Preparation, Characterization and Antibacterial Property Analysis of Cellulose Nanocrystals (CNC) and Chitosan Nanoparticles Fine-Tuned Starch Film
Abstract
1. Introduction
2. Results and Discussions
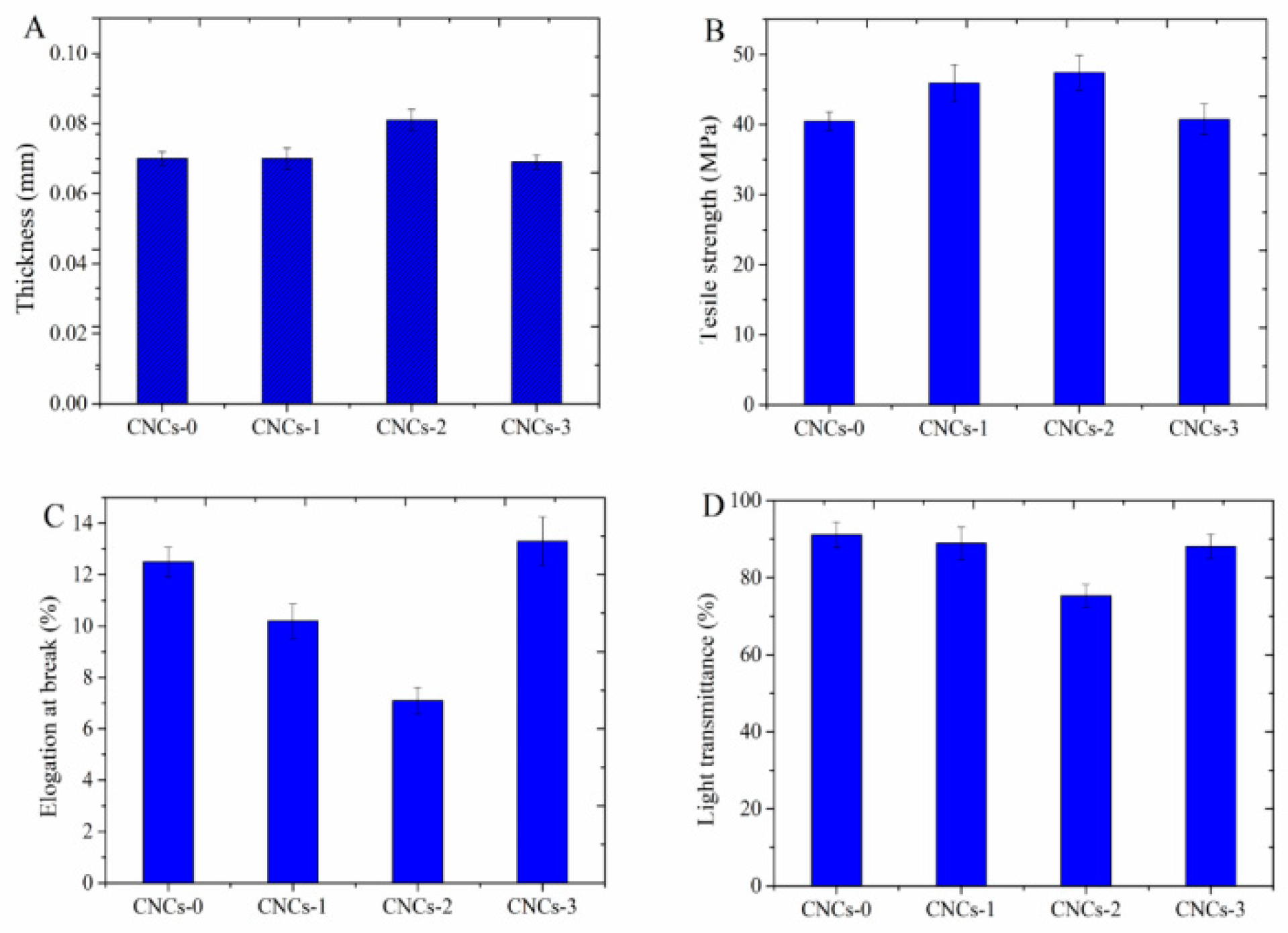
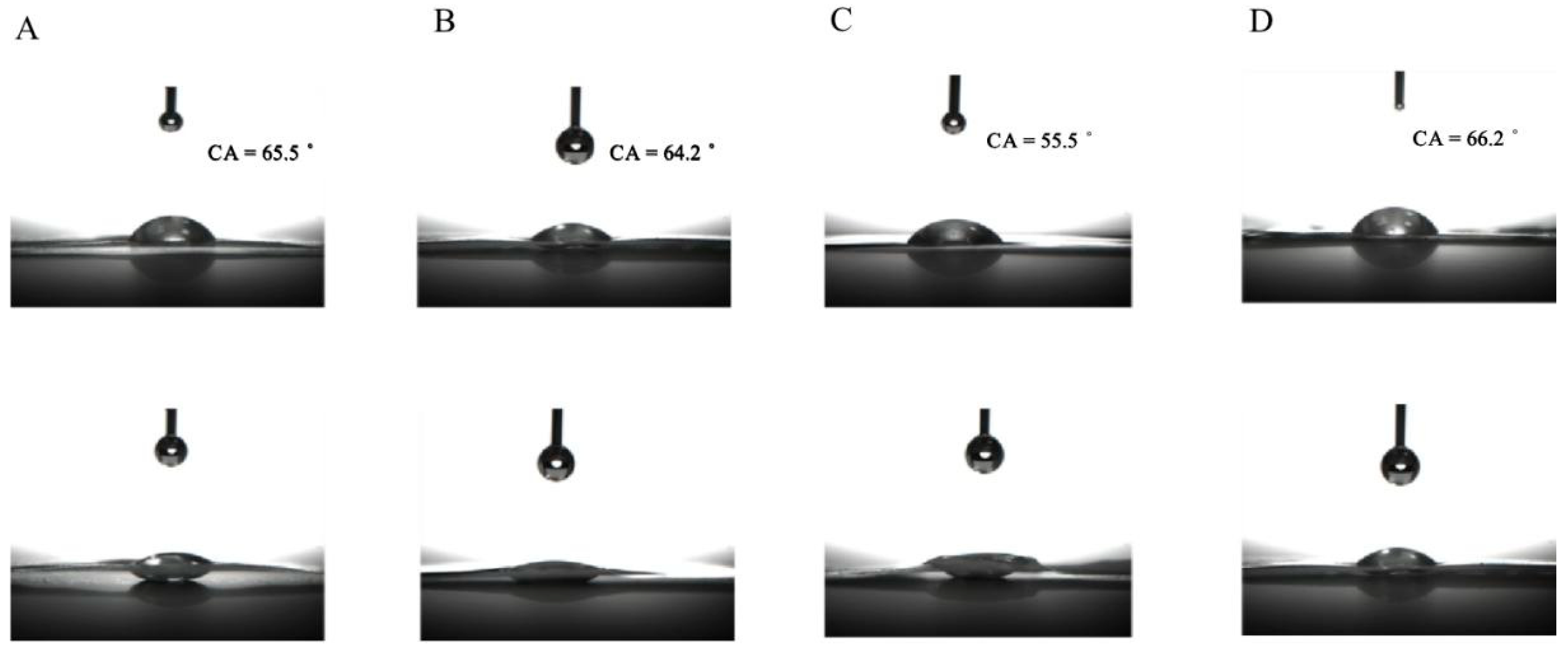
2.1. Physicochemical Properties of CNCs Nanocomposite Film
2.2. FESEM Observation of CNCs Nanocomposite Film
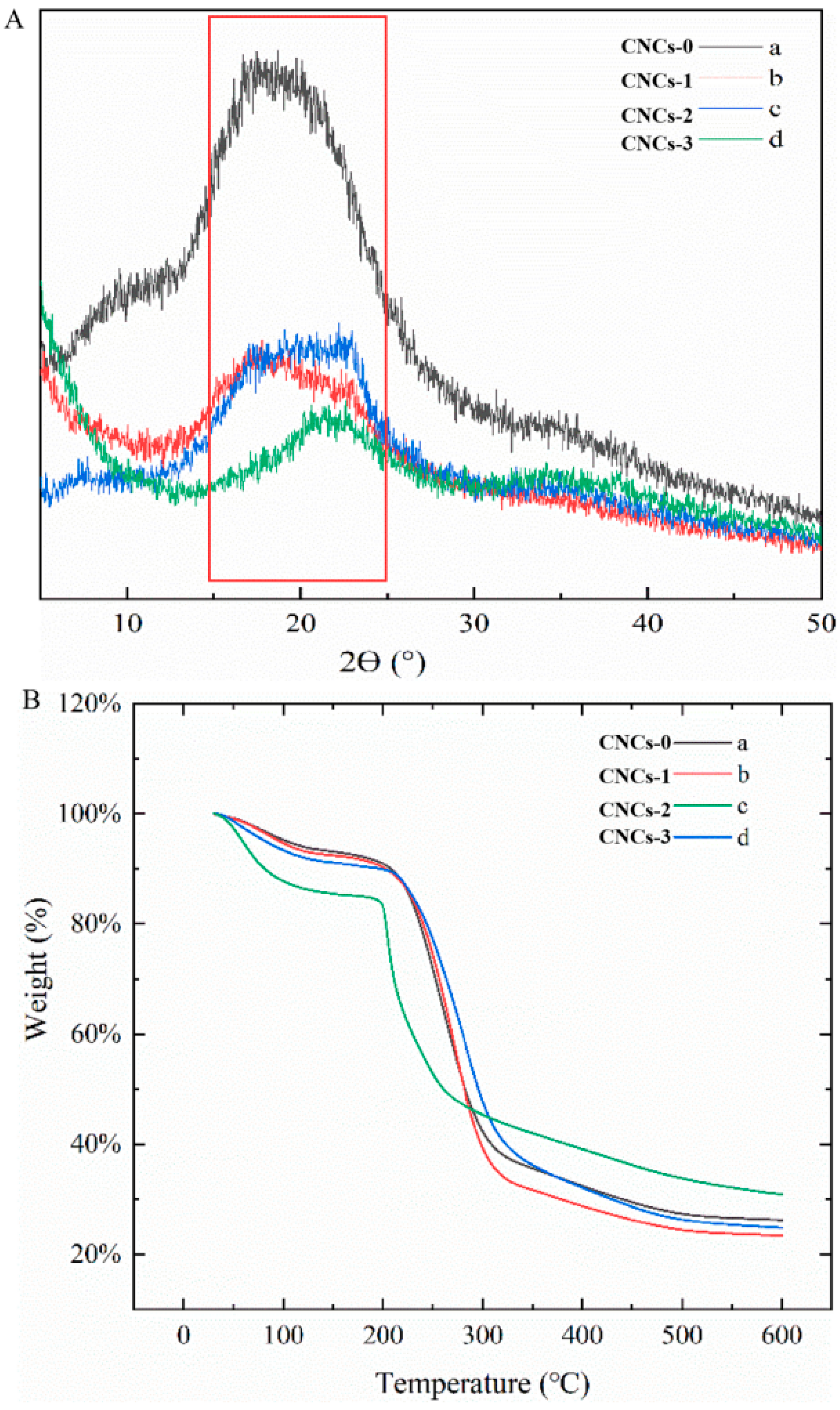
2.3. XRD Analysis of CNCs Nanocomposite Film
2.4. TGA Analysis of CNCs Nanocomposite Film
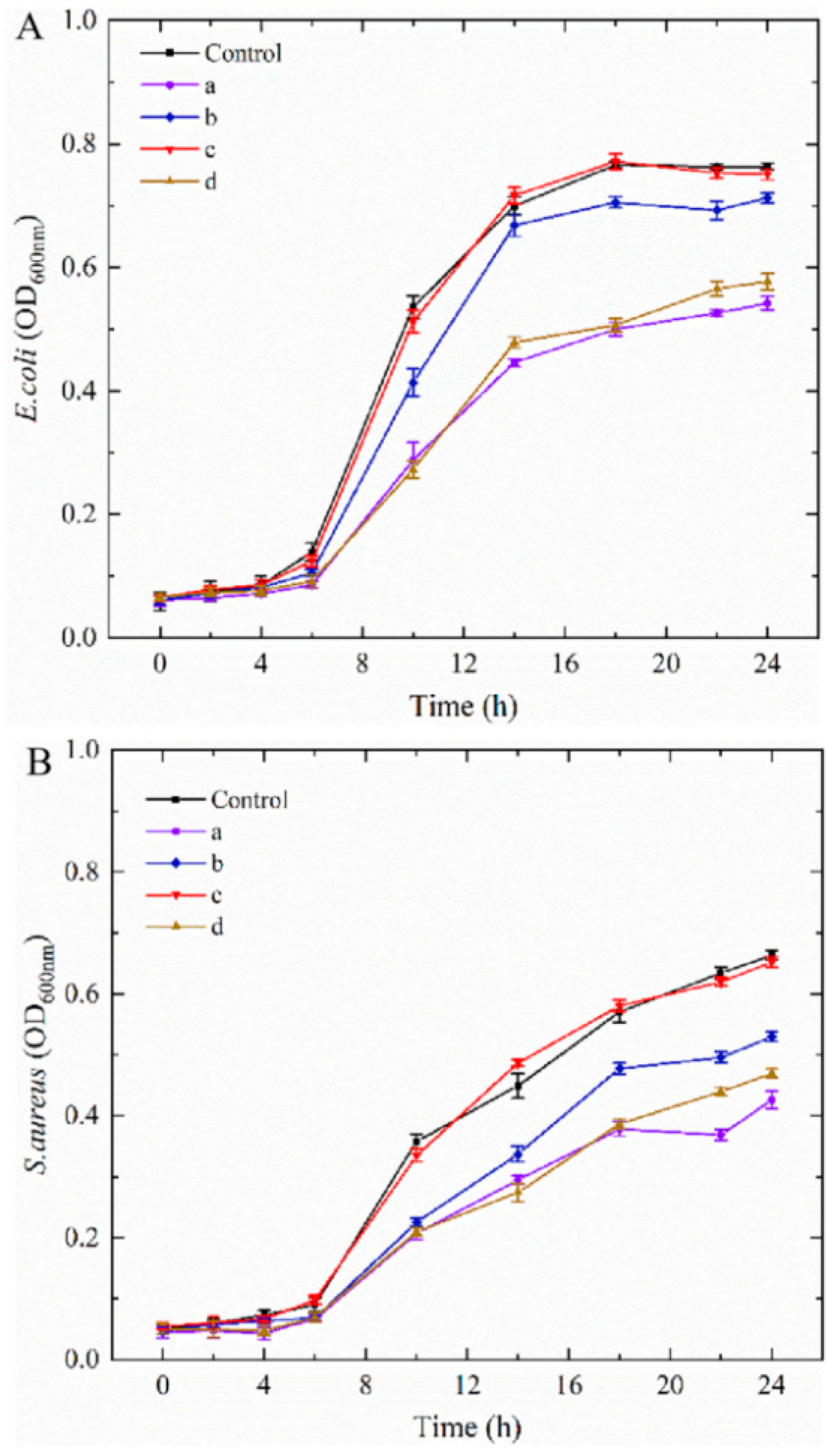
2.5. Antibacterial Performance of CNCs Nanocomposite Film
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of CNCs from MCC
3.3. Preparation of CS NPs Solutions
3.4. Preparation of Nanocomposite Film with CNCs Incorporated CS NPs in PS Matrix
3.5. Physicochemical Analysis of CNCs Nanocomposite Film
3.6. Field Emission Scanning Electron Microscope (FE-SEM)
3.7. Structural Analysis of CNCs Nanocomposite Film
3.8. Determination of Bacterial Growth Rate Using CNCs Nanocomposite Film
3.9. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Yadav, M.; Chiu, F.C. Cellulose nanocrystals reinforced κ-carrageenan based UV resistant transparent bionanocomposite film for sustainable packaging applications. Carbohydr. Polym. 2019, 211, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.R.; Souza, A.G.; Quispe, Y.M.; Rosa, D.S. Essential oils loaded-chitosan nanocapsules incorporation in biodegradable starch films: A strategy to improve fruits shelf life. Int. J. Biol. Macromol. 2021, 188, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Whiteside, W.S. Nano-cellulose reinforced starch bio composite films–A review on green composites. Int. J. Biol. Macromol. 2021, 185, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chang, W.; Chen, M.; Xu, F.; Ma, J.; Zhong, F. Film-forming properties of guar gum, tara gum and locust bean gum. Food Hydrocoll. 2020, 98, 105007. [Google Scholar] [CrossRef]
- Xiao, Y.Q.; Liu, Y.N.; Kang, S.F.; Cui, M.D.; Xu, H.D. Development of ph-responsive antioxidant soy protein isolate films incorporated with cellulose nanocrystals and curcumin nanocapsules to monitor shrimp freshness. Food Hydrocoll. 2021, 120, 10683. [Google Scholar] [CrossRef]
- Nascimento, K.M.; Cavalheiro, J.B.; Netto, A.; Scapim, M.; Bergamasco, R. Properties of alginate films incorporated with free and microencapsulated stryphnodendron adstringens extract (barbatimo). Food Packaging Shelf. 2021, 28, 100637. [Google Scholar] [CrossRef]
- Azucena Castro-Yobal, M.; Contreras-Oliva, A.; Saucedo-Rivalcoba, V.; Rivera-Armenta, J.L.; Hernández-Ramírez, G.; SalinasRuiz, J.; Herrera-Corredor, A. Evaluation of physicochemical properties of film-based alginate for food packing applications. e-Polymers 2021, 21, 82–95. [Google Scholar] [CrossRef]
- Rodrigues, M.V.; Marangon, C.A.; Martins, V.; Plepis, A. Chitosan/gelatin films with jatobá resin: Control of properties by vegetal resin inclusion and degree of acetylation modification. Int. J. Biol. Macromol. 2021, 182, 1737–1745. [Google Scholar] [CrossRef]
- Kyriakidou, A.; Makris, D.P.; Lazaridou, A.; Biliaderis, C.G.; Fabiano-Tixier, A.S. Physical properties of chitosan films containing pomegranate peel extracts obtained by deep eutectic solvents. Foods 2021, 10, 1262. [Google Scholar] [CrossRef]
- Qi, H.S.; Chang, C.Y.; Zhang, L.N. Properties and applications of biodegradable transparent and photoluminescent cellulose films prepared via a green process. Green Chem. 2009, 11, 177–184. [Google Scholar] [CrossRef]
- Pracella, M.; Mura, C.; Galli, G. Polyhydroxyalkanoate nanocomposites with cellulose nanocrystals as biodegradable coating and packaging materials. ACS Appl. Nano. Mater. 2021, 4, 260–270. [Google Scholar] [CrossRef]
- Sun, J.H.; Jiang, H.X.; Wu, H.B.; Tong, C.L.; Pang, J.; Wu, C.H. Multifunctional bionanocomposite film based on konjac glucomannan/chitosan with nano-ZnO and mulberry anthocyanin extract for active food packaging. Food Hydrocoll. 2020, 107, 105942. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Alrashedee, F.; Emran, K.A.; Al-Abdulkarim, H. Development of some magnetic metal-organic framework nano composites for pharmaceutical applications. Inorg. Chem. Commun. 2022, 138, 109251. [Google Scholar] [CrossRef]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.B.; Nguyen, D.H.H.; Nguyen, H.V.H. Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria x ananassa Duch.). Postharvest Biol. Technol. 2020, 162, 111103. [Google Scholar] [CrossRef]
- Zhang, H.C.; Jung, J.; Zhao, Y.Y. Preparation, characterization and evaluation of antibacterial activity of catechins and catechins-Zn complex loaded β-chitosan nanoparticles of different particle sizes. Carbohydr. Polym. 2016, 137, 82–91. [Google Scholar] [CrossRef]
- Alqahtani, F.Y.; Aleanizy, F.S.; Tahir, E.E.; Alquadeib, B.T.; Alsarra, I.A.; Alanazi, J.S.; Abdelhady, H.G. Preparation, characterization, and antibacterial activity of diclofenac-loaded chitosan nanoparticles. Saudi Pharm. J. 2019, 27, 82–87. [Google Scholar] [CrossRef]
- Pan, C.; Qian, J.; Fan, J.; Guo, H.; Gou, L.; Yang, H.; Liang, C. Preparation nanoparticle by ionic cross-linked emulsified chitosan and its antibacterial activity. Colloids Surf. A 2019, 568, 362–370. [Google Scholar] [CrossRef]
- Foged, C.; Brodin, B.; Frokjaer, S.; Sundblad, A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharmaceut. 2005, 298, 315–322. [Google Scholar] [CrossRef]
- Das, R.; Tom, L.; Sharma, P.R.; Chi, K.; Hsiao, B.S. Nanocellulose for sustainable water purification. Chem. Rev. 2022, 122, 8936–9031. [Google Scholar] [CrossRef]
- Deng, Z.L.; Jung, J.; Simonsen, J.; Wang, Y.; Zhao, Y.Y. Cellulose nanocrystal reinforced chitosan coatings for improving the storability of postharvest pears under both ambient and cold storages. J. Food Sci. 2017, 82, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.; Hamzeh, Y.; Kaboorani, A.; Abdulkhani, A. Influence of cellulose nanocrystal on strength and properties of low density polyethylene and thermoplastic starch composites. Ind. Crops Prod. 2018, 115, 298–305. [Google Scholar] [CrossRef]
- Kumar, A.; Lee, Y.; Kim, D.; Rao, K.M.; Kim, J.; Park, S.; Haider, A.; Lee, D.H.; Han, S.S. Effect of crosslinking functionality on microstructure, mechanical properties, and in vitro cytocompatibility of cellulose nanocrystals reinforced poly (vinyl alcohol)/sodium alginate hybrid scaffolds. Int. J. Biol. Macromol. 2017, 95, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Chaichi, M.; Hashemi, M.; Badii, F.; Mohammadi, A. Preparation and characterization of a novel bionanocomposite edible film based on pectin and crystalline nanocellulose. Carbohydr. Polym. 2017, 157, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.C.; Feng, M.M.; Chen, S.S.; Shi, W.Z.; Wang, X.C. Incorporation of lysozyme into cellulose nanocrystals stabilized β-chitosan nanoparticles with enhanced antibacterial activity. Carbohydr. Polym. 2020, 236, 115974. [Google Scholar] [CrossRef]
- Deng, Z.L.; Jung, J.; Simonsen, J.; Zhao, Y.Y. Effect of cellulose nanocrystals Pickering emulsion incorporated chitosan coating on storability of postharvest Bartlett pears (Pyrus communis) during high humidity long-term cold storage. Food Hydrocoll. 2018, 84, 229–237. [Google Scholar] [CrossRef]
- Song, M.; Hu, X.; Gu, T.; Zhang, W.X.; Deng, Z.L. Nanocelluloses affixed nanoscale Zero-valent iron (nZVI) for nickel removal: Synthesis, characterization and mechanisms. J. Environ. Chem. Eng. 2022, 10, 107466. [Google Scholar] [CrossRef]
- Sahraee, S.; Ghanbarzadeh, B.; Milani, J.M.; Hamishehkar, H. Development of gelatin bionanocomposite film containing chitin and ZnO nanoparticles. Food Bioprocess Technol. 2017, 10, 1–13. [Google Scholar] [CrossRef]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and characterization of edible chitosan/olive oil emulsion films. Carbohydr. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Gopi, S.; Geethamma, V.G.; Kalarikkal, N.; Thomas, S. Cellulose nanofiber vs nanocrystals from pineapple leaf fiber: A comparative studies on reinforcing efficiency on starch nanocomposites. Macromol. Symp. 2018, 380, 1–7. [Google Scholar] [CrossRef]
- González, K.; Guaresti, O.; Palomares, T.; Alonso-Varona, A.; Eceiza, A.; Gabilondo, N. The role of cellulose nanocrystals in biocompatible starch-based clicked nanocomposite hydrogels. Int. J. Biol. Macromol. 2020, 143, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, X.; Jin, R.; Li, D. Preparation and characterization of nanocomposite films containing starch and cellulose nanofibers. Ind. Crops Prod. 2018, 123, 654–660. [Google Scholar] [CrossRef]
- Bahar, E.; Ucar, N.; Onen, A.; Wang, Y.J.; Oksüz, M.; Ayaz, O.; Ucar, M.; Demir, A. Thermal and mechanical properties of polypropylene nanocomposite materials reinforced with cellulose nanowhiskers. J. Appl. Polym. Sci. 2012, 125, 2882–2889. [Google Scholar]
- Morais, J.P.S.; Rosa, M.F.; Filho, M.M.S.; Nascimento, L.D.; Nascimento, D.M.; Cassales, R. Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydr. Polym. 2013, 91, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.P.; Rhim, J.W. Characterization of bionanocomposite film prepared with agar and paper-mulberry pulp nanocellulose. Carbohydr. Polym. 2014, 110, 480–488. [Google Scholar] [CrossRef]
- Henrique, M.A.; Neto, W.P.F.; Silvério, H.A.; Martins, D.F.; Gurgel, L.V.A.; da Silva Barud, H.; de Morais, L.C.; Pasquini, D. Kinetic study of the thermal decomposition of cellulose nanocrystals with different polymorphs, cellulose I and II, extracted from different sources and using different types of acids. Ind. Crops Prod. 2015, 76, 128–140. [Google Scholar] [CrossRef]
- Nessi, V.; Falourd, X.; Maigret, J.E.; Cahier, K.; D’Orlando, A.; Descamps, N.; Lourdin, D. Cellulose nanocrystals-starch nanocomposites produced by extrusion: Structure and behavior in physiological conditions. Carbohydr. Polym. 2019, 225, 115123. [Google Scholar] [CrossRef]
- Liu, D.; Dong, Y.; Bhattacharyya, D.; Sui, G. Novel sandwiched structures in starch/cellulose nanowhiskers (CNWs) composite films. Chem. Commun. 2017, 4, 5–9. [Google Scholar] [CrossRef]
- Wang, S.F.; Shen, L.; Zhang, W.D.; Tong, Y.J. Preparation and mechanical properties of chitosan/carbon nanotubes composites. Biomacromolecules 2005, 6, 3067–3072. [Google Scholar] [CrossRef]
- Marett, J.; Aning, A.; Foster, E.J. The isolation of cellulose nanocrystals from pistachio shells via acid hydrolysis. Ind. Crops Prod. 2017, 109, 869–874. [Google Scholar] [CrossRef]
- Zhang, H.C.; Qian, Y.N.; Chen, S.S.; Zhao, Y.Y. Physicochemical charateristics and emulsificaiton properties of cellulose nanocrystals stabilized O/W Pickering emulsions with high -OSO3− groups. Food Hydrocoll. 2019, 96, 267–277. [Google Scholar] [CrossRef]
- Prado, K.S.; Spinacé, M.A.S. Isolation and characterization of cellulose nanocrystals from pineapple crown waste and their potential uses. Int. J. Biol. Macromol. 2019, 122, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Dehnad, D.; Mirzaei, H.; Emam-Djomeh, Z.; Jafari, S.; Dadashi, S. Thermal and antimicrobial properties of chitosan-nanocellulose films for extending shelf life of ground meat. Carbohydr. Polym. 2014, 109, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, R.A.; Salmieri, S.; Tien, C.L.; Riedel, B.; Bouchrd, J.; Chauve, G.; Tan, V.; Kamal, M.R.; Lacroix, M. Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan nanocomposite film. Carbohydr. Polym. 2012, 90, 1601–1608. [Google Scholar] [CrossRef]
- Marroquin, J.B.; Rheea, K.Y.; Park, S.J. Chitosan nanocomposite film: Enhanced electrical conductivity, thermal stability, and mechanical properties. Carbohydr. Polym. 2013, 92, 1783–1791. [Google Scholar] [CrossRef]
- Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R.; Montero, B. Processing and characterization of polyols plasticized-starch reinforced with microcrystalline cellulose. Carbohydr. Polym. 2016, 149, 83–93. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; Mccarron, P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf. B 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Al-Farhan, B.S.; Abou El-Ezz, D.; Abd–El Sayed, M.A.; Zikry, M.M.; Abu-Dief, A.M. Green biogenic synthesis of silver nanoparticles using aqueous extract ofmoringa oleifera: Access to a powerful antimicrobial, anticancer, pesticidal and catalytic agents. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1422–1435. [Google Scholar] [CrossRef]
- Moritz, M.; Geszkemoritz, M. The newest achievements in synthesis, imobilization and practical applications of antibacterial nanoparticles. Chem. Eng. J. 2013, 228, 596–613. [Google Scholar] [CrossRef]
- Zhang, H.C.; Jung, J.; Zhao, Y.Y. Preparation and characterization of cellulose nanocrystals films incorporated with essential oil loaded β-chitosan beads. Food Hydrocoll. 2017, 69, 164–172. [Google Scholar] [CrossRef]
- Baek, J.; Wahid-Pedro, F.; Kim, K.; Tam, K.C. Phosphorylated-CNC/modified-chitosan nanocomplexes for the stabilization of Pickering emulsions. Carbohydr. Polym. 2019, 206, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Zhao, Y.Y. Effect of molecular weight, acid, and plasticizer on the physicochemical and antibacterial properties of β-chitosan based films. J. Food Sci. 2012, 77, E127–E136. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Z.; Wu, Z.; Tan, X.; Deng, F.; Chen, Y.; Chen, Y.; Zhang, H. Preparation, Characterization and Antibacterial Property Analysis of Cellulose Nanocrystals (CNC) and Chitosan Nanoparticles Fine-Tuned Starch Film. Molecules 2022, 27, 8542. https://doi.org/10.3390/molecules27238542
Deng Z, Wu Z, Tan X, Deng F, Chen Y, Chen Y, Zhang H. Preparation, Characterization and Antibacterial Property Analysis of Cellulose Nanocrystals (CNC) and Chitosan Nanoparticles Fine-Tuned Starch Film. Molecules. 2022; 27(23):8542. https://doi.org/10.3390/molecules27238542
Chicago/Turabian StyleDeng, Zilong, Zixuan Wu, Xiao Tan, Fangkun Deng, Yaobang Chen, Yanping Chen, and Hongcai Zhang. 2022. "Preparation, Characterization and Antibacterial Property Analysis of Cellulose Nanocrystals (CNC) and Chitosan Nanoparticles Fine-Tuned Starch Film" Molecules 27, no. 23: 8542. https://doi.org/10.3390/molecules27238542
APA StyleDeng, Z., Wu, Z., Tan, X., Deng, F., Chen, Y., Chen, Y., & Zhang, H. (2022). Preparation, Characterization and Antibacterial Property Analysis of Cellulose Nanocrystals (CNC) and Chitosan Nanoparticles Fine-Tuned Starch Film. Molecules, 27(23), 8542. https://doi.org/10.3390/molecules27238542





