A Lewis Acid-Promoted Michael Addition and Ring-Expansion Cascade for the Construction of Nitrogen-Containing Medium-Sized Rings
Abstract
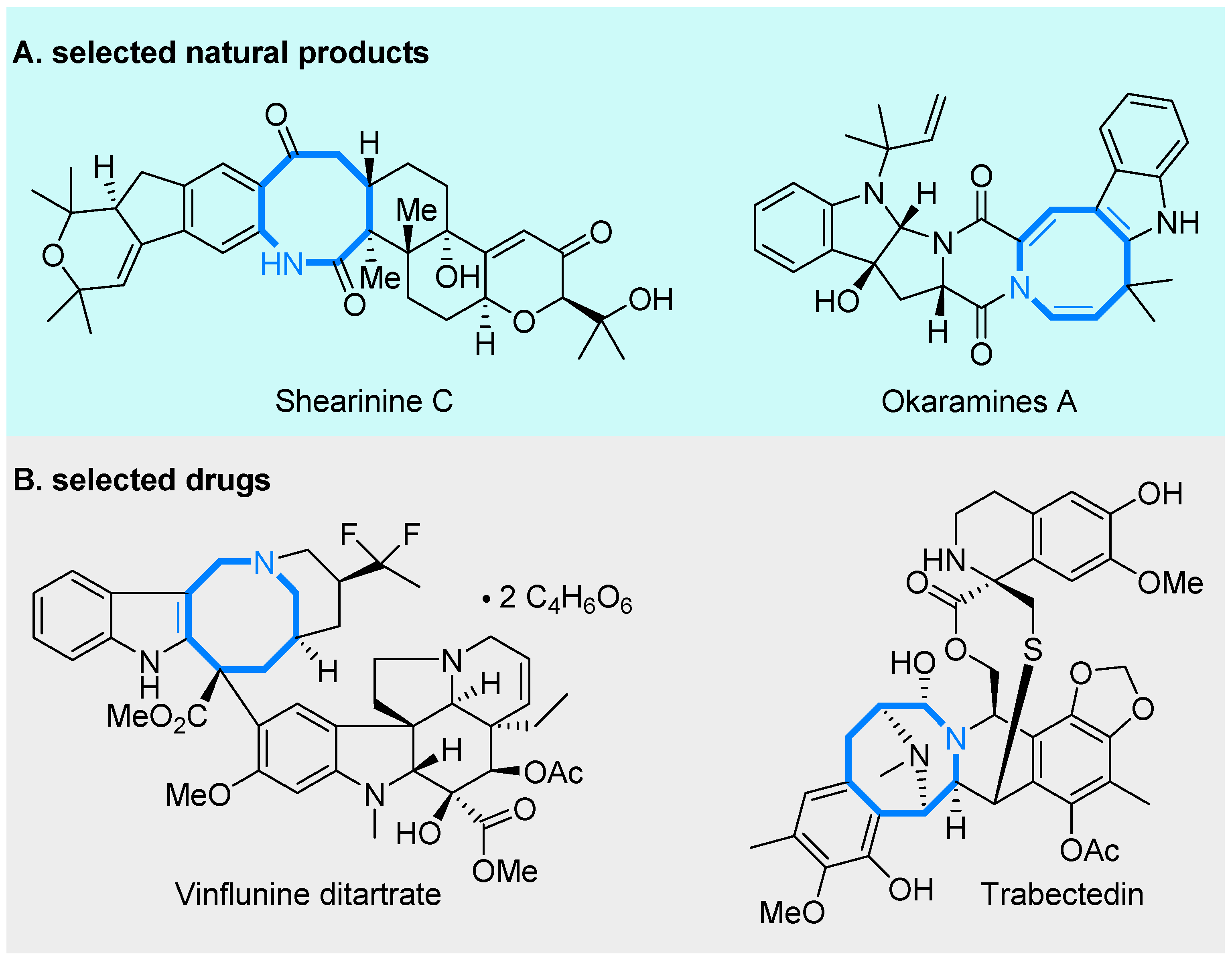
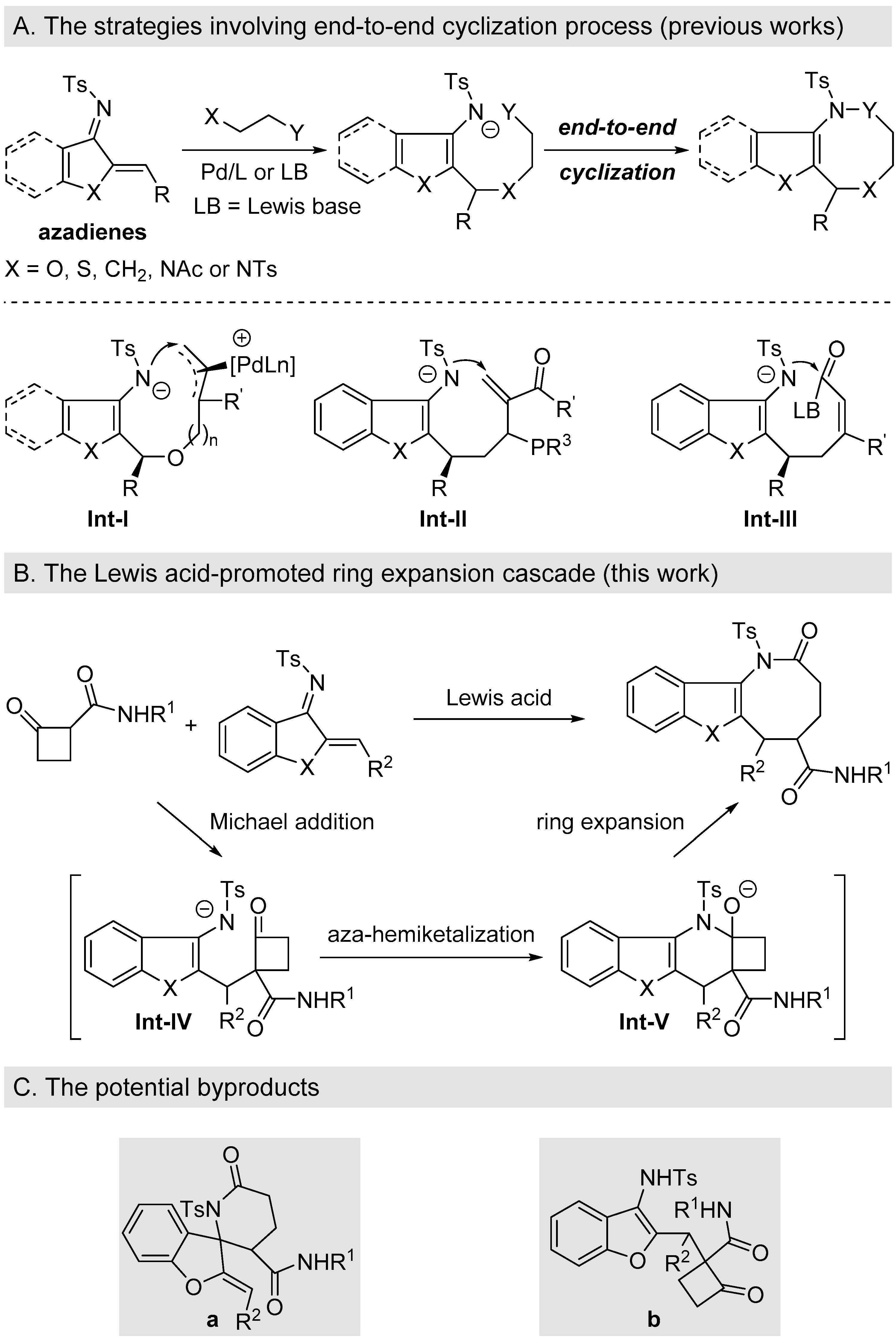
1. Introduction
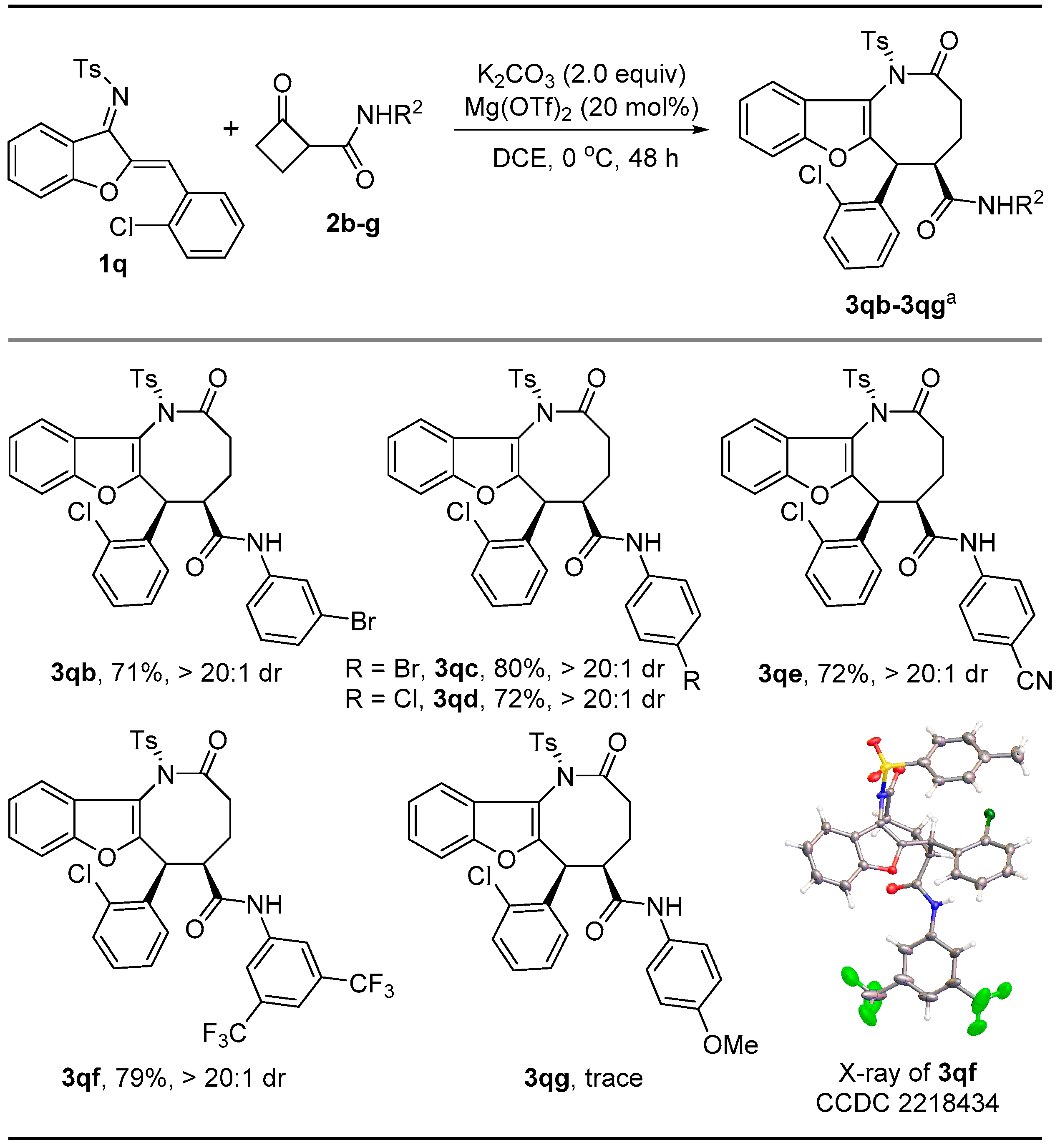
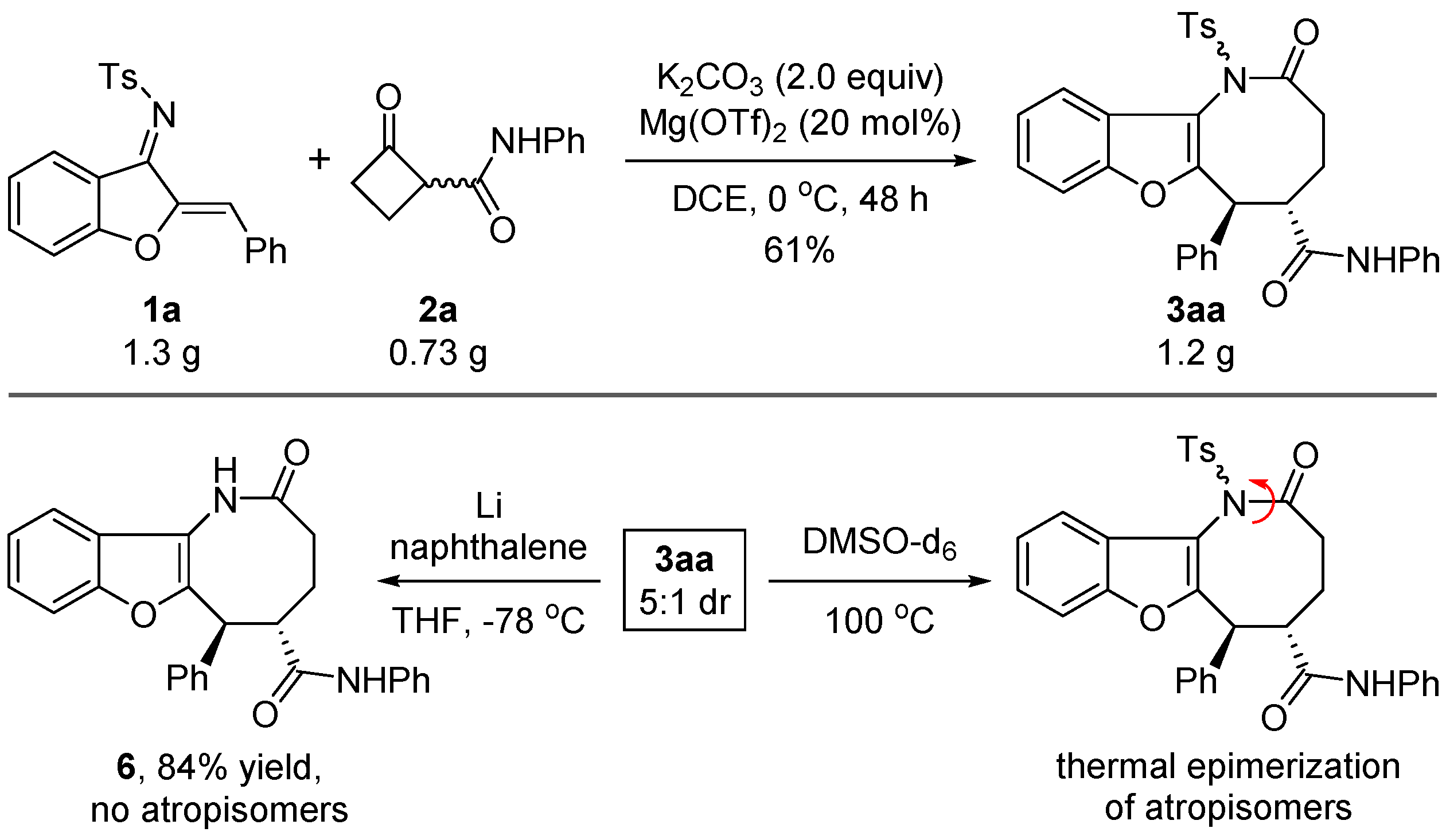
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure for the Synthesis of 3
3.2. General Procedure for the Synthesis of 6
3.3. General Procedure for the Synthesis of 7
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spring, D.R.; Krishnan, S.; Blackwell, H.E.; Schreiber, S.L. Diversity-oriented synthesis of biaryl-containing medium rings using a one bead/one stock solution platform. J. Am. Chem. Soc. 2002, 124, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Spring, D.R.; Krishnan, S.; Schreiber, S.L. Towards diversity-oriented, stereoselective syntheses of biaryl- or bis(aryl)metal-containing medium rings. J. Am. Chem. Soc. 2000, 122, 5656–5657. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Hong, B.; Ai, W.; Wang, X.; Li, H.; Lei, X. Diversity-oriented synthesis of Lycopodium alkaloids inspired by the hidden functional group pairing pattern. Nat. Commun. 2014, 5, 4614. [Google Scholar] [CrossRef]
- Zhao, C.; Ye, Z.; Ma, Z.-X.; Wildman, S.A.; Blaszczyk, S.A.; Hu, L.; Guizei, I.A.; Tang, W. A general strategy for diversifying complex natural products to polycyclic scaffolds with medium-sized rings. Nat. Commun. 2019, 10, 4015. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-J.; Li, L.-X.; Han, J.-C.; Min, L.; Li, C.-C. Recent advances in the total synthesis of natural products containing eight-membered carbocycles (2009–2019). Chem. Rev. 2020, 120, 5910–5953. [Google Scholar] [CrossRef]
- Belofsky, G.N.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. Antiinsectan alkaloids: Shearinines A-C and a new paxilline derivative from the ascostromata of Eupenicillium shearii. Tetrahedron 1995, 51, 3959–3968. [Google Scholar] [CrossRef]
- Hayashi, H.; Furutsuka, K.; Shiono, Y. Okaramines H and I, New okaramine congeners, from Aspergillus aculeatus. J. Nat. Prod. 1999, 62, 315–317. [Google Scholar] [CrossRef]
- Silvestri, R. New prospects for vinblastine analogues as anticancer agents. J. Med. Chem. 2013, 56, 625–627. [Google Scholar] [CrossRef]
- Kinghorn, A.D. Review of anticancer agents from natural products. J. Nat. Prod. 2015, 78, 2315. [Google Scholar] [CrossRef]
- Butler, M.S. The Role of Natural Product Chemistry in Drug Discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef]
- McGrath, N.A.; Brichacek, M.; Njardarson, J.T. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 2010, 87, 1348–1349. [Google Scholar] [CrossRef]
- Bauer, R.A.; Wenderski, T.A.; Tan, D.S. Biomimetic diversity-oriented synthesis of benzannulated medium rings via ring expansion. Nat. Chem. Biol. 2013, 9, 21–29. [Google Scholar] [CrossRef]
- Brown, D.G.; Wobst, H.J. A decade of FDA-approved drugs (2010–2019): Trends and future directions. J. Med. Chem. 2021, 64, 2312–2338. [Google Scholar] [CrossRef]
- Khan, A.R.; Parrish, J.C.; Fraser, M.E.; Smith, W.W.; Bartlett, P.A.; James, M.N.G. Lowering the entropic barrier for binding conformationally flexible inhibitors to enzymes. Biochemistry 1998, 37, 16839–16845. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Rezai, T.; Yu, B.; Millhauser, G.L.; Jacobson, M.P.; Lokey, R.S. Testing the Conformational Hypothesis of Passive Membrane Permeability Using Synthetic Cyclic Peptide Diastereomers. J. Am. Chem. Soc. 2006, 128, 2510–2511. [Google Scholar] [CrossRef]
- Kwon, Y.-U.; Kodadek, T. Quantitative comparison of the relative cell permeability of cyclic and linear peptides. Chem. Biol. 2007, 14, 671–677. [Google Scholar] [CrossRef]
- Guney, T.; Wenderski, T.A.; Boudreau, M.W.; Tan, D.S. Synthesis of benzannulated medium-ring lactams via a tandem oxidative dearomatization-ring expansion reaction. Chem. Eur. J. 2018, 24, 13150–13157. [Google Scholar] [CrossRef]
- Kurouchi, H.; Ohwada, T. Synthesis of medium-ring-sized benzolactams by using strong electrophiles and quantitative evaluation of ring-size dependency of the cyclization reaction rate. J. Org. Chem. 2020, 85, 876–901. [Google Scholar] [CrossRef]
- Osipyan, A.; Sapegin, A.; Novikov, A.S.; Krasavin, M. Rare medium-sized rings prepared via hydrolytic imidazoline ring expansion (HIRE). J. Org. Chem. 2018, 83, 9707–9717. [Google Scholar] [CrossRef]
- Hussain, A.; Yousuf, S.K.; Mukherjee, D. Importance and synthesis of benzannulated medium-sized and macrocyclic rings (BMRs). RSC Adv. 2014, 4, 43241–43257. [Google Scholar] [CrossRef]
- Ouyang, W.; Rao, J.; Li, Y.; Liu, X.; Huo, Y.; Chen, Q.; Li, X. Recent achievements in the rhodium-catalyzed concise construction of medium N-heterocycles, azepines and azocines. Adv. Synth. Catal. 2020, 362, 5576–5600. [Google Scholar] [CrossRef]
- Stephens, T.C.; Unsworth, W.P. Consecutive ring-expansion reactions for the iterative assembly of medium-sized rings and macrocycles. Synlett 2020, 31, 133–146. [Google Scholar] [CrossRef]
- Reyes, R.L.; Iwai, T.; Sawamura, M. Construction of medium-sized rings by gold catalysis. Chem. Rev. 2021, 121, 8926–8947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Duan, S.; Long, Y.; Li, J. Recent progress in the synthesis of medium-sized ring and macrocyclic compounds. Chin. J. Org. Chem. 2021, 41, 1878. [Google Scholar] [CrossRef]
- Yao, T.; Li, J.; Jiang, C.; Zhao, C. Recent advances for the catalytic asymmetric construction of medium-sized rings. Chem Catal. 2022, 11, 2929–2964. [Google Scholar] [CrossRef]
- Boyd, O.; Wang, G.-W.; Sokolova, O.O.; Calow, A.D.J.; Bertrand, S.M.; Bower, J.F. Electroreduction of alkyl halides to alkyl boronic esters. Angew. Chem. Int. Ed. 2019, 58, 18844–18848. [Google Scholar] [CrossRef]
- Lawer, A.; Rossi-Ashton, J.A.; Stephens, T.C.; Challis, B.J.; Epton, R.G.; Lynam, J.M.; Unsworth, W.P. Internal nucleophilic catalyst mediated cyclisation/ring expansion cascades for the synthesis of medium-sized lactones and lactams. Angew. Chem. Int. Ed. 2019, 58, 13942–13947. [Google Scholar] [CrossRef]
- Huang, L.; Dai, L.-X.; You, S.-L. Enantioselective synthesis of indole-annulated medium-sized rings. J. Am. Chem. Soc. 2016, 138, 5793–5796. [Google Scholar] [CrossRef]
- Huang, L.; Cai, Y.; Zheng, C.; Dai, L.-X.; You, S.-L. Iridium-catalyzed enantioselective synthesis of pyrrole-annulated medium-sized-ring compounds. Angew. Chem. Int. Ed. 2017, 56, 10545–10548. [Google Scholar] [CrossRef]
- Tan, T.-D.; Zhu, X.-Q.; Bu, H.-Z.; Deng, G.; Chen, Y.-B.; Liu, R.-S.; Ye, L.-W. Copper-catalyzed cascade cyclization of indolyl homopropargyl amides: Stereospecific construction of bridged aza-[n.2.1] skeletons. Angew. Chem. Int. Ed. 2019, 58, 9632–9639. [Google Scholar] [CrossRef]
- Zhang, Q.-L.; Xiong, Q.; Li, M.-M.; Xiong, W.; Shi, B.; Lan, Y.; Lu, L.-Q.; Xiao, W.-J. Palladium-catalyzed asymmetric [8+2] dipolar cycloadditions of vinyl carbamates and photogenerated ketenes. Angew. Chem. Int. Ed. 2020, 59, 14096–14100. [Google Scholar] [CrossRef]
- Kang, G.; Yamagami, M.; Vellalath, S.; Romo, D. Enantioselective synthesis of medium-sized lactams via chiral α,β-unsaturated acylammonium salts. Angew. Chem. Int. Ed. 2018, 57, 6527–6531. [Google Scholar] [CrossRef]
- Wang, N.; Gu, Q.-S.; Li, Z.-L.; Li, Z.; Guo, Y.-L.; Guo, Z.; Liu, X.-Y. Direct photocatalytic synthesis of medium-sized lactams by C−C bond cleavage. Angew. Chem. Int. Ed. 2018, 57, 14225–14229. [Google Scholar] [CrossRef]
- Lee, J.Y.; Varshnaya, R.K.; Yoo, E.J. Synthesis of chiral diazocine derivatives via a copper-catalyzed dearomative [5+3] cycloaddition. Org. Lett. 2022, 24, 3731–3735. [Google Scholar] [CrossRef]
- An, X.-T.; Du, J.-Y.; Jia, Z.-L.; Zhang, Q.; Yu, K.-Y.; Zhang, Y.-Z.; Zhao, X.-H.; Fang, R.; Fan, C.-A. Asymmetric catalytic [4+5] annulation of ortho-quinone methides with vinylethylene carbonates and its extension to stereoselective tandem rearrangement. Chem. Eur. J. 2020, 26, 3803–3809. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y. Phosphine-catalyzed sequential [4 + 3] domino annulation/allylic alkylation reaction of MBH carbonates: Efficient construction of seven-membered heterocycles. Org. Lett. 2017, 19, 5609–5612. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Gondo, S.; Nagender, P.; Uno, H.; Tokunaga, E.; Shibata, N. Access to benzo-fused nine-membered heterocyclic alkenes with a trifluoromethyl carbinol moiety via a double decarboxylative formal ring-expansion process under palladium catalysis. Chem. Sci. 2018, 9, 3276–3281. [Google Scholar] [CrossRef]
- Uno, H.; Punna, N.; Tokunaga, E.; Shiro, M.; Shibata, N. Synthesis of both enantiomers of nine-membered CF3-substituted heterocycles using a single chiral ligand: Palladium-catalyzed decarboxylative ring expansion with kinetic resolution. Angew. Chem. Int. Ed. 2020, 59, 8187–8194. [Google Scholar] [CrossRef]
- Li, Q.; Pan, R.; Wang, M.; Yao, H.; Lin, A. Ligand-controlled, palladium-catalyzed asymmetric [4+4] and [2+4] cycloadditions. Org. Lett. 2021, 23, 2292–2297. [Google Scholar] [CrossRef]
- Scuiller, A.; Liu, X.; Cordier, M.; Garrec, J.; Archambeau, A. A palladium-catalyzed oxa-(4+4)-cycloaddition strategy towards oxazocine scaffolds. Synlett 2021, 32, 981–986. [Google Scholar]
- Liu, Y.; He, Y.; Liu, Y.; Wei, K.; Guo, W. Access to azonanes via Pd-catalyzed decarboxylative [5+4] cycloaddition with exclusive regioselectivity. Org. Chem. Front. 2021, 8, 7004–7008. [Google Scholar] [CrossRef]
- Rong, Z.-Q.; Yang, L.-C.; Liu, S.; Yu, Z.; Wang, Y.-N.; Tan, Z.Y.; Huang, R.-Z.; Lan, Y.; Zhao, Y. Nine-membered benzofuran-fused heterocycles: Enantioselective synthesis by Pd-catalysis and rearrangement via transannular bond formation. J. Am. Chem. Soc. 2017, 139, 15304–15307. [Google Scholar] [CrossRef] [PubMed]
- Scuiller, A.; Karnat, A.; Casaretto, N.; Archambeau, A. Vinylcyclopropanes as all-carbon 1,5-dipoles: A reactivity switch for Palladium-catalyzed (5+4) cycloadditions. Org. Lett. 2021, 23, 2332–2336. [Google Scholar] [CrossRef]
- Yang, G.; Ke, Y.-M.; Zhao, Y. Stereoselective access to polyfunctionalized nine-membered heterocycles by sequential gold and palladium catalysis. Angew. Chem. Int. Ed. 2021, 60, 12775–12780. [Google Scholar] [CrossRef]
- Yang, L.-C.; Rong, Z.-Q.; Wang, Y.-N.; Tan, Z.Y.; Wang, M.; Zhao, Y. Construction of nine-membered heterocycles through palladium-catalyzed formal [5+4] cycloaddition. Angew. Chem. Int. Ed. 2017, 56, 2927–2931. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Yang, L.-C.; Rong, Z.-Q.; Liu, T.-L.; Liu, R.; Zhao, Y. Pd-catalyzed enantioselective [6+4] cycloaddition of vinyl oxetanes with azadienes to access ten-membered heterocycles. Angew. Chem. Int. Ed. 2018, 57, 1596–1600. [Google Scholar] [CrossRef]
- Ni, H.; Tang, X.; Zheng, W.; Yao, W.; Ullah, N.; Lu, Y. Enantioselective phosphine-catalyzed formal [4+4] annulation of α,β-unsaturated imines and allene ketones: Construction of eight-membered rings. Angew. Chem. Int. Ed. 2017, 56, 14222–14226. [Google Scholar] [CrossRef]
- Jiang, B.; Du, W.; Chen, Y.-C. Modified cinchona alkaloid-catalysed enantioselective [4+4] annulations of cyclobutenones and 1-azadienes. Chem. Commun. 2020, 56, 7257–7260. [Google Scholar] [CrossRef] [PubMed]
- Illuminati, G.; Mandolini, L. Ring closure reactions of bifunctional chain molecules. Acc. Chem. Res. 1981, 14, 95–102. [Google Scholar] [CrossRef]
- Biletskyi, B.; Colonna, P.; Masson, K.; Parrain, J.-L.; Commeiras, L.; Chouraqui, G. Small rings in the bigger picture: Ring expansion of three- and four-membered rings to access larger all-carbon cyclic systems. Chem. Soc. Rev. 2021, 50, 7513–7538. [Google Scholar] [CrossRef]
- Clarke, A.K.; Unsworth, W.P. A happy medium: The synthesis of medicinally important medium-sized rings via ring expansion. Chem. Sci. 2020, 11, 2876–2881. [Google Scholar] [CrossRef]
- Donald, J.R.; Unsworth, W.P. Ring-expansion reactions in the synthesis of macrocycles and medium-sized rings. Chem. Eur. J. 2017, 23, 8780–8799. [Google Scholar] [CrossRef]
- Palate, K.Y.; Epton, R.G.; Whitwood, A.C.; Lynam, J.M.; Unsworth, W.P. Synthesis of macrocyclic and medium-sized ring thiolactones via the ring expansion of lactams. Org. Biomol. Chem. 2021, 19, 1404–1411. [Google Scholar] [CrossRef]
- Palate, K.Y.; Yang, Z.; Whitwood, A.C.; Unsworth, W.P. Synthesis of medium-ring lactams and macrocyclic peptide mimetics via conjugate addition/ring expansion cascade reactions. RSC Chem. Biol. 2022, 3, 334–340. [Google Scholar] [CrossRef]
- Hall, J.E.; Matlock, J.V.; Ward, J.W.; Gray, K.V.; Clayden, J. Medium-ring nitrogen heterocycles through migratory ring expansion of metalated ureas. Angew. Chem. Int. Ed. 2016, 55, 11153–11157. [Google Scholar] [CrossRef]
- Costil, R.; Lefebvre, Q.; Clayden, J. Medium-sized-ring analogues of dibenzodiazepines by a conformationally induced smiles ring expansion. Angew. Chem. Int. Ed. 2017, 56, 14602–14606. [Google Scholar] [CrossRef]
- Kitsiou, C.; Hindes, J.J.; I’Anson, P.; Jackson, P.; Wilson, T.C.; Daly, E.K.; Felstead, H.R.; Hearnshaw, P.; Unsworth, W.P. The synthesis of structurally diverse macrocycles by successive ring expansion. Angew. Chem. Int. Ed. 2015, 54, 15794–15798. [Google Scholar] [CrossRef]
- Stephens, T.C.; Lodi, M.; Steer, A.M.; Lin, Y.; Gill, M.T.; Unsworth, W.P. Synthesis of cyclic peptide mimetics by the successive ring expansion of lactams. Chem. Eur. J. 2017, 23, 13314–13318. [Google Scholar] [CrossRef]
- Stephens, T.C.; Lawer, A.; French, T.; Unsworth, W.P. Iterative Assembly of Macrocyclic Lactones using Successive Ring Expansion Reactions. Chem. Eur. J. 2018, 24, 13947–13953. [Google Scholar] [CrossRef]
- Ito, T.; Tsutsumi, M.; Yamada, K.-I.; Takikawa, H.; Yamaoka, Y.; Takasu, K. Synthesis of Functionalized Medi-um-Sized trans-Cycloalkenes by 4π Electrocy-clic Ring Opening/Alkylation Sequence. Angew. Chem. Int. Ed. 2019, 58, 11836–11840. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.-L.; Rodriguez, J.; Coquerel, Y. Enantioselective organocatalytic four-atom ring expansion of cyclobutanones: Synthesis of benzazocinones. Angew. Chem. Int. Ed. 2019, 58, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yang, X.; Wang, X.; An, Q.; He, X.; Pan, H.; Zuo, Z. Photocatalytic aerobic oxidative ring expansion of cyclic ketones to macrolactones by cerium and cyanoanthracene catalysis. Angew. Chem. Int. Ed. 2021, 60, 5370–5376. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Thombare, V.J.; Charron, C.L.; Wille, U.; Hutton, C.A. Ring expansion of thiolactams via imide intermediates: An amino acid insertion strategy. Chem. Eur. J. 2021, 27, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-L.; Wang, Y.-L.; Li, W.; Gu, B.-M.; Wang, S.-W.; Luo, X.; Tian, B.-X.; Deng, W.-P. Diastereo- and enantioselective synthesis of eight-membered heterocycles via an allylation/ring expansion sequence enabled by multiple catalysis. ACS Catal. 2021, 11, 12557–12564. [Google Scholar] [CrossRef]
- Yang, W.-L.; Li, W.; Yang, Z.-T.; Deng, W.-P. Organocatalytic regiodivergent ring expansion of cyclobutanones for the enantioselective synthesis of azepino[1,2-a]indoles and cyclohepta[b]indoles. Org. Lett. 2020, 22, 4026–4032. [Google Scholar] [CrossRef]
- Zhao, C.; Xie, X.; Duan, S.; Li, H.; Fang, R.; She, X. Gold-catalyzed 1,2-acyloxy migration/intramolecular [3+2] 1,3-dipolar cycloaddtion cascade reaction: An efficient strategy for syntheses of medium-sized-ring ethers and amines. Angew. Chem. Int. Ed. 2014, 53, 10789–10793. [Google Scholar] [CrossRef]
- Gu, J.; Luo, S.; Gu, Q.; Cao, X.; Ge, Y.; Wang, C.; Yuan, C.; Wang, H. DBU-Catalyzed [3+2] Cycloaddition of Benzoaurones with 3-Homoacyl Coumarins: Synthesis of Spiro [Benzofuranone-Cyclopentane] Compounds. ChemistrySelect 2022, 7, e202201599. [Google Scholar]
- Gao, Z.; Chen, K.; Zhang, Y.; Kong, L.; Li, Y.; Ye, S. Enantioselective N-Heterocyclic Carbene-Catalyzed Synthesis of Spirocyclic Oxindole-benzofuroazepinones. J. Org. Chem. 2018, 83, 15225–15235. [Google Scholar] [CrossRef]
- Modrocká, V.; Veverková, E.; Mečiarová, M.; Šebesta, R. Bifunctional amine-squaramides as organocatalysts in michael/hemiketalization reactions of β,γ-unsaturated α-ketoesters and α,β-unsaturated ketones with 4-hydroxycoumarins. J. Org. Chem. 2018, 83, 13111–13120. [Google Scholar] [CrossRef]
- Li, N.-K.; Zhang, J.-Q.; Sun, B.-B.; Li, H.-Y.; Wang, X.-W. Chiral diphosphine–palladium-catalyzed sequential asymmetric double-Friedel-rafts alkylation and N-hemiketalization for spiro-polycyclic indole derivatives. Org. Lett. 2017, 19, 1954–1957. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Blümel, M.; Philipps, A.R.; Wang, A.; Puttreddy, R.; Rissanen, K.; Enders, D. Asymmetric synthesis of spirobenzazepinones with atroposelectivity and spiro-1,2-diazepinones by NHC-catalyzed [3+4] annulation reactions. Angew. Chem. Int. Ed. 2016, 55, 11110–11114. [Google Scholar] [CrossRef] [PubMed]
- Laina-Martín, V.; Humbrías-Martín, J.; Mas-Ballesté, R.; Fernández-Salas, J.A.; Alemán, J. Enantioselective Inverse-Electron Demand Aza-Diels–Alder Reaction: Ipso,α-Selectivity of Silyl Dienol Ethers. ACS Catal. 2021, 11, 12133–12145. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Zuo, Z. Highly Regio-, Diastereo-, and Enantioselective Synthesis of Tetrahydroazepines and Benzo[b]oxepines through Palladium-Catalyzed [4+3] Cycloaddition Reactions. Angew. Chem. Int. Ed. 2020, 59, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-Y.; Meng, F.-T.; Wang, M.; Tu, S.-J.; Hao, W.-J.; Wang, J.; Jiang, B. Gold-Catalyzed Skeletal Rearrangement of Alkenes: Regioselective Synthesis of Skeletally Diverse Tricyclic Heterocycles and Mechanistic Investigations. ACS Catal. 2021, 11, 6951–6959. [Google Scholar] [CrossRef]
- Liu, K.; Yang, J.; Li, X. Palladium-Catalyzed Diastereo- and Enantioselective [3 + 2] Cycloaddition of Vinylcyclopropanes with Azadienes: Efficient Access to Chiral Spirocycles. Org. Lett. 2021, 23, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Mailhol, D.M.; Duque, d.M.S.; Raimondi, W.; Bonne, D.; Constantieux, T.; Coquerel, Y.; Rodriguez, J. Enantioselective Organocatalytic Michael Addition of Cyclobutanones to Nitroalkenes. Adv. Synth. & Catal. 2012, 354, 3523–3532. [Google Scholar]
- He, P.; Liu, X.; Shi, J.; Lin, L.; Feng, X. Organocatalytic Sequential Michael Reactions: Stereoselective Synthesis of Multifunc-tionalized Tetrahydroindan Derivatives. Org. Lett. 2011, 13, 936–939. [Google Scholar] [CrossRef]
- Sheppard, C.I.; Taylor, J.L.; Wiskur, S.L. Silylation-Based Kinetic Resolution of Monofunctional Secondary Alcohols. Org. Lett. 2011, 13, 3794–3797. [Google Scholar] [CrossRef]
- Karplus, M. Vicinal Proton Coupling in Nuclear Magnetic Resonance. J. Am. Chem. Soc. 1963, 85, 2870–2871. [Google Scholar] [CrossRef]


 | ||||
|---|---|---|---|---|
| Entry a | Solvent | Base | Lewis Acid | Yield (%) b |
| 1 | CH2Cl2 | Et3N | 16 | |
| 2 e | CH2Cl2 | DIPEA | 11 | |
| 3 f | CH2Cl2 | DMAP | 17 | |
| 4 g | CH2Cl2 | DBN | NR | |
| 5 | CH2Cl2 | Cs2CO3 | 17 | |
| 6 | CH2Cl2 | NaHCO3 | 26 | |
| 7 | CH2Cl2 | K2CO3 | 27 | |
| 8 | CH2Cl2 | KOtBu | trace | |
| 9 | CH2Cl2 | KOH | trace | |
| 10 | CH2Cl2 | K2CO3 | Mg(OTf)2 | 54 |
| 11 | CH2Cl2 | K2CO3 | Sc(OTf)2 | trace |
| 12 | CH2Cl2 | K2CO3 | Zn(OTf)2 | 41 |
| 13 | CH2Cl2 | K2CO3 | Cu(OTf)2 | 11 |
| 14 | THF | K2CO3 | Mg(OTf)2 | 57 |
| 15 h | DCE | K2CO3 | Mg(OTf)2 | 63 |
| 16 | MeCN | K2CO3 | Mg(OTf)2 | 46 |
| 17 | Toluene | K2CO3 | Mg(OTf)2 | 18 |
| 18 i | DCE | K2CO3 | Mg(OTf)2 | 78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, J.; Zhao, C. A Lewis Acid-Promoted Michael Addition and Ring-Expansion Cascade for the Construction of Nitrogen-Containing Medium-Sized Rings. Molecules 2023, 28, 1650. https://doi.org/10.3390/molecules28041650
Wang J, Li J, Zhao C. A Lewis Acid-Promoted Michael Addition and Ring-Expansion Cascade for the Construction of Nitrogen-Containing Medium-Sized Rings. Molecules. 2023; 28(4):1650. https://doi.org/10.3390/molecules28041650
Chicago/Turabian StyleWang, Jiaming, Jia Li, and Changgui Zhao. 2023. "A Lewis Acid-Promoted Michael Addition and Ring-Expansion Cascade for the Construction of Nitrogen-Containing Medium-Sized Rings" Molecules 28, no. 4: 1650. https://doi.org/10.3390/molecules28041650
APA StyleWang, J., Li, J., & Zhao, C. (2023). A Lewis Acid-Promoted Michael Addition and Ring-Expansion Cascade for the Construction of Nitrogen-Containing Medium-Sized Rings. Molecules, 28(4), 1650. https://doi.org/10.3390/molecules28041650







