1. Introduction
The biological reduction of carbon dioxide (CO
2) involves the conversion of a highly stable and chemically inert compound into more reactive and useful organic chemicals, representing a key process in the global carbon cycle that is of high relevance for combating the greenhouse effect [
1]. Among these enzymes, formate dehydrogenases (FDHs) represent a diverse group of enzymes in bacteria, archaea, and eukaryotes that have been proposed to facilitate the reversible two electron and one proton abstraction of formate to produce CO
2 by catalyzing the following redox reaction, with the equilibrium being on the side of formate oxidation [
2,
3]:
Enzymatic reduction in CO
2 to formate would allow for the storage of hydrogen as a fuel for industrial applications [
4,
5,
6] as well as carbon sequestration from the atmosphere [
1,
7], rendering these enzymes interesting targets for biotechnological utilization.
In general, most of the FDH enzymes catalyze preferentially the forward reaction of formate oxidation [
8]. The released electrons are transferred in intramolecular electron transfer reactions for the eventual reduction of one of several terminal electron acceptors. Some metal-containing enzymes, however, were described to act rather as CO
2 reductases [
9], reducing CO
2 to formate [
10]. Metal-containing FDHs belong to the family of molybdenum/tungsten cofactor-containing enzymes, binding the bis-Metal-binding pterin Guanine Dinucleotide (bis-MGD) cofactor at their active site [
11]. In the bis-MGD cofactor, the metal center is coordinated by two pterin ene-dithiolates, a sulfur atom, and either a cysteine or selenocysteine [
12]. FDHs containing the latter ligand were found to be rather oxygen-sensitive [
3,
12]. All FDHs contain two additional highly conserved residues in the active site, a histidine and an arginine [
13,
14]. The reaction of formate/CO
2 interconversion occurs at the molybdenum or tungsten metal ion in the bis-MGD cofactor, in which the metal (M) cycles between the M
VI, M
V, and M
IV oxidation states during the reaction [
15]. Close to the (bis-MGD) cofactor, a proximal [4Fe–4S] cluster is present in all metal-containing FDH enzymes [
16], which is involved in the intramolecular electron transfer reaction. In
Rhodobacter capsulatus FDH, the oxidation of formate is energetically coupled to the reduction in oxidized nicotinamide adenine dinucleotide (NAD
+) in the cytosol [
17].
While metal-dependent FDH enzymes have been studied for several decades and the
E. coli, the FdhF enzyme was among the first molybdoenzymes to be crystalized [
12]. Details of the reaction mechanism remain only poorly understood and the overall catalytic mechanism of formate oxidation is still under debate [
2,
18].
It was reported [
19,
20,
21] that the reaction of metal-containing FDHs do not constitute the characteristic oxygen atom transfer reaction, as typically catalyzed by the DMSO reductase family of Mo/W bis-MGD-containing enzymes [
22]. Instead, it has been suggested that the reaction involves only the heterolytic fission of the C-H bond facilitated through a direct hydride transfer to the terminal sulfido ligand at the molybdenum atom, with the immediate product being CO
2 and not bicarbonate [
19,
20]. This hypothesis was first proposed by Thauer et al. (1975) in the characterization of the FDH (ferredoxin: CO
2 oxidoreductase) from
Clostridium pasteurianum [
19]. It was later supported by a study from Khangulov et al. (1998) [
21] on
Escherichia coli FdhF, who investigated product formation using GC-MS with
13C-labeled formate in
18O-enriched water, which resulted in the detection of only
13CO
2 gas without any
18O atoms.
A recent study by Meneghello et al. (2021) [
20] applied an electrochemical approach to the tungsten-containing FDH from
Desulfovibrio vulgaris Hildenborough [
23] to confirm conclusions from earlier cumulative work; i.e., that the substrate of FDHs for the back reaction of CO
2 reduction is CO
2 rather than HCO
3–. However, as already pointed out by Cooper et al. (1968) [
24], it still remains possible that these observations are more a reflection of the binding of the preferred molecule to the active site and do not necessarily reflect what is happening during substrate turnover, especially since the product formation was not quantified in the assay, and by electrochemistry it cannot be determined which substrate is bound at the active site and which ligands are involved in the binding. It, therefore, might be possible that charged HCO
3− is hindered from entering the active site via the hydrophobic channel that has been identified in crystal and cryo-EM structures of FDH enzymes, while CO
2 in contrast can easily enter [
23,
25]. At the active site, CO
2 could react with H
2O to form HCO
3− which then acts as the actual substrate for the reaction, which is only formally the reduction of CO
2 [
19]. This option has, so far, been unconsidered in most respective studies [
2,
10,
13,
15,
18,
21,
23]. It should, however, attract more attention, since in some X-ray structures of FDH enzymes a water molecule was identified in the active site which is bound in the vicinity to the conserved arginine residue in the second coordination sphere [
23,
26,
27]. It is not unlikely that H
218O progression into the active site of the enzyme and its exchange with unlabeled water therein is slow, if the enzyme and active site are saturated with unlabeled water. This might be one of the reasons why Khangulov et al. did not detect an
18O-labeled CO
2 product in their approach [
21].
Here, we reinvestigated the oxygen atom transfer from H
2O
18 to formate yielding C
18O
16O and present the identification of bicarbonate as the first intermediate in the reaction of formate oxidation, before CO
2 is produced in the secondary reaction. We provide clear evidence that metal-containing FDHs catalyze a typical oxygen atom transfer (OAT) reaction. This work revises the 25-year-old hypothesis that FDHs represent an exception in the family of Mo/W-containing enzymes by catalyzing a heterolytic C-H bond cleavage reaction instead of OAT [
21]. We also show for the back reaction, that CO
2 and not bicarbonate enters the active site, likely by the proposed hydrophobic channel, but at the active site, first bicarbonate is formed likely in a carbonic anhydrase-like reaction before formate is released, as evidenced by using the enzyme soaked in H
218O labeled water and a
13CO
2 saturated buffer, which resulted in the production of H
13C
16O
18O
− labeled formate.
3. Discussion
CO
2 is a kinetically and thermodynamically stable molecule, with a high negative reduction potential value for the CO
2/HCOOH pair (highly pH dependent), all of which render its activation and reduction difficult tasks [
1]. For the biological reversible conversion of CO
2 to formate, prokaryotes and eukaryotes use FDH enzymes [
2,
3]. FDHs are a heterogeneous and broadly distributed group of enzymes that evolved to be part of diverse metabolic pathways, most notably the generation of energy from formate oxidation by coupling it to the reduction in several terminal electron acceptors, or the reduction in CO
2 into formate catalyzed by some prokaryotic organisms. FDHs belong to two major classes, the metal-dependent and the metal-independent ones [
9]. The metal-dependent ones are only present in prokaryotes and were shown to catalyze the oxidation of formate with higher catalytic efficiencies as compared to the metal-independent enzymes [
13]. In particular, metal-dependent enzymes are much better catalysts for the reduction in CO
2, and for a long time it was believed that metal-independent enzymes are not even able to catalyze the back reaction [
10]. Metal-dependent enzymes belong to the class of Mo- or W-containing enzymes bearing the bis-MGD cofactor [
11]. This class of Mo- and W-containing enzymes was first shown by Holm and coworkers in the 1980s to catalyze classical oxygen atom transfer (OAT) reactions [
42]. In OATs, the oxygen atom from water is transferred to the substrate which is oxidized, or in the opposite direction, from the substrate to yield water; these reactions are coupled to the reversible transfer of two electrons and two protons in the course of the transformation cycles [
22]. The electrons are directly transferred to the Mo/W metal ion of the cofactor and the metals cycle between the Mo/W
IV and Mo/W
VI oxidation states, with Mo/W
V as the intermediate state. FDH enzymes were considered to be an exception in the group of molybdenum- and tungsten-containing enzymes for catalyzing direct hydride abstraction from the parent carbon atom instead of an oxygen atom transfer [
2,
13,
18,
21]. Such exceptional behavior was mainly proposed based on a report by Khangulov et al. in 1998 [
21], concluding that the immediate product of formate oxidation is CO
2 and not bicarbonate. They used an experimental setup with
18O-labeled water and
13C-labeled formate and observed only
13C
16O
2 as an initial product of the reaction. This experiment and its outcome have never really been questioned since and resulted in the proposal of numerous mechanisms for FDH-catalyzed formate oxidation without considering any OAT transitions [
2,
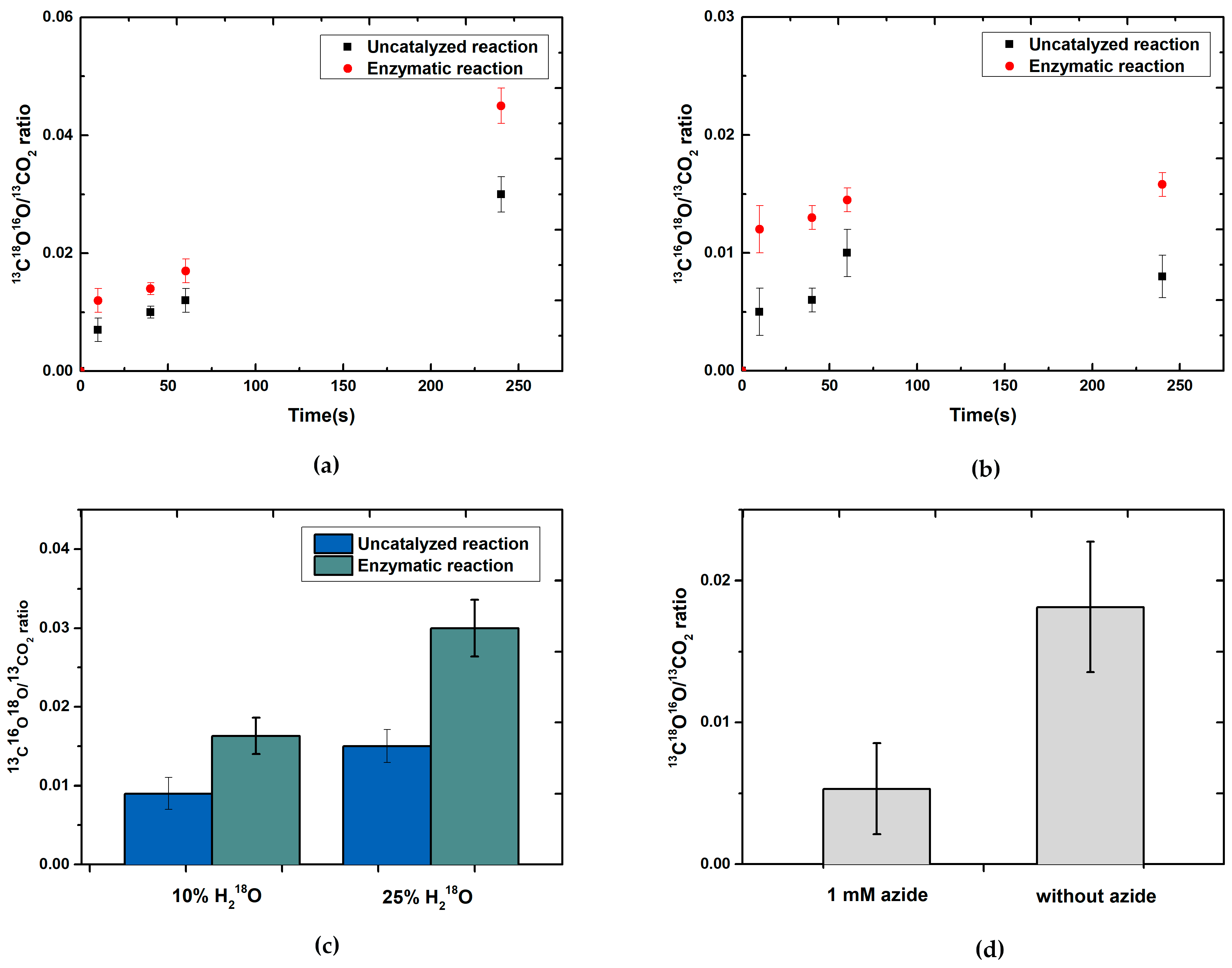
10]. However, a uniformly accepted, undisputed reaction mechanism has not been put forward as of yet. Since the enzyme in the Khangulov et al. experiment was inhibited by relatively high amounts of azide [
21], which was used to slow down the reaction, we decided to reinvestigate the experiment in the absence of azide. We used
R. capsulatus FDH instead of
E. coli FDH, an enzyme that we characterized in detail before and which is much more oxygen-tolerant compared to the
E. coli FdhF enzyme [
17]. This enabled us to use low/no azide concentrations with the enzyme for the experiments. We performed the formate oxidation assay at 10 °C to particularly slow down the secondary reaction of non-enzymatic CO
2 hydration [
24]. Our data clearly show that after a reaction time of 10 s, labeled
13C
18O
16O was readily detectable, demonstrating that the oxygen of H
218O water is, in fact, inserted into the product. The enzyme-catalyzed reaction was much faster as compared to the secondary hydration of CO
2 under our experimental conditions, as evidenced by respective control reactions.
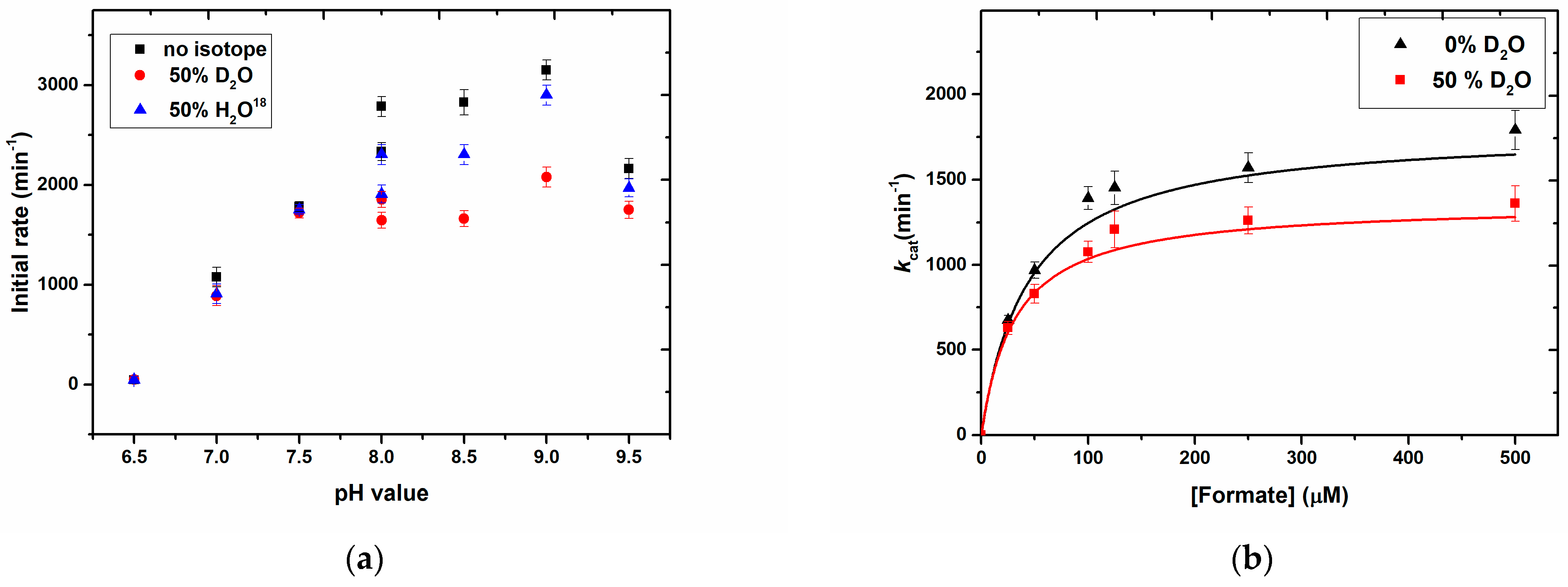
We further show solvent kinetic isotope effects on the reactions using D
2O, H
218O, and DCOO
–, confirming the impact of water on the substrate transformation rates and, therefore, a mechanistic role of H
2O. It has been shown previously that the D of DCOO
– of formate is transferred to the sulfido ligand on the Mo-centre [
15,
30,
32], with which our results are entirely in accordance. To accurately determine the rate-limiting step of the reaction, more detailed investigations will be necessary during future studies. In our experimental setup, we worked with a 50% H
218O saturated buffer which reliably gave rise to the observed solvent kinetic isotope effect.
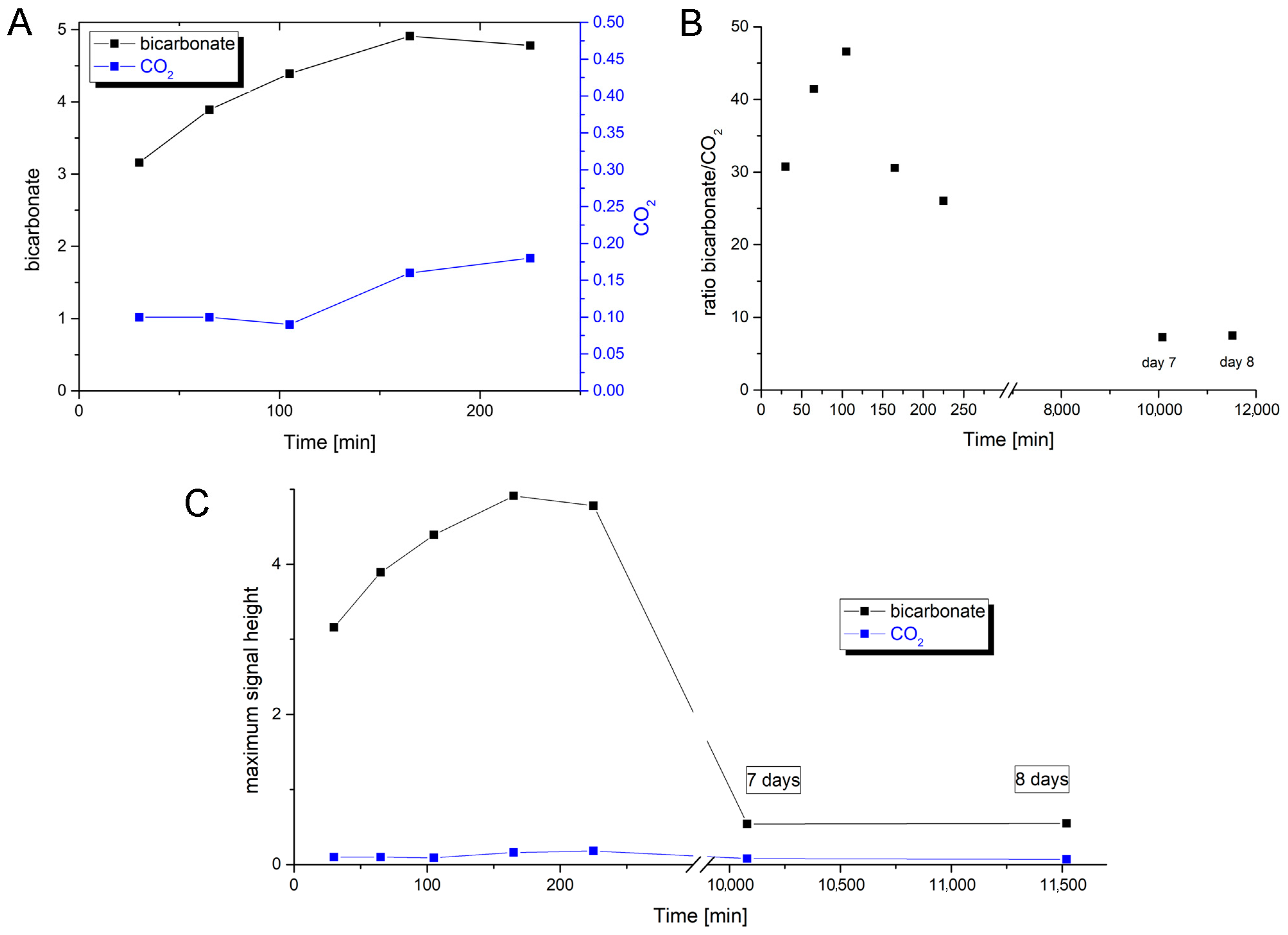
To further confirm the insertion of oxygen into H
13COO
–-labeled formate and to determine the formed product intermediate, we used NMR as a detection method. Since the reaction was too fast and the
13C-NMR measurements were relatively time-intense, we had to slow down the reaction with azide. The reaction was further performed at 5 °C to also decelerate the non-enzymatic hydration of CO
2. The first product that was detected by NMR in substantial abundance was bicarbonate, the formation of which already began to decrease before the abundance of CO
2 increased up to its maximum reaching even an oversaturation of the solution. In a previous report, the same observations were made, detecting bicarbonate as the first intermediate by NMR [
43]. The first data point, however, was drawn only after 25 min, which impedes disentangling the still quite fast enzymatic reaction and the subsequent secondary reaction of the CO
2/HCO
3– equilibrium at pH 9.0. Therefore, in order to receive even more coherent data, we used
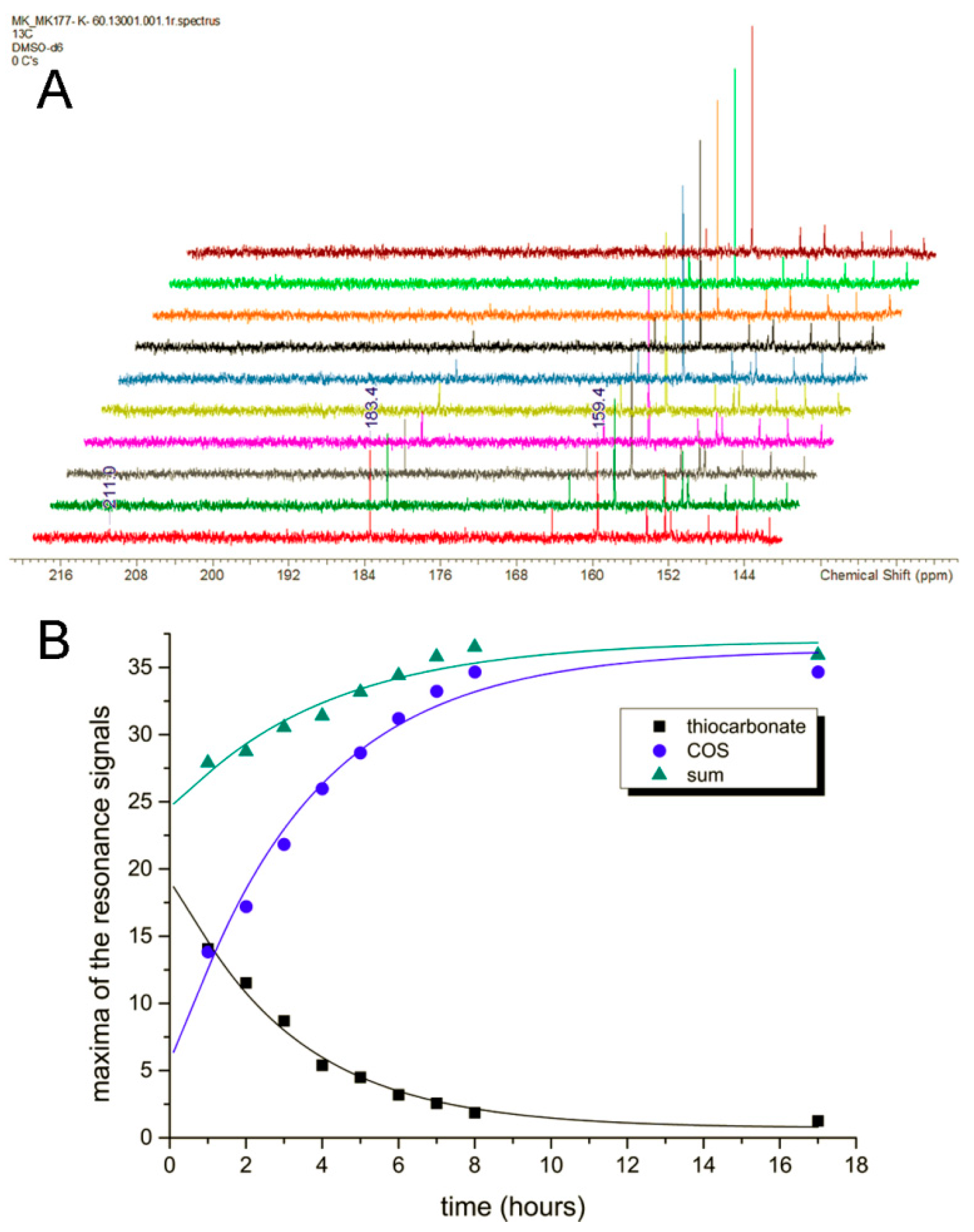
13C-labeled thioformate in the experiment, which was shown to be a suitable substrate of
R. capsulatus FDH in the bisubstrate kinetic experiments. When
13C-labeled thioformate was used in the NMR experiment with low azide concentrations (remnants of protein preparation), thiocarbonate was clearly detected as the first intermediate, the abundance of which then decreased while COS abundance increased steadily. Here, thiocarbonate is detected instead of thiobicarbonate, based on the fact that thiobicarbonate is more acidic than bicarbonate, less stable at this pH, and easily deprotonated. This clearly confirms the oxygen atom transfer with bicarbonate/thiocarbonate as reaction intermediates of the reaction, before CO
2 or COS are formed in a secondary follow-up reaction inside the enzyme and/or a non-enzymatic secondary reaction outside the enzyme. We further investigated the back reaction of CO
2 reduction to clarify whether bicarbonate enters the enzyme (preferentially) or whether CO
2 does instead, followed by bicarbonate formation at the active site. Using a
12CO
2 saturated buffer in the presence of a H
218O saturated enzyme, we obtained H
12C
18O
16O
−labeled formate in the first 10 s of the reaction, showing that CO
2 is the primary substrate that enters the enzyme, which is then converted to a H
12C
18O
16O
2 bicarbonate at the active site (possibly in a carboanhydrase-like hydration reaction). This bicarbonate is then used as the actual substrate for the reduction to formate and water. When H
13CO
3− was used as the only substrate, no
18O-labeled formate was detected. Likely, bicarbonate is not used as a direct substrate in the back reaction because it cannot enter the enzyme through the hydrophobic CO
2 channel. Or, the entrance is hindered by the histidine, since structural changes were observed in oxidized and reduced X-ray structures [
26]. In the NMR experiment, using
13C-labeled formate, bicarbonate was detected as an immediate intermediate product and much higher in abundance than the enzyme, suggesting it may be released from the enzyme. In this reaction, an azide-inhibited enzyme was used. For carbonic anhydrase, it was reported that azide is a competitive inhibitor for bicarbonate dehydration [
44], while it is a non-competitive inhibitor for CO
2 hydration. For
E. coli FdhF, inhibition studies with azide showed that azide acts as a competitive inhibitor for formate oxidation and as a non-competitive inhibitor for the reduction in CO
2 [
30], with the inhibitor being more competent toward the oxidation of formate. For
RcFDH, it was recently shown that azide acts as a mixed-type (competitive, non-competitive) inhibitor for both formate and CO
2 [
45,
46]. It was concluded in the study of the
E. coli enzyme that the inhibitors bind differently to the reduced and oxidized forms of the enzyme. Assuming a similar inhibition mechanism for azide with bicarbonate/CO
2 in
R. capsulatus FDH, we propose that in the reaction of formate oxidation, the bicarbonate binding site is competitively blocked by azide so that the dehydration of bicarbonate is inhibited and bicarbonate is released instead of CO
2, likely through the formate channel. The formate channel might be suitable for more charged substrates, such as formate, nitrate, and bicarbonate, while the CO
2 channel is specific for CO
2 and cannot be used by bicarbonate [
25]. Redox, charge, and protonation states of the active site should all have an impact on the attraction or repulsion of charged and uncharged substrates/products. Therefore, the presence/absence of substrates, products, inhibitors, reducing, and/or oxidizing agents might have gatekeeper function(s) for either of the substrates. Further, after reduction in the enzyme with formate, conformational changes in the second coordination sphere were observed in the crystal structures of the
D. vulgaris FDH enzyme [
23] and the reinterpreted structure of the
E. coli FdhF enzyme [
47]. We, therefore, hypothesize that by the structural rearrangement in the formate reduced enzyme, the formate funnel becomes accessible for the release of bicarbonate, an exit site that is blocked by the histidine in the oxidized enzyme or inhibited enzyme. We assume that the site for CO
2 hydration and bicarbonate dehydration is close to or at the conserved arginine residue in the second coordination sphere, since in the crystal structures of the
D. vulgaris [
23] and formyl-methanofuran dehydrogenase from
Methanothermobacter wolfeii [
27], a water molecule was identified to reside in the vicinity of this residue. In previous studies, it was also speculated that azide is bound to this residue in the active site, matching our hypothesis that this is the non-competitive binding site for azide and bicarbonate [
32,
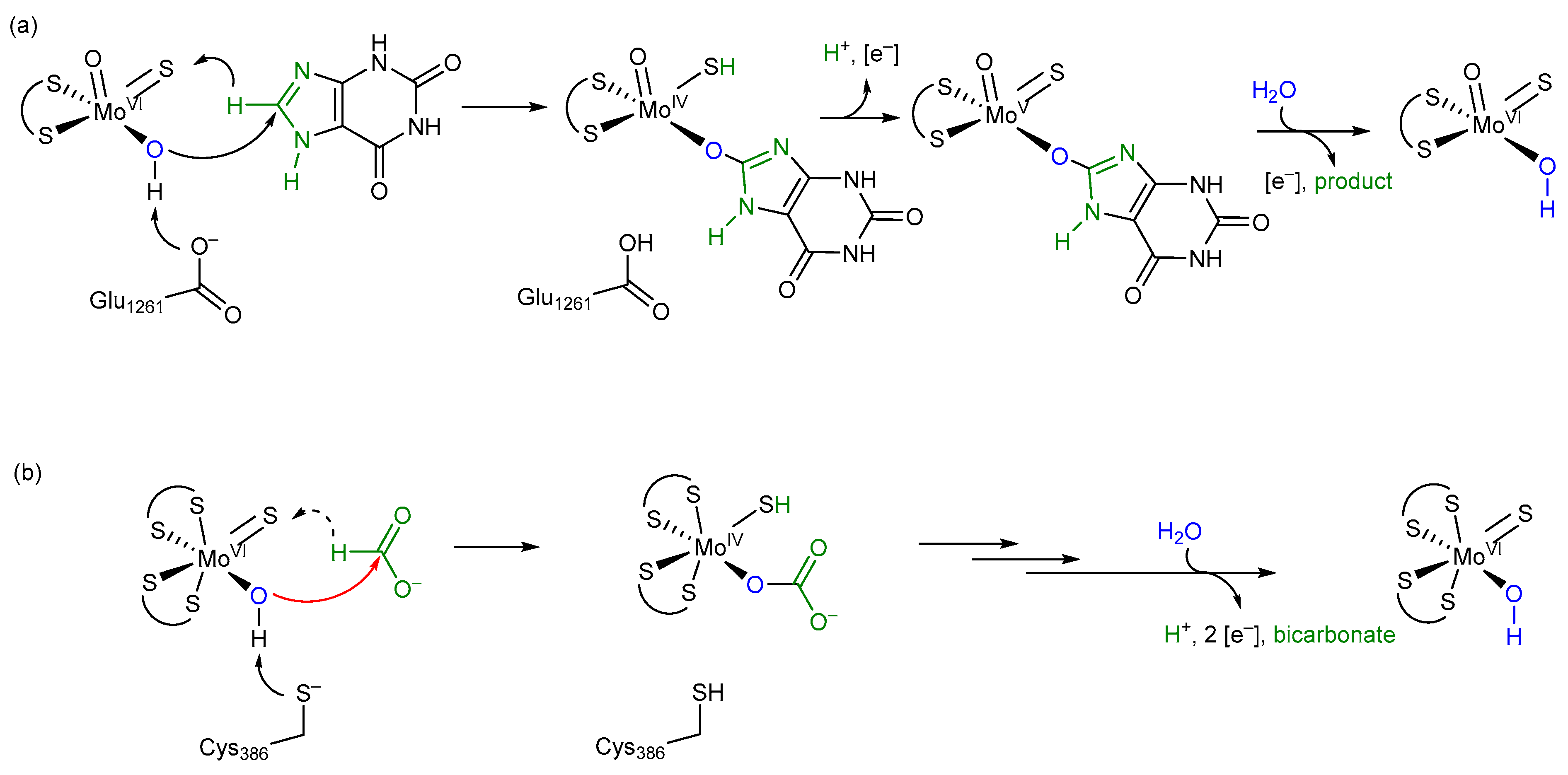
45]. Overall, we want to propose the oxygen atom transfer mechanism for the reversible oxidation of formate by metal-containing FDH enzymes, as shown in
Figure 8, which shows similarities to well-accepted enzyme mechanisms, such as the one for xanthine oxidase. We think that all metal-containing FDHs would work after this mechanism and that there are no differences in W- or SeCys-containing enzymes
Considering the difference between formate and xanthine at the site of enzymatic transformation being only the presence of two N functionalities versus two oxygen functionalities and a relatively similar bonding situation thereof, the oxygen atom transfer mechanism of FDH is tentatively proposed to resemble that of XO (xanthine oxidase), with the –OH groups of the initial species stemming from water [
22] (
Figure 8). All protonation or deprotonation states of substrates, amino acids, and ligands will be directly dependent on the concentration of protons in the active site, i.e., its pH value. For a more detailed mechanistic insight into the actual individual transformative steps, a number of further experiments will need to be carried out.
The release of the bicarbonate product, one proton, and two electrons from the inner active site composition regenerates the species with which this cycle starts. The key step of this proposed mechanism constitutes a typical and common oxygen atom transfer, as implied by our presented new data and as proposed for xanthine oxidase. This mechanism is in accordance with all undisputed experimental data available, stoichiometrically balanced and, hence, coherent.
In our mechanism, we propose that after formate binding the amino acid ligand at the Mo atom is displaced by water, which might be facilitated by a conformational change in the enzyme upon formate binding. The displacement of the cysteine ligand in
R. capsulatus FDH has been shown by us in previous studies by iodoacetamide labeling of the nitrate-inhibited and formate-reduced enzyme and by EXAFS studies [
13,
14]. The iodoacetamide labeling of the selenocysteine ligand was also shown for the
E. coli enzyme [
13]. However, not all enzymes are inhibited by iodoacetamide, e.g., the
D. vulgaris Hildenborough enzyme [
23]. In this enzyme, no carboxamidomethyl labeling of the selenocysteine ligand was observed. Notably, in this experiment, a nitrate and a glycerol-inhibited enzyme were used, and in particular glycerol might interfere with the accessibility of iodoacetamide to this enzyme [
23]. Further, selenocysteine-containing enzymes are more oxygen sensitive, and it remains possible that after the displacement of the selenocysteine ligand, the selenocysteine is easily oxidized and the oxidized SeO
2 or SeO
3 species do not react with iodoacetamide. We also do not exclude that distinct FDH enzymes have different accessibility for iodoacetamide, e.g., by variations of amino acids in the formate-binding funnel. EXAFS studies of the
R. capsulatus enzyme further proved displacement of the cysteine residue in the formate-reduced enzyme by an oxygen atom (which can be the one from water as shown in this study). In the EXAFS data of the azide or cyanate-inhibited enzyme, instead of cysteine sulfur a light atom was observed to be bound to the molybdenum center [
28], which we assigned to be an oxygen atom from water rather than a C or N atom from the inhibitors which are proposed to not directly bind to molybdenum. The binding of water observed in the EXAFS studies supports our oxygen atom transfer mechanism. In contrast, for the
E. coli FdhF and
D. vulgaris enzymes, EXAFS and crystallographic studies did not support displacement of the selenocysteine ligand [
23,
48]. We consider it likely that the
E. coli enzyme was oxidatively damaged and the sulfido ligand was lost in the enzyme preparation for the EXAFS studies, while in the formate reduced structure of the
D. vulgaris enzyme, the oxidation state of the molybdenum atom is not clear and the crystalized enzyme might be in the re-oxidized Mo
VI state after product release since neither the product nor the substrate was present in the structure. Often, EPR studies are considered as an argument for the oxidation state-dependent active site structure of the enzyme. However, the Mo
V active site constitutes an intermediate state after product release and one electron oxidation in which the amino acid ligand is quite likely to rebind again to the molybdenum atom which otherwise would be coordinatively unsaturated [
18,
49]. In previous studies, several groups proposed a hydride transfer mechanism with the formate being bound within the second coordination sphere of the active site metal [
15,
50]. One of the arguments used by the authors to support their mechanism was that the second p
Ka value of formic acid (i.e., the one for C-H dissociation) disfavors a proton abstraction and the resulting carbanion would be unstable. In our mechanism, the formate is directly bound to the molybdenum atom through an oxido function derived from a water molecule, so a carbanion would not be formed after proton abstraction [
2]. Nevertheless, in our mechanism, we leave it open whether the hydrogen of formate is transferred as a hydride or in a proton-coupled electron transfer reaction to the sulfido ligand, forming the SH group and the reduced Mo
IV (i.e., we do not propose the direction in which the electrons or electron pairs move when entering the transition state). The sulfido group as a hydride (or proton) acceptor has been well-established in XDH enzymes [
51] and was proven in FDH enzymes by EPR studies to contain a strongly coupled, solvent exchangeable and substrate-derived proton with a hyperfine constant of 20–30 MHz, which is consistent with the hydrogen atom from the formate being transferred to this ligand in the first coordination sphere of the molybdenum upon reduction. This observation is also consistent with our model reaction mechanism; however, it does not prove that the H-atom is transferred as a hydride.
Meneghello et al. (2021) [
20] recently chose an electrochemical approach with the tungsten-containing FDH from
Desulfovibrio vulgaris Hildenborough for confirming the substrate of FDHs for the back reaction of CO
2 reduction being CO
2 rather than HCO
3−, as previously and repeatedly concluded before. However, we want to point out that product formation was not measured in this study and that in their experimental setup, the enzymes might have been washed away; therefore, their results need to be taken with caution. As already emphasized by Cooper et al. (1968) [
24] and confirmed by our investigation, respective studies do not necessarily reflect what is happening directly at the active site. We, therefore, propose that for the reduction of CO
2 the intermediate ionic species HCO
3− is hindered from entering the reduced active site via the hydrophobic CO
2 channel that has been identified in crystal and cryo-EM structures, while CO
2 instead can easily enter. This is still consistent with the report by Meneghello et al. (2021) [
20]. At the active site, CO
2 then reacts with H
2O to form HCO
3− which subsequently provides the direct substrate for the back reaction resulting in formate and water formation through oxygen atom transfer from bicarbonate. In conclusion, we also want to emphasize that mechanistic studies of metal-containing FDH enzymes need to be performed in the absence of any inhibitor under strictly anaerobic conditions.
4. Materials and Methods
4.1. Chemicals and Reagents
Elemental potassium was purchased from Sigma-Aldrich (St. Louis, MI, USA).
13C-labeled formic acid (
13C, 99%) was purchased from Cambridge Isotope Laboratories, Inc. (Tewksbury, MA, USA). Phenol was purchased from Fisher Scientific (Waltham, MA, USA). POCl
3 was purchased from Acros (Geel, Belgium). Penta fluorobenzyl bromide (PFBBr) was purchased from Alfa Aesar (Haverhill, MA, USA). Potassium hydrogenphosphate and potassium dihydrogenphosphate were purchased from Sigma-Aldrich. Potassium dithioformate was prepared according to a literature method [
52].
4.2. Synthesis of Monothioformate
First, the starting materials KHS and phenyl formate had to be synthesized.
(i) Synthesis of potassium hydrogen sulfide (KHS). Potassium hydrogen sulfide, according to literature reports, is most commonly synthesized by the reaction between a solution of potassium sulfide with excess dihydrogen sulfide. However, the such-synthesized potassium hydrogen sulfide is not completely pure. To avoid water and any other impurities, the following modified procedure was used to synthesize dry and pure potassium hydrogen sulfide. A total of 12 mL of dry ethanol was charged in a pre-evacuated flask which was equipped with a stirrer and bubbler under nitrogen. The flask was cooled to −78 °C (a mixture of isopropanol and liquid nitrogen was used as a cooling bath). Then, 1.7 g of potassium was inserted into the flask gradually and cautiously in small portions over a period of 2 h. When all of the potassium particles had dissolved, dry gaseous dihydrogen sulfide which was produced through a Kipps apparatus and dried over CaCl2, which was passed through the reaction mixture for about 4 h. As soon as the passing of dihydrogen sulfide started, the formation of a white precipitate could be observed. The passing of dihydrogen sulfide was continued until the end of precipitation. Then, the excess gaseous dihydrogen sulfide was removed by bubbling nitrogen through the solution. The solid compound was dried under a vacuum for 3 h. Elemental analysis (calcd). S 44.43, H 1.397; found S 43.11, H 1.80; IR (KBr): ν cm–1 = 2520 (w), 2100 (br), 1650 (s), 1400 (br), 1250 (w), 1150 (m), 1000 (s), and 700 (w).
(ii) Phenyl formate was synthesized with small modifications to a literature procedure [
53]. A total of 1.2 eq of
13C-labeled formic acid (21.2 mmol, 0.975 g) was mixed with 1 eq of phenol (17.67 mmol, 1.66 g) in a three-neck round bottom flask under a nitrogen atmosphere. The reaction mixture was stirred for 3 h at 80 °C under nitrogen. Then, the reaction mixture was cooled to room temperature and 0.33 eq of POCl
3 (5.83 mmol, 0.89 g) was added slowly and dropwise. The reaction mixture was poured slowly into a solution of sodium bicarbonate in ice water. The resultant solution was then extracted three times with diethyl ether (40 mL). The combined organic phases were dried over sodium sulfate, filtered, and the solvent was removed from the filtrate with a rotary evaporator at a low temperature to obtain crude phenyl formate. Phenyl formate is not stable and should be used immediately for the following step.
1HNMR (CDCl
3, 400MHz, 298 K): δ: 8.37 ppm (1H, CH), δ: 7.43 ppm (2H, CH-Ar), δ: 7.26 ppm (1H, CH-Ar), δ: 7.07 ppm (2H, CH-Ar);
13CNMR (CDCl
3, 400MHz, 298 K): δ: 161.12 ppm (1C, CH), δ: 155.54 ppm (1C, C), δ: 115.75 ppm (2C, CH), δ: 130 ppm (2C, CH), and δ: 121.10 ppm (C, CH).
(iii) Potassium monothioformate was synthesized with small modifications to a literature procedure. A total of 1 eq of phenyl formate (18 mmol, 2.20 g) was mixed with 1 eq of dry KHS (18 mmol, 1.30 g) in a Schlenk flask under nitrogen. The Schlenk flask was placed into an ultrasonic bath at 0 °C for 2 h. Then, 2 mL of dry methanol was added to the Schlenk flask and the mixture was stirred for another 2 h. After the addition of 20 mL of dry diethyl ether, the reaction mixture started to form a precipitate. After precipitation stopped, anaerobic filtration was carried out under a nitrogen atmosphere. The precipitate was washed with small amounts of cold methanol and dried under a vacuum to yield a fine pale yellow powder. 1HNMR (CDCl3, 400MHz, 298 K): δ: 9.8 ppm and δ: 10.4 ppm (1H, CH) and 13CNMR (CDCl3, 400MHz, 298 K): δ: 211.11 ppm (1C, CH). Two resonances were detected due to the tautomerization effect. MS (ESI): m/z = 101.4 [M] H13COSK. IR (KBr): νmax = 2580 (C-H stretching), 1630 (C=O stretching), 1390 (C-H bending), 812 (C-S). Elemental analysis (calcd). C 11.99, H 1.01, and S 32.00; found C 11.96 and H 1.54; S 31.41.
4.3. NMR Spectroscopy
NMR spectra were recorded on a Bruker Avance II 300 spectrometer (300, 75, and 121.5 MHz, respectively). Chemical shifts (δ) are given in parts per million (ppm) using solvent signals as a reference (DMSO-d6 1H: δ = 2.50 ppm; 13C: δ = 39.52 ppm; CD3OD 1H: δ = 3.31 ppm; 13C: δ = 49.15 ppm) relative to external tetramethylsilane (δ = 0 ppm).
4.4. Preparation of the Time-Dependent NMR Measurement Sample for the Enzymatic Reaction of RcFDH with 13C-Labeled Sodium Formate
In order to prepare the nicotinamidadenindinucleotide stock (150 mM), 99.5 mg of NAD+ was dissolved in 1 mL of a tris(hydroxymethyl)aminomethane buffer (75 mM, pH: 9). For preparing the sodium azide stock (50 mM), 3.25 mg of sodium azide was dissolved in 1 mL of a buffer. After the preparation of the stocks, 290 µL of NAD+ stock was charged in a new vial. Then, 100 µL of labeled sodium formate (1.62 M; 11.2 mg in buffer at pH 9), 24 µL of sodium azide, and 150 µL of a buffer were added, respectively. Finally, and immediately before the NMR measurement series started, 60 µL of an enzyme (360 µM; for preparation see below) was inserted into the reaction mixture. A total of 0.5 mL of the prepared mixture was transferred quickly to the NMR tube. Deuterated methanol (CD3OD) was used as the internal standard in an insert tube (i.e., not mixed with the sample solution). The 13C time-dependent measurements were started immediately without filtration of the sample at 5 °C and run in automation overnight.
4.5. Preparation of the Time-Dependent NMR Measurement Sample for the Enzymatic Reaction of RcFDH with a 13C-Labeled Monothioformate
NAD+ stock solution was prepared as described above. A total of 290 µL of NAD+ stock was charged in a new vial. Then, 100 µL of monothioformate (1.12 M; 11.2 mg in buffer at pH 9) and 150 µL of water were added. Finally, 60 µL of an enzyme (360 µM) was added to the sample mixture immediately before the measurements started. A total of 0.5 mL of the prepared mixture was transferred quickly to the NMR tube. Deuterated DMSO-d6 (dimethylsulfoxide) was used as the internal standard in an insert tube (i.e., not mixed with the sample solution). The 13C time-dependent measurements were started immediately without filtration of the sample solution at room temperature (25 °C operation temperature in the NMR laboratory) allowing for continuous monitoring of the transformations.
4.6. Control Measurements for the Thioformate Substrate Series
The same procedure as described above was followed with regard to sample preparation, except that one component or more were left out (
Table 4). The total volume of the reaction mixture was 600 µL in all cases, of which 5 mL was transferred to the NMR tube. The solution volume with the ingredient not included in the control mixture was replaced by the buffer to reach the total volume of 600 µL.
In none of the control condition experiments could any progress of the enzymatic reaction be observed. The resonances at 183.4 ppm for thiocarbonate and 159.4 ppm for COS remained absent. Although NAD
+ has many
13C-NMR signals in a broad range of the spectrum, these do not interfere with the signals of the enzymatic transformation of interest. In contrast to all other components of the reaction, FDH enzyme concentration is so low that no respective signals could be observed. Tris(hydroxymethyl)aminomethane (THAM) was used for the preparation of the buffer (pH = 9), which has two resonances in the
13C-NMR spectrum (56.14 ppm and 61.92 ppm). An additional coupling
13C-measurement (4 h duration) for the reaction mixture bearing all components proved that the intermediate at 183.4 ppm is not showing any splitting in the
13C {H} coupled NMR. This means that there is no proton in scalar coupling with this carbon atom, which further supports an assignment to the thiocarbonate dianion (
Figure S5).
4.7. The Expression and Purification of R. capsulatus FDH
R. capsulatus FDH was expressed in E.coli MC1061 cells containing plasmids pTHfds05 and pTHfds07 obtained from a previous study [
17] and purified according to the published procedure [
25]. The enzyme was stored in a 75 mM Kpi buffer containing 10 mM sodium azide, pH 7.5, and before usage of the enzyme in the assays, the buffer was exchanged with a 75 mM Kpi buffer, pH 7.5, using PD-10 desalting columns (Sephadex G-25 M; Amersham Biosciences) to remove azide.
4.8. 13C-Labeled Formate Oxidation by RcFDH in the Presence of 18O-Labeled Water
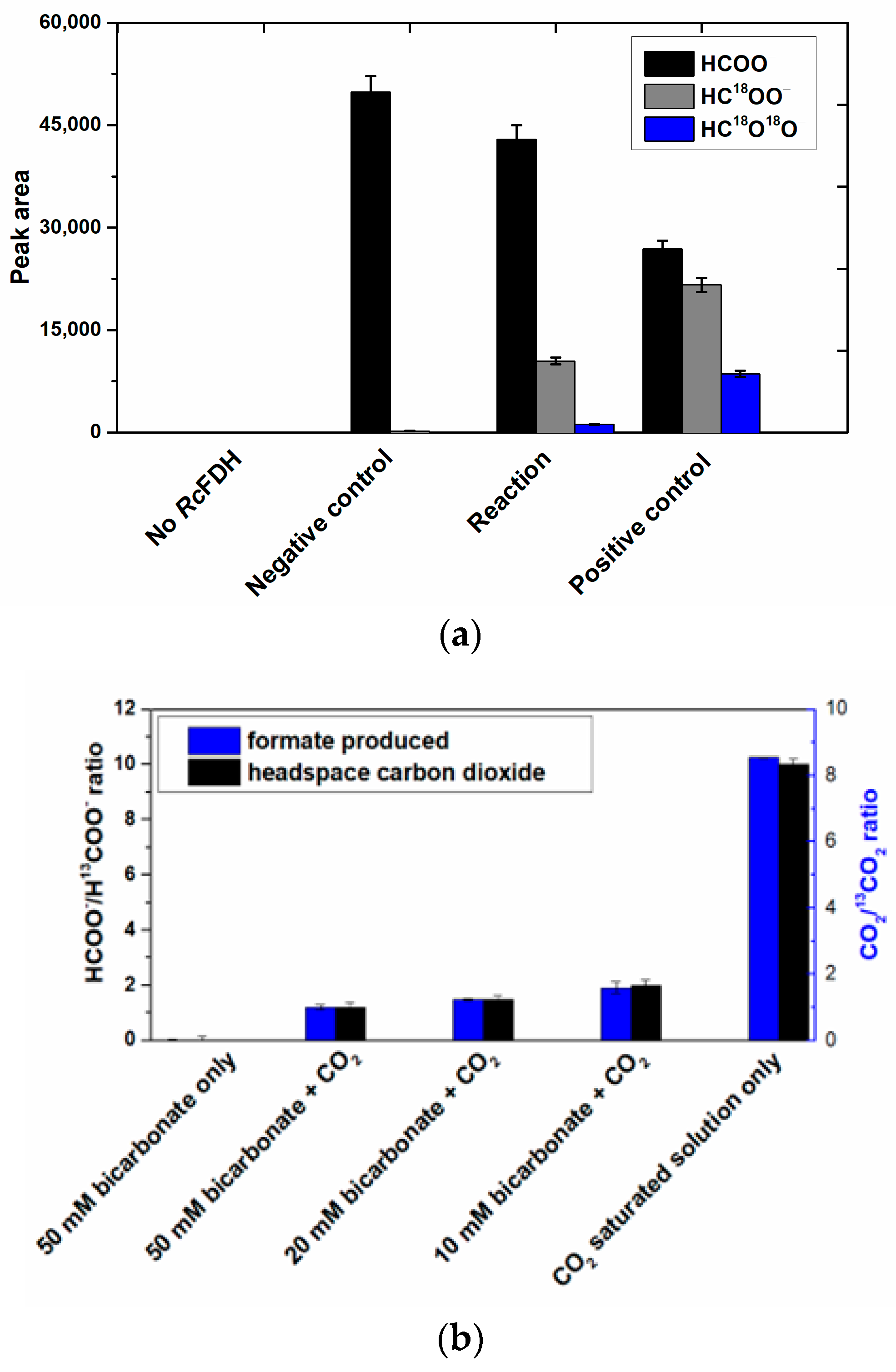
The oxidation of 13C-labeled formate by RcFDH in the presence of 18O-labeled water was carried out to analyze the resultant CO2 isotope composition. The reaction mixture of 100 µL was prepared in a glass insert containing a GC vial by mixing 10 mM 13C-labeled formate and 5 mM NAD+ anaerobically in 100 mM Tris-HCl, pH 9. R. capsulatus FDH, pre-mixed with 18O-labeled water, was anaerobically transferred to a syringe. The final concentration of the enzyme and 18O-containing water were 10–30 µM and 10–50%, respectively. The reaction was started by injecting the assay mixture into the closed GC vial at 10 °C. For control reactions, up to 100 mM NaH13CO3 was used as a 13CO2 source. All the reagents were pre-incubated at the required temperatures beforehand. Headspace samples were taken from the vial by the autosampler after the indicated time intervals. The isotopic composition of produced 13CO2 was analyzed by using GC-MS QP2010 SE (Schimadzu) modified for headspace samples. Sample volumes of 1–5 µL were used in the DB-WAX UI column (30 m × 0.32 mm × 0.25 µm, Agilent). The injection temperature was kept at 200 °C. The temperature program for analysis consisted of (i) 30 °C for 3 min; (ii) from 30 °C to 200 °C at 30 °C/min; (iii) 200 °C for 1 min; and (iv) from 200 °C to 30 °C at 30 °C/min.
For MS analysis, the selected ion monitoring (SIM) mode was used due to its high sensitivity. A detector voltage of +0.3 kV relative to tuning with the ion source and interface temperature of 200 °C was applied each. The MS analysis method was used to look for
m/
z values corresponding to the
13C
16O
16O,
13C
16O
18O, and C
13O
18O
18 isotopomers of CO
2. Different concentrations of NaH
13CO
3 solutions were used to confirm the correct
m/
z values and to create a calibration curve (
Figure S9).
4.9. Derivatization and Analysis of the Formate Using GC-MS
The formate was derivatized by modifying a previous method [
54] using 2,3,4,5,6-pentafluoro-benzylbromide(PFBBr). A total of 100 µL of enzyme-free samples were mixed with 50 µL of a 325 mM phosphate buffer, pH 8.5. To this, 365 µL of 100 mM PFBBr solution prepared in acetone was added. The solution was vortexed for 1 min and heated at 60 °C for 20 min. After cooling down to room temperature, 500 µL of
n-hexane was added and vortexed for 1 min. Phases were separated by centrifugation at 13,000 rpm for 1 min and the upper organic phase was carefully pipetted into a 2 mL insert containing GC vials. The samples were analyzed by using GC-MS QP2010 SE (Schimadzu) modified for headspace samples. Sample volumes of 1 µL were used in the DB-WAX UI column (30 m × 0.32 mm × 0.25 µm, Agilent).
4.10. The Solvent Kinetic Isotope Effect Using Pre-Steady State Kinetics
The solvent kinetic isotope effect for the reductive half-reaction was studied using deuterated water (D
2O) in comparison to H
2O. An SX-20 stopped-flow spectrophotometer (Applied Photophysics, Inc., Leatherhead, UK) was used to study the reaction of the formate using purified R. capsulatus FDH. A total of 5 µM of FDH was anaerobically equilibrated in a 100 mM Kpi buffer, pH/pD = 7.9, to remove the azide present from the purification. pD of the deuterated buffer was adjusted 0.4 units higher in the pH meter (pD = pH + 0.4). Glucose oxidase (10 IU) and catalase (100 IU) were mixed with enzymes to remove the residual oxygen from the stopped-flow cell. The preparation was filled into a 10 mL glass syringe anaerobically. The second syringe was filled with the anaerobically prepared formate or deuterated formate solution (5 mM) in the same buffer and was containing 5 mM glucose. Since the stopped-flow instrument was not in an anaerobic chamber, additional modifications from Applied Photophysics, Inc. were adopted. Additional nitrogen was continuously purged around the syringe housings of the instrument. The water bath was purged with nitrogen for 30 min to remove oxygen. Both photomultiplier tube (PMT) and photodiode array (PDA) detectors were used alternatively depending on the type of experiment. The path length used for the experiments, unless mentioned otherwise, was 1 cm. The reaction of the formate with as-isolated
R. capsulatus FDH was performed as described by Niks et al. (2016) [
15] at 10 °C. The formate was used at saturating concentrations and enzyme concentrations used were between 2.5–15 µM, depending on the experiment. In the case of the PMT detector, changes at 450 nm were followed. In the case of the PDA detector, spectra were recorded between 280–700 nm wavelengths. The curves obtained at 450 nm were fitted to the sum of three exponentials using non-linear least square regression analysis using the following equation:
where
n represents the number of kinetic phases. Data analysis was performed using ProData Viewer 4.2.0 (Applied Photophysics, Inc.).
4.11. The Solvent Kinetic Isotope Effect at Different pH Values
The effect of solvent isotopes (D2O and H218O) at different pH was determined anaerobically by following the formation of NADH at 340 nm. A 100 mM phosphate buffer was used at pH values between 6.0 and 7.5 and a 100 mM Tris HCl buffer was used at pH values between 8.0 and 9.0. A D2O/H2O or H218O/H2O ratio of 0.5 was chosen for all the measurements by adding 50% D2O, H218O, or H2O to the assay mixture. The reaction volume of 100 µL contained 10 mM of formate, 2 mM NAD+, and the indicated buffer. The reaction was started by adding 25 nM FDH to the cuvette, followed by photometric detection at 340 nm for 60 s.
4.12. Steady State Kinetics
RcFDH kinetic assays were measured anaerobically using a UV-2401PC spectrophotometer (Shimadzu Europa, Duisburg, Germany) and following the formation of NADH at 340 nm (εNADH = 6220 m−1·cm−1). Steady state kinetics for monothioformate were determined by varying the concentrations of monothioformate (0.5–20mM) and NAD (0.05–2mM). In the case of formate, the concentration varied both for formate and NAD and was 0.05–2mM. The assay was always started with the addition of RcFDH (4–8 nM) and the NADH formation was monitored for 60 s. Data obtained were fitted with the Hill function ([y = Vmax × Xn/(Kmn + Xn)], n = 1) and Origin software (OriginPro 8.1G SR1; OriginLab Corporation, Northampton, MA, USA).