Abstract
One mononuclear Mn(III) complex [MnIIIL(H2O)(MeCN)](ClO4) (1) and one hetero-binuclear complex [(CuIILMnII(H2O)3)(CuIIL)2](ClO4)2·CH3OH (2) have been synthesized with the Schiff base ligand (H2L = N,N′-bis(3-methoxysalicylidene)-1,2-phenylenediamine). Single crystal X-ray structural analysis manifests that the Mn(III) ion in 1 has an octahedral coordination structure, whereas the Mn(II) ion in 2 possesses a trigonal bipyramidal configuration and the Cu(II) ion in 2 is four-coordinated with a square-planar geometry. Electrochimerical catalytic investigation demonstrates that the two complexes can electrochemically catalyze water oxidation and CO2 reduction simultaneously. The coordination environments of the Mn(III), Mn(II), and Cu(II) ions in 1 and 2 were provided by the Schiff base ligand (L) and labile solvent molecules. The coordinately unsaturated environment of the Cu(II) center in 2 can perfectly facilitate the catalytic performance of 2. Complexes 1 and 2 display that the over potentials for water oxidation are 728 mV and 216 mV, faradaic efficiencies (FEs) are 88% and 92%, respectively, as well as the turnover frequency (TOF) values for the catalytic reduction of CO2 to CO are 0.38 s−1 at −1.65 V and 15.97 s−1 at −1.60 V, respectively. Complex 2 shows much better catalytic performance for both water oxidation and CO2 reduction than that of complex 1, which could be owing to a structural reason which is attributed to the synergistic catalytic action of the neighboring Mn(III) and Cu(II) active sites in 2. Complexes 1 and 2 are the first two compounds coordinated with Schiff base ligand for both water oxidation and CO2 reduction. The finding in this work can offer significant inspiration for the future development of electrocatalysis in this area.
1. Introduction
With the continuous development of humans, the overuse of fossil fuels and the increased emissions of CO2 have induced a series of energy shortages and environmental crises [1,2,3,4,5]. To achieve the goal of carbon neutral [6,7,8,9], conversion of CO2 into fuels and other chemical sources with high energy density is a very promising strategy [10,11,12,13], which requires the systematic development of advanced electrochemical catalysts. Water oxidation and CO2 reduction reactions are two important half-reactions in the classical artificial photosynthesis system, where water oxidation can provide protons and electrons for CO2 conversion [14,15,16]. However, both water oxidation and CO2 reduction reactions have high kinetic and thermodynamic barriers [17,18,19,20]. Therefore, how to develop cheap, durable, and efficient bifunctional electrochemical catalysts for water oxidation and CO2 reduction has become an increasingly important research topic.
Homogeneous catalysis has been widely used in CO2 reduction and water oxidation in recent years due to the high activity, facilely adjustable structure and property, and easily clarifying catalytic mechanism [21,22,23,24,25,26,27,28,29,30]. However, so far, only a few complexes have been reported for homogeneously catalyzing both water oxidation and CO2 reduction. What has been done in this area to this day is as below. In 2012, Meyer’s team reported a molecular electrochemical catalyst based on Ru(II), which can catalyze CO2 reduction and water oxidation simultaneously in a CH3CN–H2O mixture for the first time [31]. In 2017, Colbran’s group successfully synthesized a Ru(II) complex [(Butpy)RuII(phenCO2)][PF6] for both CO2 reduction and water oxidation, in which the 1,10-phenanthroline-2-carboxylate ligand was of the greatest significance for the electrocatalysis [32]. In 2021, Wang’s group developed a mononuclear copper complex [CuIIL12](ClO4)2·2CH3OH (L1 = methoxy-di-pyridin-2-ylmethanol) as a bifunctional electrochemical catalyst for CO2 reduction and water oxidation, of which the turnover frequencies (TOFs) were 2.99 s−1 and 9.20 s−1, respectively [33]. Later, they reported a multinuclear cobalt complex (Et3NH)2[CoIII2CoII(OH2)(pda)5] (H2pda = 2,6-pyridinedicarboxylic acid) as an excellent homogeneous electrochemical catalyst for water oxidation and CO2 reduction, where the labile aqua ligand played an important role in improving the catalytic performance [34]. Except for the above works, most of the coordination complexes and other materials in this area are heterogeneous catalysts [35,36,37,38,39]. For instance, Lin’s group introduced metal complexes with dicarboxylic acid functional groups into the highly stable and porous ZrⅣ6O4(OH)4(bpdc)6(UiO-67) backbone, achieving excellent performance for water oxidation and CO2 reduction [37]. Nam’s group reported the use of N-heterocyclic carbines as persistent connectors for the immobilization of Rubpy complex-based catalyst for photoelectrochemical CO2 reduction and water oxidation with high efficiency [38]. In 2016, Morlanés et al. reported the electrolysis of H2O/CO2 to O2/CO with CoIIFPc as a catalyst immobilized on the carbon electrode, in which the negative induction effect of the fluorine substituent reduces the affinity of the metal center for CO, thus enhancing CO2 reduction [39]. In order to simulate the photosynthesis in the natural ecosystem, what is still a huge challenge is how to integrate CO2 reduction and water oxidation in a homogeneous catalytic system with high efficiency.
Schiff base ligand features the ability to easily regulate the molecular and electronic structures of complexes, and tune their physicochemical properties [40]. Hence, Schiff base is a type of versatile ligand, which has been widely applied in various fields such as biological [41,42,43,44,45], analytical [46], and catalytic [47,48,49,50,51] fields. Recently, many Schiff base complexes have been utilized in electrocatalysis for CO2 reduction or water oxidation. In 2018, Mojtaba et al. reported a novel chromium complex [CrIII(L2-2H)Cl] (L2 is a salen-type Schiff base) with a TOF of 49.7 s−1 at an overpotential of 426 mV for water oxidation [52]. In 2022, Mo’s group anchored iron and cobalt ions in the vacancies of the triazine-based Schiff base network, resulting in excellent oxygen evolution reaction (OER) performance with an overpotential of 288 mV [53]. In 2020, Gurpreet and coworkers investigated the activity of mono- and dinuclear complexes of Cr, Mn, Fe, Co, and Ni with macrocyclic Schiff base cuprolic ligand for catalytic CO2 reduction, which could efficiently convert CO2 to methane at a potential of −0.24 V [54]. In 2021, Bonetto et al. studied the carbon dioxide reduction properties of five Fe(III) complexes of Schiff-base-containing donor sites N2O2, which show the overpotential of Fe(salen) of 910 mV, Kcat of 50,000 s−1 [55]. Nevertheless, as yet, there has been no reported Schiff base complex acting as bifunctional electrochemical catalyst for CO2 reduction and water oxidation.
Based the reasons above, herein, we have rationally designed and successfully obtained two novel non-noble transition metal coordination complexes: one mononuclear MnIII complex [MnIIIL(H2O)(MeCN)](ClO4) (1) and one hetero-binuclear MnII–CuII complex [(CuIILMnII(H2O)3)(CuIIL)2](ClO4)2·CH3OH (2) with the same Schiff base ligand L, and explored their homogeneous electrocatalytic properties for water oxidation and CO2 reduction. The special coordination environments of 1 and 2 enable both of them to catalyze water oxidation and CO2 reduction simultaneously with high catalytic performance.
Complexes 1 and 2 are the first two compounds coordinated with Schiff base ligand for both water oxidation and CO2 reduction. The complexes 1 and 2 show the electrocatalytic activity for water oxidation with TOFs of 3.66 s−1 and 7.88 s−1 with the overpotentials of 728 mV and 216 mV, meanwhile the catalytic reduction of CO2 to CO with the TOF values of 0.38 s−1 at −1.65 V and 15.97 s−1 at −1.60 V, respectively, which are higher than or comparable with those of reported complexes (Table S2) [31,32,33,34]. Through great efforts, we used the same Schiff base ligand L to obtain mononuclear MnIII complex 1 and hetero-binuclear MnII–CuII complex 2 for accurately comparing their catalytic behaviors. Complex 2 was found to exhibit superior catalytic performance compared to 1 due to the synergistic catalytic action of the Mn(II) and Cu(II) metal centers in 2. This research supplies an efficient electrocatalytic system for both water oxidation and CO2 reduction with high efficiency, stability, and selectivity under homogeneous conditions, which can offer significant inspiration for the future development of electrocatalysis in this area.
2. Results and Discussion
2.1. Characterization of the Crystal Structures
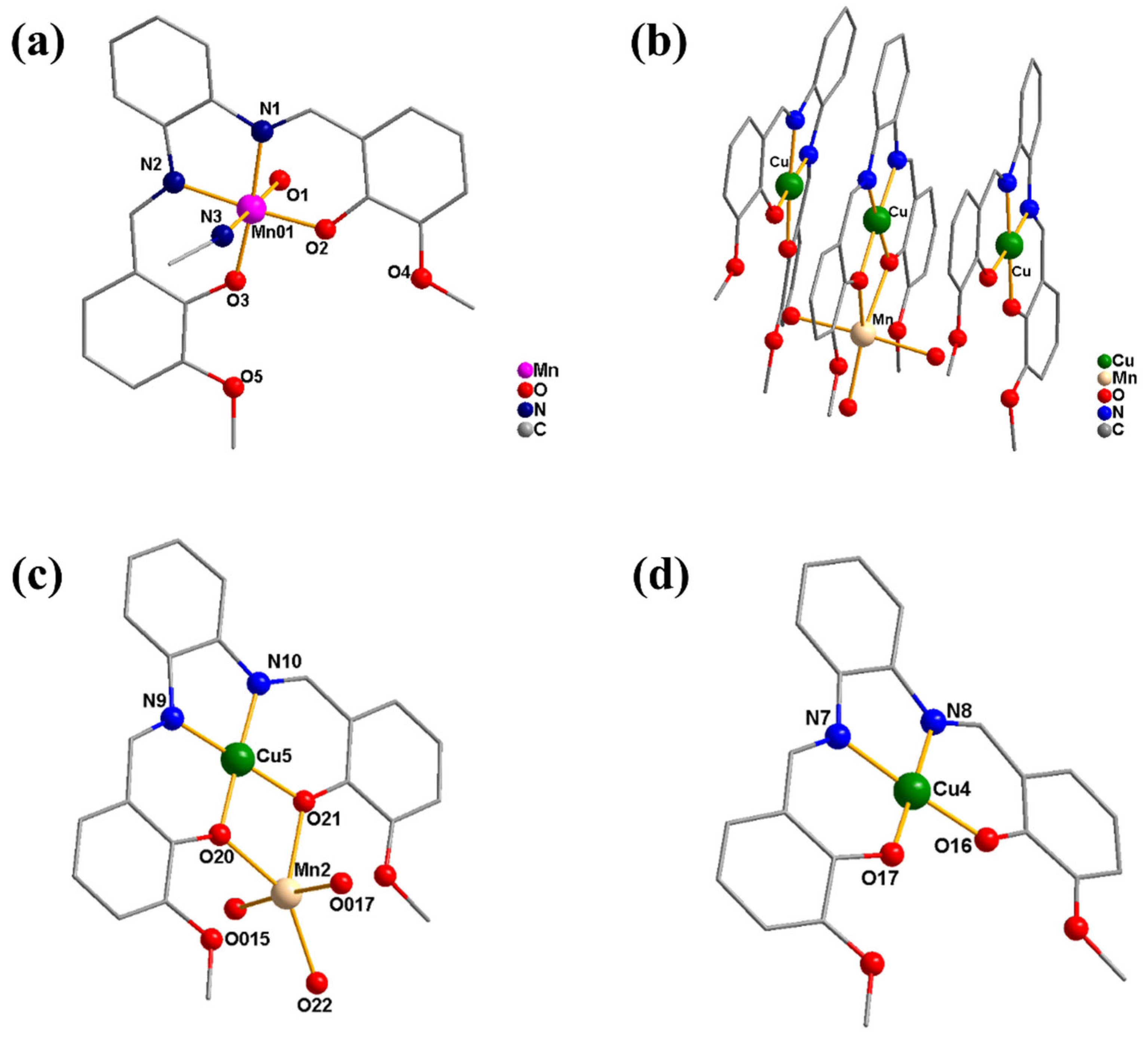
Single-crystal X-ray diffraction analysis demonstrates that 1 and 2 crystallize in the monoclinic system with space group P121/c1 and triclinic crystal system with P-1 space group, respectively (Tables S3 and S4). As illustrated in Figure 1, complex 1 is a mononuclear complex, of which the metal center Mn is six-coordinated with two N atoms and two O atoms from the deprotonated Schiff base ligand (L), as well as one O atom and one N atom from two labile solvent ligands of water and acetonitrile, respectively, forming a distorted octahedral coordination structure. With a particular configuration, complex 2 consists of three almost planar parts, of which a hetero-binuclear Mn–Cu cation moiety is sandwiched between two identical mononuclear copper natural moieties connected by π–π stacking interaction. In the hetero-binuclear Mn–Cu cation, Cu is four-ligated by two O atoms and two N atoms from the Schiff base ligand L, leading to a square-planar coordination geometry, meanwhile, Mn is five-coordinated by two O atoms from L and three O atoms from water ligands with a distorted trigonal bipyramid configuration. Additionally, the Mn and Cu centers are linked together by two µ2-O bridges with a distance of ~3.216 Å. In the two identical mononuclear copper parts, the Cu center is in coplanar configuration chelated with two O atoms and two N atoms from the ligand L. According to the bond valence sum calculations, the manganese ion in complex 1 is in a +3 oxidation state, while in complex 2, the valence states of the three copper ions are all +2 and the manganese ion is +2 [56]. As shown in Figure S3, the magnetic moment (µeff) of complex 1 at room temperature is around 5.0 µB, which suggests that the manganese ion should be in the +3 oxidation state. It may be due to the oxidation of the MnII ion in the air during the reaction process, which is similar to the other reported mononuclear Schiff base manganese complexes (Scheme S1) [57]. However, the MnII ion is not oxidized in the hetero-binuclear Mn–Cu system of complex 2, which resembles the other reported Schiff base Mn–Cu complexes (Scheme S1) [58,59].
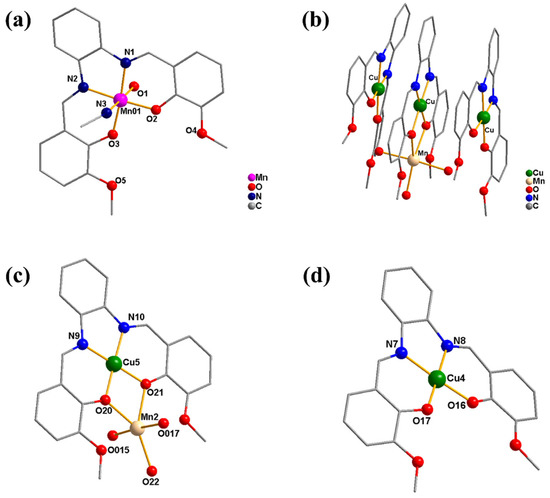
Figure 1.
Crystal structures of complex 1 (a) and 2 ((b): the whole part of 2; (c): the middle Mn–Cu cation moiety in 2; (d): the natural Cu moiety in 2) (hydrogen atoms, solvents, and anions have been omitted for clarity).
2.2. Electrochemical Properties under Atmosphere of Ar
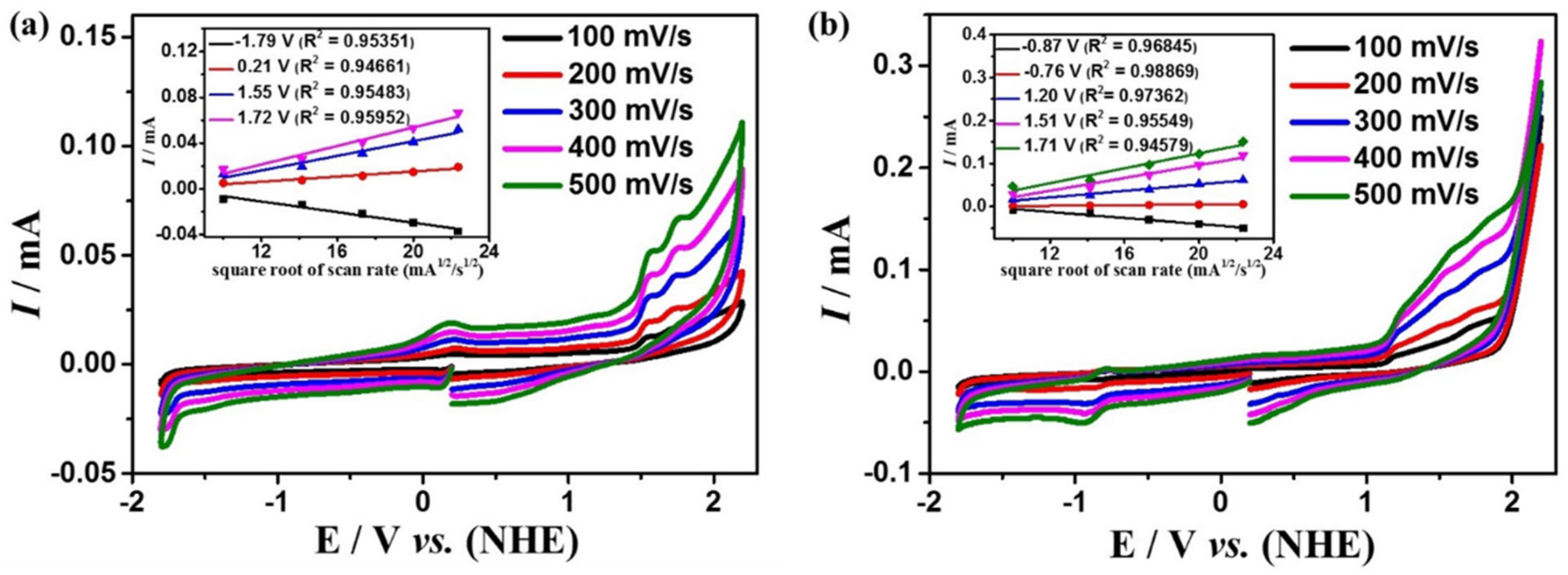
Figure 2 shows the cyclic voltammograms (CVs) of complexes 1 and 2 in the DMF solution at the scan rates ranging from 100 to 500 mV s−1 under an argon atmosphere (all potentials are relative to the NHE electrode).
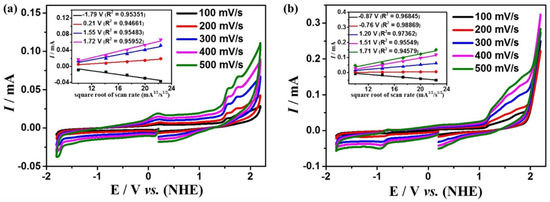
Figure 2.
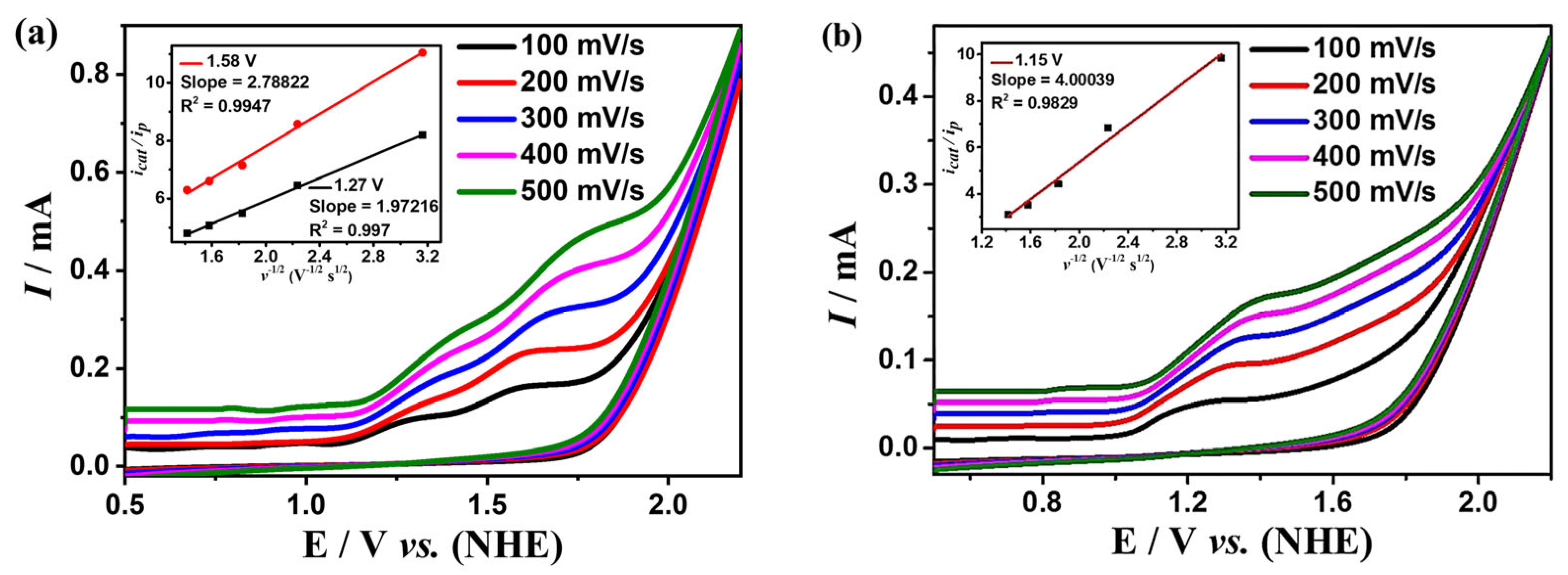
Cyclic voltammogram of 0.5 mM complexes 1 (a) and 2 (b) in the DMF solution, the sweep speed range is 100–500 mV s−1. The insets of (a,b) are the linear relationship between the irreversible peak current and the square root of the scan rate mA1/2/s1/2 at different potentials for 1 and 2, respectively.
While scanning towards the cathode, complexes 1 and 2 exhibit irreversible reduction peaks at Ep = −1.79 V and −0.87 V, respectively. When scanning towards the anode potential, complex 1 shows three irreversible oxidation peaks at Ep = 0.21 V, 1.55 V, 1.72 V (Figure 2a), and complex 2 shows four irreversible oxidation peaks at Ep = −0.76 V, 1.20 V, 1.51 V, 1.71 V (Figure 2b). The peak potentials of both complexes are linearly related to the square root of their scan rates, indicating that the processes are all controlled by diffusion (the insets of Figure 2).
2.3. Electrochemical Properties for Water Oxidation
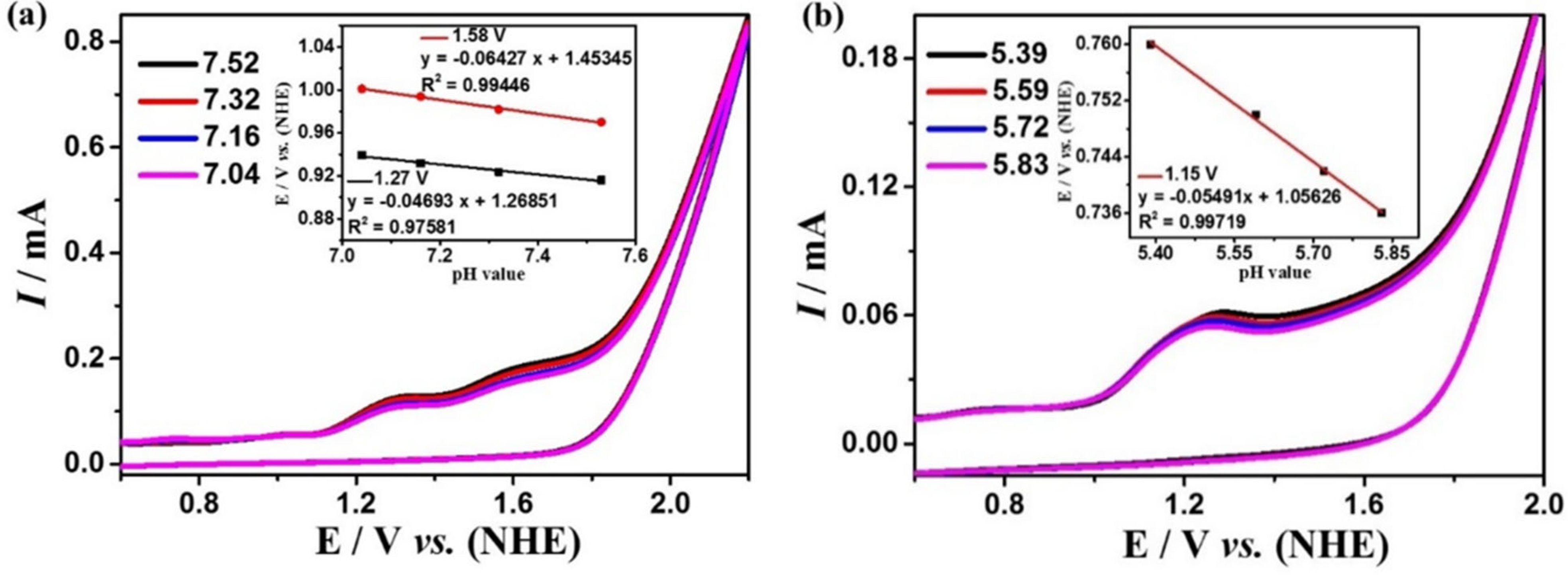
The electrocatalytic water oxidation performances of complexes 1 and 2 were studied by cyclic voltammetry in DMF:water (3:7 v/v) solution with 0.2 M phosphate buffer at different pH values. Complex 1 exhibits the largest current density at pH = 7.52, and there are two strongly enhanced irreversible oxidation peaks at 1.27 and 1.58 V (Figure 3a). While complex 2 exhibits the largest current density at pH = 5.39, and there is a strongly enhanced irreversible oxidation peak at 1.15 V (Figure 3b). In addition, during electrolysis at these potentials, many bubbles can be observed on the surface of the GC electrode. The generation of oxygen can be detected by the Ocean Optics NeoFox-GT oxygen sensor. These experimental results indicate that complexes 1 and 2 can catalyze the oxidation of water to produce O2 at these potentials. Furthermore, the water oxidation onset potentials of the two complexes are linearly dependent on pH values (the insets of Figure 3) with the slopes of 47 mV pH−1 and 64 mV pH−1 for complex 1 as well as −55 mV pH−1 for complex 2, respectively, which indicate that the catalytic water oxidation reactions should be all a PCET (proton-coupled electron transfer) process, which is characteristic for a 1e−/1H+-coupled redox process according to the Nernst equation [60]. According to the Equations (1) and (2), the overpotentials for electrocatalytic water oxidation of complex 1 are 411 and 728 mV, and for complex 2 is 216 mV, which is lower than many previously reported great coordination catalysts [61,62,63,64,65].
𝐸𝑡ℎ𝑒𝑜𝑟𝑦 (𝑂2/𝐻2𝑂) = 1.23 − 0.059 𝑝𝐻
𝜂 = 𝐸𝑟𝑒𝑎𝑙 (𝑂2/𝐻2𝑂) − 𝐸𝑡ℎ𝑒𝑜𝑟𝑦 (𝑂2/𝐻2𝑂)
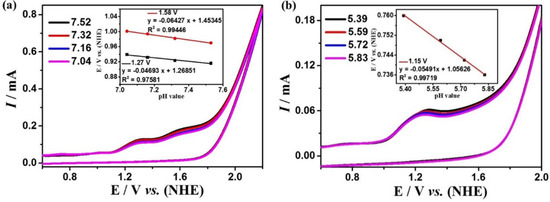
Figure 3.
Cyclic voltammograms of 0.5 mM complexes 1 (a) and 2 (b) in DMF:water (3:7 v/v) solution with 0.2 M phosphate buffer at different pH (scan rate 100 mV s−1). The insets of (a) and (b) are the relationships of onset potentials for electrocatalytic water oxidation with pH values.
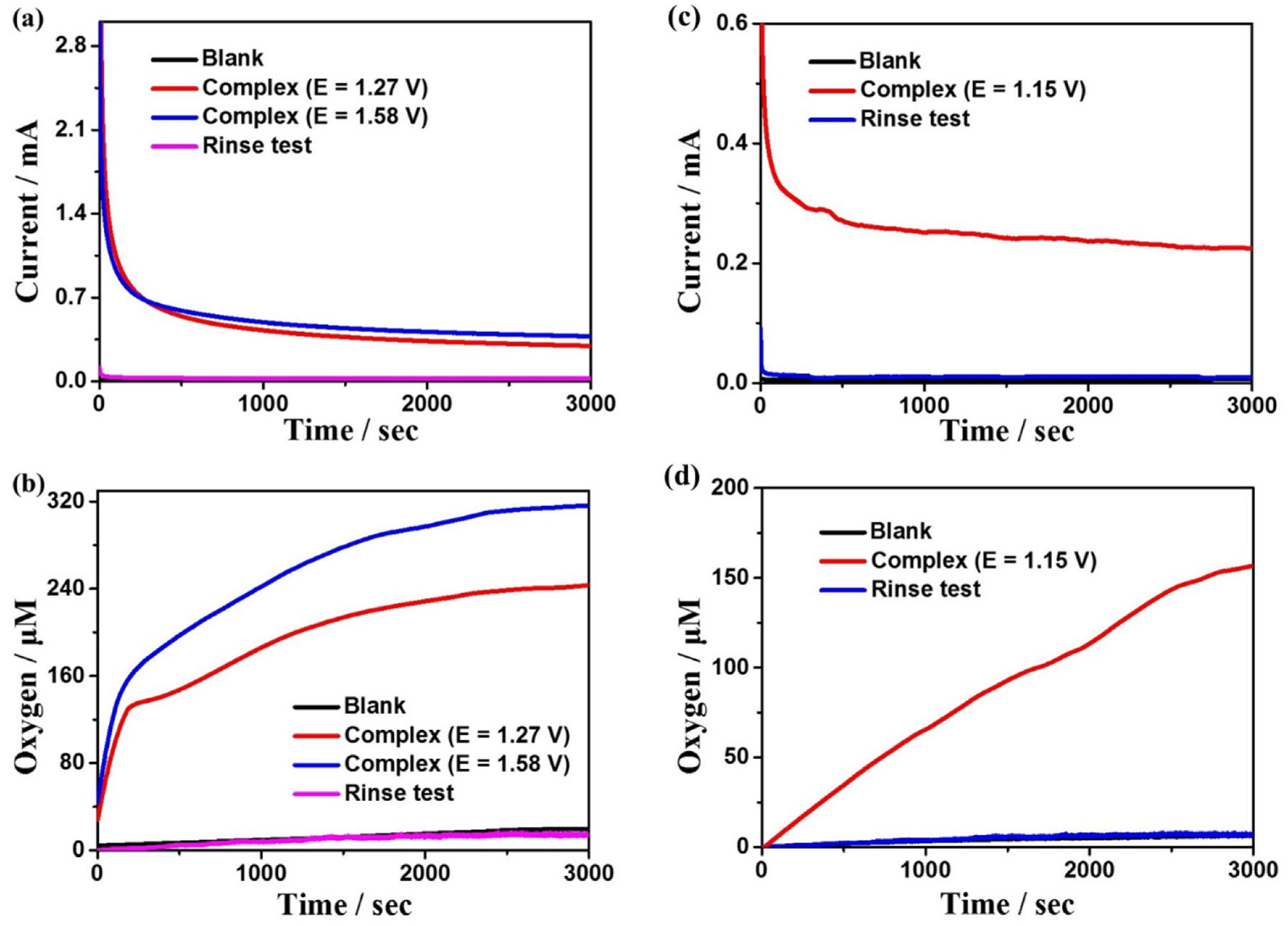
In order to further evaluate the electrocatalytic reactivity of complexes 1 and 2 for water oxidation, the controlled potential electrolysis (CPE) experiments were carried out in DMF:water (3:7 v/v) solution with 0.2 M phosphate as buffer at the potentials of 1.27 and 1.58 V for complex 1 at pH = 7.52, and at the potential of 1.15 V for complex 2 at pH = 5.39, respectively, using F-doped tin oxide (FTO) as the working electrode. In the meantime, to determine the generated oxygen, the oxygen sensor was used to record the oxygen density in the solution in situ (the oxygen density in the headspace of the electrolytic cell is below the detection limit of the sensor). It is found that the oxygen content and detected current density in the blank solution are close to zero without complexes (Figure 4, black lines), indicating that no catalysis occurred with no complex 1 or 2 dissolved in the solution. With 1 or 2 dissolved in the system, the current density and oxygen content increase sharply, indicating that the two complexes are excellent electrochemical catalysts for water oxidation at some particular pH values. Throughout an electrolysis of 3600 s at the applied potentials of 1.27 V and 1.58 V for complex 1, the observed dissolved oxygen concentrations are 238 and 320 μM (Figure 4b), while the current densities are 0.31 and 0.38 mA cm−2, respectively (Figure 4a). Based on the experimental results, the Faraday efficiencies (FEs) are calculated to be 84% and 88%, respectively. What is more, throughout an electrolysis of 3600 s at the applied potential of 1.15 V for complex 2, the observed dissolved oxygen concentration is 157 μM (Figure 4d), while the current density is 0.22 mA cm−2 (Figure 4c), and the FE is 92%. Moreover, the current densities of both complexes tend to be stable during electrolysis, indicating that they remain stable during the electrolysis process. Additionally, the rinse tests can also verify the high stability of the two catalysts (Figure 4). In addition, to further explore the stability of the complexes during electrocatalytic water oxidation (Figure S4), in situ UV–Vis spectroelectrochemical detections were performed for the two complexes. The spectrums detected every 500 s are almost the same for both 1 and 2, confirming that the two complexes remain stable during the catalytic processes.
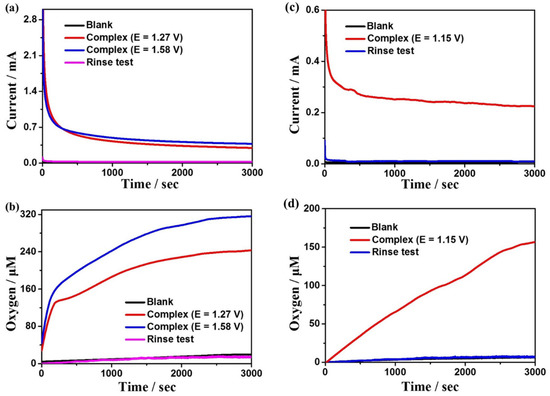
Figure 4.
(a) CPEs for a solution containing 0.5 mM complex 1 and no complex 1 on the FTO working electrode (1.0 cm−2). (b) Dissolved oxygen concentration curves of complex 1 during electrolysis. (c) CPEs for a solution containing 0.5 mM complex 2 and no complex 2 on the FTO working electrode (1.0 cm−2). (d) Dissolved oxygen concentration curves of complex 2 during electrolysis.
In addition to the above studies, the relationship between the concentrations of complexes 1 and 2 and the peak currents is also explored for kinetic studies. The peak currents raise continuously with the increase of the concentration of the complexes, which shows linear relationships, indicating that the rate-determining steps of the water oxidation processes for the two complexes are all in the first order (Figure S5). To further investigate the kinetic information of water oxidation catalyzed by complexes 1 and 2, the CV curves of the two complexes at different scan rates were determined at the optimal pH (Figure 5). The reaction rate coefficient kcat was calculated based on Equation (3), which can be roughly considered as the turnover frequency (TOF) of the catalytic reaction. As shown in the insets of Figure 5, TOFs are calculated to be 1.92 s−1 and 3.66 s−1 for complex 1 at the potentials of 1.27 V and 1.58 V, respectively, and 7.88 s−1 for complex 2 at 1.15 V, which is higher than many other great catalysts (Table S5). Complex 2 shows much greater catalytic performance for water oxidation than that of complex 1, which could be due to the unique coordination sphere of 2 and the cooperative catalytic effect between the manganese and copper ions in complex 2.
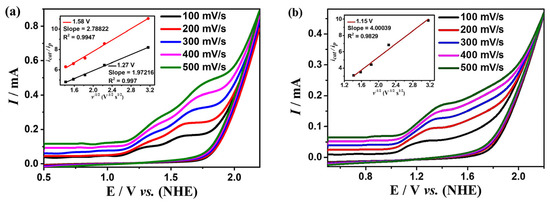
Figure 5.
Cyclic voltammogram of 0.5 mM complexes 1 (a) and 2 (b) in DMF:H2O (3:7 v/v) solution containing 0.2 M phosphonate buffer, the sweep speed range is 100–500 mV s−1. The inset is the relationships of the values of icat/ip with the inverse function of square root of sweep velocity.
In this equation, icat is the catalytic current, ip is the peak current measured without substrate, ncat is the number of electrons involved in the catalytic reaction, F is the Faraday constant, R is the general gas constant, T is the Kelvin unit temperature, v is the scan rate.
2.4. Electrochemical Properties for Carbon Dioxide Reduction
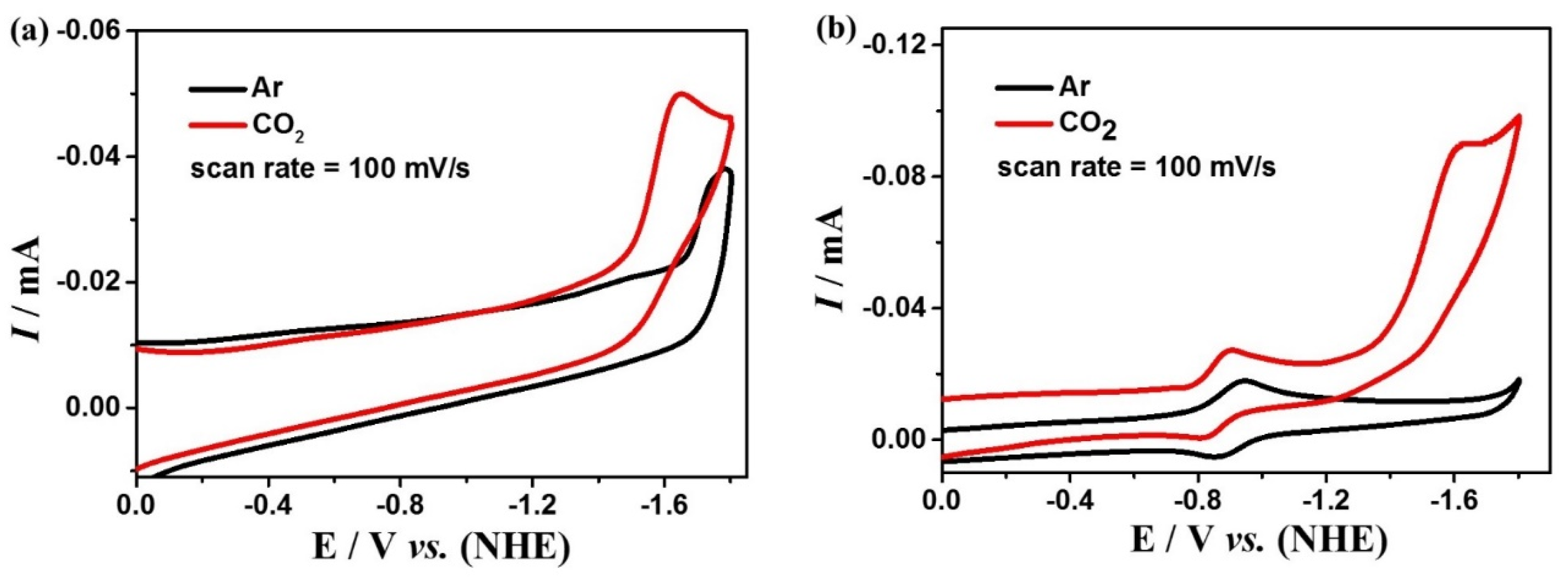
To investigate the performance of the electrocatalytic CO2 reduction of complexes 1 and 2, cyclic voltammetry and controlled potential electrolysis (CPE) experiments were performed using the glassy carbon (GC) and FTO electrode, respectively, in a DMF solution containing 0.1 M nBu4NPF6 under a saturated CO2 atmosphere. Cyclic voltammetric curves under an Ar and CO2 atmosphere at the same scan rate show significant enhancement at the reduction peaks around −1.65 V for complex 1 and −1.60 V for complex 2 (Figure 6), indicating that both complexes can catalyze CO2 reduction. Furthermore, by testing the CVs of the two complexes at different scan rates, it is found that the peak currents are linearly related to the square root of the scan rates (Figure S6), indicating that the catalytic CO2 reduction processes by complexes 1 and 2 are both diffusion controlled. In addition, the CV plots at different scan rates are well reproducible and no new reduction peaks appeared, which further affirms the high stability of the two complexes during catalysis. Additionally, the relationship between the concentrations of the complexes and the peak potentials was investigated by CVs (Figure S7). It is found that the concentrations of 1 and 2 exhibit a linear relationship with the peak currents, revealing that the rate-determining steps for the two catalytic reactions are all in the first order.
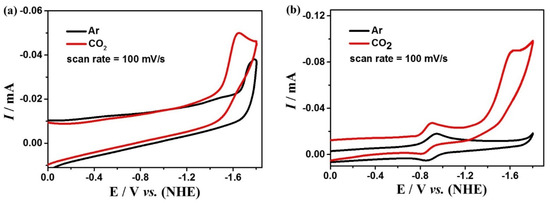
Figure 6.
Cyclic voltammetry of 0.5 mM complexes 1 (a) and 2 (b) in the DMF solution under CO2 (red) and Ar (black) at glassy carbon.
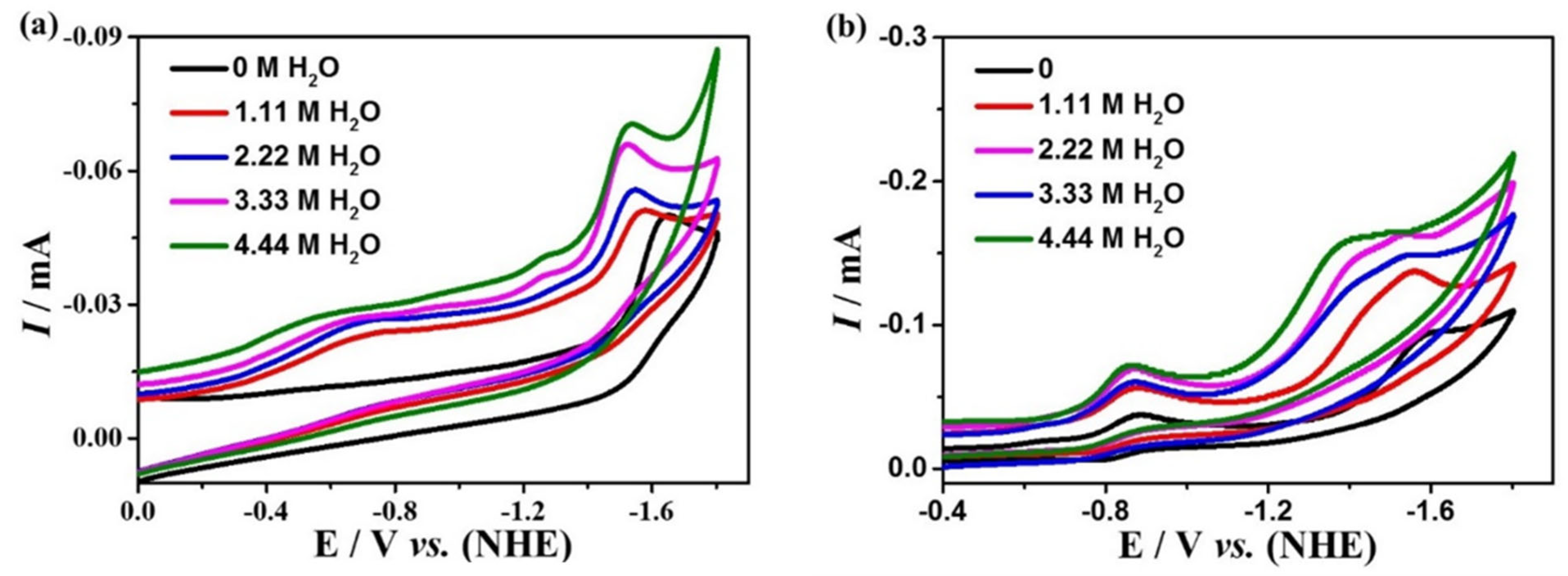
The effect of proton source on the electrocatalytic CO2 reduction of the complexes was further investigated by adding distilled water to 0.1 M nBu4NPF6 DMF, which is shown in Figure 7. The current densities for complexes 1 and 2 raise with the increasing amount of water added, indicating that the proton source could improve the catalytic activity of both complexes. The TOFs of 1 and 2 for electrocatalytic CO2 reduction are obtained based on Equation (4), which are 0.38 s−1 for complex 1 at the potential of −1.65 V, and 15.97 s−1 for complex 2 at the potential of −1.60 V, which is higher than many other reported catalysts (Table S6).
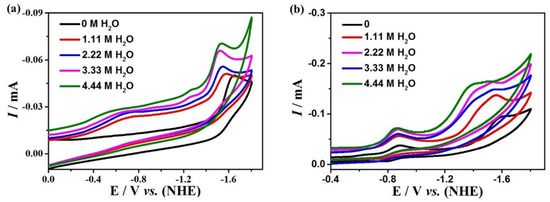
Figure 7.
Cyclic voltammetry of 0.5 mM complexes 1 (a) and 2 (b) in different concentrations of H2O in 0.1 M nBu4NPF6 DMF solution.
In this formula, F is the Faraday constant (96,485 C mol−1), v is the scan rate (0.1 V s−1), np is the number of electrons participating in the noncatalytic redox reaction (np = 1), R is the gas constant (8.314 J K−1 mol−1), T is the temperature (298.15 K), ncat is the number of electrons involved in the catalytic reaction (ncat = 2, reducing CO2 to CO), and ip and icat are the peak currents under Ar and CO2, respectively.
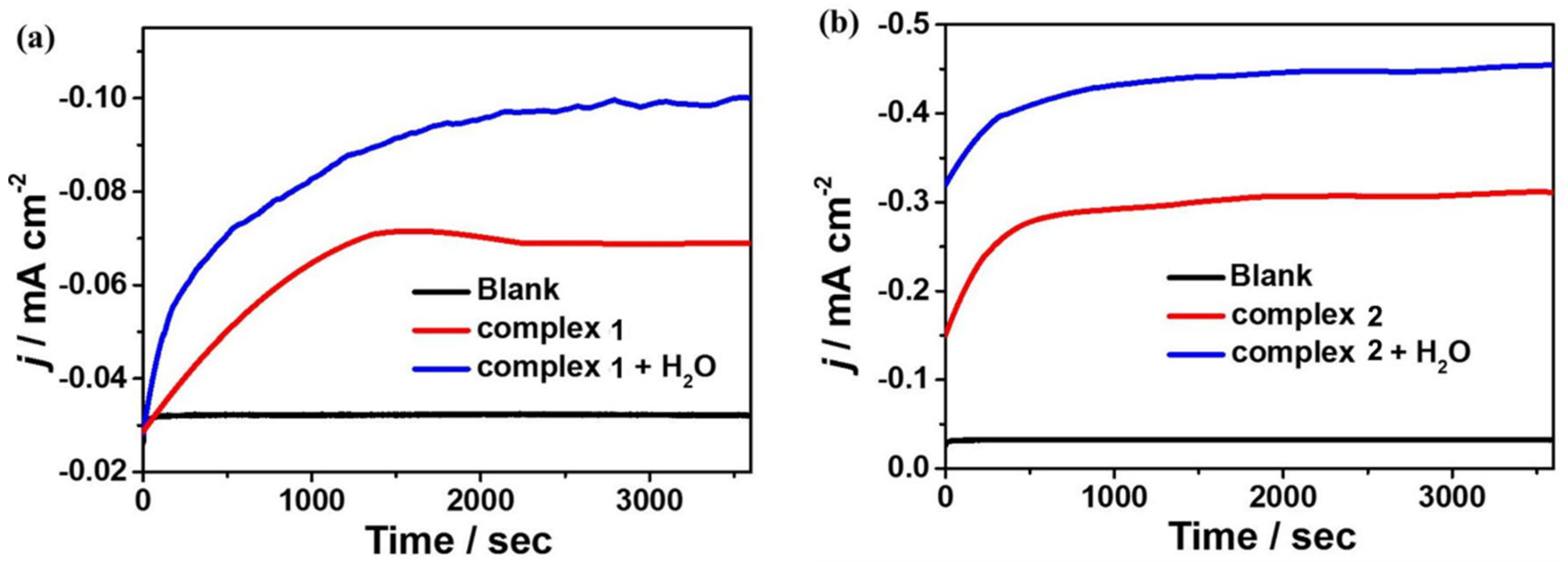
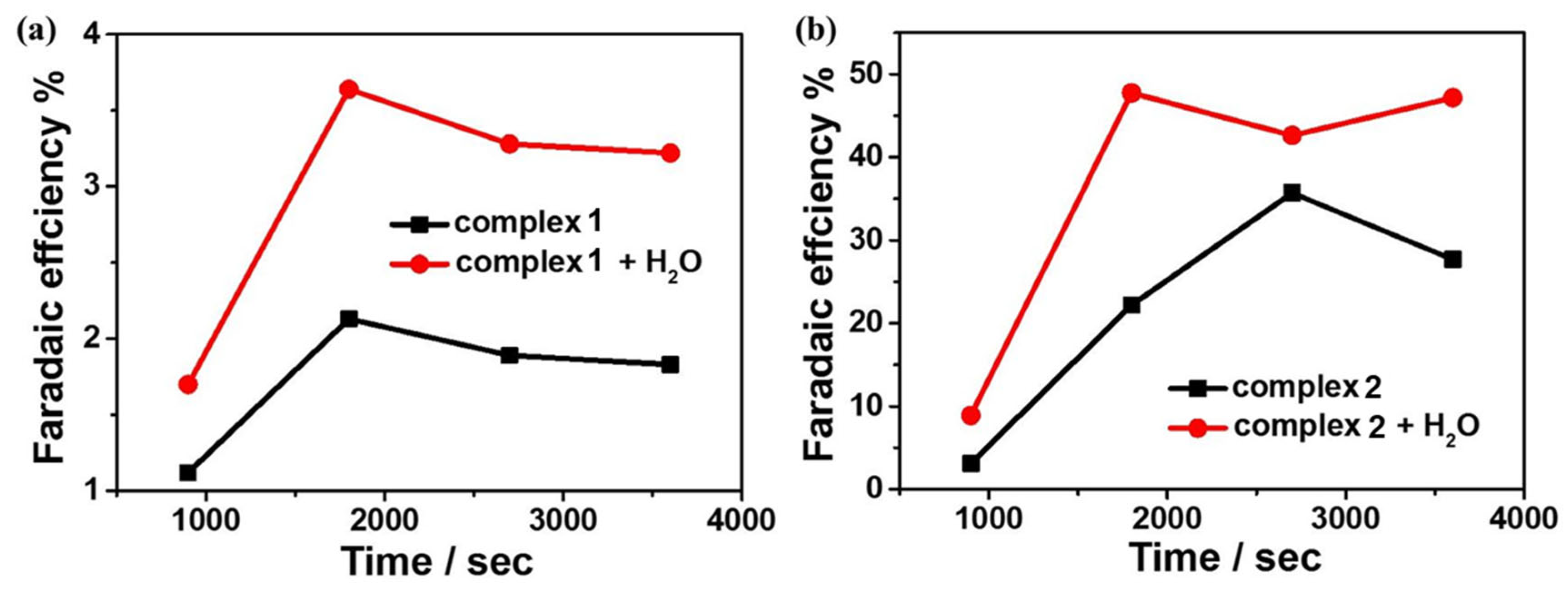
To further investigate the reactivity of 1 and 2 for electrocatalytic CO2 reduction, controlled potential electrolysis experiments (CPEs) were performed in 0.1 M nBu4NPF6 DMF solution under a CO2-saturated atmosphere using FTO as the working electrode, and the electrolysis products were analyzed by gas chromatography. The current density of complex 1 can reach −0.069 mA cm−2 during the electrolysis of 3600 s at the applied potential of −1.65 V. After the introduction of water as a proton source, the current density can reach −0.10 mA cm−2 (Figure 8a). At the applied potential of −1.60 V, the current density of complex 2 can reach −0.31 mA cm−2 during 3600 s of electrolysis and −0.45 mA cm−2 after the addition of water (Figure 8b). Based on gas chromatography analysis, it is revealed that complexes 1 and 2 can electrocatalyze CO2 reduction to CO. The FEs are 2% and 36% for complexes 1 and 2, respectively, and up to 4% and 48% after the addition of 4.5 M water (Figure 9). Furthermore, to further investigate the stability of the complexes during electrocatalytic carbon dioxide reduction (Figure S8), in situ UV–Vis spectroelectrochemistry was carried out for the two complexes. The spectra are almost the same during 3600 s catalysis, confirming that 1 and 2 stay stable during the catalytic process. Notably, complex 2 displays better catalytic behavior for CO2 reduction than that of complex 1, which could be because of the special configuration of complex 2 determined by the Schiff base ligand together with the synergistic catalytic effect between the adjacent Mn and Cu active sites in the complex.
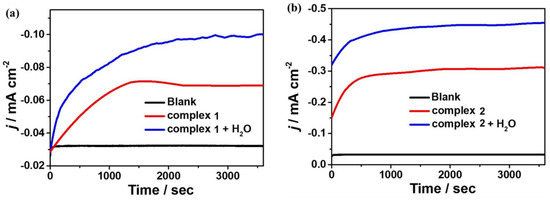
Figure 8.
CPE curves of 0.5 mM complexes 1 (a) and 2 (b) in 0.1 M nBu4NPF6 DMF solution with and without H2O and 0.1 M nBu4NPF6 DMF solution without complex 1 or 2.
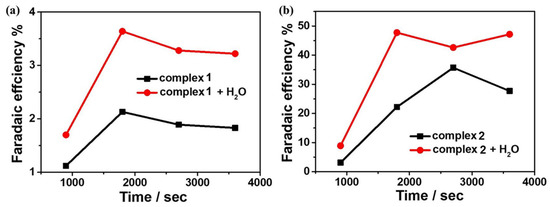
Figure 9.
(a) Faraday efficiency of carbon monoxide produced by 0.5 mM complex 1 in the presence/absence of H2O in DMF solution; (b) Faraday efficiency of carbon monoxide produced by 0.5 mM complex 2 in the presence/absence of H2O in DMF solution.
3. Materials and Methods
3.1. Materials and Characterization
o-Vanillin (C8H8O3, AR) was purchased from Aladdin Biochemical Technology Co., Ltd., (Shanghai, China). 1,2-diaminobenzene (C6H8N2, AR) was purchased from Shanghai Macklin Biochemical Co., Ltd. Manganese(II) Perchlorate (Mn(ClO4)2·6H2O, AR) was purchased from Energy Chemical Reagent Co., Ltd., (Shanghai, China). Cupric Acetate Monohydrate (Cu(CH3COO)2·H2O, AR), Acetic Acid (CH3COOH, AR), methanol (CH3OH, AR), and N,N-Dimethylformamide (C3H7NO, AR) were purchased from Sinopharm Chemical Reagent Co., Ltd., (Shanghai, China). Deionized water was utilized in all experiments. Unless specified otherwise, all chemicals were used as received without purification.
Elemental analyses (C, H, and N) of complexes were performed on a model 2400 PerkinElmer analyzer. The infrared spectra (2 wt% sample in KBr pellets) were recorded on a Nicolet 170SX spectrometer in the 4000–400 cm−1 region. UV–Vis absorption spectra were recorded on TU-1800. Single crystal was mounted on a Bruker SMART APEX II CCD X-ray single-crystal diffractometer, and all data were collected at 173 K with graphite monochromated MoKa radiation (λ = 0.71073 Å) in I > 2σ(I) diffraction spots and reduced by the SAINT program, and absorption corrections were applied using the program SADABS [66]. Structures for 1 and 2 were solved by direct methods using the SHELXS-2014 package and refined with SHELXL-2014/7 [67]. CCDC numbers for the complexes 1 and 2 are 2069590 and 2069498, respectively.
3.2. Synthesis
3.2.1. Synthesis of N,N′-bis(3-Methoxysalicylidene)-1,2-Phenylenediamine (H2L)
The pro-ligand H2L N,N′-(1,2-phenylene)-bis(3-methoxysalicylaldimine) was synthesized according to the literature procedure [68].
The synthesis scheme for H2L and the metal complexes 1 and 2 was illustrated in Scheme S1.
3.2.2. Synthesis of [MnIIIL(H2O)(MeCN)](ClO4) (1)
In a 50 mL flask, the ligand H2L (0.0376 g, 0.1 mmol) was dissolved in 5 mL of methanol, which was then added dropwise to Mn(ClO4)2·6H2O (0.0362 g, 0.1 mmol) in 10 mL of acetonitrile solution. Afterwards, the reaction mixture was stirred for 2 h at 25 °C, and then filtered. X-ray quality crystals were grown by vapor diffusion of diethyl ether into the resulted filtrate at 25 °C. The large quantity reddish-brown crystals were obtained after one week, which were washed with diethyl ether and dried in vacuum at 25 °C, affording 0.0260 g of complex 1 (72% yield, based on Mn). Elemental analysis of Calc. (Found) for C24H23ClMnN3O9: C, 48.78 (48.99); H, 3.74 (3.91); N, 7.53 (7.14). IR (KBr disk, cm−1): 619, 1086, 1257, 1434, 1598, 1648, 2343 (Figure S1).
3.2.3. Synthesis of [(CuIILMnII(H2O)3)(CuIIL)2](ClO4)2·CH3OH (2)
In a 50 mL conical flask, Cu(CH3COO)2·H2O (0.0200 g, 0.1 mmol) was first dissolved in 1 mL H2O, which was added dropwise to the ligand H2L (0.0376 g, 0.1 mmol) dissolved in 10 mL of methanol solution. Afterwards, the reaction mixture was stirred for 2 h at 25 °C. Then, Mn(ClO4)2·6H2O (0.0362 g, 0.1 mmol) dissolved in a solution of methanol (5 mL) was added dropwise to the above resulted mixture, which was stirred for 2 h at 25 °C, and then filtered. X-ray quality crystals were grown by vapor diffusion of diethyl ether into the resulted filtrate at 25 °C. The large quantity brownish-yellow crystals were obtained after one week, which were washed with diethyl ether and dried in vacuum at 25 °C, affording 0.0221 g of complex 2 (61% yield, based on Mn). Elemental analysis of Calc. (Found) for C67H64Cl2Cu3MnN6O24: C, 48.33 (48.62); H, 3.94 (3.87); N, 5.43 (5.08). IR (KBr disk, cm−1): 410, 524, 669, 1093, 1257, 1446, 1604, 1680, 2461 (Figure S2).
3.2.4. Electrochemical Measurement and Electrolytic Product Analysis
All electrochemical experiments were tested by a CHI660E electrochemical analyzer to study their electrocatalytic properties and performed in a single chamber three-electrode cell. The electrocatalytic water oxidation properties of the complexes were measured in DMF:water (3:7 v/v) solution with 0.2 M phosphate buffer, and the electrocatalytic CO2 reduction properties were measured in 0.1 M nBu4NPF6 DMF solution with distilled water as proton sources. The solution was purged with high purity argon gas for 30 min prior to each electrochemical test to eliminate air interference.
For the cyclic voltammetry (CV) experiments, the glassy carbon electrode, Ag/AgCl electrode, and platinum wire electrode were used as the working electrode, reference electrode, and counter electrode, respectively. The glassy carbon electrode was polished several times with polishing powder, then washed with anhydrous ethanol and deionized water, and finally dried before use.
For the controlled potential electrolysis experiments (CPE), the working electrodes were replaced with F-doped tin oxide (FTO) conductive glass substrates (1 × 1 cm, effective surface area of 1.0 cm2), which were immersed in ethanol solution containing 5% NaOH for several hours before use, and then washed with water, ethanol, and water in turn. For water oxidation, the pH was adjusted using 0.1 M sodium phosphate and 0.1 M phosphoric acid. For CO2 reduction, distilled water was added to 0.1 M nBu4NPF6 DMF solution to tune the concentration of proton sources.
The oxygen concentration generated during the water oxidation electrolysis process was detected using the Ocean Optics NeoFox-GT oxygen sensor, and the carbon monoxide concentration generated during CO2 reduction was detected using the Shimadzu GC-2014.
4. Conclusions
In this work, we have successfully isolated two novel non-noble transition metal complexes with the same Schiff base ligand L: mononuclear MnIII complex [MnIIIL(H2O)(MeCN)](ClO4) (1) and hetero-binuclear MnII–CuII complex [(CuIILMnII(H2O)3)(CuIIL)2](ClO4)2·CH3OH (2). The structures of the two complexes are studied by X-ray crystallography, which reveals that the MnIII center in 1 is six-coordinated, while in complex 2, the sphere of CuII center is in the square-planar coordination sphere and the MnII center is in a five coordination environment. The peculiar coordination environments of 1 and 2 make them capable of homogeneous electrocatalytic water oxidation and CO2 reduction simultaneously. Complexes 1 and 2 show water oxidation reactivity with the overpotentials of 728 mV and 216 mV, FEs of 88% and 92%, respectively, while the TOF values for the catalytic reduction of CO2 to CO are 0.38 s−1 at −1.65 V and 15.97 s−1 at −1.60 V, respectively. Complexes 1 and 2 are the first two compounds coordinated with Schiff base ligand as bifunctional electrocatalysts in this field. By comparison, complex 2 displays much greater catalytic performance than that of 1 and many other reported complexes. The excellent catalytic activity of complex 2 could be due to the special configuration of complex 2 determined by Schiff base ligand together with the synergistic catalytic effect between the adjacent Mn and Cu active sites in the complex. This research supplies a facile strategy of the synergistic catalytic effect between two different centers for investigating efficient non-noble transition metal complexes as bifunctional electrochemical catalysts for water oxidation and CO2 reduction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28031074/s1, Scheme S1. The synthesis scheme for H2L and the metal complexes 1 and 2 under study. Figure S1. IR spectrum of the complex 1. Figure S2. IR spectrum of the complex 2. Figure S3. The magnetic moment (µeff) of complex 1. Figure S4. In situ UV–Vis spectra of complex 1 in DMF:H2O (3:7 v/v) solution containing 0.2 M phosphonate buffer solution at 1.27 V (a) and 1.58 V (b), and in situ UV–Vis spectra of complex 2 in DMF:H2O (3:7 v/v) solution containing 0.2 M phosphonate buffer at 1.15 V (c). Figure S5. Cyclic voltammograms of different concentrations of 0.5 mM complex 1 (a) and 2 (b) in DMF:H2O (3:7 v/v) solution containing 0.2 M phosphonate buffer at optimal pH (scanning rate is 100 mV s−1). The inset is the relationships of catalytic currents with concentrations. Figure S6. Cyclic voltammetry of 1 (a) and 2 (b) in DMF under CO2 recorded at scan rates range from 100 to 500 mV s−1. The inset is the relationships of the peak currents with the square root of the scan rates. Figure S7. Cyclic voltammetry of complexes 1 (a) and 2 (b) in the 0.1 M nBu4NPF6 DMF solution under CO2 in different concentrations (the scanning rate is 100 mV s−1). The inset is the relationship between complex concentration and reduction peak current. Figure S8. In situ UV–Vis spectra of complex 1 in 0.1 M nBu4NPF6 DMF at −1.65 V (a) and in situ UV–Vis spectra of complex 2 in 0.1 M nBu4NPF6 DMF solution at −1.60 V (b). Table S1. Nomenclature table for all the special symbols in the text. Table S2. Comparison of different parameters of the reported similar homogeneous catalysts in recent years [31,32,33,34]. Table S3. Crystallographic data and structure correction parameters of complexes 1 and 2. Table S4. The main key length and key angle of the complex 1 and 2. Table S5. Comparison of different parameters of complexes for water oxidation in recent years [69,70,71,72]. Table S6. Comparison of different parameters of complexes for carbon dioxide reduction in recent years [34,73,74,75,76,77].
Author Contributions
Conceptualization, X.Z., J.L., H.J. and M.L.; data curation, X.Z.; formal analysis, J.L.; funding acquisition, M.W.; investigation, X.Z., J.L., H.J. and M.W.; methodology, X.Z. and J.L.; project administration, M.W.; resources, M.L.; supervision, J.L., H.J. and M.W.; writing—original draft, X.Z.; writing—review and editing, X.Z. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key R&D Program of China, grant No. 2022YFA1502902 and National Natural Science Foundation of China, grant No. 21601171.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gurney, K.R.; Mendoza, D.L.; Zhou, Y.Y.; Fischer, M.L.; Miller, C.C.; Geethakumar, S.; de la Rue du Can, S. High resolution fossil FuelCombustion CO2 emission fluxes for combustionthe united states. Environ. Sci. Technol. 2009, 43, 5535–5541. [Google Scholar] [CrossRef]
- Hove, S.; Tursoy, T. An investigation of the environmental Kuznets curve in emerging economies. J. Clean. Prod. 2019, 236, 117628.1–117628.9. [Google Scholar] [CrossRef]
- Yaseen, M.; Rawat, S.K.; Kumar, M. Cattaneo-Christov heat flux model in Darcy-Forchheimer radiative flow of MoS2-SiO2/kerosene oil between two parallel rotating disks. J. Therm. Anal. Calorim. 2022, 147, 10865–10887. [Google Scholar] [CrossRef]
- Yaseen, M.; Rawat, S.K.; Shafiq, A.; Kumar, M.; Nonlaopon, K. Analysis of Heat Transfer of Mono and Hybrid Nanofluid Flow between Two Parallel Plates in a Darcy Porous Medium with Thermal Radiation and Heat Generation/Absorption. Symmetry 2022, 14, 1943. [Google Scholar] [CrossRef]
- Gumber, P.; Yaseen, M.; Rawat, S.K.; Kumar, M. Heat transfer in micropolar hybrid nanofluid flow past a vertical plate in the presence of thermal radiation and suction/injection effects. Partial. Differ. Equ. Appl. Math. 2022, 5, 100240. [Google Scholar] [CrossRef]
- Becker, S.; Bouzdine-Chameeva, T.; Jaegler, A. The carbon neutrality principle: A case study in the French spirits sector. J. Clean. Prod. 2020, 274, 122739. [Google Scholar] [CrossRef]
- Pickering, B.; Lombardi, F.; Pfenninger, S. Diversity of Options to Reach Carbon-Neutrality Across the Entire European Energy System. Joule 2022, 6, 1253–1276. [Google Scholar] [CrossRef]
- Li, Z.S.; Yue, Y.; Peng, J.C.; Luo, Z.M. Phase engineering two-dimensional nanostructures for electrocatalytic hydrogen evolution reaction. Chin. Chem. Lett. 2023, 34, 107119. [Google Scholar] [CrossRef]
- Zou, X.H.; Li, Z.S.; Xie, Y.X.; Wu, H.; Lin, S. Phosphorus-doping and addition of V2O5 into Pt/graphene resulting in highly-enhanced electro-photo synergistic catalysis for oxygen reduction and hydrogen evolution reactions. Int. J. Hydrogen Energy 2020, 45, 30647–30658. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, T.; Sun, L.; Nsanzimana, J.M.V.; Fisher, A.C.; Wang, X. Selective electrochemical reduction of CO2 to ethylene on Nanopores-Modified copper electrodes in aqueous solution. ACS Appl. Mater. Inter. 2017, 9, 32782–32789. [Google Scholar] [CrossRef]
- Yue, Y.; Zou, X.H.; Shi, Y.D.; Cai, J.N.; Xiang, Y.X.; Li, Z.S.; Lin, S. A low crystallinity CuO-SnO2/C catalyst for efficient electrocatalytic reduction of CO2. J. Electroanal. Chem. 2023, 928, 117089. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z.S.; Liu, Y.; Zou, X.H.; Yin, L.W.; Lin, S. A cost-effective indium/carbon catalyst for highly efficient electrocatalytic reduction of CO2 to HCOOH. Sustain. Energ. Fuels 2021, 5, 5798–5803. [Google Scholar] [CrossRef]
- Ye, Y.Z.; Liu, Y.; Li, Z.S.; Zou, X.H.; Wu, H.; Lin, S. Highly selective and active Cu-In2O3/C nanocomposite for electrocatalytic reduction of CO2 to CO. J. Colloid Interf. Sci. 2021, 586, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Adhikary, S.D.; Kapurwan, S.; Konar, S. En route to artificial photosynthesis: The role of polyoxometalate based photocatalysts. J. Mater. Chem. A 2022, 10, 13152–13169. [Google Scholar] [CrossRef]
- Wang, J.W.; Zhong, D.C.; Lu, T.B. Artificial photosynthesis: Catalytic water oxidation and CO2 reduction by dinuclear non-noble-metal molecular catalysts. Coord. Chem. Rev. 2018, 377, 225–236. [Google Scholar] [CrossRef]
- Yoshino, S.; Takayama, T.; Yamaguchi, Y.; Iwase, A.; Kudo, A. CO2 Reduction Using Water as an Electron Donor over Heterogeneous Photocatalysts Aiming at Artificial Photosynthesis. Acc. Chem. Res. 2022, 55, 966–977. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, H.Q.; Zhang, W.; Cao, R. Mononuclear first-row transition-metal complexes as molecular catalysts for water oxidation. Chin. J. Catal. 2018, 39, 228–244. [Google Scholar] [CrossRef]
- Yang, M.J.; Li, J.; Ke, G.L.; Liu, B.Y.; Dong, F.Q.; Yang, L.; He, H.C.; Zhou, Y. WO3 homojunction photoanode: Integrating the advantages of WO3 different facets for efficient water oxidation. J. Energy Chem. 2021, 56, 37–45. [Google Scholar] [CrossRef]
- Yang, B.P.; Liu, K.; Li, H.J.W.; Liu, C.X.; Fu, J.W.; Li, H.M.; Huang, J.E.; Ou, P.; Alkayyali, T.; Cai, C.; et al. Accelerating CO2 Electroreduction to Multicarbon Products via Synergistic Electric–Thermal Field on Copper Nanoneedles. J. Am. Chem. Soc. 2022, 144, 3039–3049. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Vali, A.; de Brito, J.F.; Boldrin Zanoni, M.V. Naming Photoelectrochemical Processes: Why Thermodynamics Holds the Key. ACS Energy Lett. 2021, 6, 2198–2201. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, Q.; Xia, R.; Meyer, T.J. CO2 Reduction: From Homogeneous to Heterogeneous Electrocatalysis. Acc. Chem. Res. 2020, 53, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Franco, F.; Rettenmaier, C.; Jeon, H.S.; Roldan, C.B. Transition metal-based catalysts for the electrochemical CO 2 reduction: From atoms and molecules to nanostructured materials. Chem. Soc. Rev. 2020, 49, 6884–6946. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, J.R.; Guntern, Y.T.; Mensi, M.; Buonsanti, R. Molecular tunability of surface-functionalized metal nanocrystals for selective electrochemical CO2 reduction. Chem. Sci. 2019, 10, 10356–10365. [Google Scholar] [CrossRef]
- Ye, S.; Ding, C.; Li, C. Artificial photosynthesis systems for catalytic water oxidation. Adv. Inorg. Chem. 2019, 74, 3–59. [Google Scholar]
- Lee, W.T.; Munoz, S.B.; Dickie, D.A.; Smith, J.M. Ligand Modification Transforms a Catalase Mimic into a Water Oxidation Catalyst. Angew. Chem. Int. Ed. 2015, 53, 9856–9859. [Google Scholar] [CrossRef]
- Liu, H.B.; Yang, L.; Qiao, K.W.; Zeng, X.F.; Huang, Y.; Zheng, L.R.; Cao, D.P. A new concept analogous to homogeneous catalysis to construct in-situ regenerative electrodes for long-term oxygen evolution reaction. Nano Energy 2020, 76, 105115. [Google Scholar] [CrossRef]
- Cui, M.; Qian, Q.L.; Zhang, J.J.; Wang, Y.; Asare, B.; Bernard, B.; Liu, H.Z.; Han, B.X. Liquid fuel synthesis via carbon dioxde hydrogenation by coupling homogeneous and heterogeneous catalysis. Chem 2021, 7, 726–737. [Google Scholar] [CrossRef]
- Narouz, M.R.; De La Torre, P.; An, L.; Chang, C.J. Multifunctional Charge and Hydrogen-Bond Effects of Second-Sphere Imidazolium Pendants Promote Capture and Electrochemical Reduction of CO2 in Water Catalyzed by Iron Porphyrins. Angew. Chem. Int. Ed. 2022, 61, e202207666. [Google Scholar] [CrossRef]
- Zhanaidarova, A.; Jones, S.C.; Despagnet-Ayoub, E.; Pimentel, B.R.; Kubiak, C.P. Re(tBu-bpy)(CO)3Cl Supported on Multi-Walled Carbon Nanotubes Selectively Reduces CO2 in Water. J. Am. Chem. Soc. 2019, 141, 17270–17277. [Google Scholar] [CrossRef]
- Xu, Q.; Li, H.; Chi, L.; Zhang, L.G.; Wan, Z.; Ding, Y.; Wang, J.D. Identification of homogeneous [Co4(H2O)4(HPMIDA)2(PMIDA)2]6- as an effective molecular-light-driven water oxidation catalyst. Appl. Catal. B Environ. 2017, 202, 397–403. [Google Scholar] [CrossRef]
- Chen, Z.F.; Concepcion, J.J.; Brennaman, M.K.; Kang, P.; Norris, M.R.; Hoertz, P.G.; Meyer, T.J. Splitting CO2 into CO and O2 by a single catalyst. Proc. Natl. Acad. Sci. USA 2012, 109, 15606–15611. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Ezzedinloo, L.; Bhadbhade, M.; Bucknall, M.P.; Colbran, S.B. Strategic design of a ruthenium catalyst for both CO2 reduction and H2O oxidation: The electronic influence of the co-ligands. Chem. Commun. 2017, 53, 10006. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.N.; Xie, W.J.; Zhang, D.M.; Fan, Y.H.; Cui, L.S.; Wang, M. A mononuclear copper complex as bifunctional electrocatalyst for CO2 reduction and water oxidation. J. Electroanal. Chem. 2021, 886, 115106. [Google Scholar] [CrossRef]
- Yin, X.M.; Zhang, S.F.; Wang, J.M.; Li, J.J.; Chen, F.F.; Yao, S.; Fan, Y.H.; Wang, M. Bioinspired cobalt molecular electrocatalyst for water oxidation coupled with carbon dioxide reduction. Appl. Organomet. Chem. 2021, 35, e6371. [Google Scholar] [CrossRef]
- Zhang, P.F.; Wu, D.; Yang, G.P.; Wang, Y.Y. Metal-Organic Frameworks as Heterogeneous Electrocatalysts for Water Splitting and CO2 Fixation. Cryst. Growth Des. 2021, 21, 3123–3142. [Google Scholar] [CrossRef]
- Seal, N.; Karthick, K.; Singh, M.; Kundu, S.; Neogi, S. Mixed- ligand-devised anionic MOF with divergent open Co(II)-nodes as chemo-resistant, bi-functional material for electrochemical water oxidation and mild-condition tandem CO2 fixation. Chem. Eng. J. 2022, 429, 132301. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Z.G.; de Krafft, K.E.; Lin, W.B. Doping Metal-Organic Frameworks for Water Oxidation, Carbon Dioxide Reduction, and Organic Photocatalysis. J. Am. Chem. Soc. 2021, 133, 13445–13454. [Google Scholar] [CrossRef]
- Jun, H.; Choi, S.; Lee, J.B.; Nam, Y.S. Plasmonic Heterostructure Functionalized with a Carbene-Linked Molecular Catalyst for Sustainable and Selective Carbon Dioxide Reduction. ACS Appl. Mater. Interfaces 2020, 12, 33817–33826. [Google Scholar] [CrossRef]
- Morlanes, N.; Takanabe, K.; Rodionov, V. Simultaneous Reduction of CO2 and Splitting of H2O by a Single Immobilized Cobalt Phthalocyanine Electrocatalyst. ACS Catal. 2016, 6, 3092–3095. [Google Scholar] [CrossRef]
- Li, T.T.; Chen, Y.; Li, F.M.; Zhao, W.L.; Wang, C.J.; Lv, X.J.; Xu, Q.Q.; Fu, W.F. Efficient Water Oxidation Catalyzed by Mononuclear Ruthenium(II) Complexes Incorporating Schiff Base Ligands. Chem. Eur. J. 2014, 20, 8054–8061. [Google Scholar] [CrossRef]
- Shebl, M.; Khalil, S.M.E.; Ahmed, S.A.; Medien, H.A.A. Synthesis, spectroscopic characterization and antimicrobial activity of mono-, bi- and tri-nuclear metal complexes of a new Schiff base ligand. J. Mol. Struct. 2010, 980, 39–50. [Google Scholar] [CrossRef]
- Singh, K.; Barwa, M.S.; Tyagi, P. Synthesis, characterization and biological studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes with bidentate Schiff bases derived by heterocyclic ketone. Eur. J. Med. Chem. 2006, 41, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, F.; Sayeed, F.; Muddassir, M.; Photochem, J. Synthesis of new chiral heterocyclic Schiff base modulated Cu(II)/Zn(II) complexes: Their comparative binding studies with CT-DNA, mononucleotides and cleavage activity. Photobiol. B 2011, 103, 166–179. [Google Scholar] [CrossRef]
- Zeynali, H.; Keypour, H.; Hosseinzadeh, L.; Gable, R.W. The Non-Templating Synthesis of Macro-Cyclic Schiff Base Ligands Containing Pyrrole and Homopiperazine and Their Binuclear Nickel(II), Cobalt(II) and Mononuclear Platinum(II) Complexes: X-Ray Single Crystal and Anticancer Studies. J. Mol. Struct. 2021, 1244, 130956. [Google Scholar] [CrossRef]
- Al-Serwi, R.H.; Othman, G.; Attia, M.A.; Enan, E.T.; El-Sherbiny, M.; Mahmoud, S.; Elsherbiny, N. Enhancement of cisplatin cytotoxicity by Cu(II)-Mn(II) Schiff base tetradentate complex in human oral squamous cell carcinoma. Molecules 2020, 25, 4688. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Yang, X.; Ma, Y.; Wang, C.; Hao, W.; Shi, D.; Schipper, D. Construction of an Octanuclear Zn(II)-Yb(III) Schiff Base Complex for the Nir Luminescent Sensing of Nitrofuran Antibiotics. Chin. J. Chem. 2021, 39, 2083–2087. [Google Scholar] [CrossRef]
- Abu-Surrah, A.; Ibrahim, K.A.; Abdalla, M.Y.; Issa, A.A. Pentacoordinated iron(II) complexes with 2,6-bis[(imino)ethyl]pyridine-Schiff base ligands as new catalyst systems mediated atom transfer radical polymerization of (meth)acrylate monomers. J. Polym. Res. 2011, 18, 59–66. [Google Scholar] [CrossRef]
- Gupta, K.C.; Sutar, A.K. Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Rodionova, L.I.; Smirnov, A.V.; Borisova, N.E.; Khrustalev, V.N.; Moiseeva, A.A.; Grünert, W. Binuclear cobalt complex with Schiff base ligand: Synthesis, characterization and catalytic properties in partial oxidation of cyclohexane. Inorg. Chim. Acta 2012, 392, 221–228. [Google Scholar] [CrossRef]
- Grivani, G.; Bruno, G.; Rudbari, H.A.; Khalaji, A.D.; Pourteimouri, P. Synthesis, characterization and crystal structure determination of a new oxovanadium(IV) Schiff base complex: The catalytic activity in the epoxidation of cyclooctene. Inorg. Chem. Commun. 2012, 18, 15–20. [Google Scholar] [CrossRef]
- Muthusami, R.; Ramachandran, V.; Palaniappan, M.; Arumugam, S.; Rangappan, R. Cu(II) Schiff Base Complex Functionalized Mesoporous Silica Nanoparticles as an Efficient Catalyst for the Synthesis of Questiomycin a and Photo-Fenton-Like Rhodamine B Degradation. J. Solid State Chem. 2021, 302, 122429. [Google Scholar] [CrossRef]
- Shamsipur, M.; Taherpour, A.; Sharghi, H.; Lippolis, V.; Pashabadi, A. A Low-Overpotential Nature-Inspired Molecular Chromium Water Oxidation Catalyst. Electrochim. Acta 2018, 265, 316–325. [Google Scholar] [CrossRef]
- Zhu, X.L.; He, N.; Ding, J.X.; Yue, R.M.; He, S.M.; Liu, W.T.; Liu, N.J.; Guo, R.B.; Mo, Z.L. Bimetal-anchored covalent organic frameworks derivatives for efficient alkaline electrolyte oxygen evolution. J. Alloys Compd. 2022, 924, 166442. [Google Scholar] [CrossRef]
- Kour, G.; Mao, X.; Du, A. First principles studies of mononuclear and dinuclear Pacman complexes for electrocatalytic reduction of CO2. Catal. Sci. Technol. 2021, 11, 637–645. [Google Scholar] [CrossRef]
- Bonetto, R.; Civettini, D.; Crisanti, F.; Sartorel, A. FeI Intermediates in N2O2 Schiff Base Complexes: Effect of Electronic Character of the Ligand and of the Proton Donor on the Reactivity with Carbon Dioxide. Energies 2021, 14, 5723. [Google Scholar] [CrossRef]
- Liu, W.T.; Thorp, H.H. Bond valence sum analysis of metal-ligand bond lengths in metalloenzymes and model complexes. 2. Refined distances and other enzymes. Inorg. Chem. 1993, 32, 4102–4105. [Google Scholar] [CrossRef]
- Maneiro, M.; Bermejo, M.R.; Sousa, A.; Fondo, M.; Gonzalez, A.M.; Sousa-Pedrares, A.; McAuliffe, C.A. Synthesis and structural characterization of new manganese(II) and (III) complexes. Study of their photolytic and catalase activity and x-ray crystal structure of [Mn(3-OMe,5-Br-salpn)(EtOH)(H2O)]ClO4. Polyhedron 2000, 19, 47–54. [Google Scholar] [CrossRef]
- Ganguly, S.; Mayans, J.; Ghosh, A. Modulation of Nuclearity in CuII-MnII Complexes of a N2O2 Donor Ligand Depending upon Carboxylate Anions: Structures, Magnetic Properties and Catalytic Oxidase Activities. Chem. Asian J. 2020, 15, 4055–4069. [Google Scholar] [CrossRef]
- Hazari, A.; Dutta, A. Catecholase like activity on heterometallic model complexes of Ni(II)-Mn(II) and Cu(II)-Mn(II) with N2O2 donor di-Schiff base ligands: A short review. Polyhedron 2022, 228, 116153. [Google Scholar] [CrossRef]
- Sebastian, N.; Emanuel, R.; Inke, S. Towards the continuous production of Pt-based heterogeneous catalysts using microfluidic systems. Dalton Trans. 2018, 47, 1693–1702. [Google Scholar]
- Xie, Q.; Fu, Q.; Cui, H.B.; Luo, B.H.; Liu, W.Q.; Qiao, R.; Ren, Y.J.; Chen, F.; Zhang, H.X.; Long, J.Q. Influence of the flexible tetrapyridines on electrocatalytic water oxidation by cobalt complexes. Polyhedron 2020, 189, 114731. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, Z.; Xiang, D.; Li, P.; Du, C.; Zhuang, Z.H.; Li, X.K.; Chen, W. Fe/Ni Bimetal Organic Framework as Efficient Oxygen Evolution catalyst with Low Overpotential. J. Colloid Interf. Sci. 2019, 555, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, C.; Duo, X.X.; Liu, Z.X.; Liu, C.Y.; Guo, L.Y.; Xie, A.J.; Luo, S.P. Self-assembled acicular CuCo-MOF for enhancing oxygen evolution reaction. Ionics 2020, 26, 5123–5132. [Google Scholar] [CrossRef]
- Abdullahi, I.M.; Masud, J.; Ioannou, P.C.; Ferentinos, E.; Kyritsis, P.; Nath, M. A Molecular Tetrahedral Cobalt–Seleno-Based Complex as an Efficient Electrocatalyst for Water Splitting. Molecules 2021, 26, 945. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.N.; Toma, S.H.; Hennemann, A.L.; Gonçalves, J.M.; Nakamura, M.; Araki, K.; Toyama, M.M.; Toma, H.E. A New Supramolecular Tetraruthenated Cobalt (II) Porphyrazine Displaying Outstanding Electrocatalytical Performance in Oxygen Evolution Reaction. Molecules 2022, 27, 4598. [Google Scholar] [CrossRef]
- Blatov, V.A.; O’Keeffe, M.; Proserpio, D.M. Vertex-, Face-, Point-, Schläfli-, and Delaney-Symbols in Nets, Polyhedra and Tilings: Recommended Terminology. CrystEngComm 2010, 12, 44–48. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Lam, F.; Jia, X.X.; Chan, K.S. Binucleating Ligands: Synthesis of Acyclic Achiral and Chiral Schiff Base-Pyridine and Schiff Base-Phosphine Ligands. J. Org. Chem. 1998, 28, 8414–8418. [Google Scholar]
- Hu, Q.Q.; Su, X.J.; Zhang, M.T. Electrocatalytic Water Oxidation by an Unsymmetrical Di-Copper Complex. Inorg. Chem. 2018, 57, 10481–10484. [Google Scholar] [CrossRef]
- Makhado, T.; Das, B.; Kriek, R.J.; Vosloo, H.C.M.; Swarts, A.J. Chemical and electrochemical water oxidation mediated by bis(pyrazol-1-ylmethyl)pyridine-ligated Cu(I) complexes. Sustain. Energ. Fuels 2021, 5, 2771–2780. [Google Scholar] [CrossRef]
- Liu, C.; Geer, A.M.; Webber, C.; Musgrave, C.B., III; Gu, S.; Johnson, G.; Dickie, D.A.; Chabbra, S.; Schnegg, A.; Zhou, H.; et al. Immobilization of “Capping Arene” Cobalt(II) Complexes on Ordered Mesoporous Carbon for Electrocatalytic Water Oxidation. ACS Catal. 2021, 11, 15068–15082. [Google Scholar] [CrossRef]
- Li, P.; Liu, J.B.; Han, S.; Deng, W.; Yao, Z.J. Half-sandwich Ir (III) and Rh (III) complexes as catalysts for water oxidation with double-site. Appl. Organomet. Chem. 2019, 33. [Google Scholar]
- Li, X.H.; Panetier, J.A. Mechanistic Study of Tungsten Bipyridyl Tetracarbonyl Electrocatalysts for CO2 Fixation: Exploring the Roles of Explicit Proton Sources and Substituent Effects. Top. Catal. 2022, 65, 325–340. [Google Scholar] [CrossRef]
- Wei, S.T.; Zou, H.Y.; Rong, W.F.; Zhang, F.X.; Ji, Y.F.; Duan, L.L. Conjugated nickel phthalocyanine polymer selectively catalyzes CO2-to-CO conversion in a wide operating potential window. Appl. Catal. B 2021, 284, 119739. [Google Scholar] [CrossRef]
- Chi, S.Y.; Chen, Q.; Zhao, S.S.; Si, D.H.; Wu, Q.J.; Huang, Y.B.; Cao, R. Three-dimensional porphyrinic covalent organic frameworks for highly efficient electroreduction of carbon dioxide. J. Mater. Chem. A 2022, 10, 4653–4659. [Google Scholar] [CrossRef]
- Hooe, S.L.; Dressel, J.M.; Dickie, D.A.; Machan, C.W. Highly Efficient Electrocatalytic Reduction of CO2 to CO by a Molecular Chromium Complex. ACS Catal. 2020, 10, 1146–1151. [Google Scholar] [CrossRef]
- Ahsan, H.M.; Breedlove, B.K.; Piangrawee, S.; Mian, M.R.; Fetoh, A.; Cosquer, G.; Yamashita, M. Enhancement of electrocatalytic abilities for reducing carbon dioxide: Functionalization with a redox-active ligand-coordinated metal complex. Dalton Trans. 2018, 47, 11313–11316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).