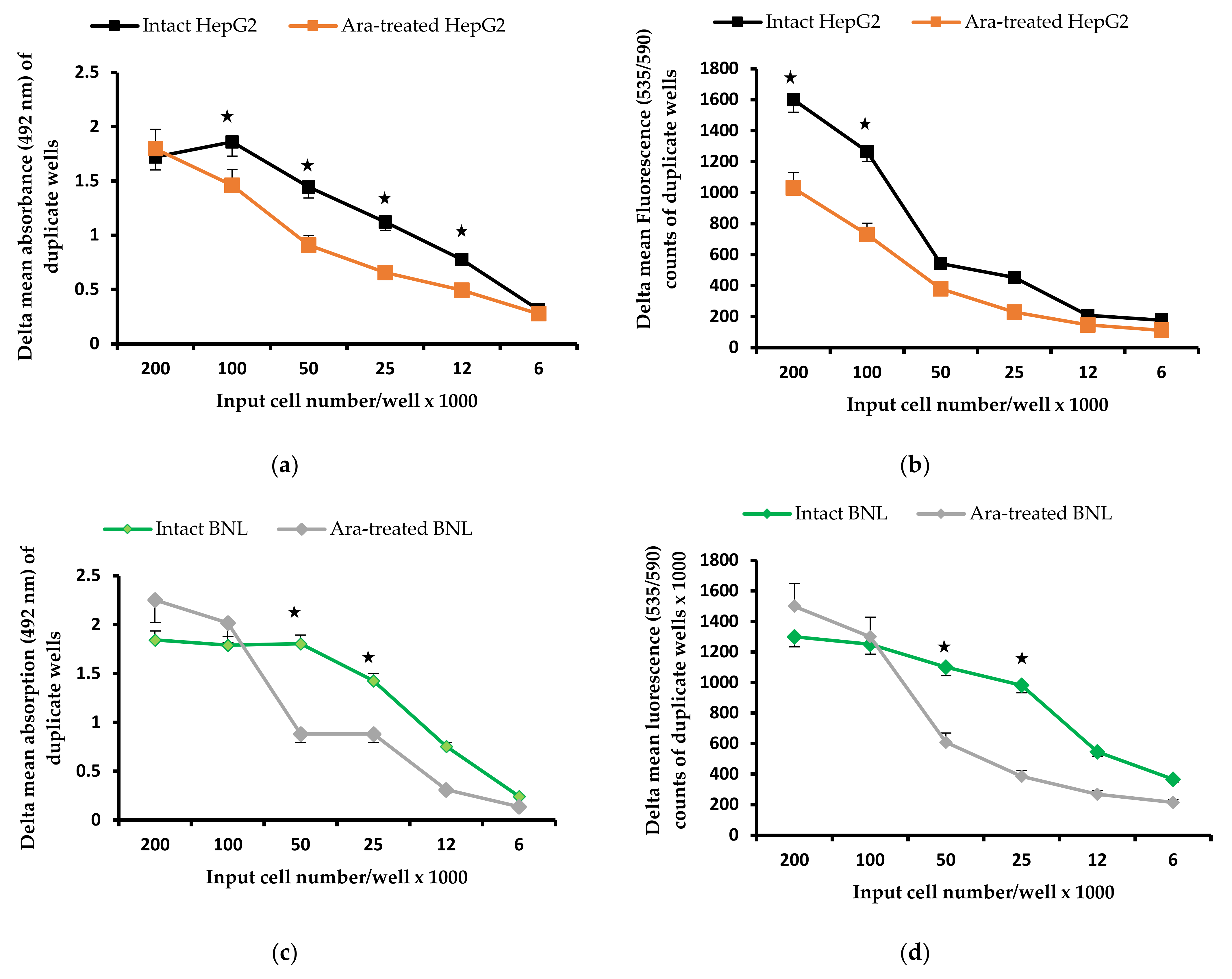
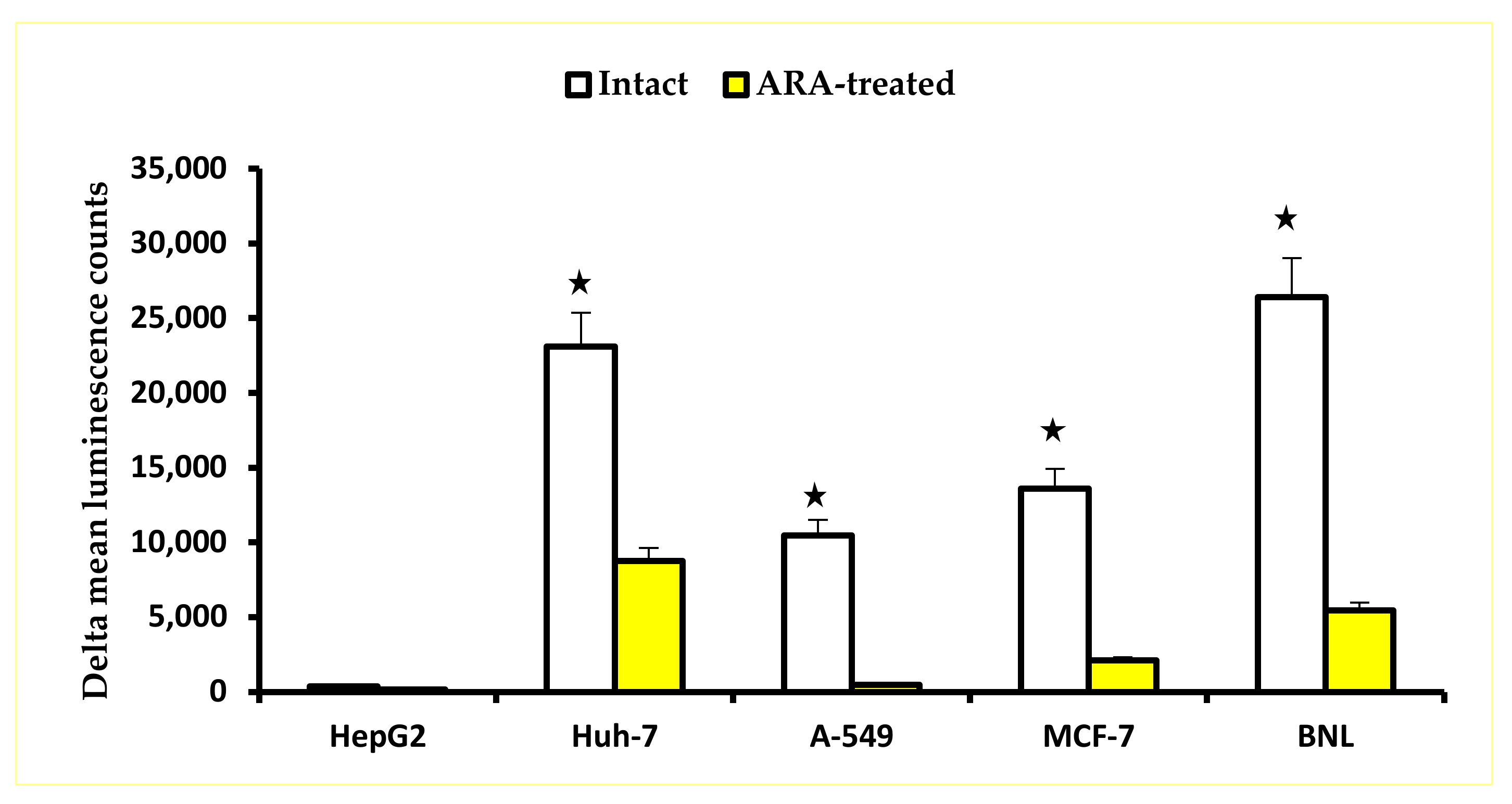
2.1. Arachidonic Acid Impact on Cell Proliferation
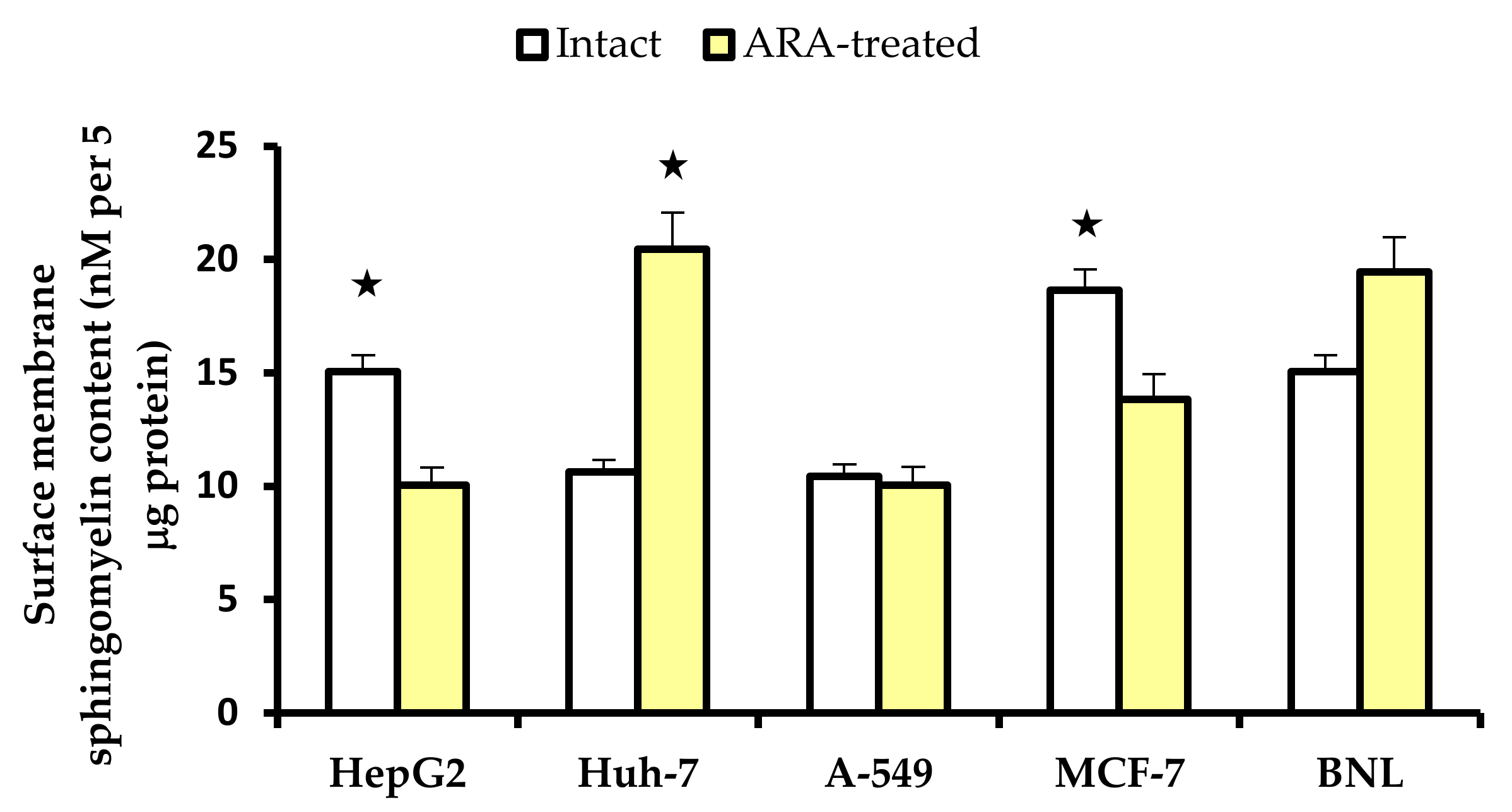
2.2. Cell Surface Membrane Sphingomyelin
2.2.1. Reactivity to Lysenin in IF
2.2.2. Cell Surface Membrane Sphingomyelin Content
2.3. Impact on Cell Surface Membrane Neutral Sphingomyelinase Activity
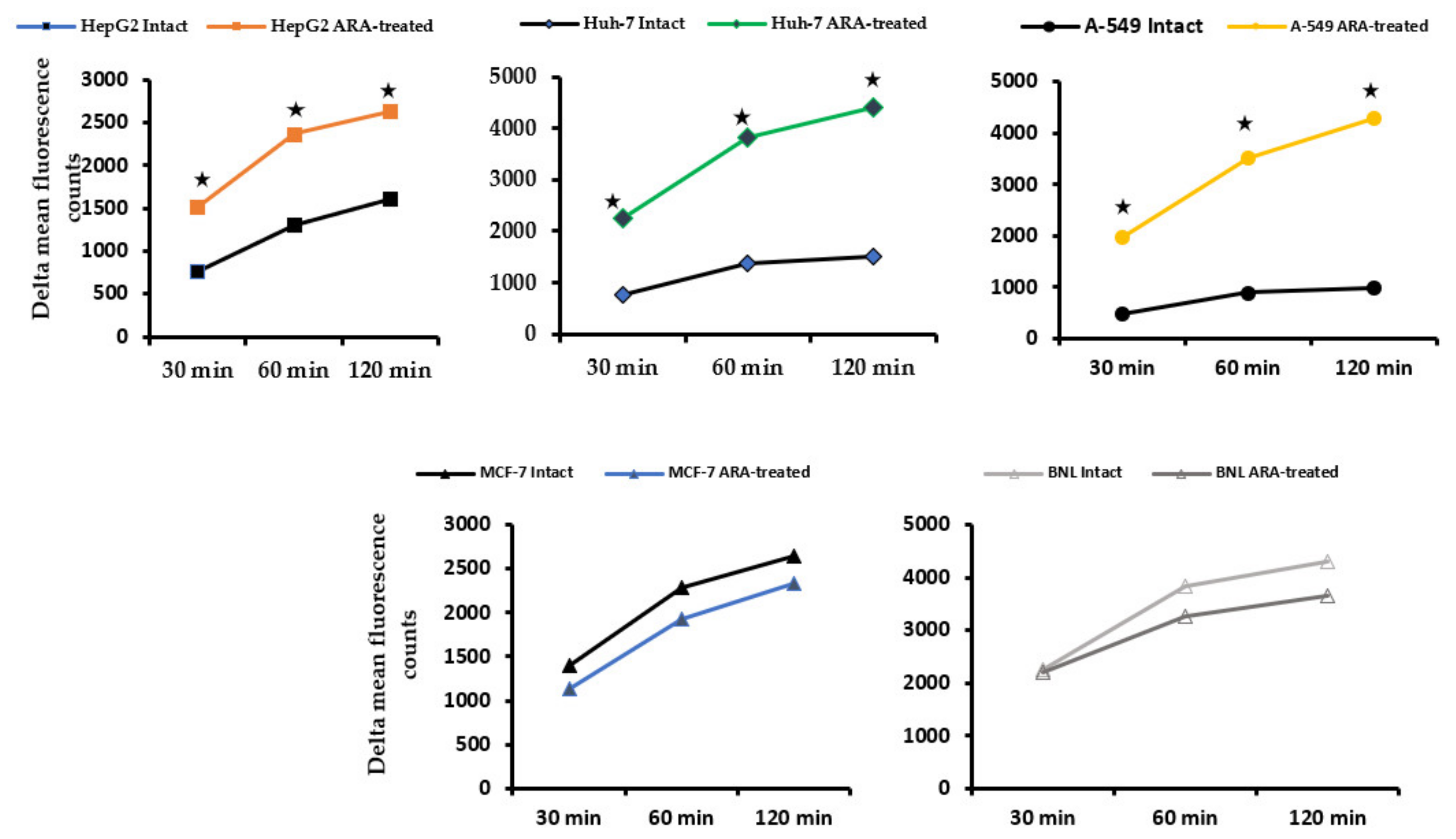
2.4. Exposure of Cell Surface Membrane β2 Microglobulin
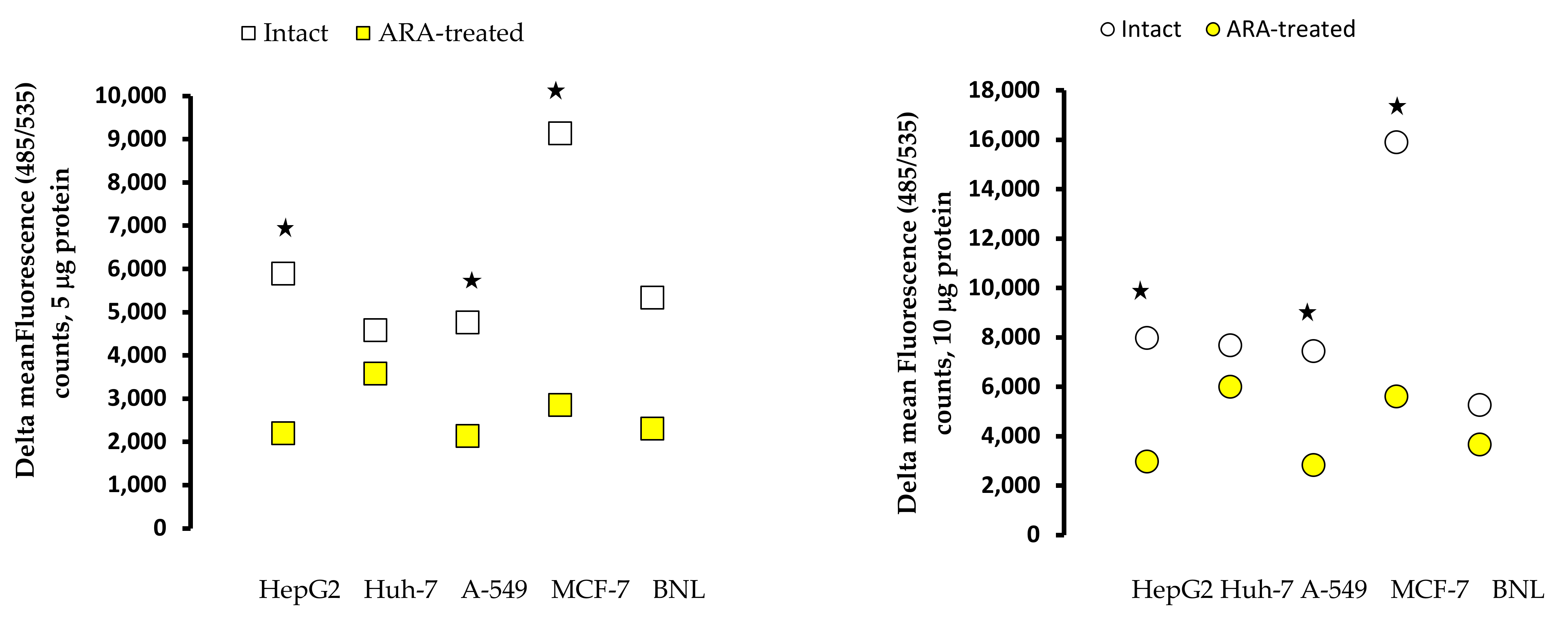
2.5. Surface Membrane and Cytoplasmic Ceramide in Intact and Treated Cells
2.6. Reactive Oxygen Species in Cell Extracts
2.7. Caspase Activity
3. Discussion
4. Materials and Methods
4.1. Tumor and Normal Cells
4.2. Arachidonic Acid Treatment
4.3. Arachidonic Acid Impact on Cell Proliferation
4.4. Preparation of Cell Surface Membrane Extracts
4.5. Preparation of Cell Homogenates Extracts
4.6. Cell Surface Membrane Sphingomyelin Content
4.6.1. Sphingomyelin Detection
4.6.2. Sphinomyelin Content
4.7. Neutral Sphingomyelinase Activity
4.8. Exposure of Cell Surface Membrane Beta 2 Microglobulin
4.9. Cell Surface Membrane and Cytoplasmic Ceramide
4.10. Reactive Oxygen Species Content in Cell Homogenate Extracts
4.11. Caspase Activity
4.12. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hyde, C.A.; Missailidis, S. Inhibition of arachidonic acid metabolism and its implication on cell proliferation and tumour-angiogenesis. Int. Immunopharmacol. 2009, 9, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Kakutani, S.; Horikawa, C.; Tokuda, H.; Kawashima, H.; Shibata, H.; Okubo, H.; Sasaki, S. Arachidonic acid and cancer risk: A systematic review of observational studies. BMC Cancer 2012, 12, 606. [Google Scholar] [CrossRef]
- Nagata, M.; Hata, J.; Hirakawa, Y.; Mukai, N.; Yoshida, D.; Ohara, T.; Kishimoto, H.; Kawano, H.; Kitazono, T.; Kiyohara, Y.; et al. The ratio of serum eicosapentaenoic acid to arachidonic acid and risk of cancer death in a Japanese community: The Hisayama Study. J. Epidemiol. 2017, 27, 578–583. [Google Scholar] [CrossRef]
- Khankari, N.K.; Banbury, B.L.; Borges, M.C.; Haycock, P.; Albanes, D.; Arndt, V.; Berndt, S.I.; Bézieau, S.; Brenner, H.; Campbell, P.T.; et al. Mendelian randomization of circulating polyunsaturated fatty acids and colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev. 2020, 29, 860–870. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J. N-6 polyunsaturated fatty acids and risk of cancer: Accumulating evidence from prospective studies. Nutrients 2020, 12, 2523. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, A.S.; Rivas-Serna, I.M.; Anoveros-Barrera, A.; Dunichand-Hoedl, A.; Bigam, D.; Khadaroo, R.G.; McMullen, T.; Bathe, O.; Putman, C.T.; Baracos, V.; et al. Depletion of essential fatty acids in muscle is associated with shorter survival of cancer patients undergoing surgery-preliminary report. Sci. Rep. 2021, 11, 23006. [Google Scholar] [CrossRef] [PubMed]
- Seviiri, M.; Law, M.H.; Ong, J.S.; Gharahkhani, P.; Nyholt, D.R.; Olsen, C.M.; Whiteman, D.C.; MacGregor, S. Polyunsaturated fatty acid levels and the risk of keratinocyte cancer: A Mendelian randomization analysis. Cancer Epidemiol. Biomarkers Prev. 2021, 30, 1591–1598. [Google Scholar] [CrossRef]
- Tu, K.; Ma, T.; Zhou, R.; Xu, L.; Fang, Y.; Zhang, C. Association between dietary fatty acid patterns and colorectal cancer risk: A large-scale case-control study in China. Nutrients 2022, 14, 4375. [Google Scholar] [CrossRef]
- Kim, H.K.; Kang, E.Y.; Go, G.W. Recent insights into dietary ω-6 fatty acid health implications using a systematic review. Food Sci. Biotechnol. 2022, 31, 1365–1376. [Google Scholar] [CrossRef]
- Booyens, J.; Engelbrecht, P.; le Roux, S.; Louwrens, C.C.; Van der Merwe, C.F.; Katzeff, I.E. Some effects of the essential fatty acids linoleic acid and alpha-linolenic acid and of their metabolites gamma-linolenic acid, arachidonic acid, eicosapentaenoic acid, docosahexaenoic acid, and of prostaglandins A1 and E1 on the proliferation of human osteogenic sarcoma cells in culture. Prostaglandins Leukot. Med. 1984, 15, 15–33. [Google Scholar] [CrossRef]
- Chow, S.C.; Sisfontes, L.; Björkhem, I.; Jondal, M. Suppression of growth in a leukemic T cell line by n-3 and n-6 polyunsaturated fatty acids. Lipids 1989, 24, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Bégin, M.E.; Ells, G.; Das, U.N.; Horrobin, D.F. Differential killing of human carcinoma cells supplemented with n-3 and n-6 polyunsaturated fatty acids. J. Natl. Cancer Inst. 1986, 77, 1053–1062. [Google Scholar] [PubMed]
- Das, U.N. Gamma-linolenic acid, arachidonic acid, and eicosapentaenoic acid as potential anticancer drugs. Nutrition 1990, 6, 429–434. [Google Scholar] [PubMed]
- Vento, R.; D’Alessandro, N.; Giuliano, M.; Lauricella, M.; Carabillò, M.; Tesoriere, G. Induction of apoptosis by arachidonic acid in human retinoblastoma Y79 cells: Involvement of oxidative stress. Exp. Eye Res. 2000, 70, 503–517. [Google Scholar] [CrossRef]
- Leaver, H.A.; Rizzo, M.T.; Whittle, I.R. Antitumour actions of highly unsaturated fatty acids: Cell signalling and apoptosis. Prostaglandins Leukot. Essent. Fatty Acids 2002, 66, 1–3. [Google Scholar] [CrossRef]
- Leaver, H.A.; Bell, H.S.; Rizzo, M.T.; Ironside, J.W.; Gregor, A.; Wharton, S.B.; Whittle, I.R. Antitumour and pro-apoptotic actions of highly unsaturated fatty acids in glioma. Prostaglandins Leukot. Essent. Fatty Acids 2002, 66, 19–29. [Google Scholar] [CrossRef]
- Monjazeb, A.M.; High, K.P.; Connoy, A.; Hart, L.S.; Koumenis, C.; Chilton, F.H. Arachidonic acid-induced gene expression in colon cancer cells. Carcinogenesis 2006, 27, 1950–1960. [Google Scholar] [CrossRef]
- Hari, A.D.; Vegi, N.G.; Das, U.N. Arachidonic and eicosapentaenoic acids induce oxidative stress to suppress proliferation of human glioma cells. Arch. Med. Sci. 2020, 16, 974–983. [Google Scholar] [CrossRef]
- Cantonero, C.; Sánchez-Collado, J.; Lopez, J.J.; Salido, G.M.; Rosado, J.A.; Redondo, P.C. Arachidonic acid attenuates cell proliferation, migration and viability by a mechanism independent on calcium entry. Int. J. Mol. Sci. 2020, 21, 3315. [Google Scholar] [CrossRef]
- Das, U.N. Tumoricidal action of cis-unsaturated fatty acids and their relationship to free radicals and lipid peroxidation. Cancer Lett. 1991, 56, 235–243. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, H.; Shen, Y.; Ni, X.; Shen, S.; Das, U.N. Polyunsaturated fatty acids trigger apoptosis of colon cancer cells through a mitochondrial pathway. Arch. Med. Sci. 2015, 11, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, M.K.; Kim, H.S.; Moon, Y.A. Arachidonic acid induces ER stress and apoptosis in HT-29 human colon cancer cells. Anim. Cells Syst. 2020, 24, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, A.; Maggiora, M.; Martinasso, G.; Cotogni, P.; Canuto, R.A.; Muzio, G. Arachidonic and docosahex aenoic acids reduce the growth of A549 human lung-tumor cells increasing lipid peroxidation and PPARs. Chem. Biol. Interact. 2007, 165, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Bocca, C.; Bozzo, F.; Martinasso, G.; Canuto, R.A.; Miglietta, A. Involvement of PPARalpha in the growth inhibitory effect of arachidonic acid on breast cancer cells. Br. J. Nutr. 2008, 100, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer. 2021, 21, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Tallima, H.; Azzazy, H.M.E.; El Ridi, R. Cell surface sphingomyelin: Key role in cancer initiation, progression, and immune evasion. Lipids Health Dis. 2021, 20, 150. [Google Scholar] [CrossRef]
- Piazzesi, A.; Afsar, S.Y.; van Echten-Deckert, G. Sphingolipid metabolism in the development and progression of cancer: One cancer’s help is another’s hindrance. Mol. Oncol. 2021, 15, 3256–3279. [Google Scholar] [CrossRef]
- Chan, T.A.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc. Natl. Acad. Sci. USA 1998, 95, 681–686. [Google Scholar] [CrossRef]
- Brash, A.R. Arachidonic acid as a bioactive molecule. J. Clin. Investig. 2001, 107, 1339–1345. [Google Scholar] [CrossRef]
- Shoji, T.; Kakiya, R.; Hayashi, T.; Tsujimoto, Y.; Sonoda, M.; Shima, H.; Mori, K.; Fukumoto, S.; Tahara, H.; Shioi, A.; et al. Serum n-3 and n-6 polyunsaturated fatty acid profile as an independent predictor of cardiovascular events in hemodialysis patients. Am. J. Kidney Dis. 2013, 62, 568–576. [Google Scholar] [CrossRef]
- Shoji, T. Serum lipids and prevention of atherosclerotic cardiovascular events in hemodialysis patients. Clin. Exp. Nephrol. 2014, 18, 257–260. [Google Scholar] [CrossRef]
- Takahashi, J.; Sakai, K.; Sato, T.; Takatsu, H.; Komatsu, T.; Mitsumura, H.; Murakami, H.; Iguchi, Y. Serum arachidonic acid levels is a predictor of poor functional outcome in acute intracerebral hemorrhage. Clin. Biochem. 2021, 98, 42–47. [Google Scholar] [CrossRef]
- Ye, Z.; Chen, J.; Du, P.; Ni, Q.; Li, B.; Zhang, Z.; Wang, Q.; Cui, T.; Yi, X.; Li, C.; et al. Metabolomics signature and potential application of serum polyunsaturated fatty acids metabolism in patients with vitiligo. Front. Immunol. 2022, 13, 839167. [Google Scholar] [CrossRef]
- Radin, N.S. Cancer progression in the kidney and prostate: Vital roles of sphingolipids in chemotherapy. Urology 2002, 60, 562–568. [Google Scholar] [CrossRef]
- Morad, S.A.; Cabot, M.C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer. 2013, 13, 51–65. [Google Scholar] [CrossRef]
- Zheng, K.; Chen, Z.; Feng, H.; Chen, Y.; Zhang, C.; Yu, J.; Luo, Y.; Zhao, L.; Jiang, X.; Shi, F. Sphingomyelin synthase 2 promotes an aggressive breast cancer phenotype by disrupting the homoeostasis of ceramide and sphingomyelin. Cell Death Dis. 2019, 10, 157. [Google Scholar] [CrossRef]
- Liu, X.T.; Chung, L.H.; Liu, D.; Chen, J.; Huang, Y.; Teo, J.D.; Han, X.D.; Zhao, Y.; Guan, F.H.X.; Tran, C.; et al. Ablation of sphingosine kinase 2 suppresses fatty liver-associated hepatocellular carcinoma via downregulation of ceramide transfer protein. Oncogenesis 2022, 11, 67. [Google Scholar] [CrossRef]
- Takanashi, Y.; Funai, K.; Sato, S.; Kawase, A.; Tao, H.; Takahashi, Y.; Sugimura, H.; Setou, M.; Kahyo, T.; Shiiya, N. Sphingomyelin(d35:1) as a novel predictor for lung adenocarcinoma recurrence after a radical surgery: A case-control study. BMC Cancer 2020, 20, 800. [Google Scholar] [CrossRef]
- Kwon, H.J.; Oh, J.Y.; Lee, K.S.; Lim, H.K.; Lee, J.; Yoon, H.-R.; Jung, J. Lipid profiles obtained from MALDI mass spectrometric imaging in liver cancer metastasis. Int. J. Anal. Chem. 2022, 2022, 6007158. [Google Scholar] [CrossRef]
- Ecker, J.; Benedetti, E.; Kindt, A.S.D.; Höring, M.; Perl, M.; Machmüller, A.C.; Sichler, A.; Plagge, J.; Wang, Y.; Zeissig, S.; et al. The colorectal cancer lipidome: Identification of a robust tumor-specific lipid species signature. Gastroenterology 2021, 161, 910–923.e19. [Google Scholar] [CrossRef]
- Arai, J.; Hayakawa, Y.; Koike, K. The colorectal cancer lipidome: Are there any differences in lipid species between right and left colorectal cancers? Gastroenterology 2022, 162, 658. [Google Scholar] [CrossRef] [PubMed]
- Boggs, J.M. Lipid intermolecular hydrogen bonding: Influence on structural organization and membrane function. Biochim. Biophys. Acta 1987, 906, 353–404. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y.; Thompson, T.E. Sphingomyelin: Biophysical aspects. Chem. Phys. Lipids 1999, 102, 29–34. [Google Scholar] [CrossRef]
- Mombelli, E.; Morris, R.; Taylor, W.; Fraternali, F. Hydrogen-bonding propensities of sphingomyelin in solution and in a bilayer assembly: A molecular dynamics study. Biophys. J. 2003, 84, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Slotte, J.P. Biological functions of sphingomyelins. Prog. Lipid Res. 2013, 52, 424–437. [Google Scholar] [CrossRef]
- Ahyayauch, H.; Arnulphi, C.; Sot, J.; Alonso, A.; Goñi, F.M. The onset of Triton X-100 solubilization of sphingomyelin/ceramide bilayers: Effects of temperature and composition. Chem. Phys. Lipids 2013, 167–168, 57–61. [Google Scholar] [CrossRef]
- Slotte, J.P. The importance of hydrogen bonding in sphingomyelin’s membrane interactions with co-lipids. Biochim. Biophys. Acta 2016, 1858, 304–310. [Google Scholar] [CrossRef]
- van Blitterswijk, W.J. Structural basis and physiological control of membrane fluidity in normal and tumor cells. Subcell. Biochem. 1988, 13, 393–413. [Google Scholar] [CrossRef]
- Koumanov, K.S.; Momchilova-Pankova, A.B.; Wang, S.R.; Infante, R. Membrane phospholipid composition, fluidity and phospholipase A2 activity of human hepatoma cell line HepG2. Int. J. Biochem. 1990, 22, 1453–1455. [Google Scholar] [CrossRef]
- Mizuno, M.; Matsuzaki, T.; Ozeki, N.; Katano, H.; Koga, H.; Takebe, T.; Yoshikawa, H.Y.; Sekiya, I. Cell membrane fluidity and ROS resistance define DMSO tolerance of cryopreserved synovial MSCs and HUVECs. Stem Cell Res. Ther. 2022, 13, 177. [Google Scholar] [CrossRef]
- Momchilova, A.; Pankov, R.; Staneva, G.; Pankov, S.; Krastev, P.; Vassileva, E.; Hazarosova, R.; Krastev, N.; Robev, B.; Nikolova, B.; et al. Resveratrol affects sphingolipid metabolism in A549 lung adenocarcinoma cells. Int. J. Mol. Sci. 2022, 23, 10870. [Google Scholar] [CrossRef]
- Barceló-Coblijn, G.; Martin, M.L.; de Almeida, R.F.; Noguera-Salvà, M.A.; Marcilla-Etxenike, A.; Guardiola-Serrano, F.; Lüth, A.; Kleuser, B.; Halver, J.E.; Escribá, P.V. Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 19569–19574. [Google Scholar] [CrossRef] [PubMed]
- Muzio, G.; Trombetta, A.; Maggiora, M.; Martinasso, G.; Vasiliou, V.; Lassen, N.; Canuto, R.A. Arachidonic acid suppresses growth of human lung tumor A549 cells through down-regulation of ALDH3A1 expression. Free Radic. Biol. Med. 2006, 40, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Murakami, M.; Furuhata, A.; Gao, S.; Yoshida, K.; Sobue, S.; Hagiwara, K.; Takagi, A.; Kojima, T.; Suzuki, M.; et al. Transcriptional regulation of neutral sphingomyelinase 2 gene expression of a human breast cancer cell line, MCF-7, induced by the anti-cancer drug, daunorubicin. Biochim. Biophys. Acta 2009, 1789, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Tanaka, K.; Hagiwara, K.; Kobayashi, M.; Hoshikawa, A.; Mizutani, N.; Takagi, A.; Kojima, T.; Sobue, S.; Ichihara, M.; et al. Transcriptional regulation of neutral sphingomyelinase 2 in all-trans retinoic acid-treated human breast cancer cell line, MCF-7. J. Biochem. 2012, 151, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Shamseddine, A.A.; Clarke, C.J.; Carroll, B.; Airola, M.V.; Mohammed, S.; Rella, A.; Obeid, L.M.; Hannun, Y.A. P53-dependent upregulation of neutral sphingomyelinase-2: Role in doxorubicin-induced growth arrest. Cell Death Dis. 2015, 6, e1947. [Google Scholar] [CrossRef]
- Bubenik, J. Tumour MHC class I downregulation and immunotherapy (Review). Oncol. Rep. 2003, 10, 2005–2008. [Google Scholar] [CrossRef]
- Garrido, F.; Aptsiauri, N. Cancer immune escape: MHC expression in primary tumours versus metastases. Immunology 2019, 158, 255–266. [Google Scholar] [CrossRef]
- Cornel, A.M.; Mimpen, I.L.; Nierkens, S. MHC class I downregulation in cancer: Underlying mechanisms and potential targets for cancer immunotherapy. Cancers 2020, 12, 1760. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer immune evasion through loss of MHC class I antigen presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef]
- Corsetto, P.A.; Cremona, A.; Montorfano, G.; Jovenitti, I.E.; Orsini, F.; Arosio, P.; Rizzo, A.M. Chemical-physical changes in cell membrane microdomains of breast cancer cells after omega-3 PUFA incorporation. Cell Biochem. Biophys. 2012, 64, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Bae, Y.S.; Lee, Y.M.; Kim, J.S.; Oh, S.H.; Kim, H.M. Sesquiterpene alcohol cedrol chemosensitizes human cancer cells and suppresses cell proliferation by destabilizing plasma membrane lipid rafts. Front. Cell Dev. Biol. 2021, 8, 571676. [Google Scholar] [CrossRef] [PubMed]
- Tani, M.; Ito, M.; Igarashi, Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell Signal. 2007, 19, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Banáth, J.; Sun, J.; Canals, D.; Hannun, Y.A.; Separovic, D. Ceramide and sphingosine-1-phosphate act as photodynamic therapy-elicited damage-associated molecular patterns: Cell surface exposure. Int. Immunopharmacol. 2014, 20, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Oskouian, B.; Saba, J.D. Cancer treatment strategies targeting sphingolipid metabolism. Adv. Exp. Med. Biol. 2010, 688, 185–205. [Google Scholar] [CrossRef]
- Echeverría, C.; Martin, A.; Simon, F.; Salas, C.O.; Nazal, M.; Varela, D.; Pérez-Castro, R.A.; Santibanez, J.F.; Valdés-Valdés, R.O.; Forero-Doria, O.; et al. In Vivo and in vitro antitumor activity of tomatine in hepatocellular carcinoma. Front. Pharmacol. 2022, 13, 1003264. [Google Scholar] [CrossRef]
- Fujikawa, K.; Shiraki, K.; Sugimoto, K.; Ito, T.; Yamanaka, T.; Takase, K.; Nakano, T. Reduced expression of ICE/caspase1 and CPP32/caspase3 in human hepatocellular carcinoma. Anticancer Res. 2000, 20, 1927–1932. [Google Scholar]
- Sun, B.H.; Zhang, J.; Wang, B.J.; Zhao, X.P.; Wang, Y.K.; Yu, Z.Q.; Yang, D.L.; Hao, L.J. Analysis of in vivo patterns of caspase 3 gene expression in primary hepatocellular carcinoma and its relationship to p21(WAF1) expression and hepatic apoptosis. World J. Gastroenterol. 2000, 6, 356–360. [Google Scholar] [CrossRef]
- Persad, R.; Liu, C.; Wu, T.T.; Houlihan, P.S.; Hamilton, S.R.; Diehl, A.M.; Rashid, A. Overexpression of caspase-3 in hepatocellular carcinomas. Mod. Pathol. 2004, 17, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, E.; Eaves, C.J. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Mechanisms of ceramide-mediated apoptosis. Adv. Exp. Med. Biol. 1997, 407, 145–149. [Google Scholar] [CrossRef]
- Andrieu-Abadie, N.; Levade, T. Sphingomyelin hydrolysis during apoptosis. Biochim. Biophys. Acta 2002, 1585, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.H.; Huang, C.N.; Hsu, S.P.; Wang, C.J. Penta-acetyl geniposide induce apoptosis in C6 glioma cells by modulating the activation of neutral sphingomyelinase-induced p75 nerve growth factor receptor and protein kinase Cdelta pathway. Mol. Pharmacol. 2006, 70, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, J. Sphingosine and ceramide signalling in apoptosis. IUBMB Life 2006, 58, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xia, Y.; Li, B.; Xu, H.; Wang, C.; Liu, Y.; Li, Y.; Li, C.; Gao, N.; Li, L. Induction of ER stress-mediated apoptosis by ceramide via disruption of ER Ca(2+) homeostasis in human adenoid cystic carcinoma cells. Cell Biosci. 2014, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- Truman, J.P.; García-Barros, M.; Obeid, L.M.; Hannun, Y.A. Evolving concepts in cancer therapy through targeting sphingolipid metabolism. Biochim. Biophys. Acta 2014, 1841, 1174–1188. [Google Scholar] [CrossRef]
- Nganga, R.; Oleinik, N.; Ogretmen, B. Mechanisms of ceramide-dependent cancer cell death. Adv. Cancer Res. 2018, 140, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Insausti-Urkia, N.; Solsona-Vilarrasa, E.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Sphingomyelinases and liver diseases. Biomolecules 2020, 10, 1497. [Google Scholar] [CrossRef]
- Taniguchi, M.; Okazaki, T. Role of ceramide/sphingomyelin (SM) balance regulated through “SM cycle” in cancer. Cell Signal. 2021, 87, 110119. [Google Scholar] [CrossRef]
- Pal, P.; Atilla-Gokcumen, G.E.; Fraso, R.J. Emerging roles of ceramides in breast cancer biology and therapy. Int. J. Mol. Sci. 2022, 23, 11178. [Google Scholar] [CrossRef] [PubMed]
- Technical Bulletin. CellTiter 96® AQ ueous One Solution Cell Proliferation Assay. Available online: https://www.promega.com/-/media/files/resources/protocols/technical-bulletins/0/celltiter-96-aqueous-one-solution-cell-proliferation-assay-system-protocol.pdf (accessed on 10 October 2022).
- Al-Nasiry, S.; Geusens, N.; Hanssens, M.; Luyten, C.; Pijnenborg, R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum. Reprod. 2007, 22, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Shakor, A.B.; Czurylo, E.A.; Sobota, A. Lysenin, a unique sphingomyelin-binding protein. FEBS Lett. 2003, 542, 1–6. [Google Scholar] [CrossRef]
- Hullin-Matsuda, F.; Murate, M.; Kobayashi, T. Protein probes to visualize sphingomyelin and ceramide phosphoethanolamine. Chem. Phys. Lipids 2018, 216, 132–141. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. 2’,7 Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic. Res. 2010, 44, 587–604. [Google Scholar] [CrossRef]
- Reiniers, M.J.; de Haan, L.R.; Reeskamp, L.F.; Broekgaarden, M.; Hoekstra, R.; van Golen, R.F.; Heger, M. Optimal use of 2’,7’-dichlorofluorescein diacetate in cultured hepatocytes. Methods Mol. Biol. 2022, 2451, 721–747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, X.; Chen, S.; Jia, W.; Ma, X.; Wang, J.; Qian, Y.; Lei, D.; Liu, H.; Pan, X. GPR12 inhibits migration and promotes apoptosis in esophageal cancer and hypopharyngeal cancer cells. Thorac. Cancer. 2021, 12, 1525–1535. [Google Scholar] [CrossRef]
- Ajibare, A.C.; Ebuehi, O.A.T.; Adisa, R.A.; Sofidiya, M.O.; Olugbuyiro, J.A.O.; Akinyede, K.A.; Iyiola, H.A.; Adegoke, Y.A.; Omoyuri, S.I.; Ekpo, O.E. Fractions of Hoslundia opposita Vahl and hoslundin induced apoptosis in human cancer cells via mitochondrial-dependent reactive oxygen species (ROS) generation. Biomed. Pharmacother. 2022, 153, 113475. [Google Scholar] [CrossRef]
- Markworth, J.F.; Mitchell, C.J.; D’Souza, R.F.; Aasen, K.M.M.; Durainayagam, B.R.; Mitchell, S.M.; Chan, A.H.C.; Sinclair, A.J.; Garg, M.; Cameron-Smith, D. Arachidonic acid supplementation modulates blood and skeletal muscle lipid profile with no effect on basal inflammation in resistance exercise trained men. Prostaglandins Leukot. Essent. Fatty Acids 2018, 128, 74–86. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Abercrombie, M. Contact inhibition and malignancy. Nature 1979, 281, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. A revisited concept: Contact inhibition of growth. From cell biology to malignancy. Exp. Cell Res. 2017, 359, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Roycroft, A.; Mayor, R. Molecular basis of contact inhibition of locomotion. Cell Mol. Life Sci. 2016, 73, 1119–1130. [Google Scholar] [CrossRef]
- Kummer, D.; Steinbacher, T.; Thölmann, S.; Schwietzer, M.F.; Hartmann, C.; Horenkamp, S.; Demuth, S.; Peddibhotla, S.S.D.; Brinkmann, F.; Kemper, B.; et al. A JAM-A-tetraspanin-αvβ5 integrin complex regulates contact inhibition of locomotion. J. Cell Biol. 2022, 221, e202105147. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tallima, H.; El Ridi, R. Mechanisms of Arachidonic Acid In Vitro Tumoricidal Impact. Molecules 2023, 28, 1727. https://doi.org/10.3390/molecules28041727
Tallima H, El Ridi R. Mechanisms of Arachidonic Acid In Vitro Tumoricidal Impact. Molecules. 2023; 28(4):1727. https://doi.org/10.3390/molecules28041727
Chicago/Turabian StyleTallima, Hatem, and Rashika El Ridi. 2023. "Mechanisms of Arachidonic Acid In Vitro Tumoricidal Impact" Molecules 28, no. 4: 1727. https://doi.org/10.3390/molecules28041727
APA StyleTallima, H., & El Ridi, R. (2023). Mechanisms of Arachidonic Acid In Vitro Tumoricidal Impact. Molecules, 28(4), 1727. https://doi.org/10.3390/molecules28041727





