Linear-Shaped Low-Bandgap Asymmetric Conjugated Donor Molecule for Fabrication of Bulk Heterojunction Small-Molecule Organic Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
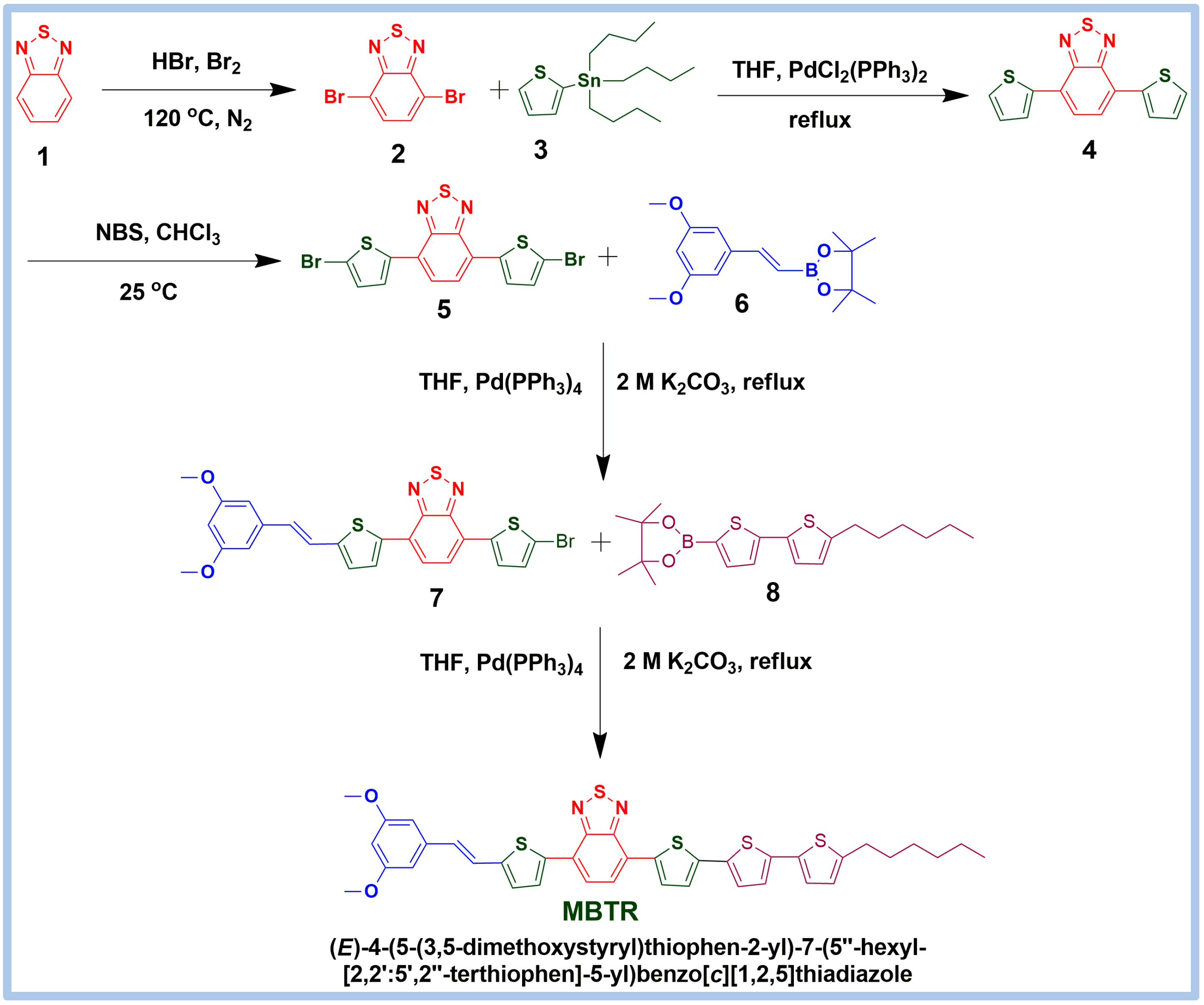
2.1. Synthesis of Small Molecule and Thermal Properties
2.2. Optical, Electrochemical, and Emission Investigations of MBTR
2.3. Photovoltaic Characteristics of MBTR
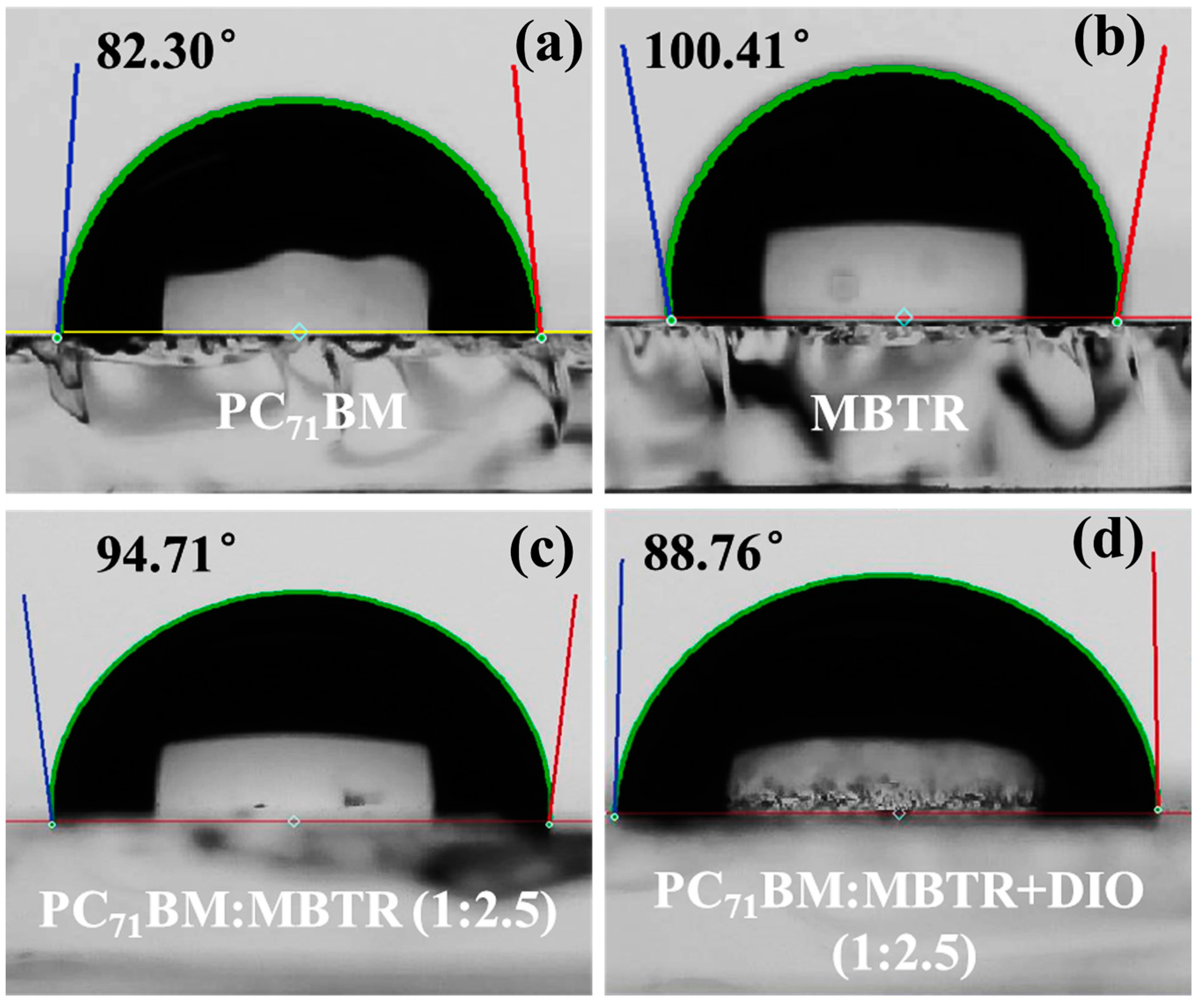
2.4. Thin-Film Morphology
3. Materials and Methods
3.1. Synthesis of (E)-4-(5-Bromothiophen-2-yl)-7-(5-(3,5-dimethoxystyryl)thiophen-2-yl)–benzo[c][1,2,5]thiadiazole (Compound 7)
3.2. Synthesis of (E)-4-(5-(3,5-Dimethoxystyryl)thiophen-2-yl)-7-(5″-hexyl-[2,2′:5′,2″-terthiophen]-5-yl)benzo[c][1,2,5]thiadiazole (MBTR)
3.3. Characterization
3.4. Device Fabrication of BHJ OSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, Z.; Wang, X.; Zheng, N.; Saparbaev, A.; Zhang, J.; Xiao, C.; Lei, S.; Zheng, X.; Zhang, M.; Li, Y.; et al. Over 17% Efficiency All-Small-Molecule Organic Solar Cells Based on an Organic Molecular Donor Employing a 2D Side Chain Symmetry Breaking Strategy. Energy Environ. Sci. 2022, 15, 4338–4348. [Google Scholar] [CrossRef]
- Zhou, D.; Li, Y.; Zhang, H.; Zheng, H.; Shen, X.; You, W.; Hu, L.; Han, L.; Tong, Y.; Chen, L. N-Type Small Molecule Electron Transport Materials with D-A-D Conjugated Core for Non-Fullerene Organic Solar Cells. Chem. Eng. J. 2023, 452, 139260. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, F.R.; Qi, F.; Heumüller, T.; Distler, A.; Egelhaaf, H.J.; Li, N.; Chow, P.C.Y.; Brabec, C.J.; Jen, A.K.Y.; et al. Renewed Prospects for Organic Photovoltaics. Chem. Rev. 2022, 122, 14180–14274. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.W.; Palomares, E. Photo-Induced Charge Carrier Recombination Kinetics in Small Molecule Organic Solar Cells and the Influence of Film Nanomorphology. Adv. Energy Mater. 2017, 7, 1601509. [Google Scholar] [CrossRef]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-Generation Organic Photovoltaics Based on Non-Fullerene Acceptors. Nat. Photonics 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Jiang, J.M.; Raghunath, P.; Lin, H.K.; Lin, Y.C.; Lin, M.C.; Wei, K.H. Location and Number of Selenium Atoms in Two-Dimensional Conjugated Polymers Affect Their Band-Gap Energies and Photovoltaic Performance. Macromolecules 2014, 47, 7070–7080. [Google Scholar] [CrossRef]
- Radu (Călugăr), A.I.; Antohe, V.A.; Iftimie, S.; Radu, A.; Filipescu, M.; Ion, L.; Dinescu, M.; Antohe, Ş. On the Physical and Photo-Electrical Properties of Organic Photovoltaic Cells Based on 1,10-Phenanthroline and 5,10,15,20-Tetra(4-Pyridyl)-21H,23H-Porphine Non-Fullerene Thin Films. Appl. Surf. Sci. 2020, 531, 147332. [Google Scholar] [CrossRef]
- Ahmad, M.; Amelot, D.; Cruguel, H.; Patil, B.R.; Ahmadpour, M.; Giangrisostomi, E.; Ovsyannikov, R.; Silly, M.G.; Dudy, L.; Madsen, M.; et al. Unveiling the Energy Alignment across Ultrathin 4P-NPD Hole Extraction Interlayers in Organic Solar Cells. ACS Appl. Energy Mater. 2022, 5, 5018–5025. [Google Scholar] [CrossRef]
- Guan, M.; Tao, W.; Xu, L.; Qin, Y.; Zhang, J.; Tan, S.; Huang, M.; Zhao, B. An Asymmetric Small-Molecule Donor Enables over 18% Efficiency in Ternary Organic Solar Cells. J. Mater. Chem. A 2022, 10, 9746–9752. [Google Scholar] [CrossRef]
- Li, C.; Zhou, J.; Song, J.; Xu, J.; Zhang, H.; Zhang, X.; Guo, J.; Zhu, L.; Wei, D.; Han, G.; et al. Non-Fullerene Acceptors with Branched Side Chains and Improved Molecular Packing to Exceed 18% Efficiency in Organic Solar Cells. Nat. Energy 2021, 6, 605–613. [Google Scholar] [CrossRef]
- Radu, A.I.; Antohe, V.A.; Iftimie, S.; Antohe, I.; Filipescu, M.; Radu, A.; Coman, D.; Stîngescu, M.L.; Dinescu, M.; Antohe, Ş. Study of a New Composite Based on SnO2 Nanoparticles—P3HT:PC71BM Co-Polymer Blend, Used as Potential Absorber in Bulk Heterojunction Photovoltaic Cells. Mater. Today Commun. 2022, 33, 104757. [Google Scholar] [CrossRef]
- Gao, H.H.; Sun, Y.; Li, S.; Ke, X.; Cai, Y.; Wan, X.; Zhang, H.; Li, C.; Chen, Y. An All Small Molecule Organic Solar Cell Based on a Porphyrin Donor and a Non-Fullerene Acceptor with Complementary and Broad Absorption. Dye. Pigment. 2020, 176, 108250. [Google Scholar] [CrossRef]
- Speller, E.M.; Clarke, A.J.; Luke, J.; Lee, H.K.H.; Durrant, J.R.; Li, N.; Wang, T.; Wong, H.C.; Kim, J.S.; Tsoi, W.C.; et al. From Fullerene Acceptors to Non-Fullerene Acceptors: Prospects and Challenges in the Stability of Organic Solar Cells. J. Mater. Chem. A 2019, 7, 23361–23377. [Google Scholar] [CrossRef]
- Gupta, A.; Ali, S.; Jameel, M.A.; Evans, R.A.; Kaur, N.; Sharma, G.D.; Li, J.L. Improvement of Charge Mobility and Photovoltaic Performance of Small Molecule Oligothiophene Donors through Self-Assembly. Dye. Pigment. 2022, 207, 110718. [Google Scholar] [CrossRef]
- Abdullah; Kim, E.B.; Akhtar, M.S.; Ameen, S. Highly Stable Bulk Heterojunction Organic Solar Cells Based on Asymmetric Benzoselenadiazole-Oriented Organic Chromophores. Int. J. Energy Res. 2022, 46, 7825–7839. [Google Scholar] [CrossRef]
- Çakal, D.; Ercan, Y.E.; Önal, A.M.; Cihaner, A. Effect of Fluorine Substituted Benzothiadiazole on Electro-Optical Properties of Donor-Acceptor-Donor Type Monomers and Their Polymers. Dye. Pigment. 2020, 182, 108622. [Google Scholar] [CrossRef]
- Kan, B.; Li, M.; Zhang, Q.; Liu, F.; Wan, X.; Wang, Y.; Ni, W.; Long, G.; Yang, X.; Feng, H.; et al. A Series of Simple Oligomer-like Small Molecules Based on Oligothiophenes for Solution-Processed Solar Cells with High Efficiency. J. Am. Chem. Soc. 2015, 137, 3886–3893. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Lin, H.; Yu, X.; Li, M.; Du, X.; Luo, J.; Yang, G.; Zheng, C.; Tao, S. Efficient and Stable Ternary Organic Solar Cells Using Liquid Crystal Small Molecules with Multiple Synergies. ACS Appl. Energy Mater. 2022, 5, 12809–12816. [Google Scholar] [CrossRef]
- Kim, H.; Reddy, M.R.; Kim, H.; Choi, D.; Kim, C.; Seo, S.Y. Benzothiadiazole-Based Small-Molecule Semiconductors for Organic Thin-Film Transistors and Complementary-like Inverters. Chempluschem 2017, 82, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Bao, X.; Andersen, C.P.; Didriksen, C.B.; Wang, J.; Lin, M.C.; Cao, Z.; Yu, D. Fluorination on Electron-Deficient Units of Benzothiadiazole-Based Donor-Acceptor Conjugated Polymers for Novel Fullerene-Based Organic Solar Cells. Sol. Energy 2021, 220, 864–872. [Google Scholar] [CrossRef]
- Abdullah; Akhtar, M.S.; Kim, E.B.; Fijahi, L.; Shin, H.S.; Ameen, S. A Symmetric Benzoselenadiazole Based D–A–D Small Molecule for Solution Processed Bulk-Heterojunction Organic Solar Cells. J. Ind. Eng. Chem. 2020, 81, 309–316. [Google Scholar] [CrossRef]
- Zhang, M.; Gu, Y.; Guo, X.; Liu, F.; Zhang, S.; Huo, L.; Russell, T.P.; Hou, J. Efficient Polymer Solar Cells Based on Benzothiadiazole and Alkylphenyl Substituted Benzodithiophene with a Power Conversion Efficiency over 8%. Adv. Mater. 2013, 25, 4944–4949. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.M.H.; Yu, H.; Luo, S.; Wang, Z.; Qi, Z.; Zhou, W.; Arunagiri, L.; Chang, Y.; Yao, H.; Ade, H.; et al. Incorporation of Alkylthio Side Chains on Benzothiadiazole-Based Non-Fullerene Acceptors Enables High-Performance Organic Solar Cells with over 16% Efficiency. J. Mater. Chem. A 2020, 8, 23239–23247. [Google Scholar] [CrossRef]
- Sun, Y.; Seifter, J.; Huo, L.; Yang, Y.; Hsu, B.B.Y.; Zhou, H.; Sun, X.; Xiao, S.; Jiang, L.; Heeger, A.J. High-Performance Solution-Processed Small-Molecule Solar Cells Based on a Dithienogermole-Containing Molecular Donor. Adv. Energy Mater. 2015, 5, 1400987. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Yang, Z.; Liu, Z.; Zhang, K.; Yang, L.; Peng, Q.; Zhu, W.; Liu, Y. An Effective Heteroatom-Substituted Strategy on Photovoltaic Properties of D(A-Ar)2 Small Molecules for Efficient Organic Solar Cells. Dye. Pigment. 2019, 170, 107595. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Z.; Si, W.; Sun, Q.; Cai, G.; Li, Y.; Jia, Y.; Lu, X.; Xu, W.; Zhang, S.; et al. Organic Photovoltaic Catalyst with Extended Exciton Diffusion for High-Performance Solar Hydrogen Evolution. J. Am. Chem. Soc. 2022, 144, 12747–12755. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Lapis, A.A.M.; Da Silva Júnior, E.N.; Dupont, J. 2,1,3-Benzothiadiazole and Derivatives: Synthesis, Properties, Reactions, and Applications in Light Technology of Small Molecules. European J. Org. Chem. 2013, 228–255. [Google Scholar] [CrossRef]
- Gamage, P.L.; Udamulle Gedara, C.M.; Ma, Z.; Bhadran, A.; Gunawardhana, R.; Bulumulla, C.; Biewer, M.C.; Stefan, M.C. Incorporation of Selenopheno[3,2-b]Pyrrole into Benzothiadiazole-Based Small Molecules for Organic Field-Effect Transistors. ACS Appl. Electron. Mater. 2021, 3, 5335–5344. [Google Scholar] [CrossRef]
- Xu, T.; Chang, Y.; Yan, C.; Yang, Q.; Kan, Z.; Singh, R.; Kumar, M.; Li, G.; Lu, S.; Duan, T. Fluorinated Oligothiophene Donors for High-Performance Nonfullerene Small-Molecule Organic Solar Cells. Sustain. Energy Fuels 2020, 4, 2680–2685. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, Y.J.; Su, Y.W.; Lin, Y.C.; Lin, H.K.; Chen, H.C.; Wang, H.C.; Li, J.X.; Wu, K.H.; Wei, K.H. Enhancing Performance of Ternary Blend Photovoltaics by Tuning the Side Chains of Two-Dimensional Conjugated Polymer. Org. Electron. 2019, 71, 185–193. [Google Scholar] [CrossRef]
- Lin, Y.C.; Su, Y.W.; Li, J.X.; Lin, B.H.; Chen, C.H.; Chen, H.C.; Wu, K.H.; Yang, Y.; Wei, K.H. Energy Transfer within Small Molecule/Conjugated Polymer Blends Enhances Photovoltaic Efficiency. J. Mater. Chem. A 2017, 5, 18053–18063. [Google Scholar] [CrossRef]
- Du, J.; Biewer, M.C.; Stefan, M.C. Benzothiadiazole Building Units in Solution-Processable Small Molecules for Organic Photovoltaics. J. Mater. Chem. A 2016, 4, 15771–15787. [Google Scholar] [CrossRef]
- Sun, Y.; Welch, G.C.; Leong, W.L.; Takacs, C.J.; Bazan, G.C.; Heeger, A.J. Solution-Processed Small-Molecule Solar Cells with 6.7% Efficiency. Nat. Mater. 2012, 11, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Fijahi, L.; Shaheer Akhtar, M.; Abdullah; Kim, E.-B.; Seo, H.-K.; Shin, H.-S.; Ameen, S. Investigation of Newly Designed Asymmetric Chromophore in View of Power Conversion Efficiency Improvements for Organic Solar Cells. Mater. Lett. 2020, 260, 126865. [Google Scholar] [CrossRef]
- Alam, S.; Akhtar, M.S.; Abdullah; Kim, E.B.; Shin, H.S.; Ameen, S. New Energetic Indandione Based Planar Donor for Stable and Efficient Organic Solar Cells. Sol. Energy 2020, 201, 649–657. [Google Scholar] [CrossRef]
- Agneeswari, R.; Kim, D.; Park, S.W.; Jang, S.; Yang, H.S.; Shin, I.; Jeong, J.H.; Tamilavan, V.; Jung, Y.K.; Park, S.H. Influence of Thiophene and Furan π–Bridge on the Properties of Poly(Benzodithiophene-Alt-Bis(π–Bridge)Pyrrolopyrrole-1,3-Dione) for Organic Solar Cell Applications. Polymer 2021, 229, 123991. [Google Scholar] [CrossRef]
- Zhang, Q.; Kan, B.; Liu, F.; Long, G.; Wan, X.; Chen, X.; Zuo, Y.; Ni, W.; Zhang, H.; Li, M.; et al. Small-Molecule Solar Cells with Efficiency over 9%. Nat. Photonics 2014, 9, 35–41. [Google Scholar] [CrossRef]
- Garcias-Morales, C.; Romero-Borja, D.; Maldonado, J.L.; Roa, A.E.; Rodríguez, M.; García-Merinos, J.P.; Ariza-Castolo, A. Small Molecules Derived from Thieno[3,4-c]Pyrrole-4,6-Dione (TPD) and Their Use in Solution Processed Organic Solar Cells. Molecules 2017, 22, 1607. [Google Scholar] [CrossRef]
- Abdullah; Kim, E.B.; Akhtar, M.S.; Shin, H.S.; Ameen, S. Benzoselenadiazole-Core Asymmetric D-A-A Small Molecule for Solution Processed Bulk Heterojunction Organic Solar Cells. Int. J. Energy Res. 2020, 44, 12100–12111. [Google Scholar] [CrossRef]
- Rajkumar, B.; Khanam, L.; Koukaras, E.N.; Sharma, G.D.; Singh, S.P.; Lochab, B. Cardanol- And Guaiacol-Sourced Solution-Processable Green Small Molecule-Based Organic Solar Cells. ACS Sustain. Chem. Eng. 2020, 8, 5891–5902. [Google Scholar] [CrossRef]
- Van der Pol, T.P.A.; Li, J.; van Gorkom, B.T.; Colberts, F.J.M.; Wienk, M.M.; Janssen, R.A.J. Analysis of the Performance of Narrow-Bandgap Organic Solar Cells Based on a Diketopyrrolopyrrole Polymer and a Nonfullerene Acceptor. J. Phys. Chem. C 2021, 125, 5505–5517. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, J.; Yang, D.; Yu, K.; Li, D.; Xia, Z.; Ge, Z. Two Star-Shaped Small Molecule Donors Based on Benzodithiophene Unit for Organic Solar Cells. Chin. Chem. Lett. 2022, 33, 247–251. [Google Scholar] [CrossRef]
- Keshtov, M.L.; Shikin, D.Y.; Khokhlov, A.R.; Ostapov, I.E.; Alekseev, V.G.; Singh, M.K.; Sharma, G.D. Dithieno[2,3-e:3′,2′-g]Isoindole-7,9(8H)-Dione and Dithieno[3′,2′:5,6;2″,3″:7,8]Naphtho[2,3-d]Imidazol-9(10H)-One-Based Wide Bandgap Copolymer for Efficient Polymer Solar Cells. Energy Technol. 2022, 9, 2201211. [Google Scholar] [CrossRef]
- Li, S.; Zhan, L.; Sun, C.; Zhu, H.; Zhou, G.; Yang, W.; Shi, M.; Li, C.Z.; Hou, J.; Li, Y.; et al. Highly Efficient Fullerene-Free Organic Solar Cells Operate at Near Zero Highest Occupied Molecular Orbital Offsets. J. Am. Chem. Soc. 2019, 141, 3073–3082. [Google Scholar] [CrossRef]
- Bauer, N.; Zhang, Q.; Zhao, J.; Ye, L.; Kim, J.H.; Constantinou, I.; Yan, L.; So, F.; Ade, H.; Yan, H.; et al. Comparing Non-Fullerene Acceptors with Fullerene in Polymer Solar Cells: A Case Study with FTAZ and PyCNTAZ. J. Mater. Chem. A 2017, 5, 4886–4893. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, X.; Xie, Z.; Liu, J.; Han, Y. Optimizing H-/J-Type Aggregation and Vertical Phase Separation to Improve Photovoltaic Efficiency of Small Molecule Solar Cells by Adding a Macromolecule Additive. ACS Appl. Energy Mater. 2018, 1, 6338–6344. [Google Scholar] [CrossRef]
- Zhang, M.; Zeng, M.; Chen, H.; Li, L.; Zhao, B.; Tan, S. A2-D-A1-D-A2-Type Small Molecule Acceptors Incorporated with Electron-Deficient Core for Non-Fullerene Organic Solar Cells. Sol. Energy 2020, 197, 511–518. [Google Scholar] [CrossRef]
- Duan, T.; Babics, M.; Seitkhan, A.; Firdaus, Y.; Liang, R.Z.; Cruciani, F.; Liu, S.; Lopatin, S.; Beaujuge, P.M. F-Substituted Oligothiophenes Serve as Nonfullerene Acceptors in Polymer Solar Cells with Open-Circuit Voltages >1 V. J. Mater. Chem. A 2018, 6, 9368–9372. [Google Scholar] [CrossRef]
- Lee, J.; Singh, R.; Sin, D.H.; Kim, H.G.; Song, K.C.; Cho, K. A Nonfullerene Small Molecule Acceptor with 3D Interlocking Geometry Enabling Efficient Organic Solar Cells. Adv. Mater. 2016, 28, 69–76. [Google Scholar] [CrossRef]
- Hu, D.; Tang, H.; Karuthedath, S.; Chen, Q.; Chen, S.; Khan, J.I.; Liu, H.; Yang, Q.; Gorenflot, J.; Petoukhoff, C.E.; et al. A Volatile Solid Additive Enables Oligothiophene All-Small-Molecule Organic Solar Cells with Excellent Commercial Viability. Adv. Funct. Mater. 2022, 33, 2211873. [Google Scholar] [CrossRef]
- Wienhold, K.S.; Körstgens, V.; Grott, S.; Jiang, X.; Schwartzkopf, M.; Roth, S.V.; Müller-Buschbaum, P. Effect of Solvent Additives on the Morphology and Device Performance of Printed Nonfullerene Acceptor Based Organic Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 42313–42321. [Google Scholar] [CrossRef] [PubMed]
- Abdullah; Akhtar, M.S.; Kim, E.B.; Shin, H.S.; Ameen, S. Justifying Benzoselenadiazole Acceptor Core as Organic Semiconductor for Stable Bulk-Heterojunction Organic Solar Cells at Ambient Temperature. J. Mater. 2021, 7, 1112–1121. [Google Scholar] [CrossRef]
- Li, D.; Wang, J.; Xie, L.; Ge, J.; Zhou, R.; Gu, Q.; Yang, D.; Zhang, J.; Ge, Z. Crystallinity Modulation of Donors by Heteroatom Side-Chain Engineering and Solvent Additive Achieving 14.3% All-Small-Molecule Organic Solar Cells. J. Mater. Chem. A 2022, 10, 9635–9642. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Liang, Z.; Wang, N.; Tong, J.; Yang, C.; Bao, X.; Xia, Y. Enhanced Organic Photovoltaic Performance through Modulating Vertical Composition Distribution and Promoting Crystallinity of the Photoactive Layer by Diphenyl Sulfide Additives. ACS Appl. Mater. Interfaces 2019, 11, 7022–7029. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Jing, Y.; Guo, X.; Sun, H.; Zhang, S.; Zhang, M.; Huo, L.; Hou, J. Remove the Residual Additives toward Enhanced Efficiency with Higher Reproducibility in Polymer Solar Cells. J. Phys. Chem. C 2013, 117, 14920–14928. [Google Scholar] [CrossRef]
- Zhu, X.; Lu, K.; Xia, B.; Fang, J.; Zhao, Y.; Zhao, T.; Wei, Z.; Jiang, L. Improving the Performances of Random Copolymer Based Organic Solar Cells by Adjusting the Film Features of Active Layers Using Mixed Solvents. Polymers 2016, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Dahiya, H.; Song, Y.; Liang, X.; Pandey, S.K.; Xu, H.; Sharma, G.D. Alkynyl BODIPY-Core Bridged Perylene Diimide Star-Shaped Nonfullerene Acceptors for Efficient Polymer Solar Cells. ACS Appl. Energy Mater. 2022, 5, 15624–15637. [Google Scholar] [CrossRef]
- Ion, L.; Enculescu, I.; Iftimie, S.; Ghenescu, V.; Tazlaoanu, C.; Besleaga, C.; Mitran, T.L.; Antohe, V.A.; Gugiu, M.M.; Antohe, S. Effects of Proton Irradiation on the Spectral Performance of Photovoltaic Cells Based on CdS/CdTe Thin Films. Chalcogenide Lett. 2010, 7, 521–530. [Google Scholar]
- Radu, A.; Iftimie, S.; Ghenescu, V.; Besleaga, C.; Antohe, V.A.; Bratina, G.; Ion, L.; Craciun, S.; Girtan, M.; Antohe, S. The Influence of Lif Layer and ZnO Nanoparticles Addings on the Performances of Flexible Photovoltaic Cells Based on Polymer Blends. Dig. J. Nanomater. Biostructures 2011, 6, 1141–1148. [Google Scholar]
- Antohe, S.; Iftimie, S.; Hrostea, L.; Antohe, V.A.; Girtan, M. A Critical Review of Photovoltaic Cells Based on Organic Monomeric and Polymeric Thin Film Heterojunctions. Thin Solid Films 2017, 642, 219–231. [Google Scholar] [CrossRef]
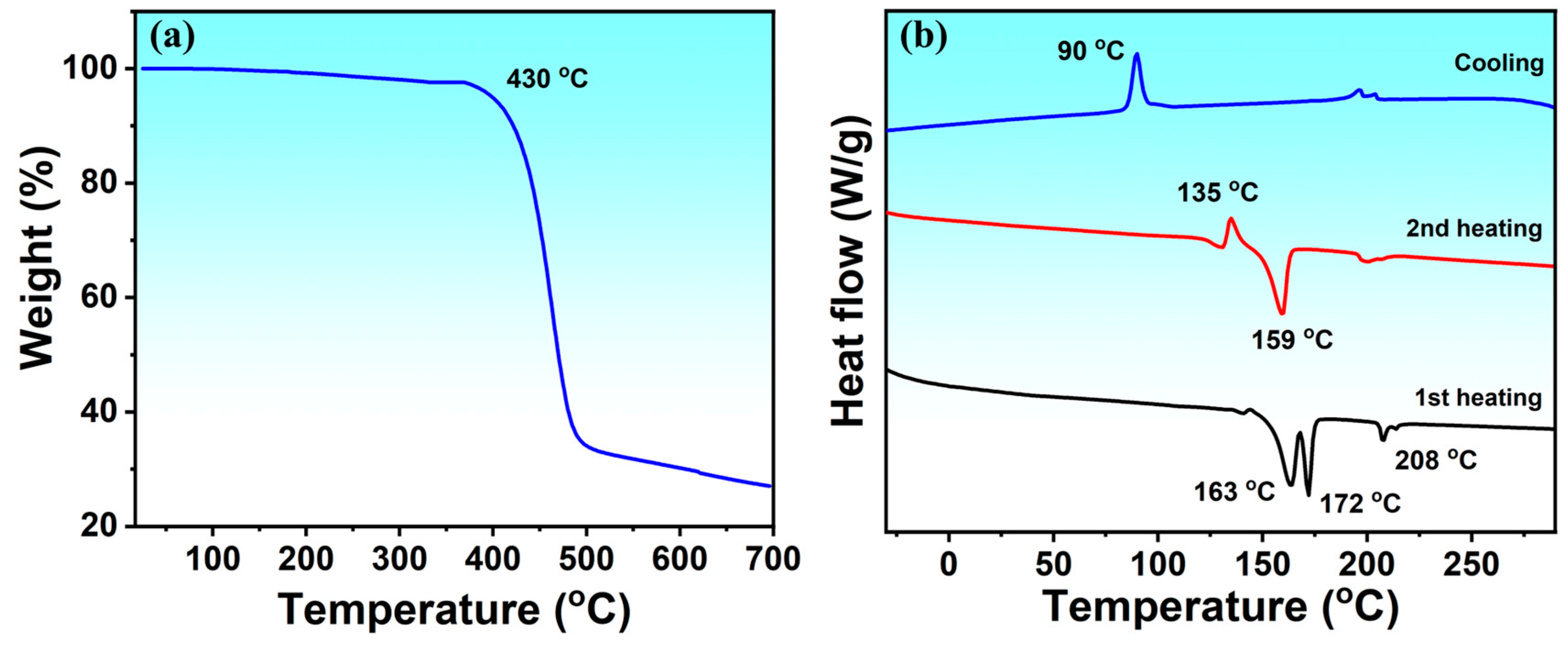
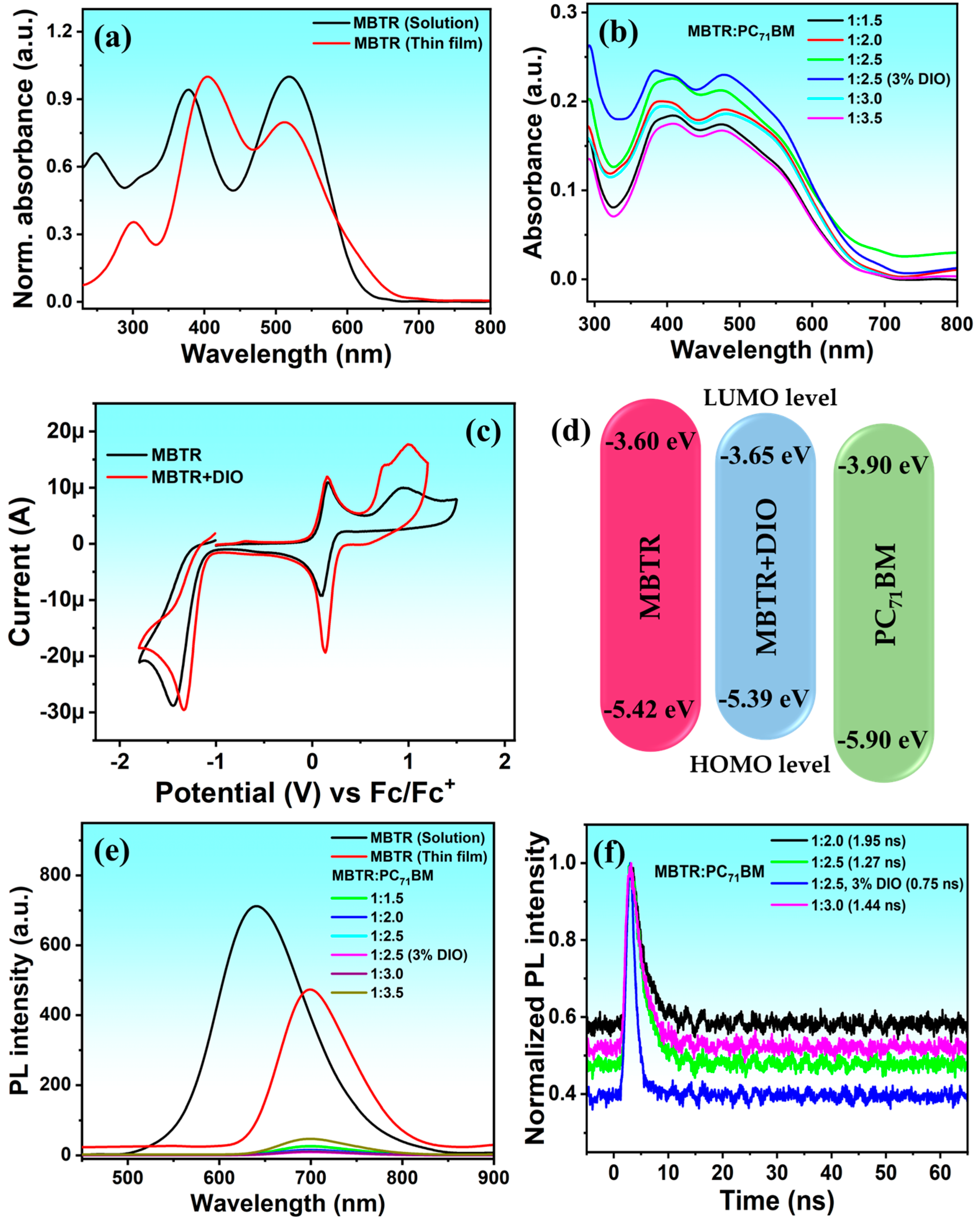


| Molecule | Td (°C) | λmax a (nm) | λmax b (nm) | Eoxonset (V) | Eredonset (V) | HOMO c (eV) | LUMO d (eV) | Egcv (eV) | Egopt (eV) |
|---|---|---|---|---|---|---|---|---|---|
| MBTR | 430 | 404, 515 | 699 | +0.62 | −1.20 | −5.42 | −3.60 | 1.81 | 1.86 |
| (w/w Ratio) | Voc (V) | Jsc (mA/cm2) | FF (%) | Rs (Ω cm2) | Rsh (Ω cm2) | PCE (%) | JscIPCE (mA/cm2) | |
|---|---|---|---|---|---|---|---|---|
| MBTR | 1:1.5 | 0.910 | 10.36 | 45.0 | 1.62 | 22.43 | 4.24 | 9.88 |
| 1:2.0 | 0.926 | 11.01 | 50.5 | 3.02 | 42.31 | 5.15 | 10.38 | |
| 1:2.5 | 0.943 | 12.63 | 59.2 | 1.59 | 60.50 | 7.05 | 12.29 | |
| 1:2.5 (3% DIO) | 1.021 | 13.78 | 62.3 | 1.20 | 69.78 | 8.76 | 13.51 | |
| 1:3.0 | 0.947 | 12.15 | 59.3 | 1.47 | 57.71 | 6.82 | 11.70 | |
| 1:3.5 | 0.859 | 9.97 | 46.1 | 2.39 | 26.91 | 3.95 | 9.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah; Lee, S.-J.; Park, J.B.; Kim, Y.S.; Shin, H.-S.; Kotta, A.; Siddiqui, Q.T.; Lee, Y.-S.; Seo, H.-K. Linear-Shaped Low-Bandgap Asymmetric Conjugated Donor Molecule for Fabrication of Bulk Heterojunction Small-Molecule Organic Solar Cells. Molecules 2023, 28, 1538. https://doi.org/10.3390/molecules28041538
Abdullah, Lee S-J, Park JB, Kim YS, Shin H-S, Kotta A, Siddiqui QT, Lee Y-S, Seo H-K. Linear-Shaped Low-Bandgap Asymmetric Conjugated Donor Molecule for Fabrication of Bulk Heterojunction Small-Molecule Organic Solar Cells. Molecules. 2023; 28(4):1538. https://doi.org/10.3390/molecules28041538
Chicago/Turabian StyleAbdullah, Sei-Jin Lee, Jong Bae Park, Yang Soo Kim, Hyung-Shik Shin, Ashique Kotta, Qamar Tabrez Siddiqui, Youn-Sik Lee, and Hyung-Kee Seo. 2023. "Linear-Shaped Low-Bandgap Asymmetric Conjugated Donor Molecule for Fabrication of Bulk Heterojunction Small-Molecule Organic Solar Cells" Molecules 28, no. 4: 1538. https://doi.org/10.3390/molecules28041538
APA StyleAbdullah, Lee, S.-J., Park, J. B., Kim, Y. S., Shin, H.-S., Kotta, A., Siddiqui, Q. T., Lee, Y.-S., & Seo, H.-K. (2023). Linear-Shaped Low-Bandgap Asymmetric Conjugated Donor Molecule for Fabrication of Bulk Heterojunction Small-Molecule Organic Solar Cells. Molecules, 28(4), 1538. https://doi.org/10.3390/molecules28041538








