Glycopolymers for Antibacterial and Antiviral Applications
Abstract
1. Introduction
2. Synthetic Glycopolymers Targeting Bacteria
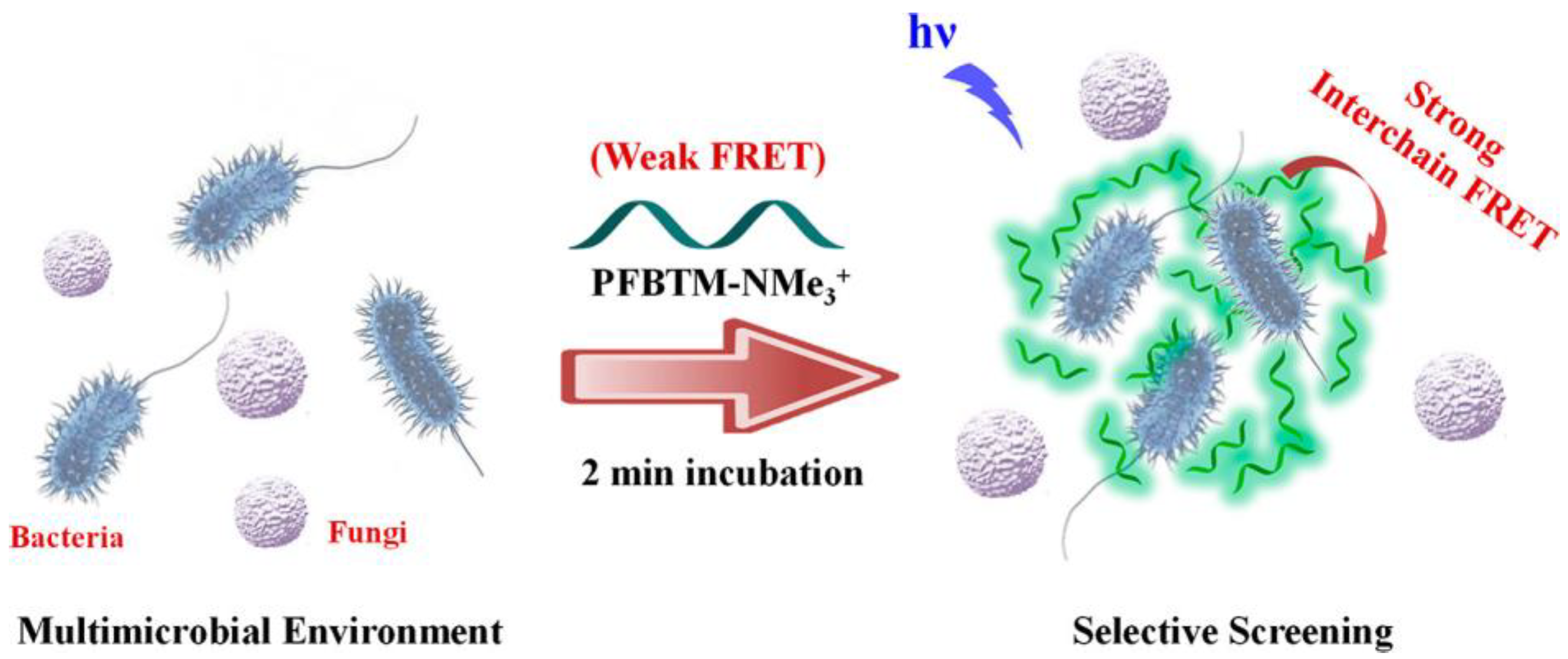
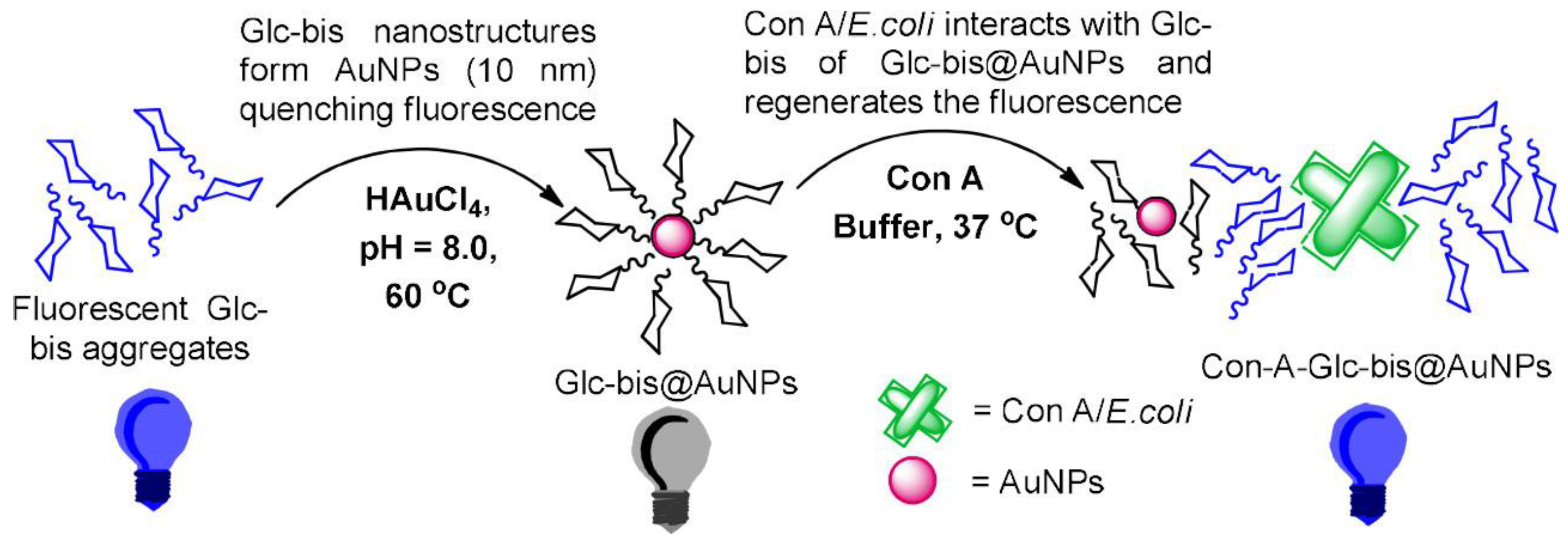
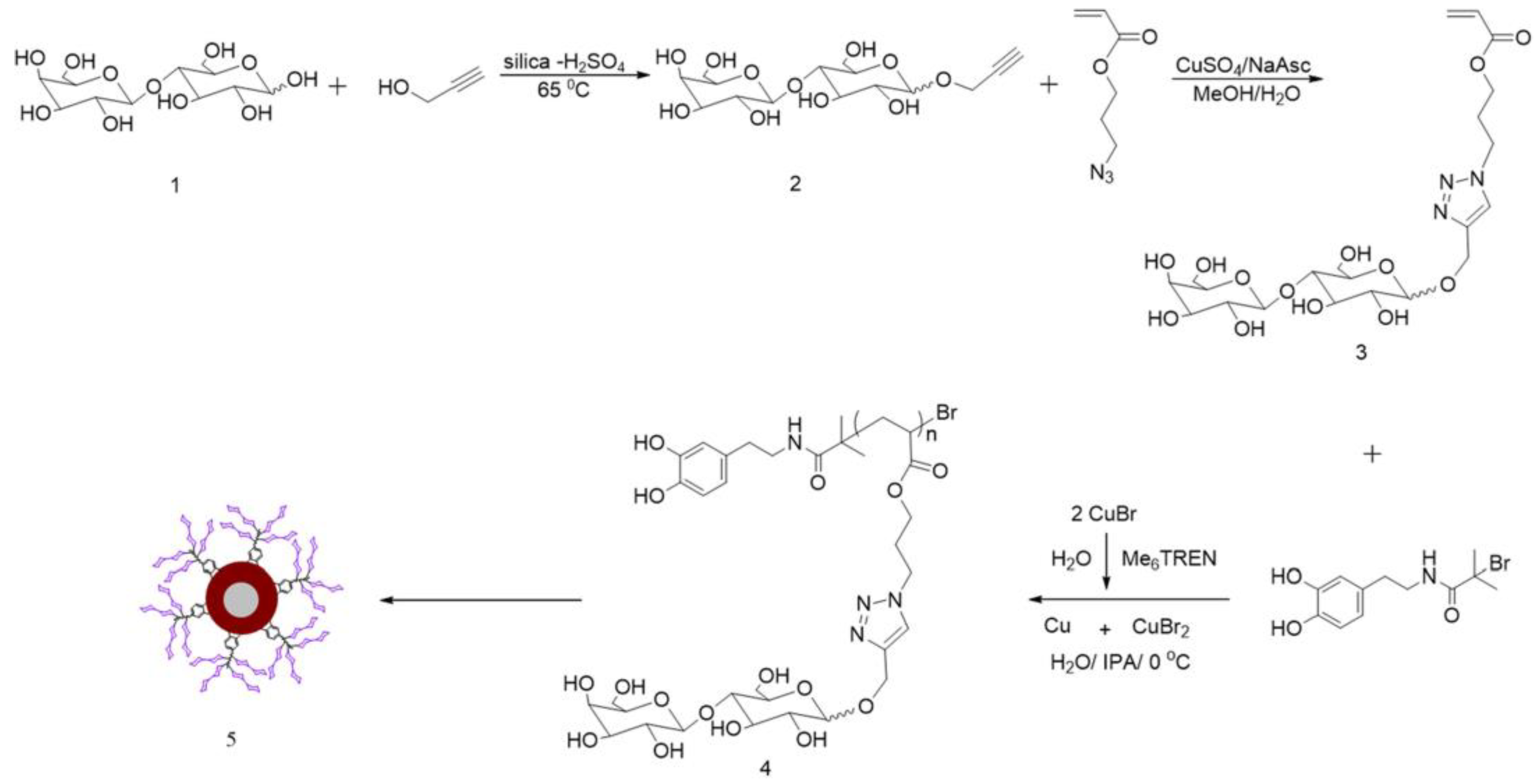
2.1. Glycopolymers for Bacterial Detection and Capture
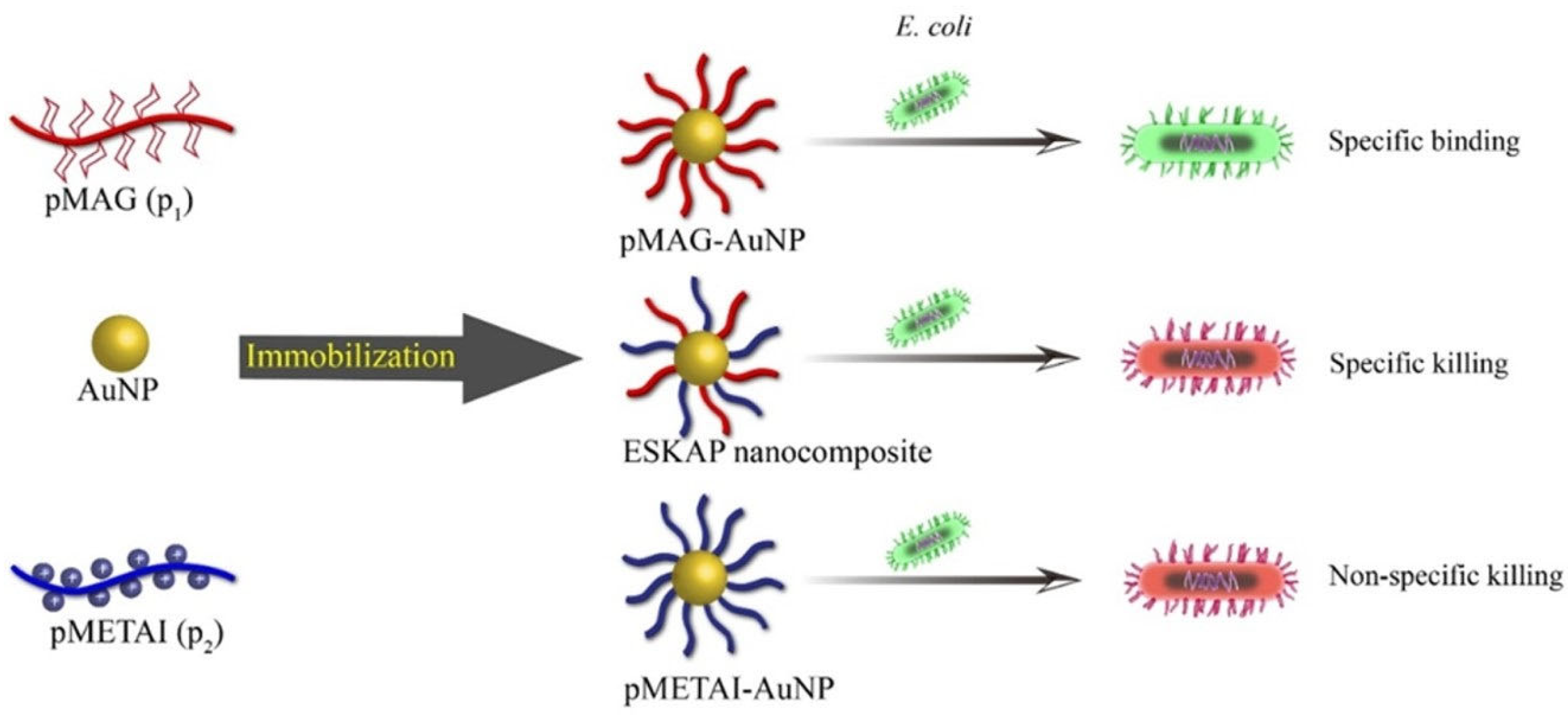
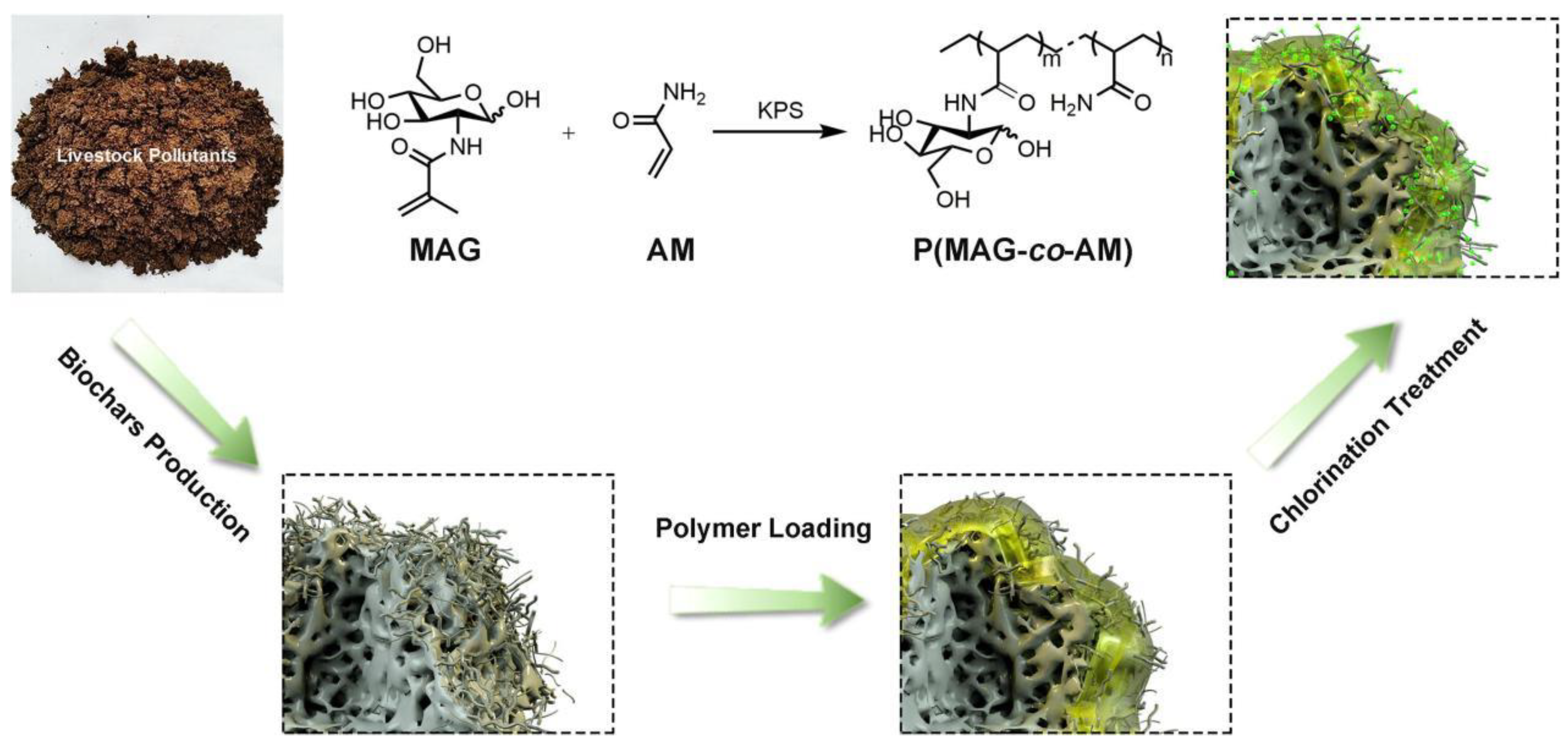
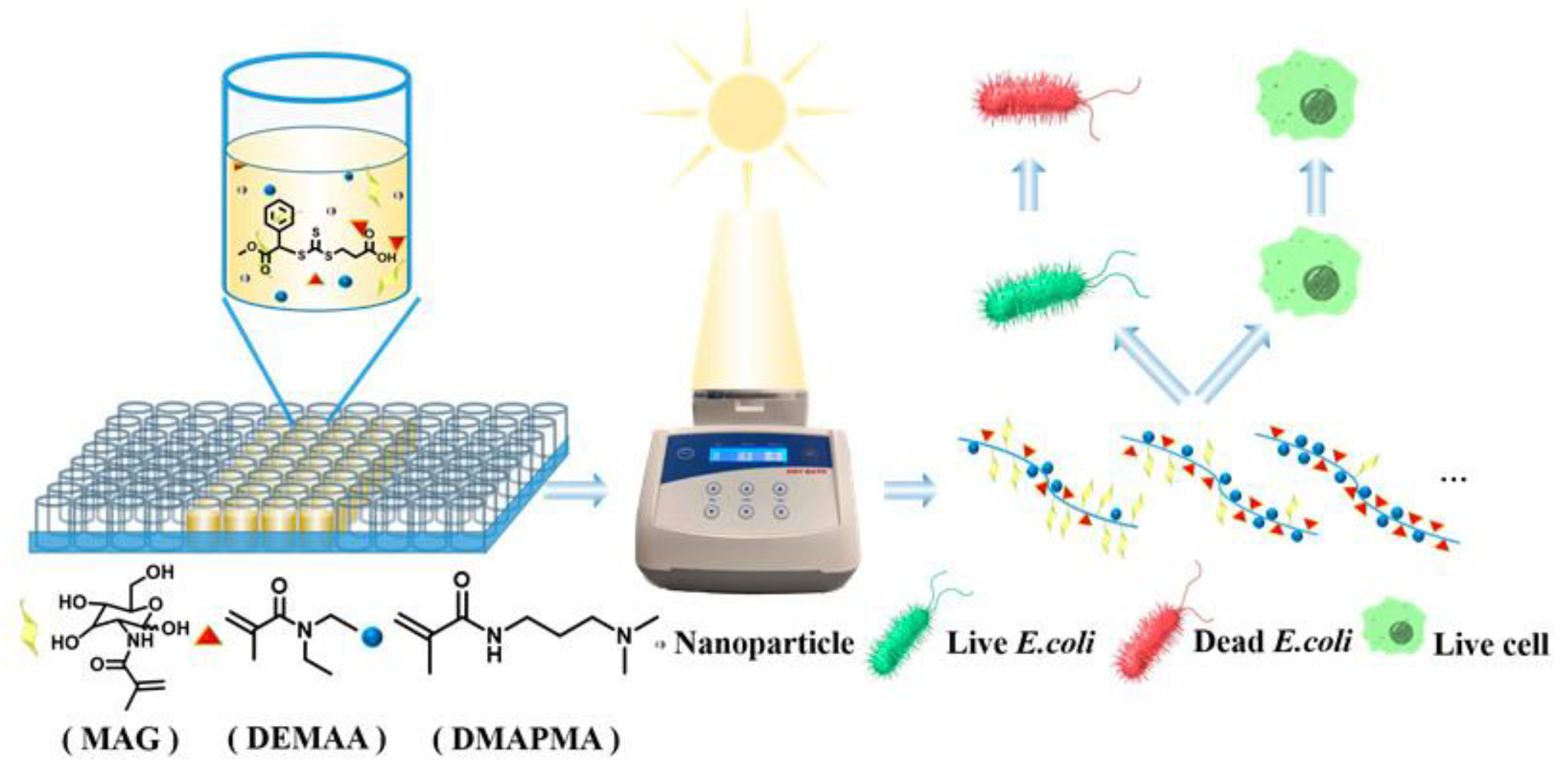
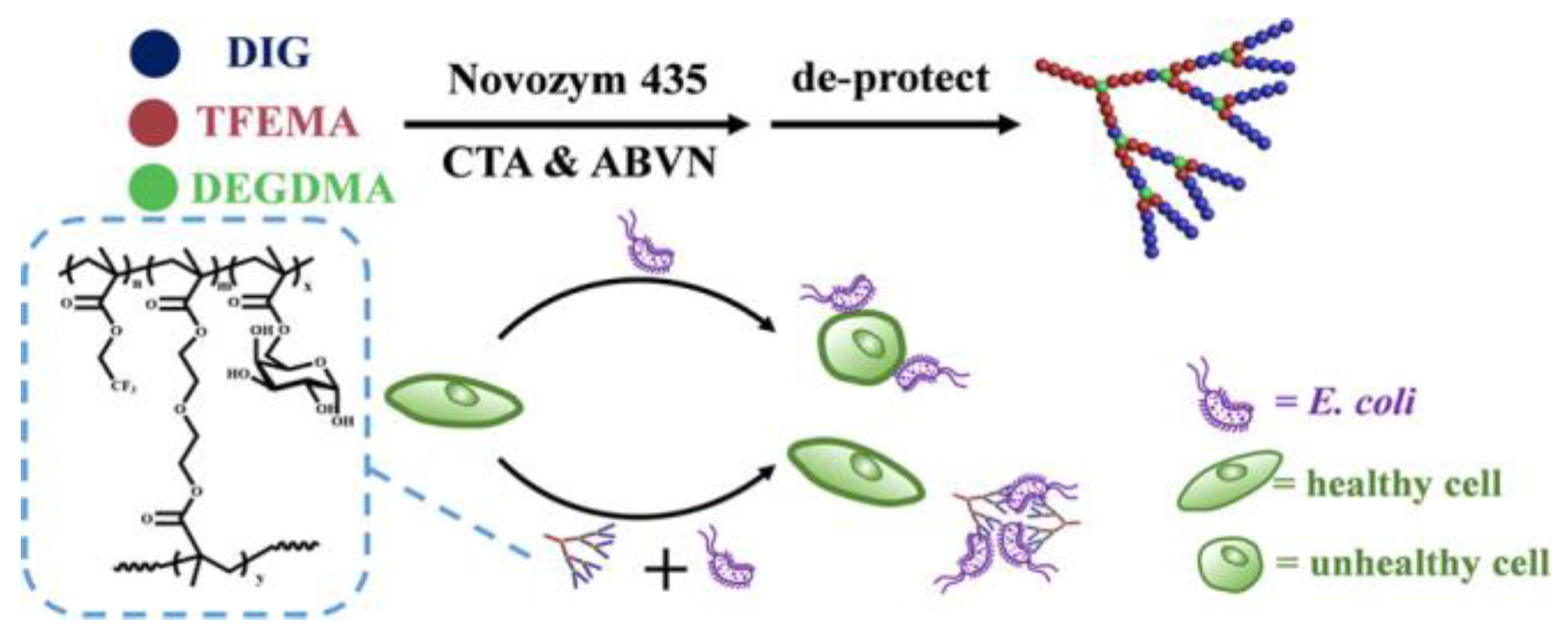
2.2. Glycopolymers for Sterilization
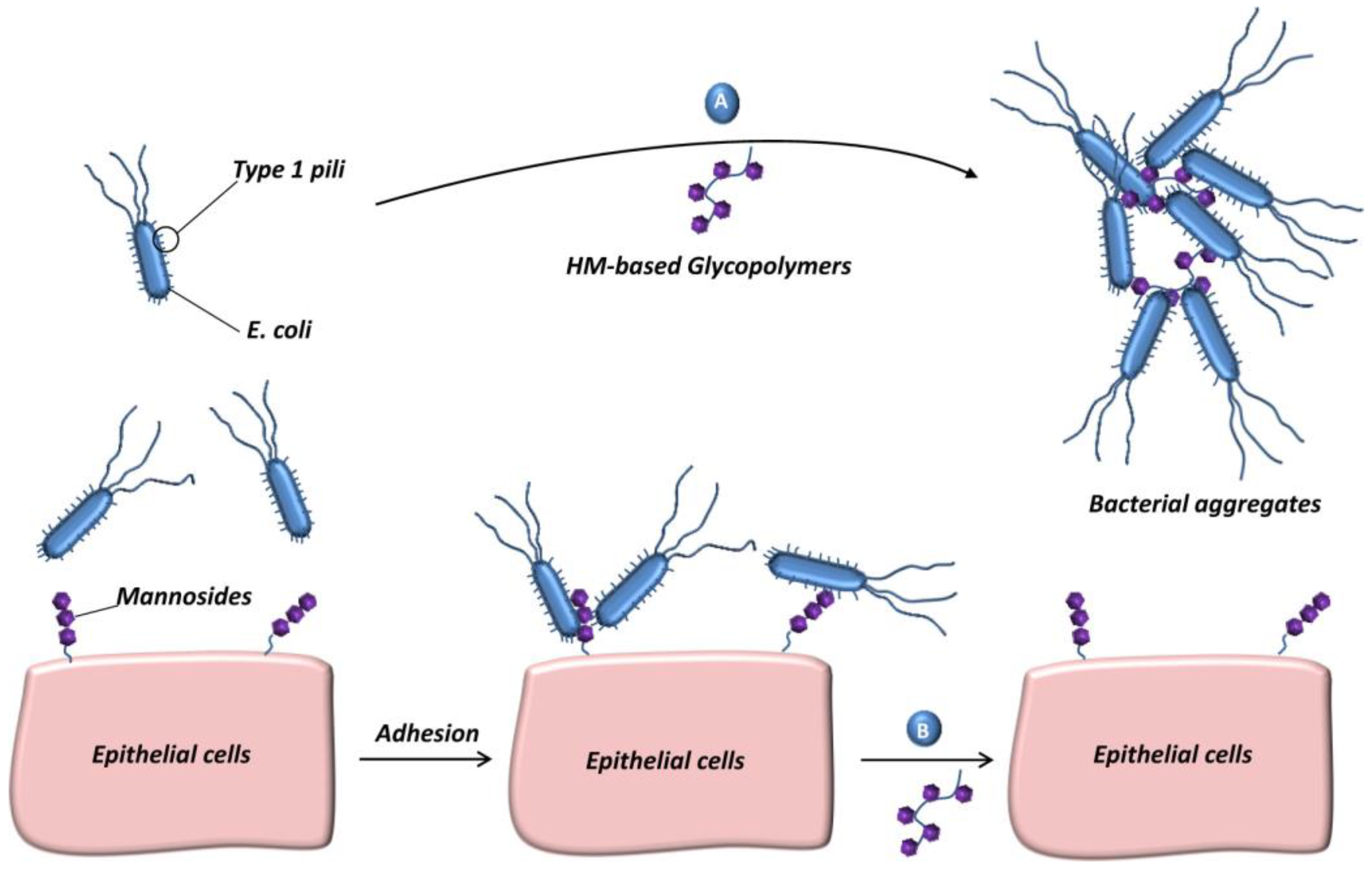
2.3. Glycopolymers for Inhibiting Bacterial Infection
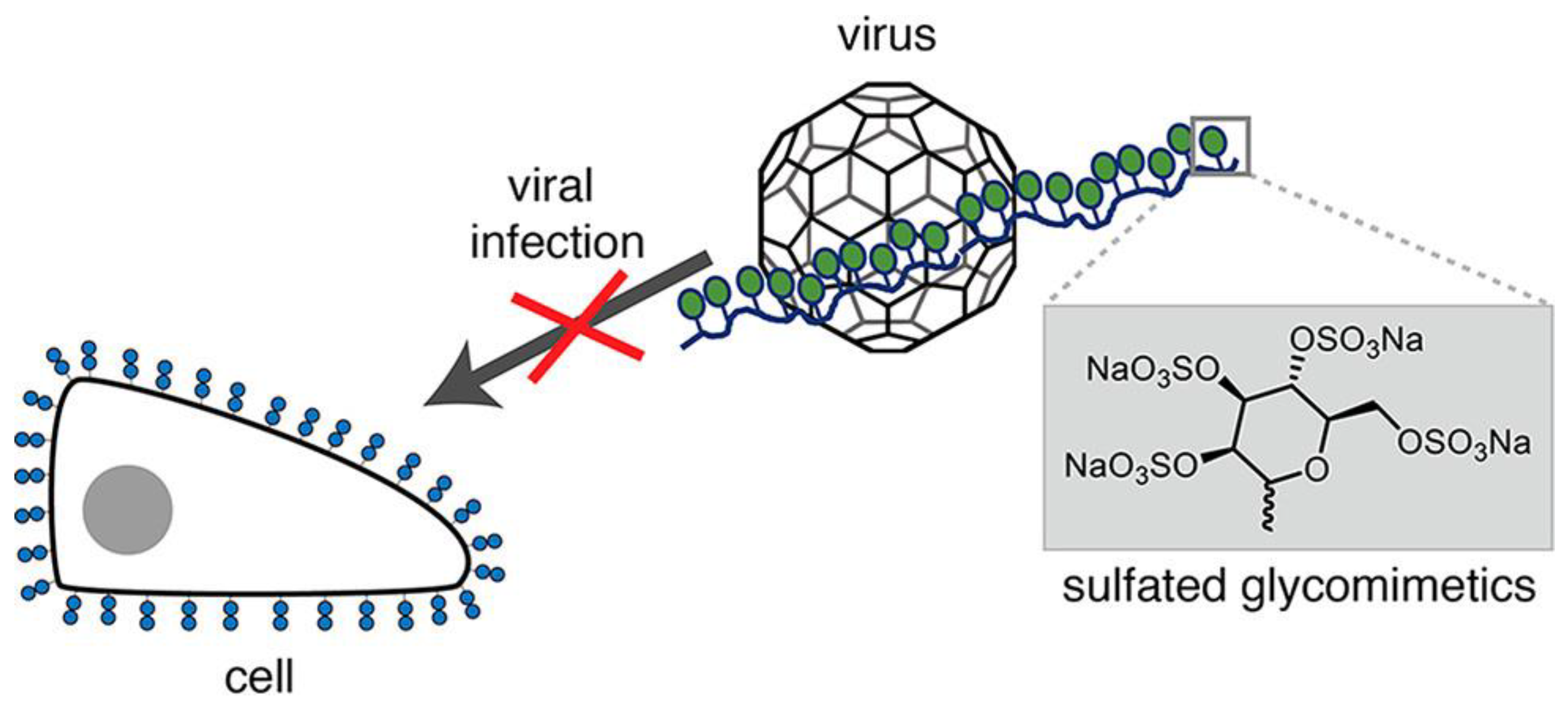
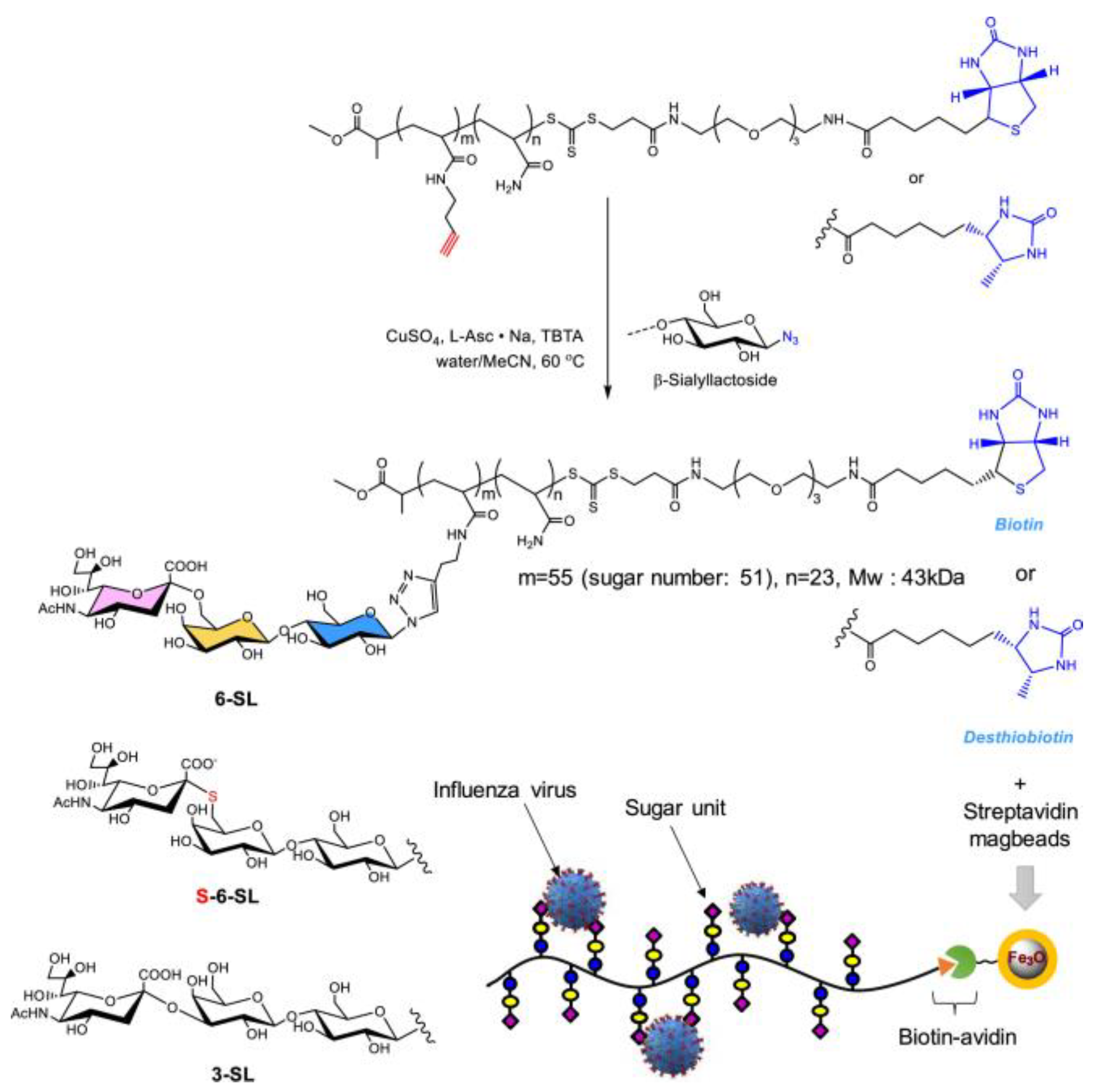
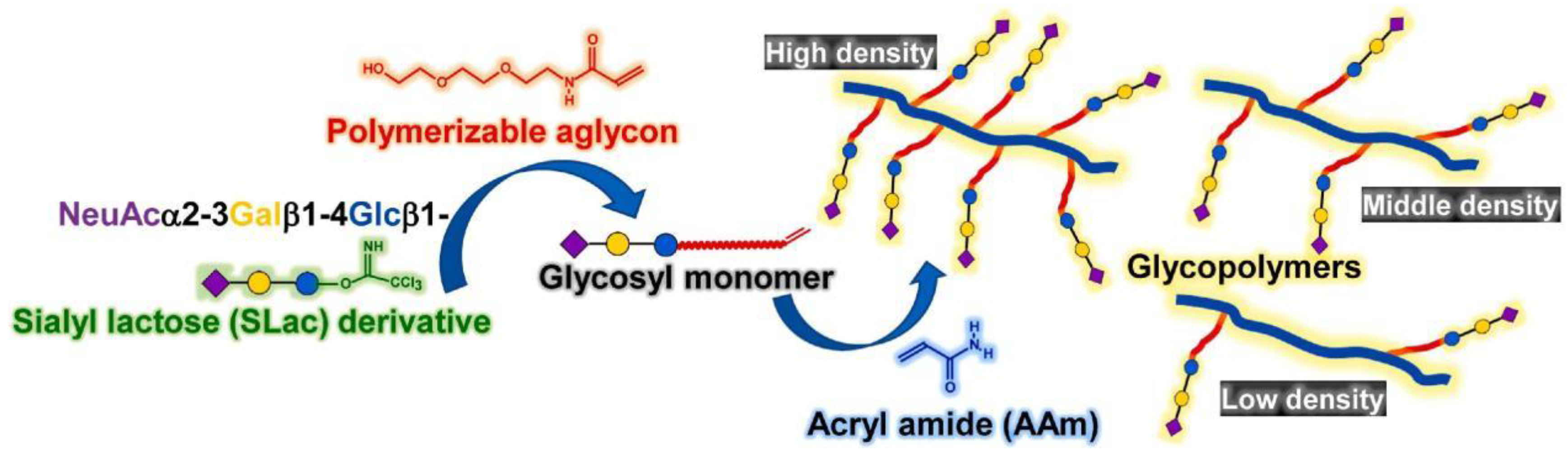
3. Glycopolymers Targeting Viruses
3.1. Glycopolymers Targeting Cell Surface Proteins
3.2. Glycopolymers Targeting Virus Surface Lectin
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus disease 2019-COVID-19. Clin. Microbiol. Rev. 2020, 33, 48. [Google Scholar] [CrossRef]
- Miura, Y.; Hoshino, Y.; Seto, H. Glycopolymer Nanobiotechnology. Chem. Rev. 2016, 116, 1673–1692. [Google Scholar] [CrossRef]
- Behren, S.; Westerlind, U. Novel approaches to design glycan-based antibacterial inhibitors. Eur. J. Org. Chem. 2022, 26, e202200795. [Google Scholar] [CrossRef]
- Mammen, M.; Choi, S.K.; Whitesides, G.M. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 1998, 37, 2755–2794. [Google Scholar] [CrossRef]
- Kiessling, L.L.; Pontrello, J.K.; Schuster, M.C. Synthetic multivalent carbohydrate ligands as effectors or inhibitors of biological processes. In Carbohydrate-Based Drug Discovery; Wong, C.-H., Ed.; Wiley: Hoboken, NJ, USA, 2003; pp. 575–608. [Google Scholar]
- Kiessling, L.L.; Gestwicki, J.E.; Strong, L.E. Synthetic multivalent ligands in the exploration of cell-surface interactions. Curr. Opin. Chem. Biol. 2000, 4, 696–703. [Google Scholar] [CrossRef]
- Ting, S.R.S.; Chen, G.J.; Stenzel, M.H. Synthesis of glycopolymers and their multivalent recognitions with lectins. Polym. Chem. 2010, 1, 1392–1412. [Google Scholar] [CrossRef]
- Gestwicki, J.E.; Cairo, C.W.; Strong, L.E.; Oetjen, K.A.; Kiessling, L.L. Influencing receptor-ligand binding mechanisms with multivalent ligand architecture. J. Am. Chem. Soc. 2002, 124, 14922–14933. [Google Scholar] [CrossRef]
- Becer, C.R. The Glycopolymer Code: Synthesis of glycopolymers and multivalent carbohydrate-Lectin interactions. Macromol. Rapid Commun. 2012, 33, 742–752. [Google Scholar] [CrossRef]
- Ladmiral, V.; Mantovani, G.; Clarkson, G.J.; Cauet, S.; Irwin, J.L.; Haddleton, D.M. Synthesis of neoglycopolymers by a combination of “click chemistry” and living radical polymerization. J. Am. Chem. Soc. 2006, 128, 4823–4830. [Google Scholar] [CrossRef]
- Nishida, Y.; Uzawa, H.; Toba, T.; Sasaki, K.; Kondo, H.; Kobayashi, K. A facile synthetic approach to L- and P-selectin blockers via copolymerization of vinyl monomers constructing the key carbohydrate modules of sialyl lewisX mimics. Biomacromolecules 2000, 1, 68–74. [Google Scholar] [CrossRef]
- Gomez-Garcia, M.; Benito, J.M.; Butera, A.P.; Mellet, C.O.; Fernandez, J.M.G.; Blanco, J.L.J. Probing carbohydrate-lectin recognition in heterogeneous environments with monodisperse cyclodextrin-based glycoclusters. J. Org. Chem. 2012, 77, 1273–1288. [Google Scholar] [CrossRef]
- Gomez-Garcia, M.; Benito, J.M.; Rodriguez-Lucena, D.; Yu, J.X.; Chmurski, K.; Mellet, C.O.; Gallego, R.G.; Maestre, A.; Defaye, J.; Fernandez, J.M.G. Probing secondary carbohydrate-protein interactions with highly dense cyclodextrin-centered heteroglycoclusters: The heterocluster effect. J. Am. Chem. Soc. 2005, 127, 7970–7971. [Google Scholar] [CrossRef]
- Vico, R.V.; Voskuhl, J.; Ravoo, B.J. Multivalent interaction of cyclodextrin vesicles, carbohydrate guests, and lectins: A kinetic investigation. Langmuir 2011, 27, 1391–1397. [Google Scholar] [CrossRef]
- Liang, C.H.; Wang, S.K.; Lin, C.W.; Wang, C.C.; Wong, C.H.; Wu, C.Y. Effects of neighboring glycans on antibody-carbohydrate interaction. Angew. Chem. Int. Ed. 2011, 50, 1608–1612. [Google Scholar] [CrossRef]
- Xue, L.L.; Xiong, X.H.; Chen, K.; Luan, Y.F.; Chen, G.J.; Chen, H. Modular synthesis of glycopolymers with well-defined sugar units in the side chain via Ugi reaction and click chemistry: Hetero vs. homo. Polym. Chem. 2016, 7, 4263–4271. [Google Scholar] [CrossRef]
- Sharon, N. Bacterial lectins, cell-cell recognition and infectious disease. FEBS Lett. 1987, 217, 145–157. [Google Scholar] [CrossRef]
- Disney, M.D.; Zheng, J.; Swager, T.M.; Seeberger, P.H. Detection of bacteria with carbohydrate-functionalized fluorescent polymers. J. Am. Chem. Soc. 2004, 126, 13343–13346. [Google Scholar] [CrossRef]
- Li, D.; Chen, J.; Hong, M.; Wang, Y.; Haddleton, D.M.; Li, G.Z.; Zhang, Q. Cationic glycopolymers with aggregation-induced emission for the killing, imaging, and detection of bacteria. Biomacromolecules 2021, 22, 2224–2232. [Google Scholar] [CrossRef]
- Hussain, S.; Lv, F.; Qi, R.; Senthilkumar, T.; Zhao, H.; Chen, Y.; Liu, L.; Wang, S. Forster resonance energy transfer mediated rapid andsynergistic discrimination of bacteria over fungi using a cationic conjugated glycopolymer. ACS Appl. Bio Mater. 2020, 3, 20–28. [Google Scholar] [CrossRef]
- Richards, S.J.; Fullam, E.; Besra, G.S.; Gibson, M.I. Discrimination between bacterial phenotypes using glyco-nanoparticles and the impact of polymer coating on detection readouts. J. Mater. Chem. B 2014, 2, 1490–1498. [Google Scholar] [CrossRef]
- Ajish, J.K.; Kanagare, A.B.; Kumar, K.S.A.; Subramanian, M.; Ballal, A.D.; Kumar, M. Self-assembled glycobis(acrylamide)-stabilized gold nanoparticles for fluorescent turn-on sensing of lectin and Escherichia coli. ACS Appl. Nano Mater. 2019, 3, 1307–1317. [Google Scholar] [CrossRef]
- Yang, Q.; Strathmann, M.; Rumpf, A.; Schaule, G.; Ulbricht, M. Grafted glycopolymer-based receptor mimics on polymer support for selective adhesion of bacteria. ACS Appl. Mater. Interfaces 2010, 2, 3555–3562. [Google Scholar] [CrossRef]
- Malakootikhah, J.; Rezayan, A.H.; Negahdari, B.; Nasseri, S.; Rastegar, H. Porous MnFe2O4@SiO2 magnetic glycopolymer: A multivalent nanostructure for efficient removal of bacteria from aqueous solution. Ecotoxicol. Environ. Saf. 2018, 166, 277–284. [Google Scholar] [CrossRef]
- Hong, M.; Miao, Z.; Xu, X.; Zhang, Q. Magnetic iron oxide nanoparticles immobilized with sugar-containing poly(ionic liquid) brushes for efficient trapping and killing of bacteria. ACS Appl. Bio Mater. 2020, 3, 3664–3672. [Google Scholar] [CrossRef]
- Ajish, J.K.; Abraham, H.M.; Subramanian, M.; Kumar, K.S.A. A reusable column method using glycopolymer-functionalized resins for capture-detection of proteins and Escherichia coli. Macromol. Biosci. 2021, 21, 2000342. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, H.T.; Yang, Y.C.; Yoon, J.; Wang, S.; Zhang, X. Supramolecular antibacterial materials for combatting antibiotic resistance. Adv. Mater. 2019, 31, 28. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.Y.K.; Liu, Q.M.; Du, J.Z. Antibacterial polymeric nanostructures for biomedical applications. Chem. Commun. 2014, 50, 14482–14493. [Google Scholar] [CrossRef]
- Yuan, Y.Q.; Liu, F.; Xue, L.L.; Wang, H.W.; Pan, J.J.; Cui, Y.C.; Chen, H.; Yuan, L. Recyclable Escherichia coli-specific-killing AuNP-polymer (ESKAP) nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 11309–11317. [Google Scholar] [CrossRef]
- Li, L.; Zhang, K.; Chen, L.; Huang, Z.; Liu, G.; Li, M.; Wen, Y. Mass preparation of micro/nano-powders of biochar with water-dispersibility and their potential application. New J. Chem. 2017, 41, 9649–9657. [Google Scholar] [CrossRef]
- Borjihan, Q.; Yao, Q.F.; Qu, H.H.; Wu, H.X.; Liu, Y.; Dong, A. Glycopolymer N-halamine-modified biochars with high specificity for Escherichia coli eradication. Chin. J. Chem. Eng. 2021, 38, 229–236. [Google Scholar] [CrossRef]
- Dalrymple, O.K.; Stefanakos, E.; Trotz, M.A.; Goswami, D.Y. A review of the mechanisms and modeling of photocatalytic disinfection. Appl. Catal. B-Environ. 2010, 98, 27–38. [Google Scholar] [CrossRef]
- Wang, B.; Shang, C.; Miao, Z.; Guo, S.; Zhang, Q. Lactose-containing glycopolymer grafted onto magnetic titanium dioxide nanomaterials for targeted capture and photocatalytic killing of pathogenic bacteria. Eur. Polym. J. 2021, 142, 110159. [Google Scholar] [CrossRef]
- Tew, G.N.; Liu, D.H.; Chen, B.; Doerksen, R.J.; Kaplan, J.; Carroll, P.J.; Klein, M.L.; DeGrado, W.F. De novo design of biomimetic antimicrobial polymers. Proc. Natl. Acad. Sci. USA 2002, 99, 5110–5114. [Google Scholar] [CrossRef]
- Crespo, L.; Sanclimens, G.; Pons, M.; Giralt, E.; Royo, M.; Albericio, F. Peptide and amide bond-containing dendrimers. Chem. Rev. 2005, 105, 1663–1681. [Google Scholar] [CrossRef] [PubMed]
- Mowery, B.P.; Lee, S.E.; Kissounko, D.A.; Epand, R.F.; Epand, R.M.; Weisblum, B.; Stahl, S.S.; Gellman, S.H. Mimicry of antimicrobial host-defense peptides by random copolymers. J. Am. Chem. Soc. 2007, 129, 15474–15476. [Google Scholar] [CrossRef]
- Gabriel, G.J.; Maegerlein, J.A.; Nelson, C.E.; Dabkowski, J.M.; Eren, T.; Nusslein, K.; Tew, G.N. Comparison of facially amphiphilic versus segregated monomers in the design of antibacterial copolymers. Chem. Eur. J. 2009, 15, 433–439. [Google Scholar] [CrossRef]
- Giuliani, A.; Rinaldi, A.C. Beyond natural antimicrobial peptides: Multimeric peptides and other peptidomimetic approaches. Cell. Mol. Life Sci. 2011, 68, 2255–2266. [Google Scholar] [CrossRef]
- Kuroda, K.; Caputo, G.A. Antimicrobial polymers as synthetic mimics of host-defense peptides. WIREs Nanomed. Nanobiotechnol. 2013, 5, 49–66. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Cerrada, M.L.; Fernández-García, M. Introduction to Antimicrobial Polymeric Materials. In Polymeric Materials with Antimicrobial Activity: From Synthesis to Applications; Muñoz-Bonilla, A., Cerrada, M., Fernández-García, M., Eds.; Royal Society of Chemistry: London, UK, 2014; pp. 1–21. [Google Scholar]
- Stach, M.; Siriwardena, T.N.; Kohler, T.; van Delden, C.; Darbre, T.; Reymond, J.L. Combining topology and sequence design for the discovery of potent antimicrobial peptide dendrimers against multidrug-resistant pseudomonas aeruginosa. Angew. Chem. Int. Ed. 2014, 53, 12827–12831. [Google Scholar] [CrossRef]
- Liu, R.H.; Chen, X.Y.; Chakraborty, S.; Lemke, J.J.; Hayouka, Z.; Chow, C.; Welch, R.A.; Weisblum, B.; Masters, K.S.; Gellman, S.H. Tuning the biological activity profile of antibacterial polymers via subunit substitution pattern. J. Am. Chem. Soc. 2014, 136, 4410–4418. [Google Scholar] [CrossRef]
- Lam, S.J.; O’Brien-Simpson, N.M.; Pantarat, N.; Sulistio, A.; Wong, E.H.H.; Chen, Y.-Y.; Lenzo, J.C.; Holden, J.A.; Blencowe, A.; Reynolds, E.C.; et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016, 1, 16162. [Google Scholar] [CrossRef]
- Namivandi-Zangeneh, R.; Kwan, R.J.; Nguyen, T.-K.; Yeow, J.; Byrne, F.L.; Oehlers, S.H.; Wong, E.H.H.; Boyer, C. The effects of polymer topology and chain length on the antimicrobial activity and hemocompatibility of amphiphilic ternary copolymers. Polym. Chem. 2018, 9, 1735–1744. [Google Scholar] [CrossRef]
- Judzewitsch, P.R.; Nguyen, T.-K.; Shanmugam, S.; Wong, E.H.H.; Boyer, C. Towards sequence-controlled antimicrobial polymers: Effect of polymer block order on antimicrobial activity. Angew. Chem. Int. Ed. 2018, 57, 4559–4564. [Google Scholar] [CrossRef]
- Pranantyo, D.; Xu, L.Q.; Hou, Z.; Kang, E.-T.; Chan-Park, M.B. Increasing bacterial affinity and cytocompatibility with four-arm star glycopolymers and antimicrobial α-polylysine. Polym. Chem. 2017, 8, 3364–3373. [Google Scholar] [CrossRef]
- Zheng, Y.; Luo, Y.; Feng, K.; Zhang, W.; Chen, G. High throughput screening of glycopolymers: Balance between cytotoxicity and antibacterial property. ACS Macro Lett. 2019, 8, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Connell, I.; Agace, W.; Klemm, P.; Schembri, M.; Mărild, S.; Svanborg, C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA 1996, 93, 9827–9832. [Google Scholar] [CrossRef]
- Nagahori, N.; Lee, R.T.; Nishimura, S.-I.; Pagé, D.; Roy, R.; Lee, Y.C. Inhibition of adhesion of Type 1 fimbriated Escherichia coli to highly mannosylated ligands. ChemBioChem 2002, 3, 836–844. [Google Scholar] [CrossRef]
- Yan, X.; Sivignon, A.; Yamakawa, N.; Crepet, A.; Travelet, C.; Borsali, R.; Dumych, T.; Li, Z.; Bilyy, R.; Deniaud, D.; et al. Glycopolymers as antiadhesives of E. coli strains inducing inflammatory bowel diseases. Biomacromolecules 2015, 16, 1827–1836. [Google Scholar] [CrossRef]
- Zheng, L.; Luo, Y.; Chen, K.; Zhang, Z.; Chen, G. Highly branched gradient glycopolymer: Enzyme-assisted synthesis and enhanced bacteria-binding ability. Biomacromolecules 2020, 21, 5233–5240. [Google Scholar] [CrossRef]
- Luo, Y.; Gu, Y.; Feng, R.; Brash, J.; Eissa, A.M.; Haddleton, D.M.; Chen, G.; Chen, H. Synthesis of glycopolymers with specificity for bacterial strains via bacteria-guided polymerization. Chem. Sci. 2019, 10, 5251–5257. [Google Scholar] [CrossRef]
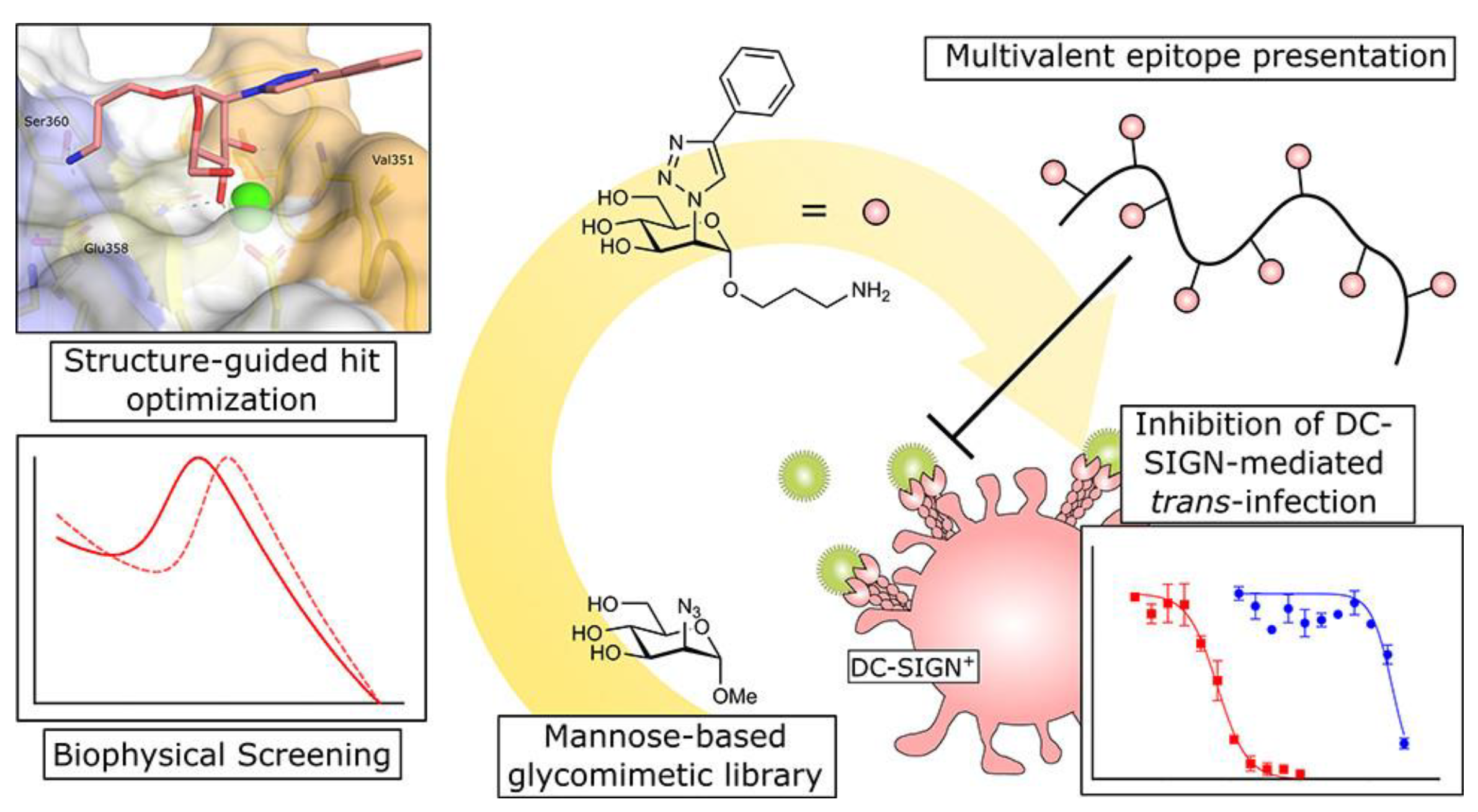
- Cramer, J.; Lakkaichi, A.; Aliu, B.; Jakob, R.P.; Klein, S.; Cattaneo, I.; Jiang, X.; Rabbani, S.; Schwardt, O.; Zimmer, G.; et al. Sweet drugs for bad bugs: A glycomimetic strategy against the DC-SIGN-mediated dissemination of SARS-CoV-2. J. Am. Chem. Soc. 2021, 143, 17465–17478. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Allen, J.D.; Wrapp, D.; Mclellan, J.S.; Crispin, M.J.S. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 2020, 369, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Becer, C.R.; Gibson, M.I.; Geng, J.; Ilyas, R.; Wallis, R.; Mitchell, D.A.; Haddleton, D.M. High-affinity glycopolymer binding to human DC-SIGN and disruption of DC-SIGN interactions with HIV envelope glycoprotein. J. Am. Chem. Soc. 2010, 132, 15130–15132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Su, L.; Collins, J.; Chen, G.; Wallis, R.; Mitchell, D.A.; Haddleton, D.M.; Becer, C.R. Dendritic cell lectin-targeting sentinel-like unimolecular glycoconjugates to release an anti-HIV drug. J. Am. Chem. Soc. 2014, 136, 4325–4332. [Google Scholar] [CrossRef]
- Peck, K.M.; Lauring, A.S. Complexities of viral mutation rates. J. Virol. 2018, 92, 8. [Google Scholar] [CrossRef]
- Sanjuan, R.; Domingo-Calap, P. Mechanisms of viral mutation. Cell. Mol. Life Sci. 2016, 73, 4433–4448. [Google Scholar] [CrossRef]
- Duffy, S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018, 16, 6. [Google Scholar] [CrossRef]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory syncytial virus: Infection, detection, and new options for prevention and treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef]
- Suthar, M.S.; Diamond, M.S.; Gale, M. West nile virus infection and immunity. Nat. Rev. Microbiol. 2013, 11, 115–128. [Google Scholar] [CrossRef]
- Jung, K.; Saif, L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015, 204, 134–143. [Google Scholar] [CrossRef]
- Xu, D.; Esko, J.D. Demystifying heparan sulfate–protein interactions. Annu. Rev. Biochem. 2014, 83, 129–157. [Google Scholar] [CrossRef]
- Katoh, M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (review). Int. J. Mol. Med. 2016, 38, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-L.; Ye, X.-S. Synthetic glycans and glycomimetics: A promising alternative to natural polysaccharides. Chem. Eur. J. 2018, 24, 6696–6704. [Google Scholar] [CrossRef] [PubMed]
- Soria-Martinez, L.; Bauer, S.; Giesler, M.; Schelhaas, S.; Materlik, J.; Janus, K.; Pierzyna, P.; Becker, M.; Snyder, N.L.; Hartmann, L.; et al. Prophylactic antiviral activity of sulfated glycomimetic oligomers and polymers. J. Am. Chem. Soc. 2020, 142, 5252–5265. [Google Scholar] [CrossRef] [PubMed]
- Lees, W.J.; Spaltenstein, A.; Kingery-Wood, J.E.; Whitesides, G.M. Polyacrylamides bearing pendant.alpha.-sialoside groups strongly inhibit agglutination of erythrocytes by influenza A virus: Multivalency and steric stabilization of particulate biological systems. J. Med. Chem. 1994, 37, 3419–3433. [Google Scholar] [CrossRef]
- Liu, H.-P.; Meng, X.; Yu, Q.; Tao, Y.-C.; Xu, F.; He, Y.; Yu, P.; Yang, Y. Synthesis of S-sialyl polymers as efficient polyvalent influenza inhibitors and capturers. J. Carbohydr. Chem. 2018, 37, 18–29. [Google Scholar] [CrossRef]
- Li, G.; Ma, W.; Mo, J.; Cheng, B.; Shoda, S.I.; Zhou, D.; Ye, X.S. Influenza virus precision diagnosis and continuous purification enabled by neuraminidase-resistant glycopolymer-coated microbeads. ACS Appl. Mater. Interfaces 2021, 13, 46260–46269. [Google Scholar] [CrossRef]
- Nagao, M.; Fujiwara, Y.; Matsubara, T.; Hoshino, Y.; Sato, T.; Miura, Y. Design of glycopolymers carrying sialyl oligosaccharides for controlling the interaction with the influenza virus. Biomacromolecules 2017, 18, 4385–4392. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kaneshima, T.; Adachi, R.; Sasaki, J.; Hashiguchi, T.; Koyama, T.; Matsushita, T.; Hatano, K. Preparation of glycopolymers having sialyl alpha2 --> 3 lactose moieties as the potent inhibitors for mumps virus. Bioorgan. Med. Chem. Lett. 2021, 52, 128389. [Google Scholar] [CrossRef]
- Nagao, M.; Matsubara, T.; Hoshino, Y.; Sato, T.; Miura, Y. Topological design of star glycopolymers for controlling the interaction with the influenza virus. Bioconjug. Chem. 2019, 30, 1192–1198. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Chang, H.-Y.; Lu, J.-K.; Huang, Y.-C.; Harroun, S.G.; Tseng, Y.-T.; Li, Y.-J.; Huang, C.-C.; Chang, H.-T. Self-assembly of antimicrobial peptides on gold nanodots: Against multidrug-resistant bacteria and wound-healing application. Adv. Funct. Mater. 2015, 25, 7189–7199. [Google Scholar] [CrossRef]
- Li, Y.; Ma, X.; Zhang, J.; Pan, X.; Li, N.; Chen, G.; Zhu, J. Degradable Selenium-Containing Polymers for Low Cytotoxic Antibacterial Materials. ACS Macro Lett. 2022, 11, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xu, Z.; Dai, X.; Zeng, Y.; Wei, Y.; He, X.; Yan, L.-T.; Tao, L. De Novo Design of Entropy-Driven Polymers Resistant to Bacterial Attachment via Multicomponent Reactions. J. Am. Chem. Soc. 2021, 143, 17250–17260. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S.; O’Garra, A.; Sher, A.; Wack, A. Host-directed immunotherapy of viral and bacterial infections: Past, present and future. Nat. Rev. Immunol. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, T.R.; Wells, T.J.; Souza-Fonseca-Guimaraes, F. Towards efficient immunotherapy for bacterial infection. Trends Microbiol. 2022, 30, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, W.; Yang, Y.; Zhao, Q.; Yang, C.; Jia, X.; Liu, Y.; Zhou, M.; Zeng, W.; Huang, X.; et al. Developing next-generation protein-based vaccines using high-affinity glycan ligand-decorated glyconanoparticles. Adv. Sci. 2022, 2204598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, L.; Li, Z.; Zhang, W.; Luo, F.; Chu, Y.; Chen, G. Glycocalyx-mimicking nanoparticles improve anti-PD-L1 cancer immunotherapy through reversion of tumor-associated macrophages. Biomacromolecules 2018, 19, 2098–2108. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, S.; Liu, B.; Yu, Y.; Zhao, Z.A.; Wang, C.; Liu, Z.; Chen, G.; Chen, H. Take immune cells back on track: Glycopolymer-engineered tumor cells for triggering immune response. ACS Macro Lett. 2019, 8, 337–344. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, R.; Heng, X.; Liu, X.; Chen, G. Glycopolymers for Antibacterial and Antiviral Applications. Molecules 2023, 28, 985. https://doi.org/10.3390/molecules28030985
Mei R, Heng X, Liu X, Chen G. Glycopolymers for Antibacterial and Antiviral Applications. Molecules. 2023; 28(3):985. https://doi.org/10.3390/molecules28030985
Chicago/Turabian StyleMei, Ruoyao, Xingyu Heng, Xiaoli Liu, and Gaojian Chen. 2023. "Glycopolymers for Antibacterial and Antiviral Applications" Molecules 28, no. 3: 985. https://doi.org/10.3390/molecules28030985
APA StyleMei, R., Heng, X., Liu, X., & Chen, G. (2023). Glycopolymers for Antibacterial and Antiviral Applications. Molecules, 28(3), 985. https://doi.org/10.3390/molecules28030985





