Electro-Oxidative C3-Selenylation of Pyrido[1,2-a]pyrimidin-4-ones
Abstract
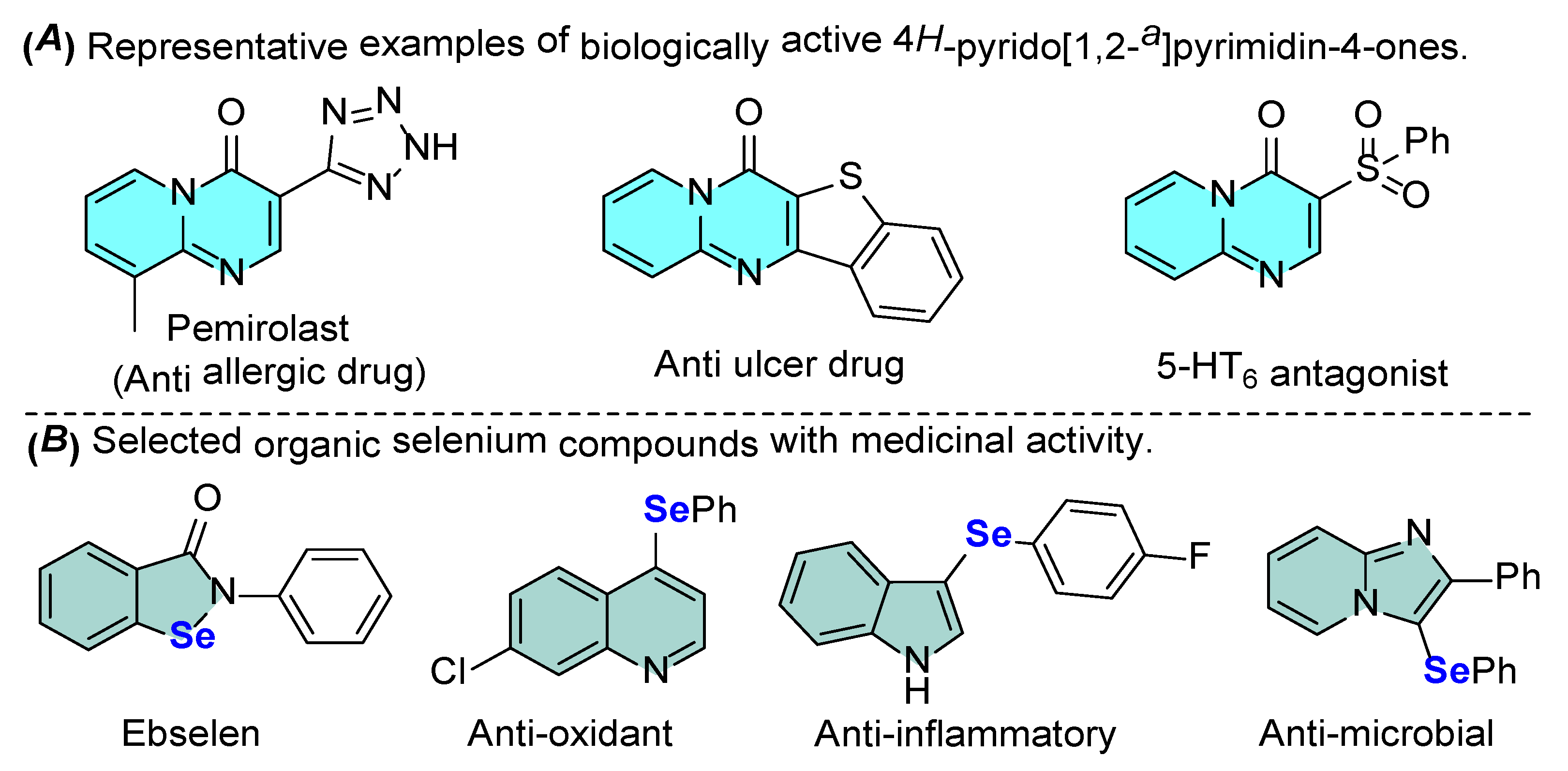
1. Introduction
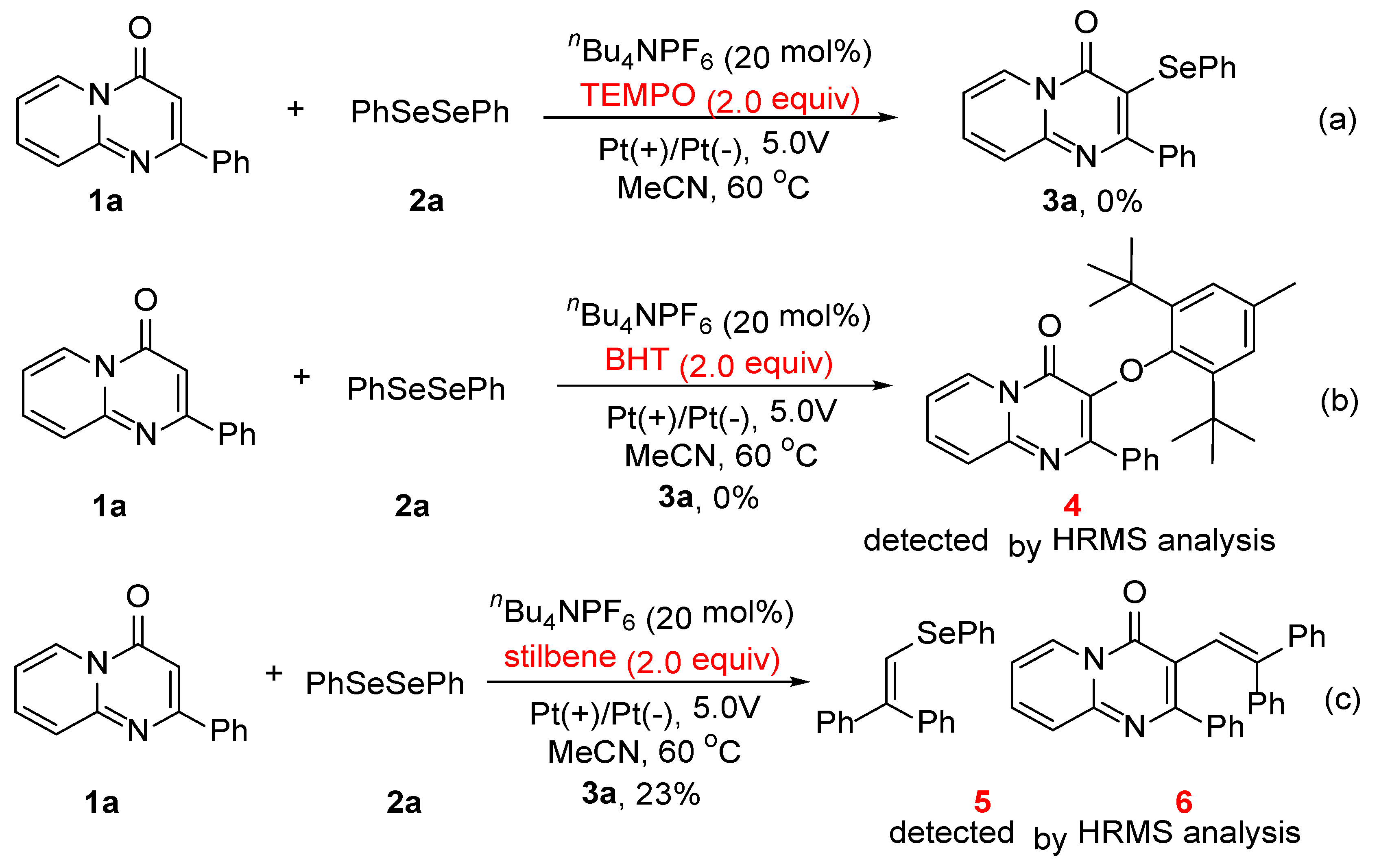
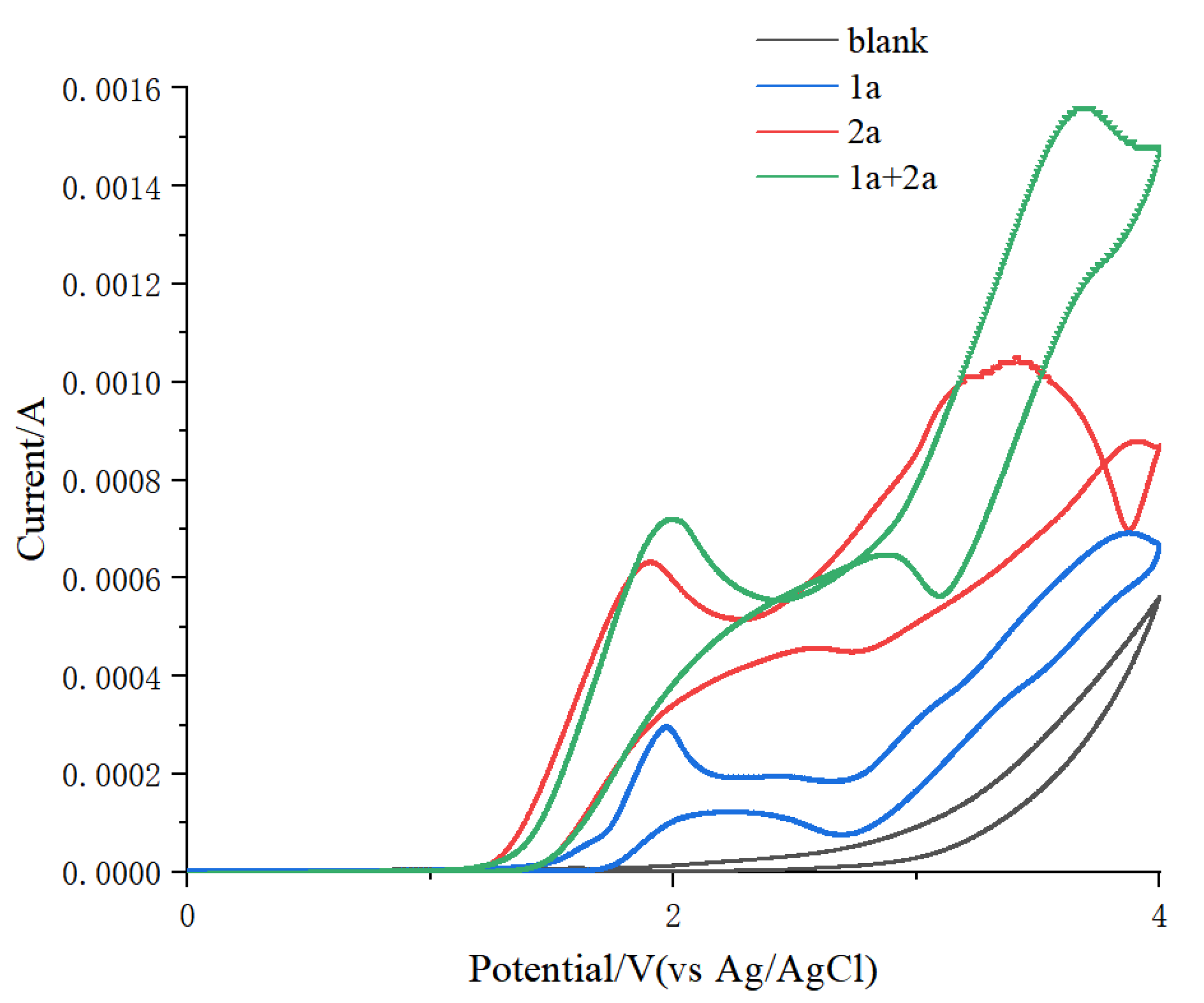
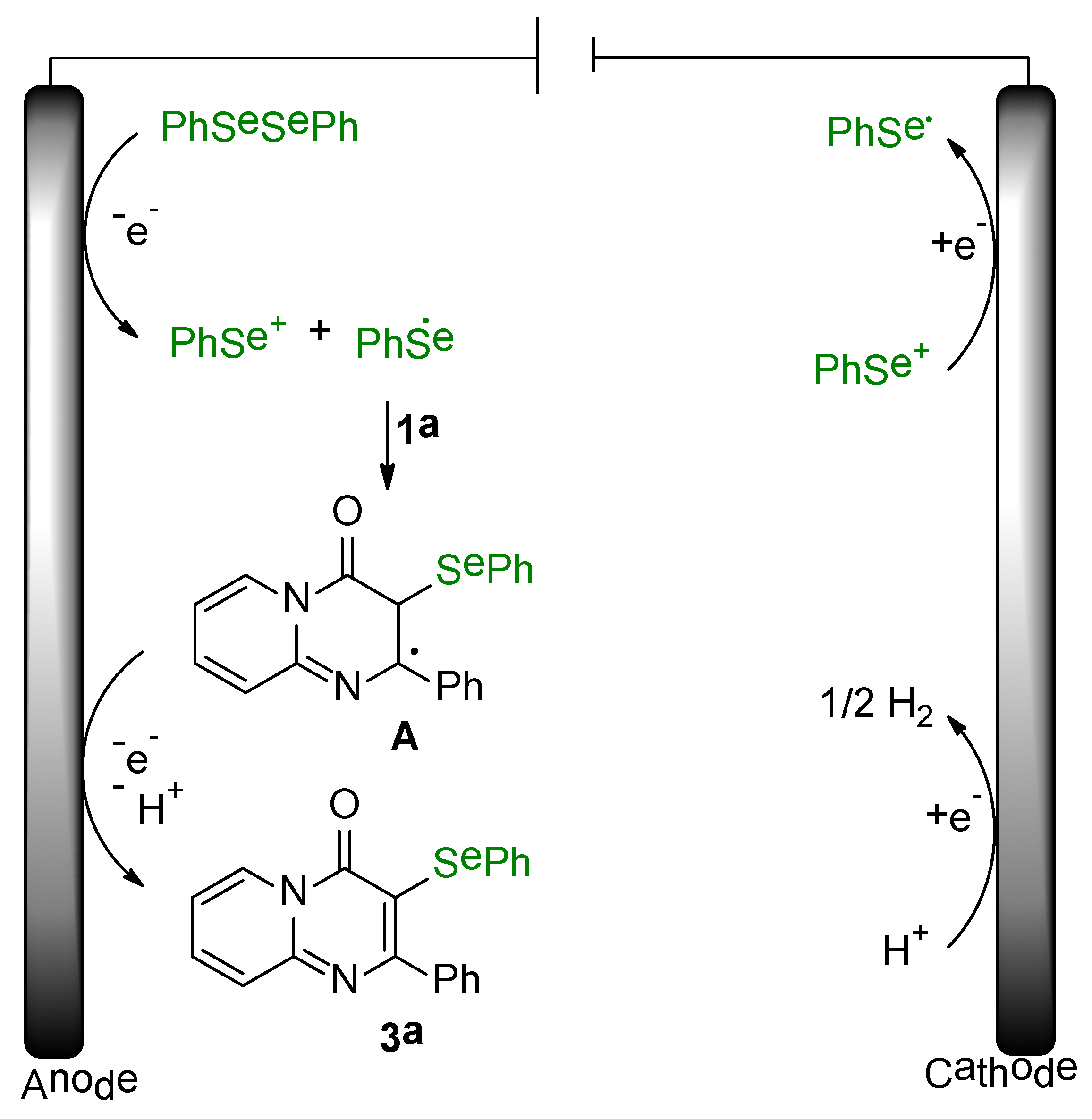
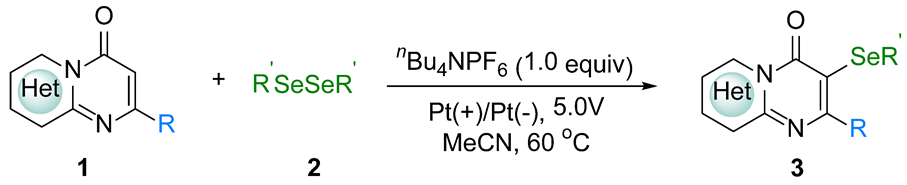
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Instruments
3.2. General Procedure for the Synthesis of 1
3.3. The General Procedure for the Synthesis of 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Henkel, T.; Brunne, R.M.; Müller, H.; Reichel, F. Statistical Investigation into the Structural Complementarity of Natural Products and Synthetic Compounds. Angew. Chem. Int. Ed. 1999, 38, 643–647. [Google Scholar] [CrossRef]
- Hili, R.; Yudin, A.K. Making Carbon-Nitrogen Bonds in Biological and Chemical Synthesis. Nat. Chem. Biol. 2006, 2, 284–287. [Google Scholar] [CrossRef]
- McGrath, N.A.; Brichacek, M.; Njardarson, J.T. A Graphical Journey of Innovative Organic Architectures that Have Improved Our Lives. J. Chem. Educ. 2010, 87, 1348–1349. [Google Scholar] [CrossRef]
- Roma, G.; Cinone, N.; Di Braccio, M.; Grossi, G.; Leoncini, G.; Signorello, M.G.; Carotti, A. Synthesis, Antiplatelet Activity and Comparative Molecular Field Analysis of Substituted 2-Amino-4H-pyrido[1,2-a]pyrimidin-4-ones, Their Congeners and Isosteric Analogues. Bioorg. Med. Chem. 2000, 8, 751–768. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Kuru, N.; Ohtsuka, M.; Yokomizo, Y.; Sakamoto, A.; Kawato, H.; Yoshida, K.; Ohta, T.; Hoshino, K.; Akimoto, K.; et al. MexAB-OprM Specific Efflux Pump Inhibitors in Pseudomonas Aeruginosa. Part 4: Addressing the Problem of Poor Stability Due to Photoisomerization of an Acrylic Acid Moiety. Bioorg. Med. Chem. Lett. 2004, 14, 2493–2497. [Google Scholar] [CrossRef]
- Nakayama, K.; Kawato, H.; Watanabe, J.; Ohtsuka, M.; Yoshida, K.; Yokomizo, Y.; Sakamoto, A.; Kuru, N.; Ohta, T.; Hoshino, K.; et al. MexAB-OprM Specific Efflux Pump Inhibitors in Pseudomonas Aeruginosa. Part 3: Optimization of Potency in the Pyridopyrimidine Series through the Application of a Pharmacophore Model. Bioorg. Med. Chem. Lett. 2004, 14, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Ishida, Y.; Ohtsuka, M.; Kawato, H.; Yoshida, K.I.; Yokomiza, Y.; Ohta, T.; Hoshino, K.; Otani, T.; Kurosaka, Y.; et al. MexAB-OprM Specific Efflux Pump Inhibitors in Pseudomonas Aeruginosa. Part 2: Achieving Activity in Vivo through the Use of Alternative Scaffolds. Bioorg. Med. Chem. Lett. 2003, 13, 4205–4208. [Google Scholar] [CrossRef]
- Fenton, C.; Scott, L.J. Risperidone: A Review of its Use in the Treatment of Bipolar Mania. CNS Drugs 2005, 19, 429–444. [Google Scholar] [CrossRef]
- Sadashiva, C.T.; Chandra, J.N.N.S.; Ponnappa, K.C.; Gowda, T.V.; Rangappa, K.S. Synthesis and Efficacy of 1-[Bis(4-fluorophenyl)-methyl]piperazine Derivatives for Acetylcholinesterase Inhibition, as a Stimulant of Central Cholinergic Neurotransmission in Alzheimer’s Disease. Bioorg. Med. Chem. Lett. 2006, 16, 3932–3936. [Google Scholar] [CrossRef]
- La Motta, C.; Sartini, S.; Mugnaini, L.; Simorini, F.; Taliani, S.; Salerno, S.; Marini, A.M.; Settimo, F.D.; Lavecchia, A.; Novellino, E.; et al. Pyrido[1,2-a]pyrimidin-4-one Derivatives as a Novel Class of Selective Aldose Reductase Inhibitors Exhibiting Antioxidant Activity. J. Med. Chem. 2007, 50, 4917–4927. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Zhang, Q.; Yu, J.; Li, J.; Xie, Z.; Cao, H. Regioselective C3 Alkenylation of 4-H-pyrido[1,2-a]pyrimidin-4-ones via Palladium-Catalyzed C-H Activation. Chem.–Asian J. 2014, 9, 2436–2439. [Google Scholar] [CrossRef] [PubMed]
- Guchhait, S.K.; Priyadarshani, G. Pd-Catalyzed Ag(I)-Promoted C3-Arylation of Pyrido[1,2-a]pyrimidin-4-ones with Bromo/Iodo-Arenes. J. Org. Chem. 2015, 80, 8482–8488. [Google Scholar] [CrossRef]
- Chen, Z.-W.; Wen, Y.-L.; Ding, H.; Luo, G.-T.; Ye, M.; Liu, L.-X.; Xue, J. Silver-Catalyzed Highly Efficient Synthesis of Pyrido[1,2-a]pyrimidin-4-ones from 2-Aminopyridines and Alkynoates. Tetrahedron Lett. 2017, 58, 13–16. [Google Scholar] [CrossRef]
- Hoang, G.L.; Zoll, A.J.; Ellman, J.A. Three-Component Coupling of Aldehydes, 2-Aminopyridines, and Diazo Esters via Rhodium(III)-Catalyzed Imidoyl C-H Activation: Synthesis of Pyrido[1,2-a]pyrimidin-4-ones. Org. Lett. 2019, 21, 3886–3890. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-J.; Liu, Y.-Y.; Wen, C.-P.; Wan, J.-P. Electrochemical Enaminone C-H Thiolation/C-N Amination Cascade for Thiazole Synthesis and its Diastereoselective Dearomatization. Green Chem. 2022, 24, 5058–5063. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, Y.-Y.; Wan, J.-P. Metal-free C(sp2)-H Perfluoroalkylsulfonylation and Configuration Inversion: Stereoselective Synthesis of α-Perfluoroalkylsulfonyl E-Enaminones. Chin. Chem. Lett. 2021, 32, 3514–3517. [Google Scholar] [CrossRef]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of Biologically Important Synthetic Organoselenium Compounds. Chem. Rev. 2001, 101, 2125–2179. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6285. [Google Scholar] [CrossRef]
- Xiao, Q.; Tong, Q.-X.; Zhong, J.-J. Recent Advances in Visible-Light Photoredox Catalysis for the Thiol-Ene/Yne Reactions. Molecules 2022, 27, 619. [Google Scholar] [CrossRef]
- Liang, R.-B.; Zhu, C.-M.; Song, P.-Q.; Zhao, L.-M.; Tong, Q.-X.; Zhong, J.-J. External Oxidant-free and Selective Thiofunctionalization of Alkenes Enabled by Photoredox-Neutral Catalysis. Org. Chem. Front. 2022, 9, 4536–4541. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, H.; Li, J.-H.; Jian, J.-X.; Tong, Q.-X.; Zhong, J.-J. Directing-Group-Assisted Markovnikov-Selective Hydrothiolation of Styrenes with Thiols by Photoredox/Cobalt Catalysis. Org. Lett. 2021, 23, 3604–3609. [Google Scholar] [CrossRef]
- Sun, K.; Wang, X.; Lv, Y.; Li, G.; Jiao, H.; Dai, C.; Li, Y.; Zhang, C.; Liu, L. Peroxodisulfate-Involved Selenoamination of Alkenes Yielding Amidoselenide-Containing Sulfamides and Azoles. Chem. Commun. 2016, 52, 8471–8474. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Wang, X.; Fu, F.; Zhang, C.; Chen, Y.; Liu, L. Metal-Free Selenosulfonylation of Alkynes: Rapid Access to β-(seleno)vinyl Sulfones via a Cationic-Species-Induced Pathway. Green Chem. 2017, 19, 1490–1493. [Google Scholar] [CrossRef]
- Sun, K.; Shi, Z.; Liu, Z.; Luan, B.; Zhu, J.; Xue, Y. Synthesis of (E)-beta-Selenovinyl Sulfones through a Multicomponent Regio- and Stereospecific Selenosulfonation of Alkynes with Insertion of Sulfur Dioxide. Org. Lett. 2018, 20, 6687–6690. [Google Scholar] [CrossRef]
- Sun, K.; Wang, S.-N.; Feng, R.-R.; Zhang, Y.-X.; Wang, X.; Zhang, Z.-G.; Zhang, B. Copper-Catalyzed Radical Selenodifluoromethylation of Alkenes: Access to CF2-Containing γ-Lactams. Org. Lett. 2019, 21, 2052–2055. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lei, J.; Guo, S.; Zhang, Y.; Ye, Y.; Tang, S.; Sun, K. Radical Selenation of C(sp(3))-H Bonds to Asymmetric Selenides and Mechanistic Study. Chem. Commun. 2022, 58, 1526–1529. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.-L.; Tan, P.-P.; Gu, X.-W.; Sun, W.-J.; Liu, C.; Chen, J.-C.; Li, J.-Z.; Sun, K. K2S2O8/I2-Promoted Electrophilic Selenylative Cyclization to Access Seleno-Benzo[b]azepines. Org. Lett. 2022, 24, 2288–2293. [Google Scholar] [CrossRef]
- Wang, X.; Meng, J.-P.; Zhao, D.-Y.; Tang, S.; Sun, K. Synthesis and Applications of Thiosulfonates and Selenosulfonates as Free-Radical Reagents. Chin. Chem. Lett. 2023, 34, 107736. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, G.K.; Saleem, F.; Singh, A.K. Organoselenium Ligands in Catalysis. Dalton Trans. 2012, 41, 11949–11977. [Google Scholar] [CrossRef]
- Ortgies, S.; Breder, A. Oxidative Alkene Functionalizations via Selenium-π-Acid Catalysis. ACS Catal. 2017, 7, 5828–5840. [Google Scholar] [CrossRef]
- Wang, X.-W.; Miao, Z.-H.; Ma, Y.; Chen, H.-J.; Qian, H.-S.; Zha, Z.-B. One-pot Solution Synthesis of Shape-Controlled Copper Selenide Nanostructures and Their Potential Applications in Photocatalysis and Photothermal Therapy. Nanoscale 2017, 9, 14512–14519. [Google Scholar] [CrossRef] [PubMed]
- Singh, F.V.; Wirth, T. Selenium Reagents as Catalysts. Catal. Sci. Technol. 2019, 9, 1073–1091. [Google Scholar] [CrossRef]
- Shao, L.-X.; Li, Y.; Lu, J.; Jiang, X. Recent Progress in Selenium-Catalyzed Organic Reactions. Org. Chem. Front. 2019, 6, 2999–3041. [Google Scholar] [CrossRef]
- Arora, A.; Oswal, P.; Kumar Rao, G.; Kumar, S.; Kumar, A. Organoselenium Ligands for Heterogeneous and Nanocatalytic Systems: Development and Applications. Dalton Trans. 2021, 50, 8628–8656. [Google Scholar] [CrossRef]
- Hua, J.-W.; Fang, Z.; Xu, J.; Bian, M.-X.; Liu, C.-K.; He, W.; Zhu, N.; Yang, Z.; Guo, K. Electrochemical Oxidative Cyclization of Activated Alkynes with Diselenides or Disulfides: Access to Functionalized Coumarins or Quinolinones. Green Chem. 2019, 21, 4706–4711. [Google Scholar] [CrossRef]
- Guan, Z.-P.; Wang, Y.-K.; Wang, H.-M.; Huang, Y.-G.; Wang, S.-Y.; Tang, H.-D.; Zhang, H.; Lei, A.-W. Electrochemical Oxidative Cyclization of Olefinic Carbonyls with Diselenides. Green Chem. 2019, 21, 4976–4980. [Google Scholar] [CrossRef]
- Sun, K.; Wang, X.; Li, C.; Wang, H.; Li, L. Recent Advances in Tandem Selenocyclization and Tellurocyclization with Alkenes and Alkynes. Org. Chem. Front. 2020, 7, 3100–3119. [Google Scholar] [CrossRef]
- Meng, X.-J.; Zhong, P.-F.; Wang, Y.-M.; Wang, H.-S.; Tang, H.-T.; Pan, Y.-M. Electrochemical Difunctionalization of Olefines: Access to Selenomethyl-Substituted Cyclic Ethers or Lactones. Adv. Synth. Catal. 2020, 362, 506–511. [Google Scholar] [CrossRef]
- Mallick, S.; Baidya, M.; Mahanty, K.; Maiti, D.; Sarkara, S.D. Electrochemical Chalcogenation of β,γ-Unsaturated Amides and Oximes to Corresponding Oxazolines and Isoxazolines. Adv. Synth. Catal. 2020, 362, 1046–1052. [Google Scholar] [CrossRef]
- Pan, C.; Liu, P.; Wu, A.-G.; Li, M.; Wen, L.-R.; Guo, W.-S. Electrochemical-Promoted Synthesis of 2-Thiazolines via Selenylation/Cyclization of N-Allylthioamides. Chin. J. Org. Chem. 2020, 40, 2855–2862. [Google Scholar] [CrossRef]
- Kharma, A.; Jacob, C.; Bozzi, A.O.; Jardim, G.M.; Braga, A.L.; Salomão, K.; Gatto, C.C.; Silva, M.F.; Pessoa, C.; Stangier, M.; et al. Electrochemical Selenation/Cyclization of Quinones: A Rapid, Green and Efficient Access to Functionalized Trypanocidal and Antitumor Compounds. Eur. J. Org. Chem. 2020, 2020, 4474–4486. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Sun, K.; Meng, J.-P.; Zhang, B. Study on the Application of Photoelectric Technology in the Synthesis of Selenium-Containing Heterocycles. Chin. J. Org. Chem. 2021, 41, 4588–4609. [Google Scholar] [CrossRef]
- Yu, K.; Kong, X.-Q.; Yang, J.-J.; Li, G.-D.; Xu, B.; Chen, Q.-J. Electrochemical Oxidative Halogenation of N-Aryl Alkynamides for the Synthesis of Spiro[4.5]trienones. J. Org. Chem. 2021, 86, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Y.; Tang, Y.-H.; Ouyang, W.-T.; Lu, Y.-H.; Chen, J.-Y.; He, W.-M. Electrochemical Selenylation of N-Unprotected Anilines for Consturcing 4-(Organylselanyl)anilines. Chin. J. Org. Chem. 2021, 41, 4766–4772. [Google Scholar] [CrossRef]
- Ghosh, P.; Chhetri, G.; Das, S. Metal Free C-3 Chalcogenation (Sulfenylation and Selenylation) of 4H-pyrido[1,2-a]pyrimidin-4-ones. RSC Adv. 2021, 11, 10258–10263. [Google Scholar] [CrossRef]
- Ghosh, P.; Chhetri, G.; Perl, E.; Das, S. [Bis(trifluoroacetoxy)iodo]benzene Mediated C-3 Selenylation of Pyrido[1,2-a]pyrimidin-4-ones Under Ambient Conditions. Adv. Synth. Catal. 2021, 363, 2148–2156. [Google Scholar] [CrossRef]
- Kingston, C.; Palkowitz, M.D.; Takahira, Y.; Vantourout, J.C.; Peters, B.K.; Kawamata, Y.; Baran, P.S. A Survival Guide for the “Electro-curious”. Acc. Chem. Res. 2020, 53, 72–83. [Google Scholar] [CrossRef]
- Liu, J.-J.; Lu, L.-X.; Wood, D.; Lin, S. New Redox Strategies in Organic Synthesis by Means of Electrochemistry and Photochemistry. ACS Cent. Sci. 2020, 6, 1317–1340. [Google Scholar] [CrossRef]
- Sun, K.; Lei, J.; Liu, Y.-J.; Liu, B.; Chen, N. Electrochemically Enabled Intramolecular and Intermolecular Annulations of Alkynes. Adv. Synth. Catal. 2020, 362, 3709–3726. [Google Scholar] [CrossRef]
- Heard, D.M.; Lennox, A.J.J. Electrode Materials in Modern Organic Electrochemistry. Angew. Chem. Int. Ed. 2020, 59, 18866–18884. [Google Scholar] [CrossRef]
- Pollok, D.; Waldvogel, S.R. Electro-organic Synthesis—A 21st Century Technique. Chem. Sci. 2020, 11, 12386–12400. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-J.; Ang, N.W.J.; Meyer, T.H.; Qiu, Y.-A.; Ackermann, L. Organic Electrochemistry: Molecular Syntheses with Potential. ACS Cent. Sci. 2021, 7, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Novaes, L.F.T.; Liu, J.-J.; Shen, Y.-F.; Lu, L.-X.; Meinhardt, J.M.; Lin, S. Electrocatalysis as an Enabling Technology for Organic Synthesis. Chem. Soc. Rev. 2021, 50, 7941–8002. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-Z.; Liu, Y.; Liu, H.; Ma, J.-J.; He, X.; Wu, H.-F.; Li, Y.-Q.; Sun, Z.-Z.; Chu, W.-Y. Metal-Free Electrochemical Oxidative Trifluoromethylation/C(sp2)H Functionalization of Quinolinones. Tetrahedron Lett. 2020, 61, 152226. [Google Scholar] [CrossRef]
- Xu, P.; Chen, P.-Y.; Xu, H.-C. Scalable Photoelectrochemical Dehydrogenative Cross-Coupling of Heteroarenes with Aliphatic C−H Bonds. Angew. Chem. Int. Ed. 2020, 59, 14275–14280. [Google Scholar] [CrossRef]
- Hou, Z.-W.; Xu, H.-C. Electrophotocatalytic C-H Azolation of Arenes. ChemElectroChem 2021, 8, 1571–1573. [Google Scholar] [CrossRef]
- Wu, Z.-L.; Chen, J.-Y.; Tian, X.-Z.; Ouyang, W.-T.; Zhang, Z.-T.; He, W.-M. Electrochemical Regioselective Synthesis of N-substituted/unsubstituted 4-Selanylisoquinolin-1(2H)-ones. Chin. Chem. Lett. 2022, 33, 1501–1504. [Google Scholar] [CrossRef]
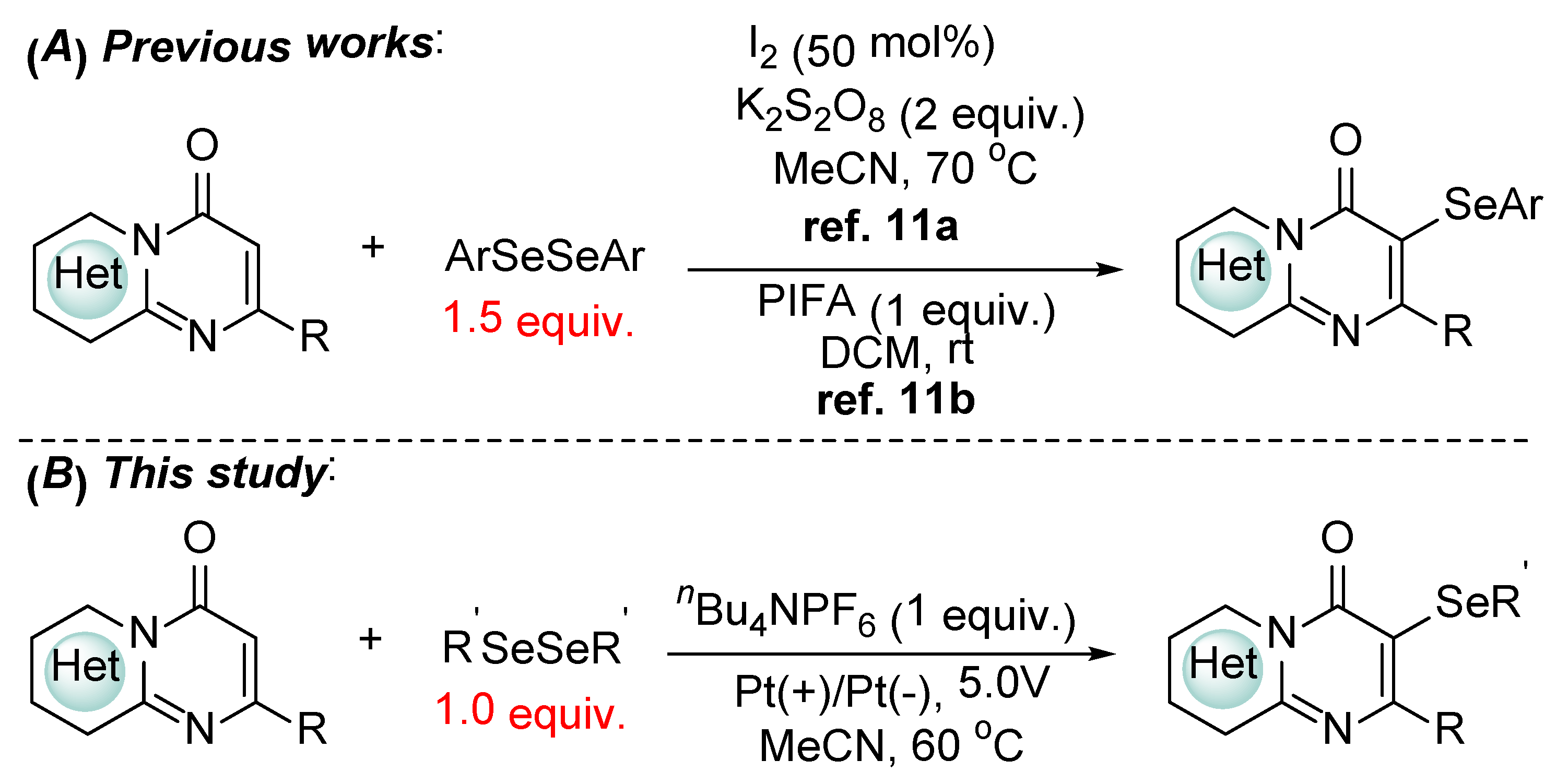

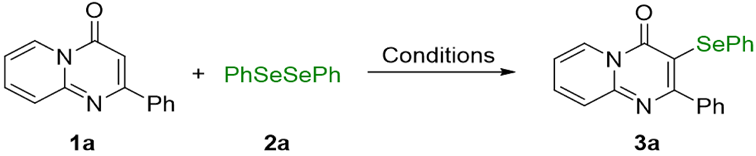 | |||||
| Entry | Electrolyte | Solvent (mL) | Electrode | Time (h) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | nBu4NBF4 | MeCN | Pt(+)/Pt(−) | 3 | 42 |
| 2 | nBu4NI | MeCN | Pt(+)/Pt(−) | 3 | 0 |
| 3 | nBu4NPF6 | MeCN | Pt(+)/Pt(−) | 3 | 66 |
| 4 | nBu4NClO4 | MeCN | Pt(+)/Pt(−) | 3 | 19 |
| 5 | nBu4NPF6 | DMF | Pt(+)/Pt(−) | 3 | 0 |
| 6 | nBu4NPF6 | DMSO | Pt(+)/Pt(−) | 3 | 0 |
| 7 | nBu4NPF6 | MeOH | Pt(+)/Pt(−) | 3 | 0 |
| 8 | nBu4NPF6 | HFIP | Pt(+)/Pt(−) | 3 | 39 |
| 9 | nBu4NPF6 | MeCN | C(+)/C(−) | 3 | 16 |
| 10 | nBu4NPF6 | MeCN | C(+)/Pt(−) | 3 | 0 |
| 11 | nBu4NPF6 | MeCN | Pt(+)/Pt(−) | 3 | Trace c |
| 12 | nBu4NPF6 | MeCN | Pt(+)/Pt(−) | 3 | 0 d |
| 13 | nBu4NPF6 | MeCN | Pt(+)/Pt(−) | 5 | 94 |
| 14 | nBu4NPF6 | MeCN | Pt(+)/Pt(−) | 5 | 0 e |
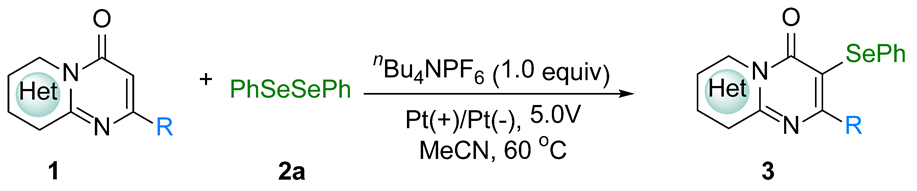 | |||||
| entry | substrate | product | entry | substrate | product |
| 1 | 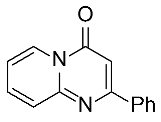 1a | 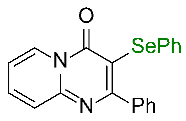 3a, 94%c | 2 | 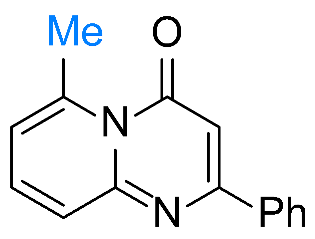 1b | 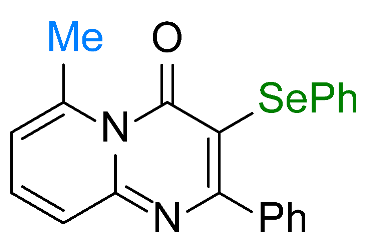 3b, 96% |
| 3 |  1c | 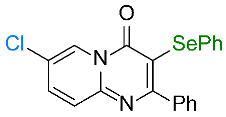 3c, 70% | 4 | 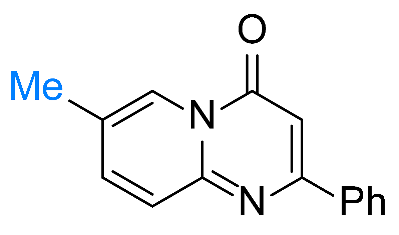 1d | 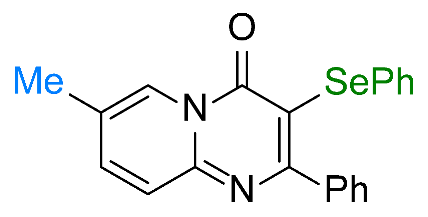 3d, 95% |
| 5 | 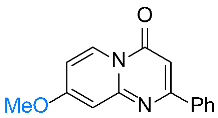 1e | 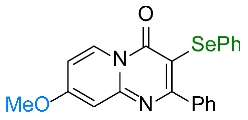 3e, 67% 3e, 67% | 6 | 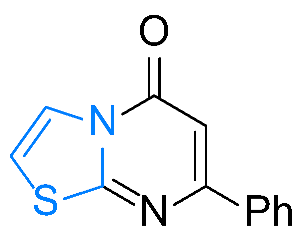 1f | 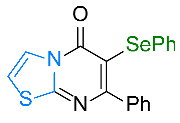 3f, 82% |
| 7 |  1g 1g | 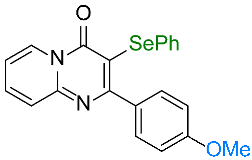 3g, 67% 3g, 67% | 8 | 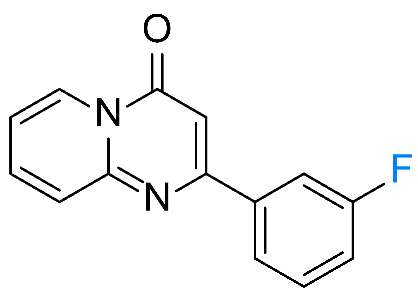 1h 1h | 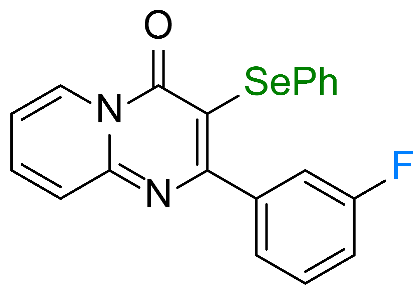 3h, 96 % 3h, 96 % |
| 9 |  1i | 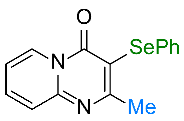 3i, 82% | 10 | 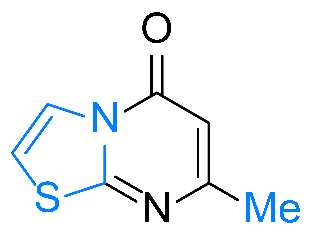 1j |  3j, 77% |
 | |||||
| entry | substrate | product | entry | substrate | product |
| 1 | 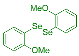 2b | 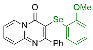 3k, 87% | 2 | 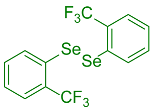 2c | 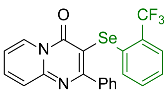 3l, 65% |
| 3 | 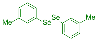 2d | 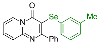 3m, 60% | 4 | 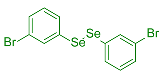 2e | 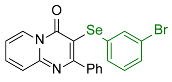 3n, 90% |
| 5 |  2f | 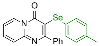 3o, 94% | 6 | 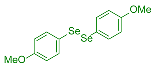 2g | 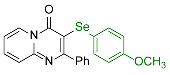 3p, 68% |
| 7 | 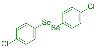 2h | 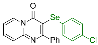 3q, 97% 3q, 97% | 8 |  2i | 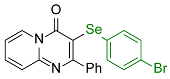 3r, 77% |
| 9 |  2j | 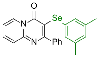 3s, 95% | 10 |  2k |  3t, 40% |
| 11 |  2l |  3u, 48% | 12 | 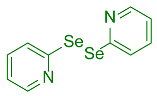 2m | 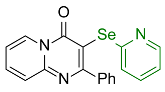 3v, 97% |
| 13 |  2n | 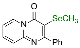 3w, 84% | 14 | 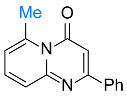 1b | 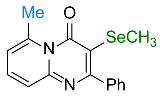 3x, 77% |
| 15 | 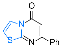 1f | 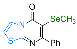 3y, 73% | 16 | 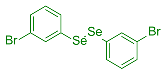 2e | 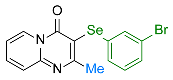 3z, 67% |
| 17 |  2o | 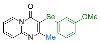 3aa, 95% | 18 |  2f | 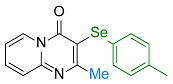 3ab, 94% |
| 19 | 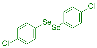 2h | 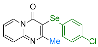 3ac, 73% | 20 | 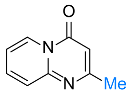 1i | 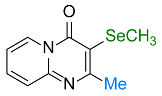 3ad, 87% |
| 21 |  1j | 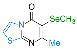 3ae, 85% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Wang, Z.; Teng, X.; Zhang, B.; Sun, K.; Wang, X. Electro-Oxidative C3-Selenylation of Pyrido[1,2-a]pyrimidin-4-ones. Molecules 2023, 28, 2206. https://doi.org/10.3390/molecules28052206
Shi J, Wang Z, Teng X, Zhang B, Sun K, Wang X. Electro-Oxidative C3-Selenylation of Pyrido[1,2-a]pyrimidin-4-ones. Molecules. 2023; 28(5):2206. https://doi.org/10.3390/molecules28052206
Chicago/Turabian StyleShi, Jianwei, Zhichuan Wang, Xiaoxu Teng, Bing Zhang, Kai Sun, and Xin Wang. 2023. "Electro-Oxidative C3-Selenylation of Pyrido[1,2-a]pyrimidin-4-ones" Molecules 28, no. 5: 2206. https://doi.org/10.3390/molecules28052206
APA StyleShi, J., Wang, Z., Teng, X., Zhang, B., Sun, K., & Wang, X. (2023). Electro-Oxidative C3-Selenylation of Pyrido[1,2-a]pyrimidin-4-ones. Molecules, 28(5), 2206. https://doi.org/10.3390/molecules28052206






