Analysis of Furan and Its Derivatives in Food Matrices Using Solid Phase Extraction Coupled with Gas Chromatography-Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of Simultaneous Separation Using GC-MS/MS
2.1.1. Evaluation of the Column
2.1.2. Ion Selection of MS/MS
2.2. Development of the SPME Arrow Method for Furan Extraction
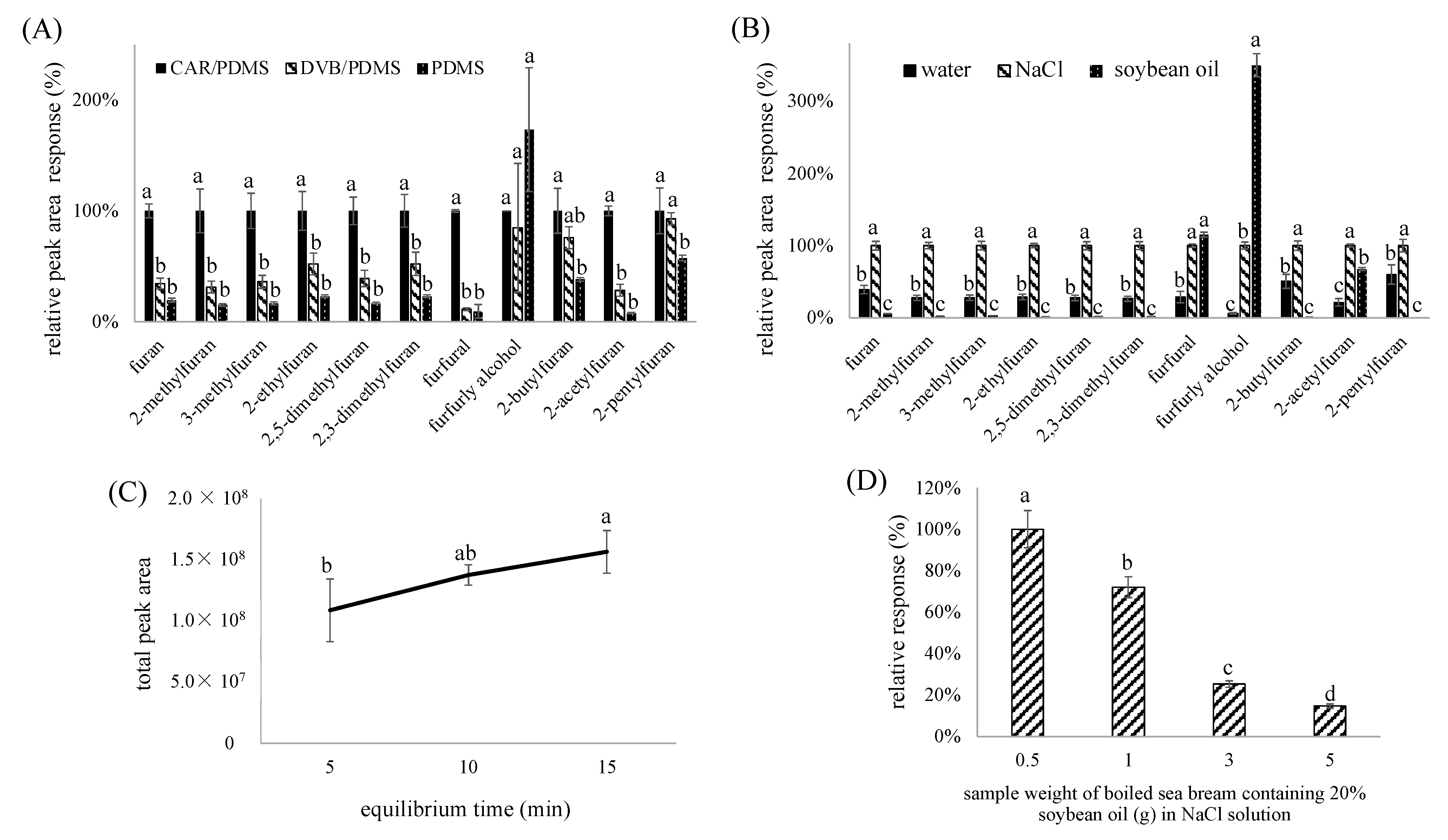
2.2.1. Evaluation of SPME Arrow Material
2.2.2. Evaluation of Extraction Efficiency by Diluted Solutions
2.2.3. Evaluation of Equilibrium Time
2.2.4. Evaluation of Ratio of Canned Oily Fish Sample to Saturated NaCl Solution
2.2.5. The Optimized Method for Analysis of Furan and Its Derivatives
2.3. Method Validation
2.3.1. Recovery
2.3.2. Precision
2.3.3. Limit of Detection (LOD) and Limit of Quantitation (LOQ)
3. Materials and Methods
3.1. Materials
3.2. Chemicals and Reagents
3.3. Instrumentation
3.4. Evaluation of Simultaneous Separation of Furan and Its 10 Derivatives by Using GC-MS/MS
3.5. Development of the SPME Arrow Method of Furan Extraction
3.5.1. Evaluation of the SPME Arrow Material, Diluted Solution and Equilibrium Time
3.5.2. Evaluation of Canned Oily Fish Sample to Saturated NaCl Solution Ratio
3.6. Method Validation
3.6.1. Recovery
3.6.2. Precision
3.6.3. Limit of Detection (LOD) and Limit of Quantitation (LOQ)
3.7. Quantitation of Furan and Its Derivatives
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IARC. Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals; IARC: Lyon, France, 1995; pp. 393–407. [Google Scholar]
- Gill, S.S.; Kavanagh, M.; Cherry, W.; Barker, M.; Weld, M.; Cooke, G.M. A 28-day gavage toxicity study in male Fischer 344 rats with 2-methylfuran. Toxicol. Pathol. 2014, 42, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Kavanagh, M.; Cherry, W.; Bourque, C.; Caldwell, D.; Wang, G.; Bondy, G. A 90-day subchronic gavage toxicity study in Fischer 344 rats with 3-methylfuran. Food Chem. Toxicol. 2018, 111, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Sujatha, P.S. Monitoring cytotoxic potentials of furfuryl alcohol and 2-furyl methyl ketone in mice. Food Chem. Toxicol. 2008, 46, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Seok, Y.J.; Kim, Y.G.; Jeong, S.Y.; Lee, J.Y.; Yoon, H.J. Furan in thermally processed foods—a review. Toxicol Res. 2015, 31, 241—53. [Google Scholar] [CrossRef] [PubMed]
- Sayre, L.M.; Arora, P.K.; Iyer, R.S.; Salomon, R.G. Pyrrole formation from 4-hydroxynonenal and primary amines. Chem. Res. Toxicol. 1993, 6, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Perez Locas, C.; Yaylayan, V.A. Origin and mechanistic pathways of formation of the parent furan—A food toxicant. J. Agric. Food Chem. 2004, 52, 6830–6836. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.T. Impact of ionizing radiation and thermal treatments on furan levels in fruit juice. J. Food Sci. 2005, 70E, 409–414. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Exploratory Data on Furan in Food: Individual Food Products. 2009. Available online: https://www.fda.gov/food/chemical-contaminants-food/furan (accessed on 1 July 2022).
- Altaki, M.S.; Santos, F.J.; Galceran, M.T. Analysis of furan in foods by headspace solid-phase microextraction-gas chromatography-ion trap mass spectrometry. J. Chromatogr. A 2007, 1146, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Liu, Q.; Jia, H.; Jiang, Y.; Nie, S.; Xie, J.; Li, C.; Xie, M. Simultaneous determination of furan and 2-alkylfurans in heat-processed foods by automated static headspace gas chromatography-mass spectrometry. LWT-Food Sci. Technol. 2016, 72, 44–54. [Google Scholar] [CrossRef]
- Condurso, C.; Cincotta, F.; Verzera, A. Determination of furan and furan derivatives in baby food. Food Chem. 2018, 250, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.; Dubois, M.; Pérez, J.F.H. Detection of furan and five alkylfurans, including 2-pentylfuran, in various food matrices. J. Chromatogr. A 2020, 1622, 461119. [Google Scholar] [CrossRef] [PubMed]
- Shirey, R.E. SPME Commercial Devices and Fibre Coatings. In Handbook of Solid Phase Microextraction; Elsevier: Oxford, UK, 2012; pp. 99–133. [Google Scholar]
- Batool, Z.; Li, L.; Xu, D.; Wu, M.; Weng, L.; Jiao, W.; Cheng, H.; Roobab, U.; Zhang, X.; Li, X.; et al. Determination of furan and its derivatives in preserved dried fruits and roasted nuts marketed in China using an optimized HS-SPME GC/ MS method. Eur. Food Res. Technol. 2020, 246, 2065–2077. [Google Scholar] [CrossRef]
- Yu, A.N.; Sun, B.G.; Tian, D.T.; Qu, W.Y. Analysis of volatile compounds in traditional smoke-cured bacon (CSCB) with different fiber coatings using SPME. Food Chem. 2008, 110, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Food and Drug Administration (TFDA). Method Validation of Food Analysis. 2013. Available online: https://www.fda.gov.tw/tc/includes/GetFile.ashx?id=f636935163435629279&type=2&cid=10975 (accessed on 1 July 2022).
- AOAC. Standard Method Performance Requirements (SMPRs®) for Furan and Alkyl Furans in Coffee, Baby Foods, Infant Formula, Cereals, and Fruit Juices. 2019. Available online: https://www.aoac.org/wp-content/uploads/2021/11/SMPR-2019_004.pdf (accessed on 1 July 2022).
- Liu, Y.T.; Tsai, S.W. Assessment of dietary furan exposures from heat processed foods in Taiwan. Chemosphere 2010, 79, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Jang, H.W.; Debnath, T.; Lee, K.G. Validation of analytical method for furan determination in eight food matrices and its levels in various foods. J. Sep. Sci. 2019, 42, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Perez-Palacios, T.; Petisca, C.; Melo, A.; Ferreira, I.M. Quantification of furanic compounds in coated deep-fried products simulating normal preparation and consumption: Optimisation of HS-SPME analytical conditions by response surface methodology. Food Chem. 2012, 135, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Food Standards Agency (FSA). Survey of Acrylimide and Furans in UK Retail Products. 2018. Available online: https://www.food.gov.uk/research/research-projects/survey-of-acrylamide-and-furan-in-uk-retail-products (accessed on 1 July 2022).
- SAS. SAS Procedures and SAS/Graph User’s Guide; SAS Institute, Inc.: Cary, NC, USA, 2019. [Google Scholar]
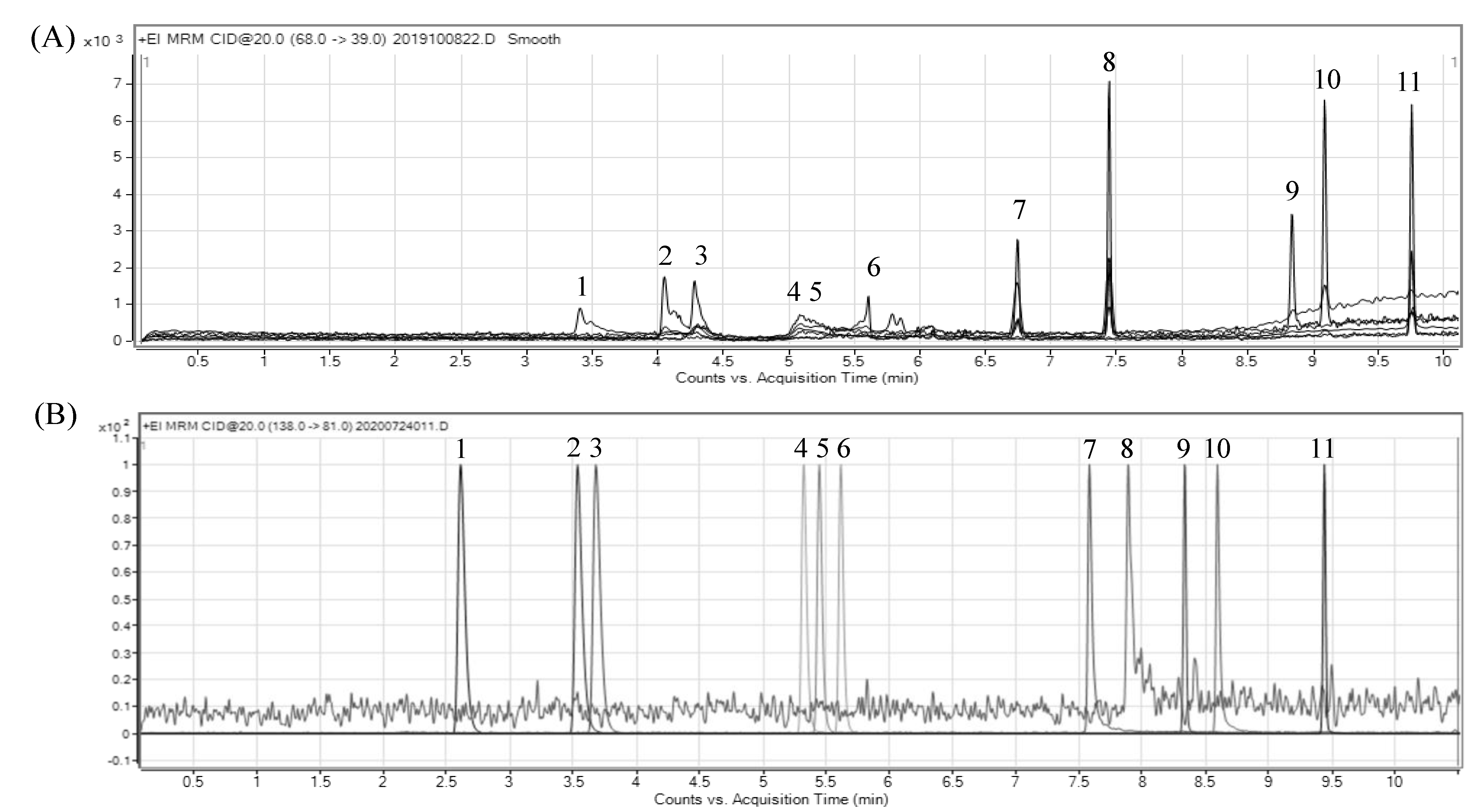
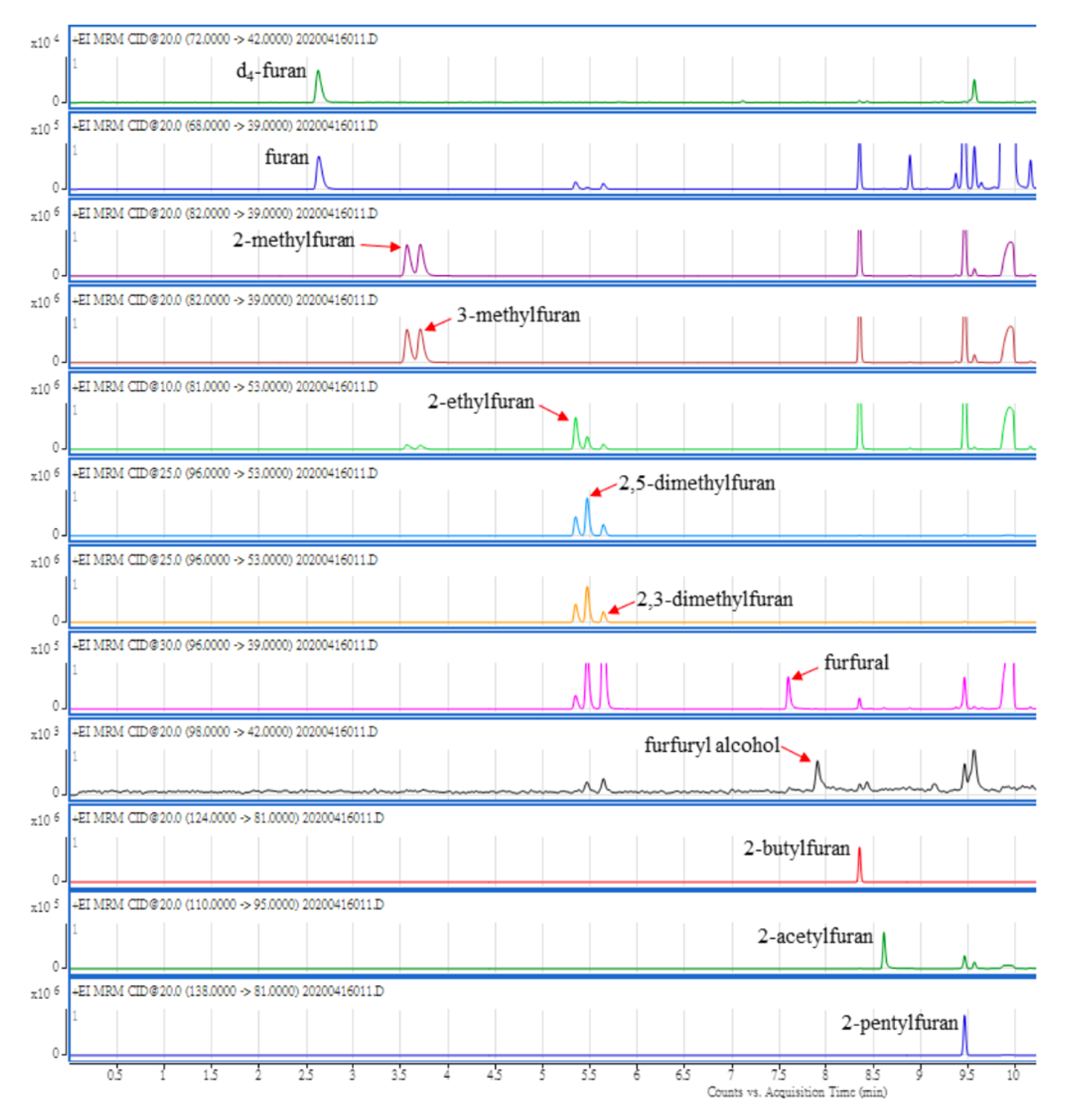
| Peak No. | Compound | Retention Time (min) | Precursor Ion (m/z) | Quantitation | Confirmation | ||
|---|---|---|---|---|---|---|---|
| Product Ion (m/z) | Collision Energy (V) | Product Ion (m/z) | Collision Energy (V) | ||||
| IS | d4-furan | 2.6 | 72 | 42 | 20 | 44 | 15 |
| 1 | furan | 2.6 | 68 | 39 | 20 | 40 | 20 |
| 2 | 2-methylfuran | 3.5 | 82 | 39 | 20 | 53 | 20 |
| 3 | 3-methylfuran | 3.7 | 82 | 39 | 20 | 53 | 20 |
| 4 | 2-ethylfuran | 5.3 | 81 | 53 | 10 | 50 | 50 |
| 5 | 2,5-dimethylfuran | 5.4 | 96 | 53 | 10 | 81 | 25 |
| 6 | 2,3-dimethylfuran | 5.6 | 96 | 53 | 10 | 81 | 25 |
| 7 | furfural | 7.6 | 96 | 39 | 30 | 68 | 20 |
| 8 | furfuryl alcohol | 7.9 | 98 | 42 | 20 | 39 | 40 |
| 9 | 2-butylfuran | 8.3 | 124 | 81 | 20 | 53 | 20 |
| 10 | 2-acetylfuran | 8.6 | 110 | 95 | 20 | 39 | 20 |
| 11 | 2-pentylfuran | 9.4 | 138 | 81 | 20 | 53 | 35 |
| Compounds | Spiking Level (ng/g) | Boiled Sea Bream Containing 20% of Soybean Oil | Fresh Pineapple | HPP Orange Juice | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recovery a (%) | Intra-Day Precision (RSD%) | Inter-Day Precision (RSD%) | Recovery (%) | Intra-Day Precision (RSD%) | Inter-Day Precision (RSD%) | Recovery (%) | Intra-Day Precision (RSD%) | Inter-Day Precision (RSD%) | ||
| furan | 1 | 106.1 ± 10.5 | 7% | 12% | 107.5 ± 2.5 | 2% | 7% | 101.7 ± 2.3 | 4% | 9% |
| 10 | 109.6 ± 1.2 | 6% | 13% | 102.0 ± 2.5 | 3% | 5% | 104.1 ± 2.0 | 6% | 7% | |
| 2-methylfuran | 1 | 101.7 ± 2.5 | 4% | 10% | 106.0 ± 2.4 | 3% | 4% | 103.9 ± 0.6 | 6% | 7% |
| 10 | 104.3 ± 0.2 | 12% | 13% | 110.1 ± 6.6 | 4% | 5% | 100.2 ± 2.1 | 5% | 11% | |
| 3-methylfuran | 1 | 95.0 ± 9.3 | 10% | 17% | 101.7 ± 10.4 | 6% | 12% | 103.9 ± 0.6 | 6% | 7% |
| 10 | 106.2 ± 2.1 | 11% | 10% | 95.6 ± 0.5 | 6% | 7% | 100.2 ± 2.1 | 5% | 11% | |
| 2-ethylfuran | 1 | 101.4 ± 5.2 | 9% | 16% | 111.4 ± 1.8 | 1% | 10% | 87.2 ± 3.3 | 6% | 6% |
| 10 | 103.5 ± 7.2 | 16% | 13% | 108.0 ± 1.8 | 2% | 8% | 96.2 ± 1.2 | 3% | 5% | |
| 2,5-dimethylfuran | 1 | 88.3 ± 1.3 | 8% | 15% | 113.9 ± 2.5 | 4% | 11% | 93.1 ± 1.8 | 9% | 11% |
| 10 | 102.5 ± 2.7 | 9% | 11% | 105.4 ± 1.2 | 5% | 10% | 102.4 ± 0.6 | 3% | 4% | |
| 2,3-dimethylfuran | 1 | 88.3 ± 1.3 | 10% | 10% | 113.9 ± 2.5 | 2% | 5% | 93.1 ± 1.8 | 7% | 14% |
| 10 | 106.4 ± 4.2 | 9% | 10% | 103.1 ± 0.8 | 2% | 10% | 90.7 ± 6.0 | 3% | 10% | |
| furfural | 50 | 100.0 ± 2.6 | 9% | 10% | 103.2 ± 11.8 | 12% | 20% | 96.8 ± 3.8 | 5% | 9% |
| 100 | 98.8 ± 14.7 | 7% | 16% | 100.9 ± 11.4 | 14% | 15% | 89.2 ± 1.8 | 9% | 7% | |
| furfuryl alcohol | 50 | 94.9 ± 2.5 | 10% | 13% | 104.1 ± 3.1 | 6% | 7% | 108.2 ± 6.0 | 10% | 13% |
| 100 | 82.1 ± 5.5 | 12% | 15% | 109.6 ± 6.3 | 6% | 8% | 84.9 ± 2.0 | 4% | 5% | |
| 2-butylfuran | 1 | 87.8 ± 9.7 | 11% | 13% | 92.2 ± 6.4 | 6% | 9% | 115.7 ± 4.1 | 4% | 10% |
| 10 | 82.1 ± 5.5 | 11% | 13% | 109.6 ± 6.3 | 7% | 12% | 84.9 ± 2.0 | 3% | 10% | |
| 2-acetylfuran | 1 | 114.6 ± 4.1 | 10% | 17% | 86.1 ± 4.9 | 10% | 16% | 86.9 ± 4.8 | 7% | 9% |
| 10 | 96.0 ± 0.1 | 5% | 8% | 98.5 ± 8.0 | 8% | 10% | 117.2 ± 0.4 | 8% | 8% | |
| 2-pentylfuran | 1 | 87.34 ± 2.1 | 11% | 19% | 98.1 ± 17.4 | 9% | 7% | 93.1 ± 1.8 | 11% | 13% |
| 10 | 75.92 ± 0.7 | 10% | 14% | 103.8 ± 7.5 | 8% | 9% | 112.9 ± 5.3 | 7% | 12% | |
| Compounds | Boiled Sea Bream Containing 20% Soybean Oil | Fresh Pineapple | HPP Orange Juice | |||
|---|---|---|---|---|---|---|
| LOD a (ng/g) | LOQ b (ng/g) | LOD (ng/g) | LOQ (ng/g) | LOD (ng/g) | LOQ (ng/g) | |
| furan | 0.024 | 0.080 | 0.004 | 0.014 | 0.006 | 0.020 |
| 2-methylfuran | 0.022 | 0.073 | 0.001 | 0.004 | 0.001 | 0.003 |
| 3-methylfuran | 0.022 | 0.073 | 0.019 | 0.063 | 0.001 | 0.003 |
| 2-ethylfuran | 0.003 | 0.010 | 0.001 | 0.003 | 0.001 | 0.003 |
| 2,5-dimethylfuran | 0.049 | 0.163 | 0.006 | 0.022 | 0.003 | 0.010 |
| 2,3-dimethylfuran | 0.094 | 0.313 | 0.002 | 0.006 | 0.007 | 0.023 |
| furfural | 0.012 | 0.040 | 0.005 | 0.019 | 0.004 | 0.013 |
| furfuryl alcohol | 0.101 | 0.337 | 0.204 | 0.675 | 0.048 | 0.160 |
| 2-butylfuran | 0.011 | 0.037 | 0.001 | 0.003 | 0.001 | 0.003 |
| 2-acetylfuran | 0.038 | 0.127 | 0.013 | 0.043 | 0.015 | 0.050 |
| 2-pentylfuran | 0.002 | 0.007 | 0.001 | 0.003 | 0.001 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsao, W.-X.; Chen, B.-H.; Lin, P.; You, S.-H.; Kao, T.-H. Analysis of Furan and Its Derivatives in Food Matrices Using Solid Phase Extraction Coupled with Gas Chromatography-Tandem Mass Spectrometry. Molecules 2023, 28, 1639. https://doi.org/10.3390/molecules28041639
Tsao W-X, Chen B-H, Lin P, You S-H, Kao T-H. Analysis of Furan and Its Derivatives in Food Matrices Using Solid Phase Extraction Coupled with Gas Chromatography-Tandem Mass Spectrometry. Molecules. 2023; 28(4):1639. https://doi.org/10.3390/molecules28041639
Chicago/Turabian StyleTsao, Wen-Xuan, Bing-Huei Chen, Pinpin Lin, Shu-Han You, and Tsai-Hua Kao. 2023. "Analysis of Furan and Its Derivatives in Food Matrices Using Solid Phase Extraction Coupled with Gas Chromatography-Tandem Mass Spectrometry" Molecules 28, no. 4: 1639. https://doi.org/10.3390/molecules28041639
APA StyleTsao, W.-X., Chen, B.-H., Lin, P., You, S.-H., & Kao, T.-H. (2023). Analysis of Furan and Its Derivatives in Food Matrices Using Solid Phase Extraction Coupled with Gas Chromatography-Tandem Mass Spectrometry. Molecules, 28(4), 1639. https://doi.org/10.3390/molecules28041639







