Abstract
Sonchus arvensis Linn. and Hemerocallis citrina Baroni. have been reported to improve body resistance. However, the underlying mechanism is not clear. In this study, Sonchus arvensis Linn. phenolic compounds (SAP) and Hemerocallis citrina Baroni. phenolic compounds (HCP) were extracted and their protective effects in Caenorhabditis elegans evaluated. SAP and HCP showed considerably different phenolic compositions. In the normal C. elegans model, HCP exhibited better effects in promoting growth than SAP. In the sucrose-incubated C. elegans model, both SAP and HCP showed positive effects against the high-sucrose-induced damage. In the stearic acid-incubated C. elegans model, both SAP and HCP improved lifespan, reproductive ability and growth, while HCP had a more evident effect than SAP on reproductive ability. The TGF-β signaling pathway was confirmed to be involved in the protective effects of SAP and HCP. The antioxidant ability of SAP was also found to be related to skn-1. Our study shows that both SAP and HCP have protective effects against high sucrose- or high stearic acid-induced damage.
1. Introduction
Carbohydrates and fats are the main sources of organic materials used for supporting the human body. A reasonable intake of carbohydrates and fats has a positive impact on human activities; however excessive intake may prove harmful to the human body, leading to the occurrence of metabolic syndrome. With an improvement in the living standards of people, dietary structure gradually shifts towards the direction of high sugar and fat consumption. Long-term intake of a diet high in sugar and fat leads to abnormal blood pressure and blood lipid levels, cognitive dysfunction, and damage to male reproductive ability. It may also make offspring vulnerable to chronic diseases, such as type 1 diabetes and reproductive disorders [1,2]. Owing to the complex background mechanism of metabolic syndrome caused by high-sugar and high-fat diets, it has become very difficult to develop effective drugs to prevent or improve metabolic syndrome. Currently, common drugs in the market are targeted to intervene in a single group of patients with metabolic syndrome. However, the disadvantage of drug therapy lies in the use of a single target, which can easily cause side effects [3]. Accordingly, the search for natural compounds as therapeutic agents that can effectively intervene in metabolic syndrome has become a research hotspot.
Sonchus arvensis Linn. is a traditional food in northern China. The extract of Sonchus arvensis Linn. has many pharmacological effects, including antioxidant, hepatoprotective, kidney-protective, antibacterial, and anti-fatigue properties [4,5,6]. The extracts of Sonchus arvensis Linn. scavenge the free radicals, which may be due to the presence of functional components include rutin, quercetin, catechin, and myricetin [7]. Previous studies have shown that Sonchus arvensis Linn. phenolic compounds (SAP) have considerable antioxidant capabilities [8]. However, its mechanism of action requires further investigation.
Hemerocallis citrina Baroni., commonly known as Citron daylily and long yellow daylily, is consumed as a vegetable in China and other Asian countries [9]. It is rich in multiple compounds, such as carbohydrates, protein, lipid, and vitamins. It also contains many volatile substances, including phenols, alcohols, and acids. Hemerocallis citrina Baroni. exerts various bioactivities including anti-gloom, anti-tumor, antioxidant, cholesterol-lowering, and anti-aging activities [10,11,12]. The strong antioxidant activity of Hemerocallis citrina Baroni. can be attributed to the action of antioxidant compounds such as phenolic compounds [13]. Hemerocallis citrina Baroni. phenolic compounds (HCP) have been shown to play many physiological functions, such as enhancing stress resistance and hepatoprotection [14,15]. HCP has effective protective properties, but the underlying mechanism is not clear.
Caenorhabditis elegans is an ideal model for studying lipid and sugar resistance because of its low cost, short lifespan, rapid reproductions cycle, and ease of observation [16,17]. In addition, the whole genome sequence of C. elegans is known, and more than 60% of the genes are homologous to human disease genes [18]. In this study, we employed a C. elegans model to investigate the protective effects of SAP and HCP in vivo.
In C. elegans, transforming growth factor-β (TGF-β) superfamily ligands participate in cell identification, growth, and development. Dbl-1 and daf-7 are the ligands belong to TGF-β superfamily and their function has been explained more clearly [19]. The C. elegans insulin signaling pathway links energy metabolism with growth, development, reproductive, longevity, and behavior [20]. This insulin signaling pathway is regulated by insulin-like peptide ligands that bind to the insulin/IGF-1 transmembrane receptor ortholog daf-2 [21]. In C. elegans, skn-1, the downstream regulator of daf-2, is required for both oxidative stress resistance and anti-aging through its accumulation in the intestinal nuclei to promote the detoxication of target genes [22].
In the present study, to better understand the bioactivity of SAP and HCP, the phenolic compounds in the SAP and HCP were analyzed using ultra-performance liquid chromatography coupled to triple-quadrupole tandem mass spectrometer (UPLC-QQQ-MS/MS). In addition, we evaluated the effects of SAP and HCP in normal C. elegans. Furthermore, the role of SAP and HCP in protecting C. elegans against sucrose or stearic acid damage was explored. The results will help investigate the mechanism of SAP and HCP and define health claims related to the consumption of Sonchus arvensis Linn. and Hemerocallis citrina Baroni.
2. Results
2.1. Extraction and Component Analysis of SAP and HCP
The yields of SAP and HCP were 23% and 39%, respectively. We used a method in the MRM mode of UPLC-QQ-MS/MS system for qualitative and quantitative analysis of phenolic compounds in SAP and HCP. The Sonchus arvensis Linn. is rich in rutin, quercetin, chlorogenic acid and other phenolic compounds [23]. Previous study showed that there are 25 polyphenols in Hemerocallis citrina Baroni [24].
As shown in Figure 1 and Figure 2, seven phenolic compounds were detected in SAP and eight phenolic compounds were detected in HCP. The phenolic compositions of SAP and HCP are summarized in Table 1. The phenolic compounds found in both SAP and HCP were rutin, chlorogenic acid, and quercetin, although their contents were extremely different. The major phenolic compounds found in SAP and HCP were chlorogenic acid and rutin, respectively. These findings indicate that SAP and HCP, with different phenolic compositions, may have different physiological activities.
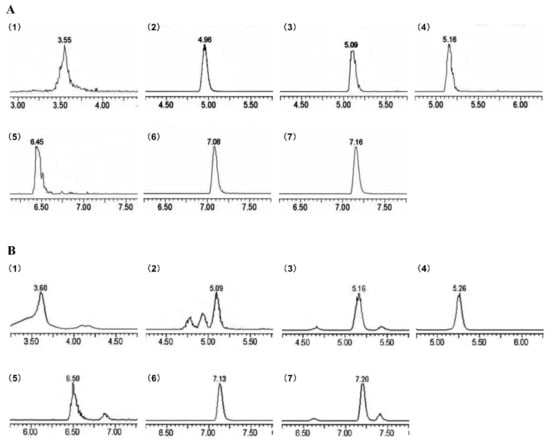
Figure 1.
MRM chromatogram of Sonchus arvensis Linn. phenolic compounds (SAP) showing the presence of seven phenolic compounds as identified. (A) MRM chromatogram of standards; (B) MRM chromatogram of SAP. Peaks: (1) chlorogenic acid, (2) scopoletin, (3) rutin, (4) hyperin, (5) quercetin, (6) apigenin, (7) chrysoeriol.
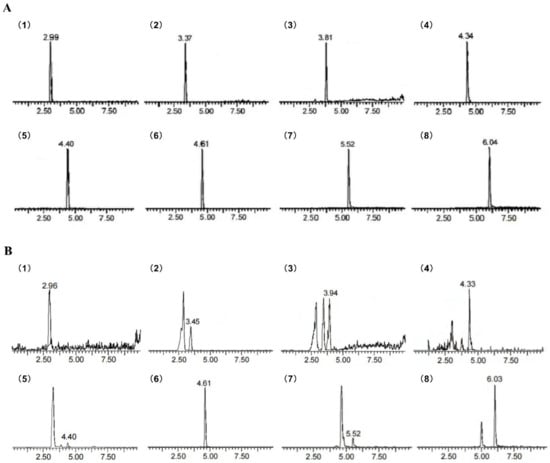
Figure 2.
MRM chromatogram of Hemerocallis citrina Baroni. phenolic compounds (HCP) showing the presence of eight phenolic compounds as identified. (A) MRM chromatogram of standards; (B) MRM chromatogram of HCP. Peaks: (1) protocatechin, (2) chlorogenic acid, (3) caffeic acid, (4) taxifolin, (5) gentisic acid, (6) rutin, (7) quercetin, (8) isorhamnetin.

Table 1.
The phenolic compositions of SAP and HCP.
2.2. Effects of SAP and HCP on the Body Length, Progeny Production and Lifespan of C. elegans
Based on the results of the preliminary experiment, C. elegans were treated with SAP at concentrations ranging from 0 to 2000 μg/mL and HCP at concentrations ranging from 0 to 1200 μg/mL. As shown in Figure 3A and Table 2, the survival curve of C. elegans treated with 500 and 1000 μg/mL SAP were obviously shifted to the right. In addition, treatment with 500 and 1000 μg/mL SAP significantly (p < 0.05) prolonged the average lifespan of C. elegans. As shown in Figure 3B and Table 2, the survival curve of 1200 μg/mL HCP was shifted to the left. Treatment with HCP at concentrations ranging from 400 to 1200 μg/mL had no significant effect on the average lifespan of C. elegans. These results indicated that between the two types of extracts, SAP exhibited a more positive effect that prolonged the lifespan of C. elegans.

Figure 3.
Effects of SAP and HCP on lifespan, reproduction, and body length of the C. elegans. (A,B): effects of SAP (A) and HCP (B) on the lifespan of C. elegans. (C,D): effects of SAP (C) and HCP (D) on the number of eggs of C. elegans on each day. (E,F): effects of SAP (E) and HCP (F) on the total number of eggs of C. elegans in the entire spawning period. (G,H): effects of SAP (G) and HCP (H) on the body length of C. elegans. Data are presented as mean ± SEM (n = 30). Values without a common letter are significantly different at p < 0.05.

Table 2.
The mean lifespan of C. elegans treated with SAP and HCP.
As shown in Figure 3C,D, after treatment with SAP or HCP, the average number of eggs laid by C. elegans on each day initially increased, and then decreased. C. elegans treated with control and different concentrations of SAP and HCP entered the spawning period on the second day and ended spawning on the sixth day. As shown in Figure 3E, after treatment with 500, 1000, and 2000 μg/mL SAP, the total number of eggs in the entire spawning period significantly (p < 0.05) increased, especially with 500 μg/mL treatments, showing the most statistically different effect among the three concentrations of SAP groups. As shown in Figure 3F, after treatment with 400 μg/mL HCP, the total number of eggs laid by C. elegans in the entire spawning period decreased, whereas treatment with 800 and 1200 μg/mL HCP significantly (p < 0.05) increased the number of eggs, and 1200 μg/mL HCP showed the most statistically different effect, increasing by 51%. Taken together, SAP and HCP improved the reproductive ability of C. elegans, especially low concentration SAP and high concentration HCP.
As shown in Figure 3G, compared with that in the control group, except for treatment with SAP at 1000 μg/mL, there was no significant difference in the length of C. elegans after treatment with the other concentrations of SAP. In the growing phase, the body length of C. elegans grew rapidly and reached a maximum of 1.2 mm on the fifth day, after treatment with SAP at 500 and 1000 μg/mL. However, treatment with 2000 μg/mL SAP brought forward the appearance of C. elegans aging. As shown in Figure 3H, as C. elegans entered the spawning period, HCP treatment rapidly promoted the growth of C. elegans, especially at a concentration of 1200 μg/mL. In addition, 800 and 1200 μg/mL HCP treatment significantly (p < 0.05) increased the maximum length of C. elegans, which reached a maximum length of 1.4 mm on the fifth day.
2.3. Effects of SAP and HCP on the Body Length, Progeny Production and Lifespan of Sucrose-incubated C. elegans
As shown in Table 3, compared to that of the control group, the average lifespan of C. elegans treated with 100 mM sucrose was significantly (p < 0.05) decreased. The average lifespan of sucrose-incubated C. elegans treated with SAP and HCP significantly (p < 0.05) increased compared with that of the control group. As shown in Figure 4A,B, compared to those of the control group, the survival curves of the HCP and SAP groups were considerably shifted to the right before the tenth day. Moreover, throughout the observation period, the survival curves of the HCP and SAP groups were obviously shifted to the right compared to those of the sucrose-incubated model group. These results indicated that the lifespan of sucrose-incubated C. elegans could be recovered by SAP and HCP treatment.

Table 3.
The mean lifespan of sucrose-incubated C. elegans treated with SAP and HCP.
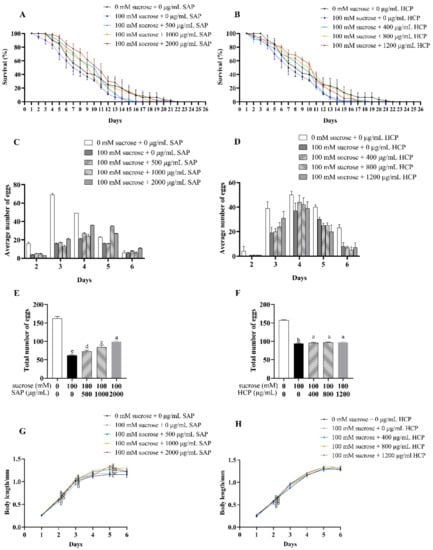
Figure 4.
Effects of SAP and HCP on lifespan, reproduction, and body length of the sucrose-incubated C. elegans. (A,B): effects of SAP (A) and HCP (B) on the lifespan of sucrose-incubated C. elegans. (C,D): effects of SAP (C) and HCP (D) on the average number of eggs of sucrose-incubated C. elegans on each day. (E,F): effects of SAP (E) and HCP (F) on the total number of eggs of sucrose-incubated C. elegans in the entire spawning period. (G,H): effects of SAP (G) and HCP (H) on the body length of sucrose-incubated C. elegans. Data are presented as mean ± SEM (n = 30). Values without a common letter are significantly different at p < 0.05.
As shown in Figure 4C,D, after treatment with 100 mM sucrose, the spawning periods were delayed by one day. A delay in the spawning period indicates inhibition of C. elegans development. As shown in Figure 4E,F, compared with the control group, the total number of eggs laid by C. elegans treated with sucrose significantly (p < 0.05) decreased. After being treated with SAP, the total number of eggs laid by sucrose-incubated C. elegans increased with increasing SAP concentrations. However, HCP treatment increased the total number of eggs laid by sucrose-incubated C. elegans without an obvious dose-response. Taken together, 100 mM sucrose caused significant damage to the reproductive ability of C. elegans, and the damage decreased with SAP and HCP treatment.
As shown in Figure 4G,H, compared with the body length in the control group, 100 mM sucrose significantly (p < 0.05) inhibited the growth of C. elegans but had no significant effect on the maximum body length of C. elegans. In the growing phase, treatment with 500 to 2000 μg/mL SAP significantly (p < 0.05) increased the body length of the sucrose-incubated C. elegans. In addition, after SAP treatment, the body length of sucrose-incubated C. elegans reached a maximum length of 1.2 mm on the fifth day, which was 1.1 times the length of the C. elegans in the model group. However, there was no significant difference in the length of sucrose-incubated C. elegans after HCP treatment.
2.4. Effects of SAP and HCP on the Body Length, Progeny Production and Lifespan of Stearic Acid-Incubated C. elegans
As shown in Figure 5 and Table 4, compared to the control group, 352 mM stearic acid treatment reduced the average lifespan of C. elegans by 2%. The average lifespan of stearic acid-incubated C. elegans treated with SAP or HCP initially increased and subsequently decreased. Treatment with 500–1000 μg/mL SAP significantly (p < 0.05) prolonged the average lifespan of stearic acid-incubated C. elegans, whereas treatment with 2000 μg/mL SAP had a weak effect on that of stearic acid-incubated C. elegans. In contrast, treatment with 400 μg/mL HCP had a weak effect on the average lifespan of stearic acid-incubated C. elegans, whereas 800–1200 μg/mL HCP significantly (p < 0.05) prolonged the lifespan of stearic acid-incubated C. elegans. This indicates that SAP or HCP treatment could improve the average lifespan of stearic acid-incubated C. elegans, although the effective concentration of the two extracts was different.
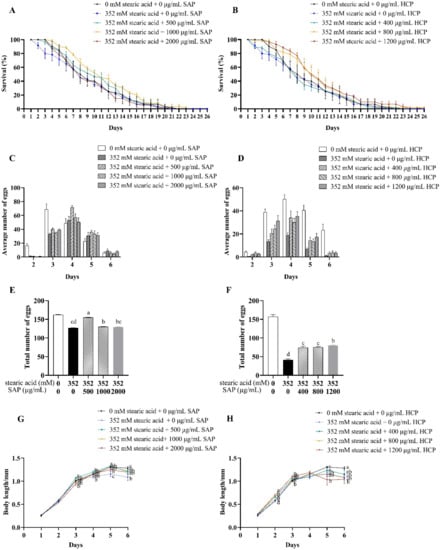
Figure 5.
Effects of SAP and HCP on life-span, reproduction, and body length of the stearic acid-incubated C. elegans. (A,B): effects of SAP (A) and HCP (B) on the lifespan of stearic acid-incubated C. elegans. (C,D): effects of SAP (C) and HCP (D) on the average number of eggs of stearic acid-incubated C. elegans on each day. (E,F): effects of SAP (E) and HCP (F) on the total number of eggs of stearic acid-incubated C. elegans in the entire spawning period. (G,H): effects of SAP (G) and HCP (H) on the body length of stearic acid-incubated C. elegans. Data are presented as mean ± SEM (n = 30). Values without a common letter are significantly different at p < 0.05.

Table 4.
The mean lifespan of stearic acid-incubated C. elegans treated with SAP and HCP.
As shown in Figure 5C–F, after treatment with 352 mM stearic acid, the spawning periods were delayed by one day, and the total number of eggs laid by C. elegans significantly (p < 0.05) decreased. As shown in Figure 5E, after treatment with 500 and 1000 μg/mL SAP, the total number of eggs laid by stearic acid-incubated C. elegans significantly (p < 0.05) increased, especially with 500 μg/mL SAP treatment. However, 2000 μg/mL SAP had no significant effect on the reproductive ability of stearic acid-incubated C. elegans. This result was similar to the lifespan of stearic acid-incubated C. elegans, indicating that when SAP was added at a concentration of 2000 μg/mL, its protective effect was weakened. As shown in Figure 5F, after treatment with 400, 800, or 1200 μg/mL HCP, the total number of eggs laid by stearic acid-incubated C. elegans significantly (p < 0.05) increased, and 1200 μg/mL HCP had the most positive effect. Taken together, the intake of 352 mM stearic acid caused significant damage to the reproductive ability of C. elegans, and the damage decreased after SAP and HCP treatment.
As shown in Figure 5G,H, treatment with 352 mM stearic acid significantly (p < 0.05) inhibited the growth of C. elegans, and reduced the maximum length by 20% on the fifth day compared with that in the control group. As shown in Figure 5G, the body length of the stearic acid-incubated C. elegans treated with 500–2000 μg/mL SAP was similar to that in the control group. In other words, SAP treatment significantly (p < 0.05) promoted the growth of stearic acid-incubated C. elegans, especially at 500 μg/mL. As shown in Figure 5H, treatment with 400–1200 μg/mL HCP significantly (p < 0.05) increased the body length of stearic acid-incubated C. elegans on the second day. In addition, 1200 μg/mL HCP had a positive effect on the length of stearic acid-incubated C. elegans on the third day. However, compared with that in the control group, 1200 μg/mL HCP treatment shortened the body length of C. elegans on the fifth and sixth days.
2.5. Effects of SAP and HCP on Gene Expressions in C. elegans
To explore the protective mechanisms of SAP and HCP, we detected the mRNA expression level of related genes (Table 5). The worms were treated with SAP (500 μg/mL) or HCP (1200 μg/mL).

Table 5.
The effect of relative genes on C. elegans.
As shown in Figure 6A, no significant difference was observedin the expression of skn-1 in normal C. elegans. It was found that 100 mM sucrose and 100 μg/L stearic acid had weak effects on the expression of skn-1. In sucrose-incubated C. elegans, no significant difference was observed in the expression of skn-1. In stearic acid-incubated C. elegans, the expression of skn-1 was significantly (p < 0.05) increased with SAP (500 μg/mL) treatment, whereas no significant difference was observed with HCP (1200 μg/mL) treatment. As shown in Figure 6B, no significant differences were observed in the expression of daf-7 in normal and sucrose-damaged C. elegans. However, both SAP (500 μg/mL) and HCP (1200 μg/mL) increase the expression of daf-7 in stearic acid-incubated C. elegans, and there was no significant difference between the effects of the two treatments. As shown in Figure 6C, SAP (500 μg/mL) treatment or HCP (1200 μg/mL) treatment had a weak effect on the expression of dbl-1 in the normal C. elegans. Treatment with 100 mM sucrose or 352 mM stearic acid significantly (p < 0.05) decreased the expression of dbl-1. In sucrose-incubated C. elegans, the expression of dbl-1 was significantly (p < 0.05) increased after HCP (1200 μg/mL) treatment, whereas no significant difference was observed with SAP (500 μg/mL) treatment. However, no significant difference was observed in the expression of dbl-1 in stearic acid-incubated C. elegans. As shown in Figure 6D, no significant difference was observed in the expression of sma-4 in normal C. elegans. Treatment with 100 mM sucrose or 352 mM stearic acid significantly (p < 0.05) decreased the expression of dbl-1. In sucrose-incubated C. elegans, the expression of sma-4 was significantly (p < 0.05) increased with HCP (1200 μg/mL) treatment, whereas no significant difference was observed with SAP (500 μg/mL) treatment. In stearic acid-incubated C. elegans, the expression of sma-4 was significantly (p < 0.05) decreased with HCP (1200 μg/mL) treatment, while no significant difference was observed with SAP (500 μg/mL) treatment.
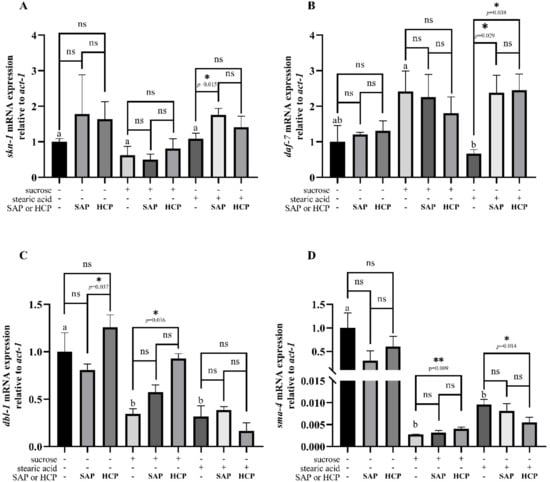
Figure 6.
Effects of SAP and HCP on gene expression of C. elegans. Worms were treated with SAP (500 μg/mL) or HCP (1200 μg/mL). (A) skn-1 mRNA expressions, (B) daf-7 mRNA expression, (C) dbl-1 mRNA expression, (D) sma-4 mRNA expression. Values are expressed as mean ± SEM. Different superscripts were considered significantly different, ANOVA followed by Tukey’s multiple-comparison test. * p <0.05; ** p < 0.01; Student’s t-test (two-tailed).
3. Materials and Methods
3.1. Reagents
Chlorogenic acid, hyperin, rutin, apigenin, quercetin, scopoletin, chrysoeriol, caffeic acid, protocatechin, isorhamnetin, taxifolin, and gentisic acid were purchased from Macklin (Shanghai, China). Methyl alcohol, formic acid, and n-Hexane were purchased from Merck Millipore. The sucrose and stearic acid were purchased from Sigma (St. Louis, MO, USA). Na2HPO4, KH2PO4, KPO4, NaCl, CaCl2, MgSO4, and NH4Cl were purchased from Beijing Lanyi Chemical Products Co. Ltd. (Beijing, China). Sucrose, stearic acid, agar, peptone, cholesterol, and DMSO were purchased from Lablead (Beijing, China).
3.2. Preparation of Phenolic Compunds from Sonchus arvensis Linn. or Hemerocallis citrina Baroni
The leaves of Sonchus arvensis Linn. were collected from Xinghua City, Jiangsu Province in China. The buds of Hemerocallis citrina Baroni. were collected from Datong City, Shanxi Province in China. The powder of Sonchus arvensis Linn. or Hemerocallis citrina Baroni. (0.2 g) was extracted using 80% methyl alcohol (5 mL) in an ultrasonic bath for 20 min (SCQ-3201, Shanghai Shengyan Ultrasonic Co., Ltd., Shanghai, China), followed by centrifugation at 7000 rpm and 4 °C (Sigma-Aldrich, Shanghai, China). The supernatant was collected and the residue was re-extracted two more times under the same conditions. The supernatant was diluted to 25 mL and mixed with n-Hexane at a 1:1 ratio. After the mixed liquid was stratified, the upper liquid was removed and lyophilized to yield partially purified Sonchus arvensis Linn. phenolic compounds (SAP) or Hemerocallis citrina Baroni. phenolic compounds (HCP) by using a vacuum freeze-dryer (Christ, Osterode, Germany). The extraction yield (%) of SAP and HCP was calculated using the following equation: extraction yield (%, w/w) = (weight of dried SAP or HCP (g)/weight of dry materials (g)) × 100%.
3.3. Phenolic Compositions Analysis of SAP and HCP
Phenolic compositions were analyzed using the UPLC-QQQ-MS/MS method. Samples were separated using ACQUITY UPLC BEH C18 (100 mm × 2.1 mm, 1.7 μm, Waters, Milford, MA, USA). The column temperature was set at 30 °C and the sample injection volume was 5 μL. Mobile phase A: methanol and mobile phase B: 0.1% formic acid in 5 mmol/L aqueous acetate were utilized with a gradient elution as follows: 0–1 min, 10% A and 90% B; 1–9 min, 10% A and 90% B; 9–11 min, 90% A and 10% B; 11–11.1 min, 100% A; 11.1–13 min, 10% A and 90% B. Post run lasted for 2 min to balance and wash the column. The flow rate was 0.25 mL/min, and eluate was monitored with a DAD detector (Waters, Milford, MA, USA) at 280 nm. Mass spectrometry data were obtained simultaneously using an electrospray ionization source in the negative ionization mode (Waters, Milford, MA, USA). The optimal source conditions were a drying gas temperature of 350 °C, nebulizer pressure of 650 L/h, and a capillary voltage of 3000 V. Phenolic compounds standards were used to identify and quantify the corresponding peaks.
3.4. C. elegans Culture and Treatment
Wild-type N2 strains were obtained from the Caenorhabditis Genetics Center (University of MN, Minneapolis, MN, USA) and maintained on nematode growth medium (NGM) plates (1.7% agar, 2.5 g/L peptone, 51 mM NaCl, 25 mM KPO4 buffer pH 6.0, 5 μg/L cholesterol, 1 mM CaCl2, 1 mM MgSO4) with Escherichia coli OP50 (Rutgers University, New Brunswick, NJ, USA) as food resource, at 20 °C. To obtain a synchronized population of worms, the eggs were collected in a certain amount of time. M9 buffer (41 mM Na2HPO4, 15 mM KH2PO4, 8.6 mM NaCl, 19 mM NH4Cl) was used to wash the eggs. Sucrose plates were prepared by adding sucrose (100 mM, sterile filtered) into NGM [28]. Stearic acid plates were prepared by adding stearic acid (352 mM, sterile filtered) into NGM [28]. SAP or HCP completely dissolved in DMSO and configured to 2 mg/mL stock solution. For assays, SAP (0, 500, 1000, and 2000 μg/mL) or HCP (0, 400, 800, and 1200 μg/mL) were added to Escherichia coli OP50 at a rate of 2%.
3.5. Body Length, Progeny Production and Lifespan Assay
The body length and number of progenies were observed under a stereomicroscope (Chongqing Optec Instrument Co., Ltd., Chongqing, China). The body length of 30 random worms in each group was measured using microscope graticules. The assay was performed in triplicate. For progeny production, 30 random worms in each group were separately transferred to a new NGM plate with Escherichia coli OP50 and grown to the L4 stage. Worms were transferred to fresh NGM plates during the reproductive period, and the remaining eggs were counted on each day. Finally, the total number of eggs laid by worms in the whole life was counted. The assay was performed in triplicate.
For lifespan, 30 worms in each group were transferred daily to fresh NGM plates from L1 stage until death. The number of surviving worms was then counted. Indicators of death included lack of movement, stress movement of the parasite, and lack of pharynx contraction after one or two attempts at gentle touch.
3.6. Quantitative Reverse Transcription-PCR
The total RNA of C. elegans samples was isolated using the RaPure Total RNA Kit (Magen, Shanghai, China) following the manufacturer’s protocol. In brief, the RLT lysis buffer was used to lyse the worms, and then total RNA was collected and purified using HiPure RNA Mini Columns. Finally, the RNA was dissolved with RNase free water. The purity and concentration of RNA were determined using a NanoDrop spectrophotometer (Thermo Fisher, Waltham, MA, USA). cDNA was synthesized using the HiScript III. All-in-one RT SuperMix Perfect for qPCR Kit (Vazyme, Nanjing, China). Relative quantitative RT-PCR was performed using Rotor-Gene Q (Qiagen, Beijing, China) with a Taq Pro Universal SYBR qPCR Master Mix Kit (Vazyme, Nanjing, China). RT-PCR amplification conditions included denaturation at 95 °C for 10 s, annealing at 55 °C for 30 s, and extension for 32 s at 72 °C. Act-1 was used for normalization. All primer pairs were synthesized by Synbio Technologies (Jiangsu, China), and the sizes of PCR products were between 100 to 300 bp. The primer sequences used are listed in Table 6. Results were analyzed using the comparative threshold cycle (Ct) method and expressed as the fold change in gene expression (2−ΔΔCt).

Table 6.
Primer squences.
3.7. Statistical Analysis
All results are presented as mean ± SEM. Statistical significance was determined using one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test or Student’s t-test (two-tailed) using SPSS version 19.0 (IBM Corporation, New York, NY, USA). Differences were considered statistically significant at p < 0.05.
4. Discussion
In this study, we detected the compositions and contents of phenolic compounds in SAP and HCP. In addition, we explored SAP and HCP on the lifespan, body length, and reproductive ability of C. elegans subjected to normal, 100 mM sucrose, or 352 mM stearic acid treatments, with the aim of identifying the impact of SAP and HCP on health. Since excessive carbohydrate and fat intake is harmful to health, 100 mM sucrose or 352 mM stearic acid was used to provide excessive nutrients for C. elegans, and the protective effects of SAP and HCP on sucrose-incubated and stearic acid-incubated C. elegans were investigated.
We detected the seven phenolic compounds of SAP and eight phenolic compounds of HCP to explore the active components of the extracts. Chlorogenic acid, the most phenolic compound presented in SAP in our study, is supposed to account for many of the beneficial anti-aging, antioxidant, anti-diabetic effects observed, as well as against cardiovascular diseases [29,30,31]. In addition, rutin, the main component of HCP, has been reported to exert antioxidant effects by controlling the expression of antioxidant enzymes [32,33]. Chlorogenic acid and rutin may play a key role separately in the protective effect of SAP and HCP in C. elegans.
Excessive sugar and lipid treatment severely shortens the lifespan, body length of the C. elegans and diminishes its reproductive ability. Treatment with SAP at different concentrations presented different degress of protective effects on normal C. elegans. In addition, treatment with 500, 1000, and 2000 μg/mL SAP significantly (p < 0.05) prolonged the lifespan, enhanced the reproductive capacity, and increased the body length of sucrose-incubated C. elegans. However, treatment with 500 and 1000 μg/mL SAP, except for 2000 μg/mL SAP, presented effective protection of stearic acid-incubated C. elegans. This may be related to the different treatment methods used in C. elegans. In other words, sugars and lipids may cause varying degrees of destruction in C. elegans [28]. Moreover, the positive effect of 500 μg/mL SAP on the reproductive capacity of stearic acid-incubated C. elegans was more pronounced than that of 1000 μg/mL SAP. Although 1200 μg/mL HCP shifted the survival curve to the left, there was no significant effect on the average lifespan of normal C. elegans. Treatment with 800, 1200 μg/mL HCP, except for 400 μg/mL HCP, had positive effects on the reproductive capacity of normal C. elegans. In addition, only 1200 μg/mL HCP significantly (p < 0.05) improved the maximum body length of normal C. elegans. Interestingly, we found that the effect of HCP on the body length of sucrose-incubated C. elegans was not as severe as that of SAP. This may be related to the differences in the phenolic compositions of SAP and HCP. All the three concentrations of HCP effectively improved the lifespan and reproductive capacity of sucrose-incubated C. elegans. Similarly, treatment with 400, 800, and 1200 μg/mL HCP significantly (p < 0.05) promoted the reproductive capacity of stearic acid-incubated C. elegans. However, only 1200 μg/mL HCP had positive effects on the lifespan and body length of stearic acid-incubated C. elegans. Overall, our results indicated that SAP and HCP protected C. elegans from sugars or lipids. By overall consideration, we used 500 μg/mL SAP and 1200 μg/mL HCP to further investigate the protective mechanism against C. elegans.
In C. elegans, the major ROS detoxification mechanisms are initiated by the transcription factor skn-1, which responds to oxidative stress [34]. Previous studies indicated that skn-1 promotes the expression of phase II detoxification genes, such as gst-4 and gst-7, which play key roles in increasing oxidative stress resistance and extending lifespan by scavenging free radicals [35]. Zheng et al. [36] reported a significant increase in the lifespan of C. elegans via activating the transcription of skn-1 after supplying chlorogenic acid. This is consistent with our findings that skn-1 mRNA expression in stearic acid-incubated C. elegans was increased with SAP treatment. The TGF-β signaling pathway is important for the regulation of stress responses in organisms [37]. A ligand for the TGF-β signaling pathway, daf-7, is related to stress resistance in C. elegans. A low expression of daf-7 indicates a harsh environment and severe stress in C. elegans [38]. Our results indicated that 352 mM stearic acid treatment significantly decreased the expression of daf-7, suggesting that high stearic acid levels caused damage to C. elegans. Treatment with SAP and HCP significantly (p < 0.05) increased daf-7 mRNA expression in stearic acid-incubated C. elegans. This was consistent with the phenotypic results that both SAP and HCP showed protective effects against stearic acid-damaged C. elegans. In the present study, we found that SAP and HCP treatments had no effects on skn-1 and daf-7 mRNA expression in sucrose-incubated C. elegans. We only evaluated the gene expression of C. elegans at the L4 stage, and further experiments are needed to investigate how SAP and HCP affect C. elegans in different environments. As a signaling ligand, dbl-1 is an important component of the TGF-β signaling pathway; the core components of the dbl-1 pathway consist of receptors (daf-4 and sma-6) and Smads (sma-2, sma-3, and sma-4) [39]. Loss of function of the signaling ligand, receptors or Smads reduces the postembryonic growth of C. elegans, leading to a smaller body size [28]. High sugar and lipid levels disrupt the metabolism of C. elegans, thereby causing damage [28]. In our study, 100 mM sucrose and 352 mM stearic acid treatments significantly (p < 0.05) reduced the expression of dbl-1 and sma-4, suggesting that high sucrose and stearic acid inhibited the development of C. elegans. HCP treatment significantly (p < 0.05) improved the expression of dbl-1 and sma-4 in sucrose-incubated C. elegans. In addition, in normal C. elegans, HCP treatment significantly (p < 0.05) improved the expression of dbl-1 compared with that in the SAP group. This was consistent with the phenotypic results showing that HCP treatment increased the maximum body length of normal C. elegans. In conclusion, we showed that SAP can effectively extend the lifespan, enhance reproductive ability, and promote growth of C. elegans under high stearic acid exposure via skn-1 and daf-7. In addition, we found that HCP can considerably increase the body length of normal C. elegans via dbl-1, and promote growth of C. elegans under stearic acid via daf-7 and sma-4.
In summary, our study demonstrated that SAP and HCP had different phenolic compositions. Additionally, we observed different degrees of protective effects of SAP and HCP treatment on C. elegans damaged by high sucrose or high stearic acid levels. Taken together, we speculate that the repair effect of SAP and HCP against damage due to high sucrose or high stearic acid is mainly manifested in the reduction of oxidative stress in C. elegans. Collectively, SAP and HCP are expected to ameliorate the damage caused by overnutrition.
Author Contributions
Conceptualization, Q.A. and Y.Z.; Data curation, Q.A. and L.Z.; Formal analysis, Q.A.; Funding acquisition, Q.M. and Y.Z.; Investigation, Q.A.; Methodology, L.Z.; Project administration, Y.Z.; Resources, X.Q., X.W. and W.W.; Software, Q.A. and L.Z.; Supervision, Q.M. and Y.Z.; Validation, Q.A. and L.Z.; Visualization, Q.A.; Writing—original draft, Q.A.; Writing—review and editing, Q.A. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the earmarked fund for CARS36.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Yeh, S.H.-H.; Shie, F.-S.; Liu, H.-K.; Yao, H.-H.; Kao, P.-C.; Lee, Y.-H.; Chen, L.-M.; Hsu, S.-M.; Chao, L.-J.; Wu, K.-W.; et al. A high-sucrose diet aggravates Alzheimer’s disease pathology, attenuates hypothalamic leptin signaling, and impairs food-anticipatory activity in APPswe/PS1dE9 mice. Neurobiol. Aging 2019, 90, 60–74. [Google Scholar] [CrossRef]
- Oshio, L.T.; Andreazzi, A.E.; Lopes, J.F.; de Sá, J.P.; Bolotari, M.; Costa, V.M.G.; Guerra, M.D.O.; Peters, V.M. A paternal hypercaloric diet affects the metabolism and fertility of F1 and F2 Wistar rat generations. J. Dev. Orig. Health Dis. 2020, 11, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Van Zwieten, P.A.; Mancia, G. Background and Treatment of Metabolic Syndrome: A Therapeutic Challenge. Semin. Cardiothorac. Vasc. Anesthesia 2006, 10, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.-Z.; Yu, X.-F.; Zhu, Z.-Y.; Zou, Z.-D. Antioxidant and antibacterial activity of six edible wild plants (Sonchus spp.) in China. Nat. Prod. Res. 2011, 25, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Torres-González, L.; Cienfuegos-Pecina, E.; Perales-Quintana, M.M.; Alarcon-Galvan, G.; Muñoz-Espinosa, L.E.; Pérez-Rodríguez, E.; Cordero-Pérez, P. Nephroprotective Effect of Sonchus oleraceus Extract against Kidney Injury Induced by Ischemia-Reperfusion in Wistar Rats. Oxidative Med. Cell. Longev. 2018, 2018, 9572803. [Google Scholar] [CrossRef]
- Yuan, T.; Wu, D.; Sun, K.; Tan, X.; Wang, J.; Zhao, T.; Ren, B.; Zhao, B.; Liu, Z.; Liu, X. Anti-Fatigue Activity of Aqueous Extracts of Sonchus arvensis L. in Exercise Trained Mice. Molecules 2019, 24, 1168. [Google Scholar] [CrossRef]
- Khan, R.A. Evaluation of flavonoids and diverse antioxidant activities of Sonchus arvensis. Chem. Cent. J. 2012, 6, 126. [Google Scholar] [CrossRef]
- Lee, J.M.; Yim, M.-J.; Choi, G.; Lee, M.S.; Park, Y.G.; Lee, D.-S. Antioxidant and Anti-inflammatory Activity of Six Halophytes in Korea. Nat. Prod. Sci. 2018, 24, 40–46. [Google Scholar] [CrossRef]
- Hou, F.; Li, S.; Wang, J.; Kang, X.; Weng, Y.; Xing, G. Identification and validation of reference genes for quantitative real-time PCR studies in long yellow daylily, Hemerocallis citrina Borani. PLoS ONE 2017, 12, e0174933. [Google Scholar] [CrossRef]
- Yang, R.-F.; Geng, L.-L.; Lu, H.-Q.; Fan, X.-D. Ultrasound-synergized electrostatic field extraction of total flavonoids from Hemerocallis citrina Baroni. Ultrason. Sonochemistry 2017, 34, 571–579. [Google Scholar] [CrossRef]
- Li, C.-F.; Chen, X.-Q.; Chen, S.-M.; Geng, D.; Liu, Q.; Yi, L.-T. Evaluation of the toxicological properties and anti-inflammatory mechanism of Hemerocallis citrina in LPS-induced depressive-like mice. Biomed. Pharmacother. 2017, 91, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Takizawa, M.; Satou, M.; Sakasai, M.; Kusuoku, H.; Nojiri, H.; Yoshizuka, N.; Hotta, M.; Kitahara, T.; Hase, T.; et al. Enhancement of Lipolytic Responsiveness of Adipocytes by Novel Plant Extract in Rat. Exp. Biol. Med. 2009, 234, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; He, Z.; Zhao, Y.; Yang, J.; Mao, L. Antioxidant properties and involved compounds of daylily flowers in relation to maturity. Food Chem. 2009, 114, 1192–1197. [Google Scholar] [CrossRef]
- Wang, J.; Hu, D.; Hou, J.; Li, S.; Wang, W.; Li, J.; Bai, J. Ethyl Acetate Fraction of Hemerocallis citrina Baroni Decreases Tert-butyl Hydroperoxide-Induced Oxidative Stress Damage in BRL-3A Cells. Oxidative Med. Cell. Longev. 2018, 2018, 1526125. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Liang, L.; Liu, H.; Yan, Y.; Zhang, Y. Exploring the Extraction Methods of Phenolic Compounds in Daylily (Hemerocallis citrina Baroni) and Its Antioxidant Activity. Molecules 2022, 27, 2964. [Google Scholar] [CrossRef]
- Shen, P.Y.; Yue, Y.R.; Zheng, J.; Park, Y. Caenorhabditis elegans: A convenient in vivo model for assessing the impact of food bioactive compounds on obesity, aging, and Alzheimer’s disease. Annu. Rev. Food Sci. Technol. 2018, 9, 1–22. [Google Scholar] [CrossRef]
- Zheng, J.; Greenway, F.L. Caenorhabditis elegans as a model for obesity research. Int. J. Obes. 2012, 36, 186–194. [Google Scholar] [CrossRef]
- Martorell, P.; Llopis, S.; González, N.; Montón, F.; Ortiz, P.; Genovés, S.; Ramón, D. Caenorhabditis elegans as a Model to Study the Effectiveness and Metabolic Targets of Dietary Supplements Used for Obesity Treatment: The Specific Case of a Conjugated Linoleic Acid Mixture (Tonalin). J. Agric. Food Chem. 2012, 60, 11071–11079. [Google Scholar] [CrossRef]
- Gumienny, T.; Savage-Dunn, C. TGF-beta signaling in C. elegans. Wormbook 2013, 1–34. [Google Scholar] [CrossRef]
- Murphy, C.T.; Hu, P.J. Insulin/insulin-like growth factor signaling in C. elegans. Wormbook Online Rev. C. Elegans Biol. 2013, 1–43. [Google Scholar] [CrossRef]
- Hua, Q.-X.; Nakagawa, S.H.; Wilken, J.; Ramos, R.R.; Jia, W.; Bass, J.; Weiss, M.A. A divergent INS protein in Caenorhabditis elegans structurally resembles human insulin and activates the human insulin receptor. Genes Dev. 2003, 17, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, X.; Wang, D.; Su, L.; Zhao, T.; Li, Z.; Lin, C.; Zhang, Y.; Huang, B.; Lu, J.; et al. O-GlcNAcylation of SKN-1 modulates the lifespan and oxidative stress resistance in Caenorhabditis elegans. Sci. Rep. 2017, 7, 43601. [Google Scholar] [CrossRef] [PubMed]
- Miyase, T.; Fukusgima, S. Studies on sesquiterpene glycosides from Sonchus oleraceus. Chem. Pharm. Bull. 1987, 35, 2869–2874. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, X.; Jiang, Y.; Yu, L.; Huang, X.; Dong, Z.; Yang, S.; He, W.; Zeng, J.; Qing, Z. Systematic identification metabolites of Hemerocallis citrina Borani by high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry combined with a screening method. J. Pharm. Biomed. Anal. 2020, 186, 113314. [Google Scholar] [CrossRef]
- Oliveira, R.P.; Abate, J.P.; Dilks, K.; Landis, J.; Ashraf, J.; Murphy, C.T.; Blackwell, T.K. Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell 2009, 8, 524–541. [Google Scholar] [CrossRef]
- Savage-Dunn, C.; Yu, L.; Gill, K.; Awan, M.; Fernando, T. Non-stringent tissue-source requirements for BMP ligand expression in regulation of body size in Caenorhabditis elegans. Genet. Res. 2011, 93, 427–432. [Google Scholar] [CrossRef]
- Wang, J.J.; Tokarz, R.; Savage-Dunn, C. The expression of tgf beta signal transducers in the hypodermis regulates body size in C. elegans. Development 2002, 129, 4989–4998. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Zhang, L.; Wang, W.; Wei, S.; Wang, J.; Che, H.; Zhang, Y. Effects of excess sugars and lipids on the growth and development of Caenorhabditis elegans. Genes Nutr. 2020, 15, 11. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of Chlorogenic Acid on Regulating Glucose and Lipids Metabolism: A Review. Evid. Based Complement. Altern. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J. Antioxidant activities of flavonoids as bioactive components of food. Biochem. Soc. Trans. 1996, 24, 790–795. [Google Scholar] [CrossRef]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.M.; Soares, M.V.; da Silva, A.F.; Machado, M.L.; Baptista, F.B.O.; da Silveira, T.L.; Arantes, L.P.; Soares, F.A.A. Neuroprotective effects of rutin on ASH neurons in Caenorhabditis elegans model of Huntington’s disease. Nutr. Neurosci. 2021, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wang, W.; Chu, W. Antioxidant and reducing lipid accumulation effects of rutin in Caenorhabditis elegans. BioFactors 2021, 47, 686–693. [Google Scholar] [CrossRef]
- An, J.H.; Vranas, K.; Lucke, M.; Inoue, H.; Hisamoto, N.; Matsumoto, K.; Blackwell, T.K. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc. Natl. Acad. Sci. USA 2005, 102, 16275–16280. [Google Scholar] [CrossRef] [PubMed]
- Fuse, Y.; Kobayashi, M. Conservation of the Keap1-Nrf2 System: An Evolutionary Journey through Stressful Space and Time. Molecules 2017, 22, 436. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-Q.; Huang, X.-B.; Xing, T.-K.; Ding, A.-J.; Wu, G.-S.; Luo, H.-R. Chlorogenic Acid Extends the Lifespan of Caenorhabditis elegans via Insulin/IGF-1 Signaling Pathway. J. Gerontol. Ser. A 2017, 72, 464–472. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, R.; Wang, D. Response of DBL-1/TGF-β signaling-mediated neuron-intestine communication to nanopolystyrene in nematode Caenorhabditis elegans. Sci. Total. Environ. 2020, 745, 141047. [Google Scholar] [CrossRef]
- Miyazono, K. Tgf-beta/smad signaling and its involvement in tumor progression. Biol. Pharm. Bull. 2000, 23, 1125–1130. [Google Scholar] [CrossRef]
- Yoshida, S.; Morita, K.; Mochii, M.; Ueno, N. Hypodermal Expression of Caenorhabditis elegans TGF-β Type I Receptor SMA-6 Is Essential for the Growth and Maintenance of Body Length. Dev. Biol. 2001, 240, 32–45. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).