Interfaces and Oxygen Vacancies-Enriched Catalysts Derived from Cu-Mn-Al Hydrotalcite towards High-Efficient Water–Gas Shift Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Hydrotalcite Samples
2.2. Catalyst Characterization
2.3. Catalytic Testing
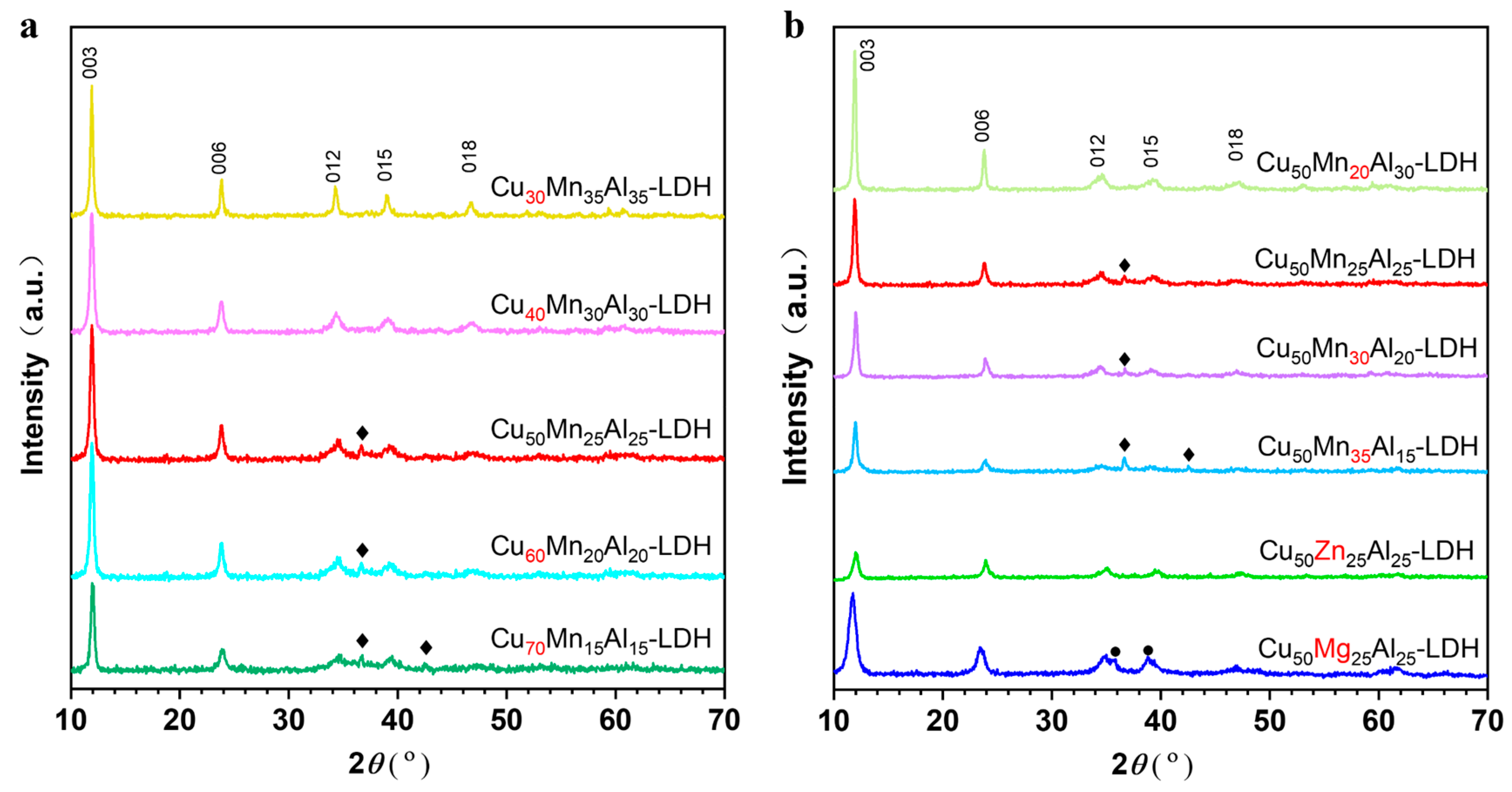
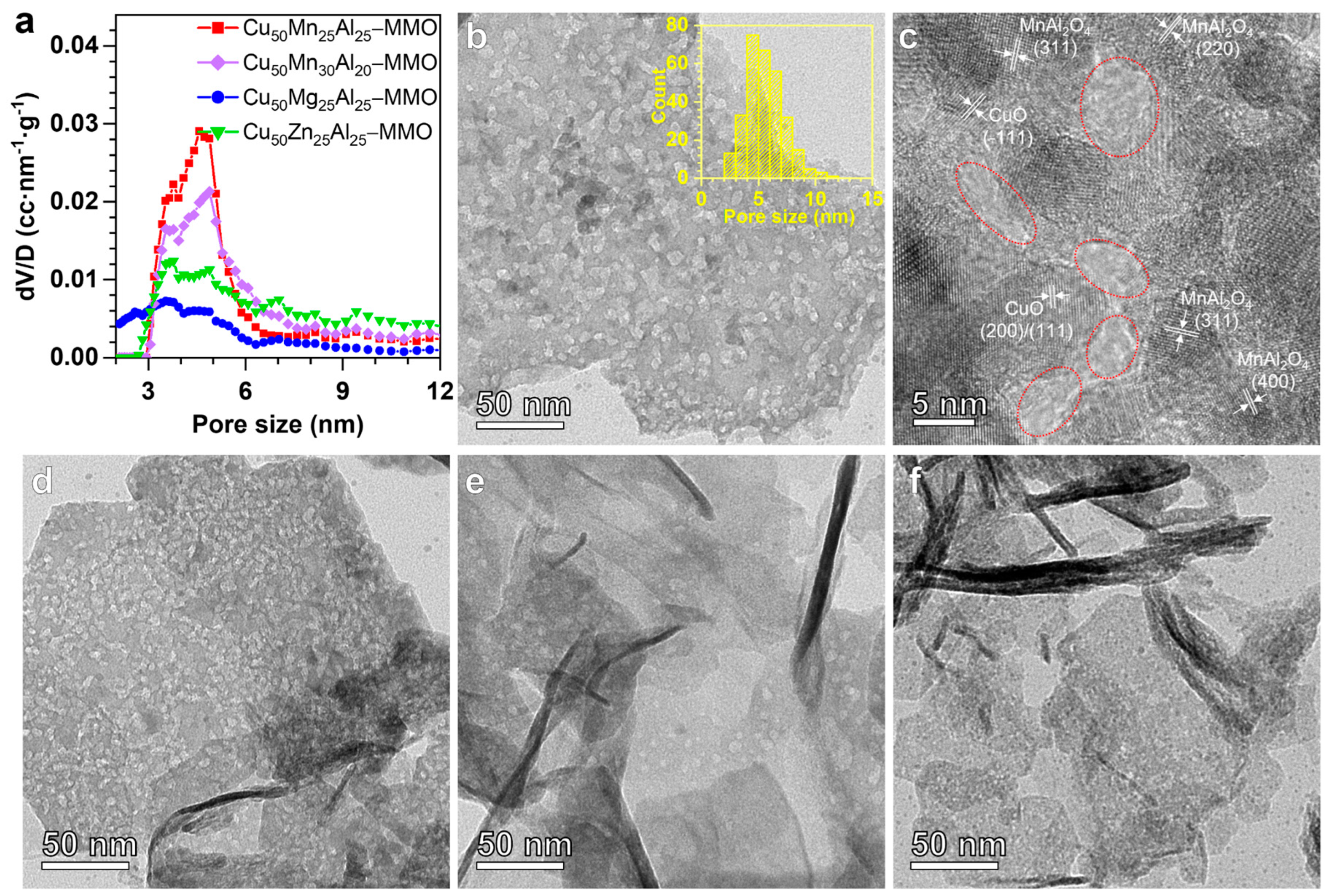
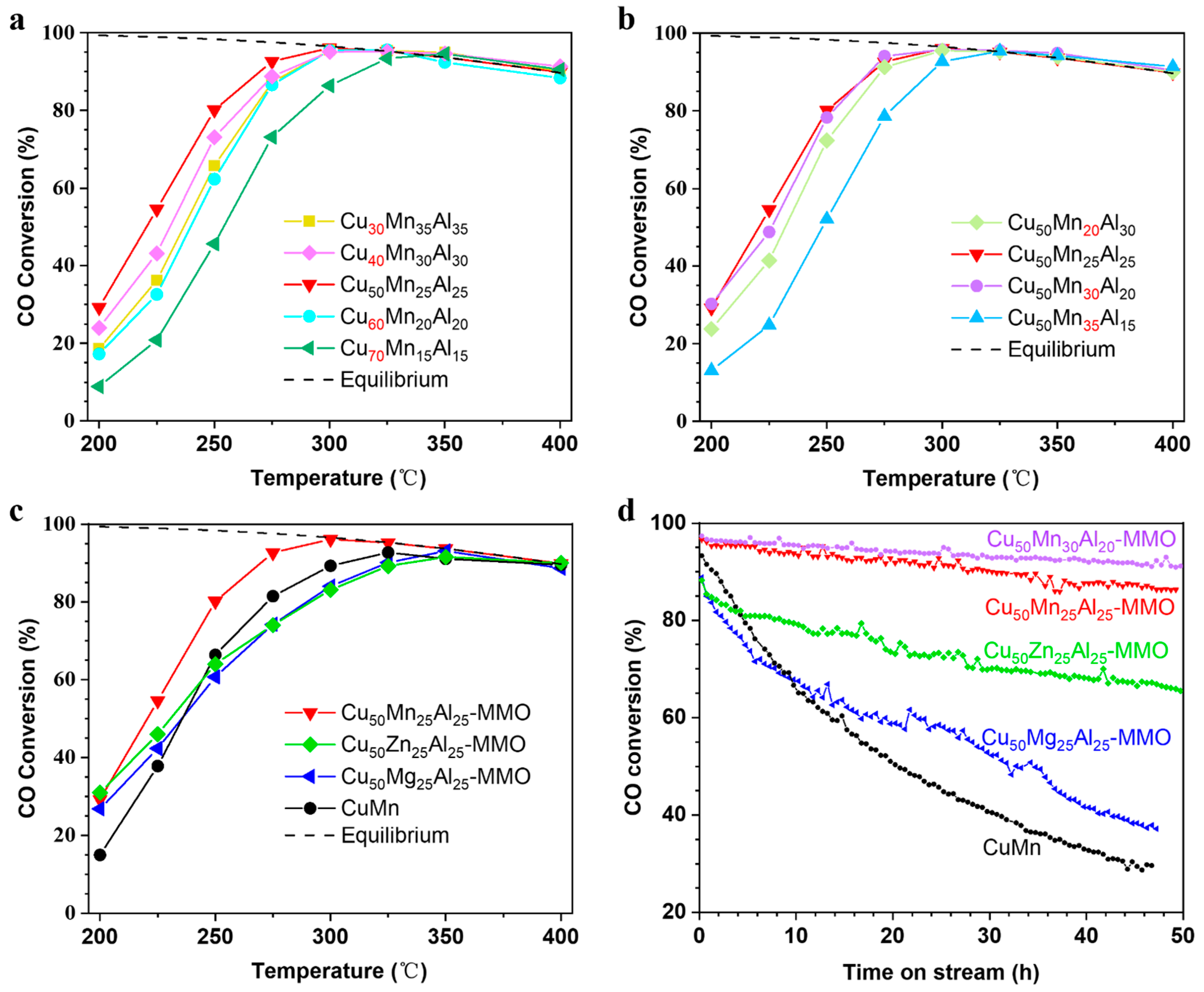
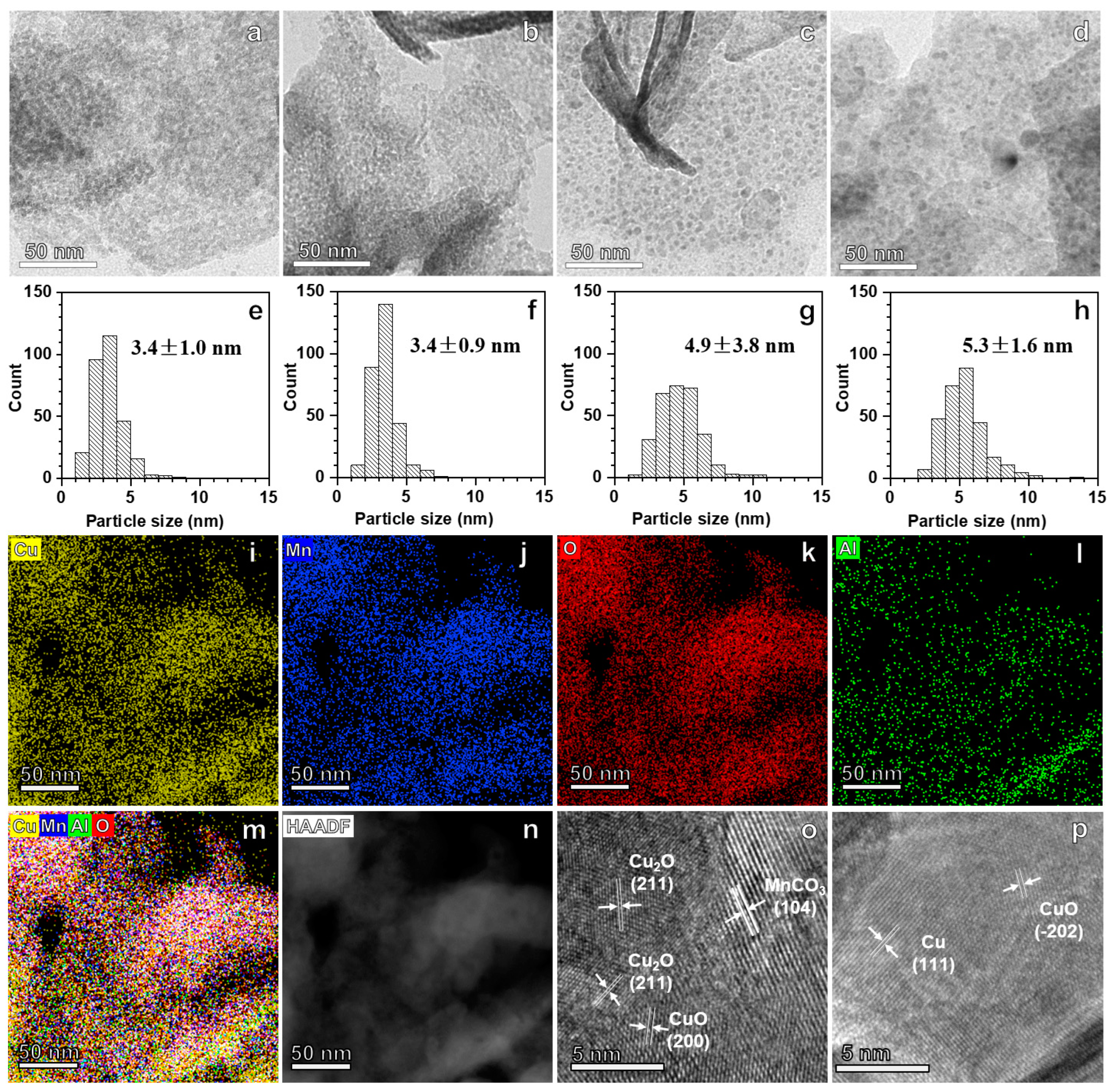
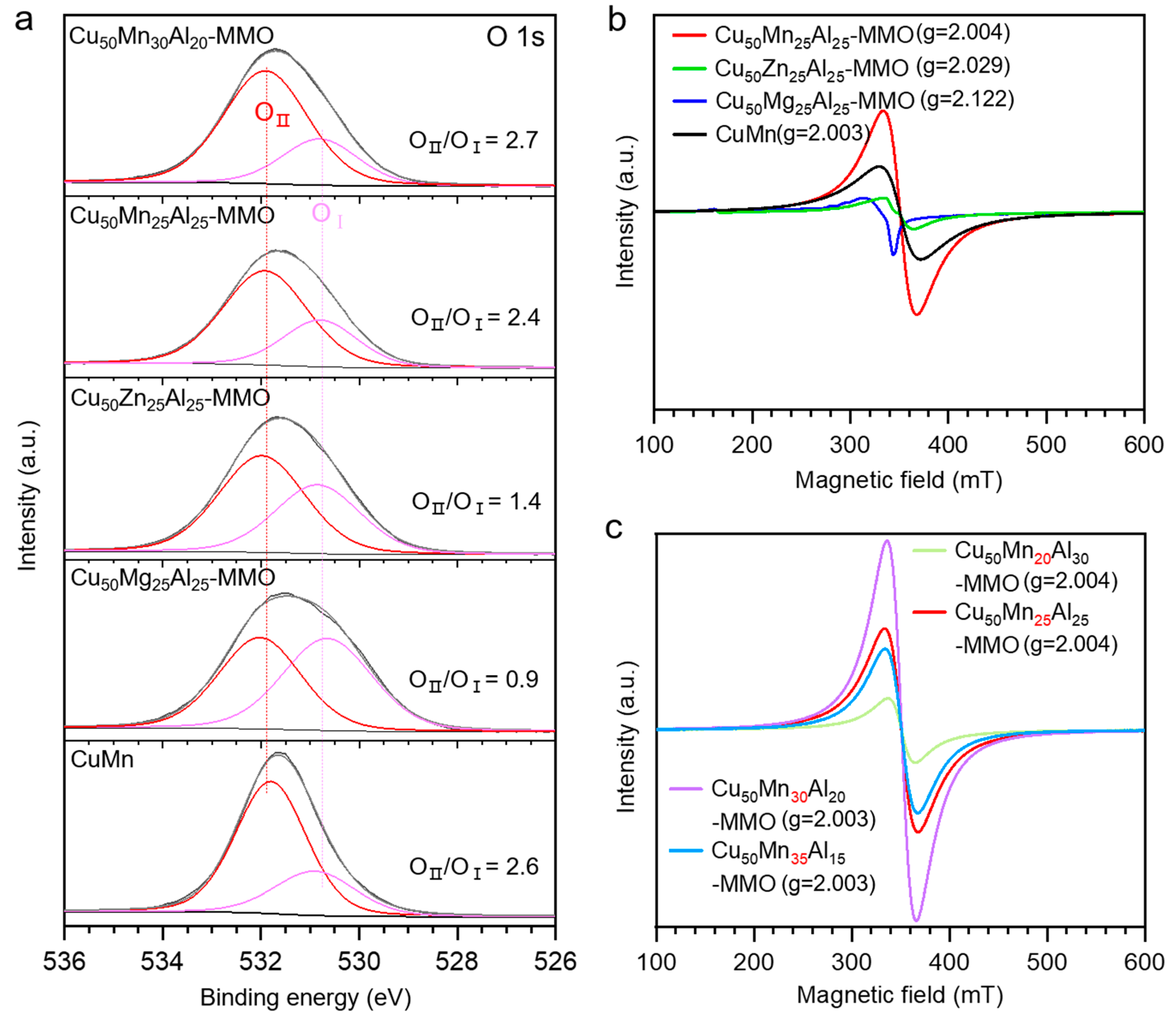
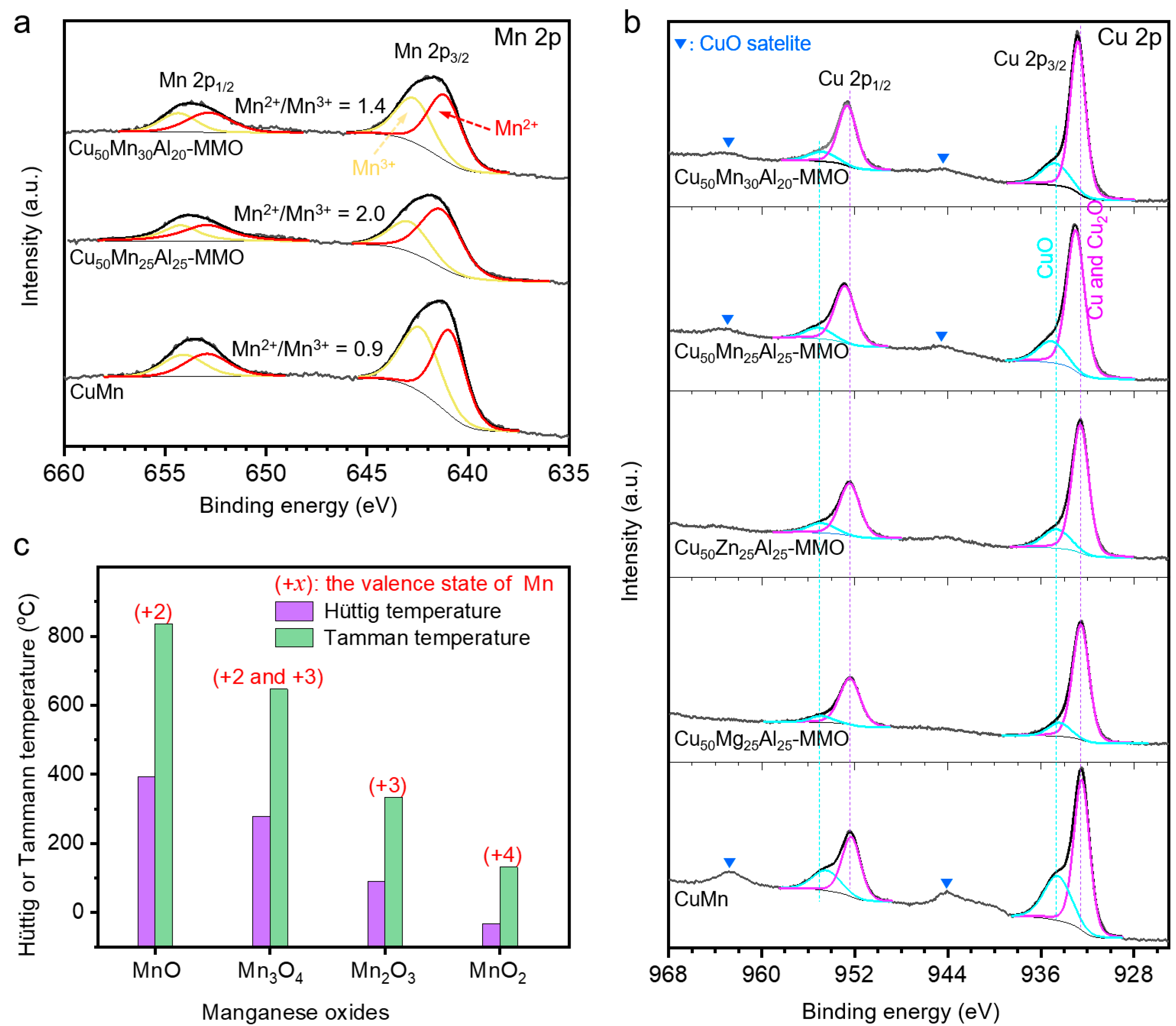
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pal, D.B.; Chand, R.; Upadhyay, S.N.; Mishra, P.K. Performance of water gas shift reaction catalysts: A review. Renew. Sustain. Energy Rev. 2018, 93, 549–565. [Google Scholar] [CrossRef]
- Sun, X.C.; Yuan, K.; Hua, W.D.; Gao, Z.R.; Zhang, Q.; Yuan, C.Y.; Liu, H.C.; Zhang, Y.W. Weakening the Metal–Support Interactions of M/CeO2 (M = Co, Fe, Ni) Using a NH3-Treated CeO2 Support for an Enhanced Water–Gas Shift Reaction. ACS Catal. 2022, 12, 11942–11954. [Google Scholar] [CrossRef]
- Baraj, E.; Ciahotný, K.; Hlinčík, T. The water gas shift reaction: Catalysts and reaction mechanism. Fuel 2021, 288, 119817. [Google Scholar] [CrossRef]
- Li, D.; Xu, S.; Cai, Y.; Chen, C.; Zhan, Y.; Jiang, L. Characterization and Catalytic Performance of Cu/ZnO/Al2O3 Water–Gas Shift Catalysts Derived from Cu–Zn–Al Layered Double Hydroxides. Ind. Eng. Chem. Res. 2017, 56, 3175–3183. [Google Scholar] [CrossRef]
- Chen, A.; Yu, X.; Zhou, Y.; Miao, S.; Li, Y.; Kuld, S.; Sehested, J.; Liu, J.; Aoki, T.; Hong, S.; et al. Structure of the catalytically active copper–ceria interfacial perimeter. Nat. Catal. 2019, 2, 334–341. [Google Scholar] [CrossRef]
- Yan, H.; Yang, C.; Shao, W.P.; Cai, L.H.; Wang, W.W.; Jin, Z.; Jia, C.J. Construction of stabilized bulk-nano interfaces for highly promoted inverse CeO2/Cu catalyst. Nat. Commun. 2019, 10, 3470. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.S.; Song, R.; Cao, T.; Luo, L.; Chen, X.; Gao, Y.; Lu, J.; Li, W.X.; Huang, W. The most active Cu facet for low-temperature water gas shift reaction. Nat. Commun. 2017, 8, 488. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Kang, J.; Yu, Z.; Tian, J.; Gong, Z.; Jia, A.; You, R.; Qian, K.; He, S.; et al. The active sites of Cu–ZnO catalysts for water gas shift and CO hydrogenation reactions. Nat. Commun. 2021, 12, 4331. [Google Scholar] [CrossRef]
- Zhu, M.; Tian, P.; Kurtz, R.; Lunkenbein, T.; Xu, J.; Schlögl, R.; Wachs, I.E.; Han, Y.F. Strong Metal–Support Interactions between Copper and Iron Oxide during the High-Temperature Water-Gas Shift Reaction. Angew. Chem. Int. Ed. 2019, 58, 9083–9087. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, S.; Yoon, H.J.; Yoon, C.W.; Lee, K.B. Water gas shift and sorption-enhanced water gas shift reactions using hydrothermally synthesized novel Cu–Mg–Al hydrotalcite-based catalysts for hydrogen production. Renew. Sustain. Energy Rev. 2021, 145, 111064. [Google Scholar] [CrossRef]
- Li, D.; Cai, Y.; Ding, Y.; Li, R.; Lu, M.; Jiang, L. Layered double hydroxides as precursors of Cu catalysts for hydrogen production by water-gas shift reaction. Int. J. Hydrogen Energy 2015, 40, 10016–10025. [Google Scholar] [CrossRef]
- Tabakova, T.; Idakiev, V.; Avgouropoulos, G.; Papavasiliou, J.; Manzoli, M.; Boccuzzi, F.; Ioannides, T. Highly active copper catalyst for low-temperature water-gas shift reaction prepared via a Cu-Mn spinel oxide precursor. Appl. Catal. A-Gen. 2013, 451, 184–191. [Google Scholar] [CrossRef]
- Tanaka, Y.; Utaka, T.; Kikuchi, R.; Takeguchi, T.; Sasaki, K.; Eguchi, K. Water gas shift reaction for the reformed fuels over Cu/MnO catalysts prepared via spinel-type oxide. J. Catal. 2003, 215, 271–278. [Google Scholar] [CrossRef]
- Su, Y.Q.; Xia, G.J.; Qin, Y.; Ding, S.; Wang, Y.G. Lattice oxygen self-spillover on reducible oxide supported metal cluster: The water–gas shift reaction on Cu/CeO2 catalyst. Chem. Sci. 2021, 12, 8260–8267. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wei, M. Layered Double Hydroxide-Based Catalysts: Recent Advances in Preparation, Structure, and Applications. Adv. Funct. Mater. 2018, 28, 1802943. [Google Scholar] [CrossRef]
- Xia, S.; Fang, L.; Meng, Y.; Zhang, X.; Zhang, L.; Yang, C.; Ni, Z. Water-gas shift reaction catalyzed by layered double hydroxides supported Au-Ni/Cu/Pt bimetallic alloys. Appl. Catal. B-Environ. 2020, 272, 118949. [Google Scholar] [CrossRef]
- Liu, N.; Xu, M.; Yang, Y.; Zhang, S.; Zhang, J.; Wang, W.; Zheng, L.; Hong, S.; Wei, M. Auδ−–Ov–Ti3+ Interfacial Site: Catalytic Active Center toward Low-Temperature Water Gas Shift Reaction. ACS Catal. 2019, 9, 2707–2717. [Google Scholar] [CrossRef]
- Ginés, M.J.L.; Amadeo, N.; Laborde, M.; Apesteguía, C.R. Activity and structure-sensitivity of the water-gas shift reaction over CuZnAl mixed oxide catalysts. Appl. Catal. A-Gen. 1995, 131, 283–296. [Google Scholar] [CrossRef]
- Lucarelli, C.; Molinari, C.; Faure, R.; Fornasari, G.; Gary, D.; Schiaroli, N.; Vaccari, A. Novel Cu-Zn-Al catalysts obtained from hydrotalcite-type precursors for middle-temperature water-gas shift applications. Appl. Clay Sci. 2018, 155, 103–110. [Google Scholar] [CrossRef]
- Nishida, K.; Atake, I.; Li, D.; Shishido, T.; Oumi, Y.; Sano, T.; Takehira, K. Effects of noble metal-doping on Cu/ZnO/Al2O3 catalysts for water–gas shift reaction: Catalyst preparation by adopting “memory effect” of hydrotalcite. Appl. Catal. A-Gen. 2008, 337, 48–57. [Google Scholar] [CrossRef]
- Santos, J.L.; Reina, T.R.; Ivanova, S.; Centeno, M.A.; Odriozola, J.A. Gold promoted Cu/ZnO/Al2O3 catalysts prepared from hydrotalcite precursors: Advanced materials for the WGS reaction. Appl. Catal. B-Environ. 2017, 201, 310–317. [Google Scholar] [CrossRef]
- Thouchprasitchai, N.; Luengnaruemitchai, A.; Pongstabodee, S. The activities of Cu-based Mg–Al layered double oxide catalysts in the water gas shift reaction. Int. J. Hydrogen Energy 2016, 41, 14147–14159. [Google Scholar] [CrossRef]
- Gabrovska, M.; Ivanov, I.; Nikolova, D.; Kovacheva, D.; Tabakova, T. Hydrogen production via water-gas shift reaction over gold supported on Ni-based layered double hydroxides. Int. J. Hydrogen Energy 2021, 46, 458–473. [Google Scholar] [CrossRef]
- Xu, M.; He, S.; Chen, H.; Cui, G.; Zheng, L.; Wang, B.; Wei, M. TiO2–x-Modified Ni Nanocatalyst with Tunable Metal–Support Interaction for Water–Gas Shift Reaction. ACS Catal. 2017, 7, 7600–7609. [Google Scholar] [CrossRef]
- Xia, S.; Dai, T.; Meng, Y.; Zhou, X.; Pan, G.; Zhang, X.; Ni, Z. A low-temperature water–gas shift reaction catalyzed by hybrid NiO@NiCr-layered double hydroxides: Catalytic property, kinetics and mechanism investigation. Phys. Chem. Chem. Phys. 2020, 22, 12630–12643. [Google Scholar] [CrossRef]
- Xi, S.; Zhang, J.; Xie, K. Low-temperature Water-gas Shift Reaction Enhanced by Oxygen Vacancies in Pt-loaded Porous Single-crystalline Oxide Monoliths. Angew. Chem. Int. Ed. 2022, 61, e202209851. [Google Scholar] [CrossRef]
- López Cámara, A.; Cortés Corberán, V.; Martínez-Arias, A.; Barrio, L.; Si, R.; Hanson, J.C.; Rodriguez, J.A. Novel manganese-promoted inverse CeO2/CuO catalyst: In situ characterization and activity for the water-gas shift reaction. Catal. Today 2020, 339, 24–31. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, D.; Li, S.; Zhang, L.; Zheng, G.; Guo, L. Layered copper manganese oxide for the efficient catalytic CO and VOCs oxidation. Chem. Eng. J. 2019, 357, 258–268. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Zhang, S.; Li, Q.; Wu, Z.; Zhang, J. Evaluation of an ε-manganese (IV) oxide/manganese vanadium oxide composite catalyst enriched with oxygen vacancies for enhanced formaldehyde removal. Appl. Catal. B-Environ. 2023, 320, 121994. [Google Scholar] [CrossRef]
- Hu, Q.; Yang, L.; Fan, G.; Li, F. Hydrogenation of biomass-derived compounds containing a carbonyl group over a copper-based nanocatalyst: Insight into the origin and influence of surface oxygen vacancies. J. Catal. 2016, 340, 184–195. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Y.; Huang, Z.; Jing, G.; Zhao, H.; Wu, X.; Zhang, S. CuMnAl–O Catalyst Synthesized via Pyrolysis of a Layered Double Hydroxide Precursor Attains Enhanced Performance for Benzene Combustion. Energy Fuel 2021, 35, 743–751. [Google Scholar] [CrossRef]
- Behrens, M.; Kasatkin, I.; Kühl, S.; Weinberg, G. Phase-Pure Cu, Zn, Al Hydrotalcite-like Materials as Precursors for Copper rich Cu/ZnO/Al2O3 Catalysts. Chem. Mater. 2010, 22, 386–397. [Google Scholar] [CrossRef]
- Comelli, N.A.; Ruiz, M.L.; Aparicio, M.S.L.; Merino, N.A.; Cecilia, J.A.; Rodríguez-Castellón, E.; Lick, I.D.; Ponzi, M.I. Influence of the synthetic conditions on the composition, morphology of CuMgAl hydrotalcites and their use as catalytic precursor in diesel soot combustion reactions. Appl. Clay Sci. 2018, 157, 148–157. [Google Scholar] [CrossRef]
- Machej, T.; Serwicka, E.M.; Zimowska, M.; Dula, R.; Michalik-Zym, A.; Napruszewska, B.; Rojek, W.; Socha, R. Cu/Mn-based mixed oxides derived from hydrotalcite-like precursors as catalysts for methane combustion. Appl. Catal. A-Gen. 2014, 474, 87–94. [Google Scholar] [CrossRef]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, B.A.T.; Eskandari, S.; Khan, U.; White, R.D.; Regalbuto, J.R. A Review of Preparation Methods for Supported Metal Catalysts. Adv. Catal. 2017, 61, 1–35. [Google Scholar]
- Fu, X.P.; Guo, L.W.; Wang, W.W.; Ma, C.; Jia, C.J.; Wu, K.; Si, R.; Sun, L.D.; Yan, C.H. Direct Identification of Active Surface Species for the Water–Gas Shift Reaction on a Gold–Ceria Catalyst. J. Am. Chem. Soc. 2019, 141, 4613–4623. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Deng, Y.; Xu, M.; Artiglia, L.; Wen, W.; Gao, R.; Chen, B.; Yao, S.; Zhang, X.; et al. A stable low-temperature H2-production catalyst by crowding Pt on α-MoC. Nature 2021, 589, 396–401. [Google Scholar] [CrossRef]
- Völs, P.; Hilbert, S.; Störr, B.; Bette, N.; Lißner, A.; Seidel, J.; Mertens, F. Methanation of CO2 and CO by (Ni,Mg,Al)-Hydrotalcite-Derived and Related Catalysts with Varied Magnesium and Aluminum Oxide Contents. Ind. Eng. Chem. Res. 2021, 60, 5114–5123. [Google Scholar] [CrossRef]
- Koo, H.M.; Ahn, C.I.; Lee, D.H.; Roh, H.S.; Shin, C.H.; Kye, H.; Bae, J.W. Roles of Al2O3 promoter for an enhanced structural stability of ordered-mesoporous Co3O4 catalyst during CO hydrogenation to hydrocarbons. Fuel 2018, 225, 460–471. [Google Scholar] [CrossRef]
- Choi, Y.; Stenger, H.G. Water gas shift reaction kinetics and reactor modeling for fuel cell grade hydrogen. J. Power Sources 2003, 124, 432–439. [Google Scholar] [CrossRef]
- Song, L.; Cao, X.; Li, L. Engineering Stable Surface Oxygen Vacancies on ZrO2 by Hydrogen-Etching Technology: An Efficient Support of Gold Catalysts for Water-Gas Shift Reaction. ACS Appl. Mater. Int. 2018, 10, 31249–31259. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, A.A.; Dumesic, J.A.; Mavrikakis, M. On the Mechanism of Low-Temperature Water Gas Shift Reaction on Copper. J. Am. Chem. Soc. 2008, 130, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Li, K.; Lu, Y.; Guo, Z.; Ni, N.; Liu, H.; Huang, Y.; Ji, H.; Wang, P. An overview of advanced methods for the characterization of oxygen vacancies in materials. TrAC-Trend Anal. Chem. 2019, 116, 102–108. [Google Scholar] [CrossRef]
- Espinós, J.P.; Morales, J.; Barranco, A.; Caballero, A.; Holgado, J.P.; González-Elipe, A.R. Interface Effects for Cu, CuO, and Cu2O Deposited on SiO2 and ZrO2. XPS Determination of the Valence State of Copper in Cu/SiO2 and Cu/ZrO2 Catalysts. J. Phy. Chem. B 2002, 106, 6921–6929. [Google Scholar] [CrossRef]
- Tahir, D.; Tougaard, S. Electronic and optical properties of Cu, CuO and Cu2O studied by electron spectroscopy. J. Phy-Condens Mater. 2012, 24, 175002. [Google Scholar] [CrossRef]
- Galtayries, A.; Bonnelle, J.P. XPS and ISS studies on the interaction of H2S with polycrystalline Cu, Cu2O and CuO surfaces. Surf. Interface Anal. 1995, 23, 171–179. [Google Scholar] [CrossRef]
- Carter, J.H.; Liu, X.; He, Q.; Althahban, S.; Nowicka, E.; Freakley, S.J.; Niu, L.; Morgan, D.J.; Li, Y.; Niemantsverdriet, J.W.; et al. Activation and Deactivation of Gold/Ceria–Zirconia in the Low-Temperature Water–Gas Shift Reaction. Angew. Chem. Int. Ed. 2017, 56, 16037–16041. [Google Scholar] [CrossRef]
- Zhu, M.; Tian, P.; Chen, J.; Ford, M.E.; Xu, J.; Wachs, I.E.; Han, Y.F. Activation and deactivation of the commercial-type CuO–Cr2O3–Fe2O3 high temperature shift catalyst. AIChE J. 2020, 66, e16846. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, P.; Cao, Z.; Campbell, C.T.; Xia, Y. The physical chemistry and materials science behind sinter-resistant catalysts. Chem. Soc. Rev. 2018, 47, 4314–4331. [Google Scholar] [CrossRef]
- Hansen, T.W.; DeLaRiva, A.T.; Challa, S.R.; Datye, A.K. Sintering of Catalytic Nanoparticles: Particle Migration or Ostwald Ripening? Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Qin, X.-T.; Yin, Y.; Teng, Y.-F.; Jin, Z.; Jia, C.-J. Promoted Cu-Fe3O4 catalysts for low-temperature water gas shift reaction: Optimization of Cu content. Appl. Catal. B: Environ. 2018, 226, 182–193. [Google Scholar] [CrossRef]
- Jin, S.; Park, Y.; Bang, G.; Vo, N.D.; Lee, C.-H. Revisiting magnesium oxide to boost hydrogen production via water-gas shift reaction: Mechanistic study to economic evaluation. Appl. Catal. B: Environ. 2020, 284, 119701. [Google Scholar] [CrossRef]
- Navasery, M.; Halim, S.A.; Lim, K.P.; Chen, S.K.; Roslan, A.S.; Abd-Shukor, R. STRUCTURE, ELECTRICAL TRANSPORT AND MAGNETO-RESISTANCE PROPERTIES OFLa5/8Ca3/8MnO3MANGANITE SYNTHESIZED WITH DIFFERENT MANGANESE PRECURSORS. Mod. Phys. Lett. B 2012, 26. [Google Scholar] [CrossRef]
- Moiseev, G.K.; Ivanovskii, A.L. Products of CuO heating in argon. Inorg. Mater. 2006, 42, 632–634. [Google Scholar] [CrossRef]
- Kosenko, A.V.; Emel’Chenko, G.A. Equilibrium phase relationships in the system Cu-O under high oxygen pressure. J. Phase Equilibria Diffus. 2001, 22, 12–19. [Google Scholar] [CrossRef]
| Samples | Designed Contents a (Cu:M:Al b) | Detected Contents (at.%) c | SBET (m2·g−1) | ||||
|---|---|---|---|---|---|---|---|
| Cu | Mn | Al | Zn | Mg | |||
| Cu30Mn35Al35-MMO | 30:35:35 | 29.9 | 34.6 | 35.5 | - | - | 122 |
| Cu40Mn30Al30-MMO | 40:30:30 | 40.2 | 29.8 | 30.0 | - | - | 117 |
| Cu50Mn25Al25-MMO | 50:25:25 | 50.6 | 25.6 | 23.8 | - | - | 88 |
| Cu60Mn20Al20-MMO | 60:20:20 | 60.8 | 19.7 | 19.5 | - | - | 74 |
| Cu70Mn15Al15-MMO | 70:15:15 | 70.4 | 15.0 | 14.7 | - | - | 71 |
| Cu50Mn20Al30-MMO | 50:20:30 | 50.7 | 19.3 | 30.1 | - | - | 72 |
| Cu50Mn30Al20-MMO | 50:30:20 | 50.8 | 29.2 | 20.0 | - | - | 73 |
| Cu50Mn35Al15-MMO | 50:35:15 | 50.9 | 34.4 | 14.7 | - | - | 62 |
| Cu50Zn25Al25-MMO | 50:25:25 | 49.3 | - | 25.3 | 25.4 | - | 67 |
| Cu50Mg25Al25-MMO | 50:25:25 | 50.9 | - | 26.2 | - | 22.8 | 58 |
| CuMn | 50:50:0 | 50.8 | 49.2 | - | - | - | 37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Xiao, Z.; Liu, P.; Wang, H.; Geng, J.; Lei, H.; Zhuo, O. Interfaces and Oxygen Vacancies-Enriched Catalysts Derived from Cu-Mn-Al Hydrotalcite towards High-Efficient Water–Gas Shift Reaction. Molecules 2023, 28, 1522. https://doi.org/10.3390/molecules28041522
Li H, Xiao Z, Liu P, Wang H, Geng J, Lei H, Zhuo O. Interfaces and Oxygen Vacancies-Enriched Catalysts Derived from Cu-Mn-Al Hydrotalcite towards High-Efficient Water–Gas Shift Reaction. Molecules. 2023; 28(4):1522. https://doi.org/10.3390/molecules28041522
Chicago/Turabian StyleLi, Hanci, Zhenyi Xiao, Pei Liu, Hairu Wang, Jiajun Geng, Huibin Lei, and Ou Zhuo. 2023. "Interfaces and Oxygen Vacancies-Enriched Catalysts Derived from Cu-Mn-Al Hydrotalcite towards High-Efficient Water–Gas Shift Reaction" Molecules 28, no. 4: 1522. https://doi.org/10.3390/molecules28041522
APA StyleLi, H., Xiao, Z., Liu, P., Wang, H., Geng, J., Lei, H., & Zhuo, O. (2023). Interfaces and Oxygen Vacancies-Enriched Catalysts Derived from Cu-Mn-Al Hydrotalcite towards High-Efficient Water–Gas Shift Reaction. Molecules, 28(4), 1522. https://doi.org/10.3390/molecules28041522





