Acute Stress-Induced Changes in the Lipid Composition of Cow’s Milk in Healthy and Pathological Animals
Abstract
1. Introduction
2. Results
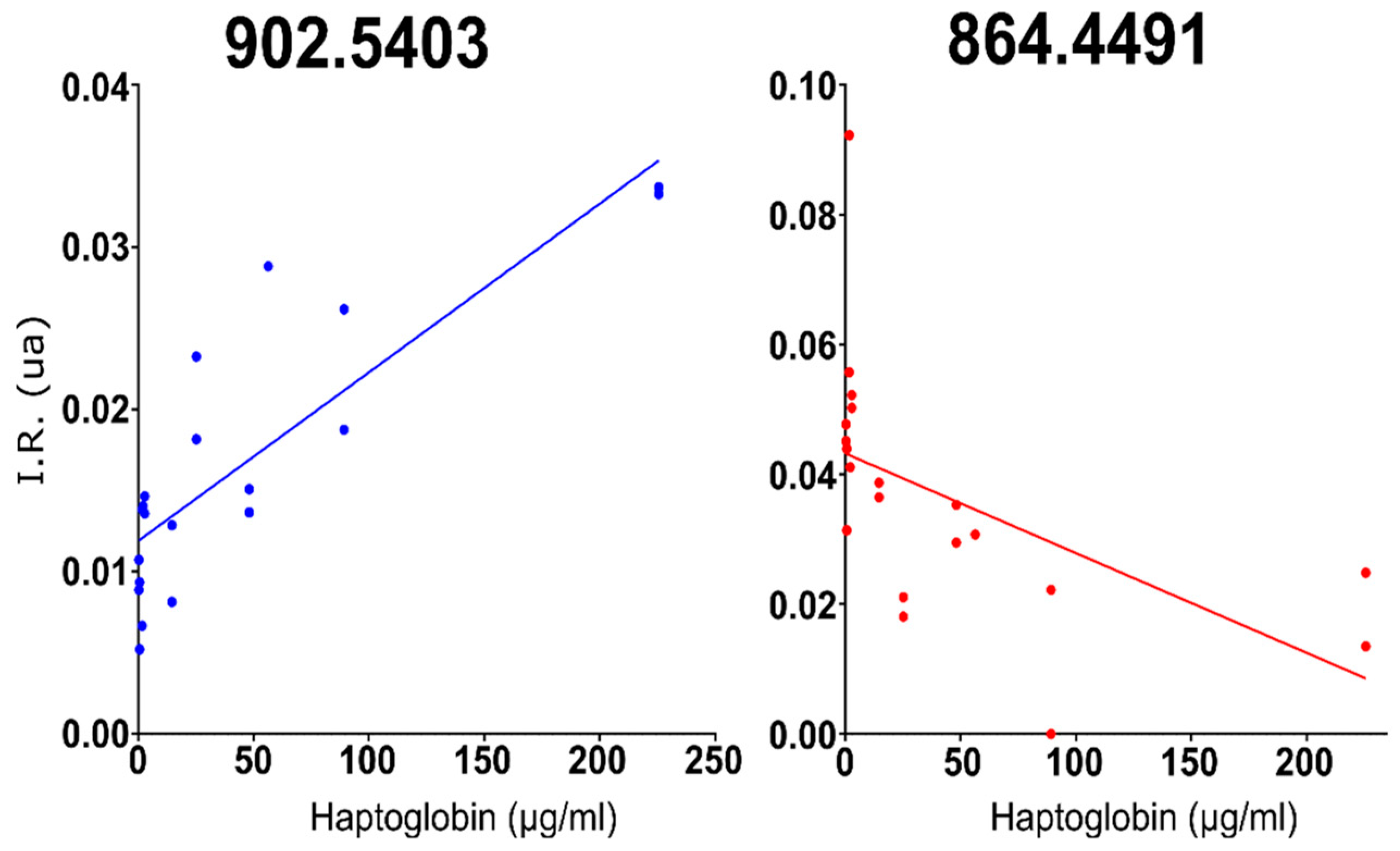
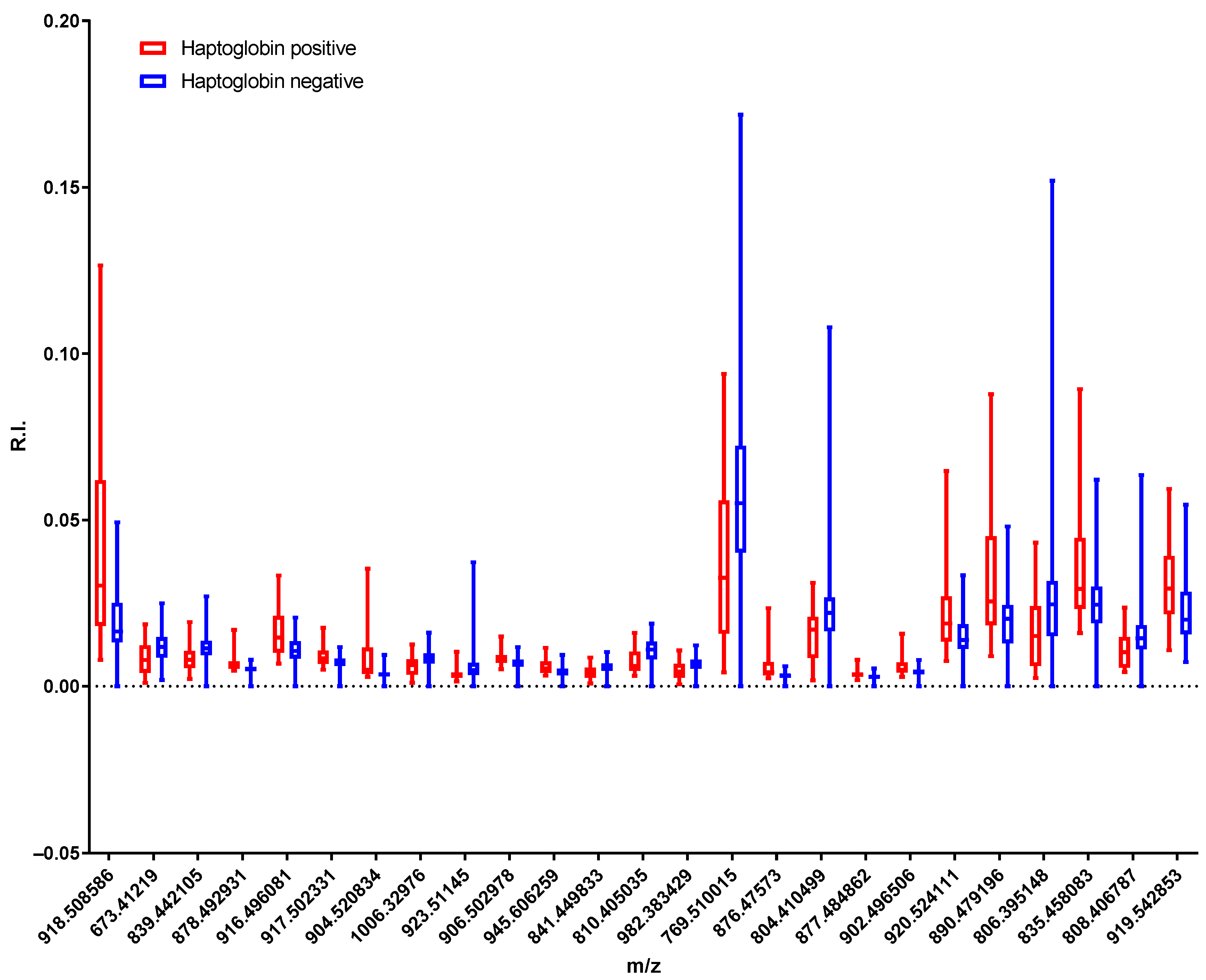
2.1. Identification of Lipid Compounds Positively or Negatively Correlated with Milk Haptoglobin Concentration
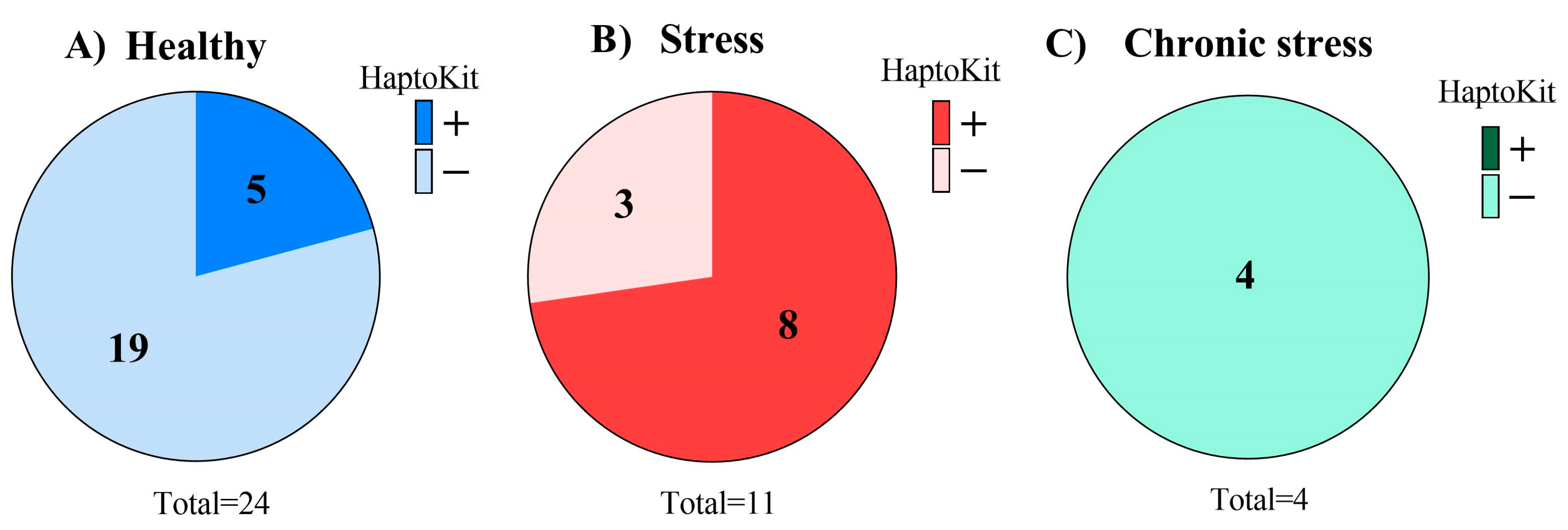
2.2. Estimation of the Prevalence of Haptoglobin in Healthy Animals, Pathological Animals, and in the Milk Tanks of Different Farms
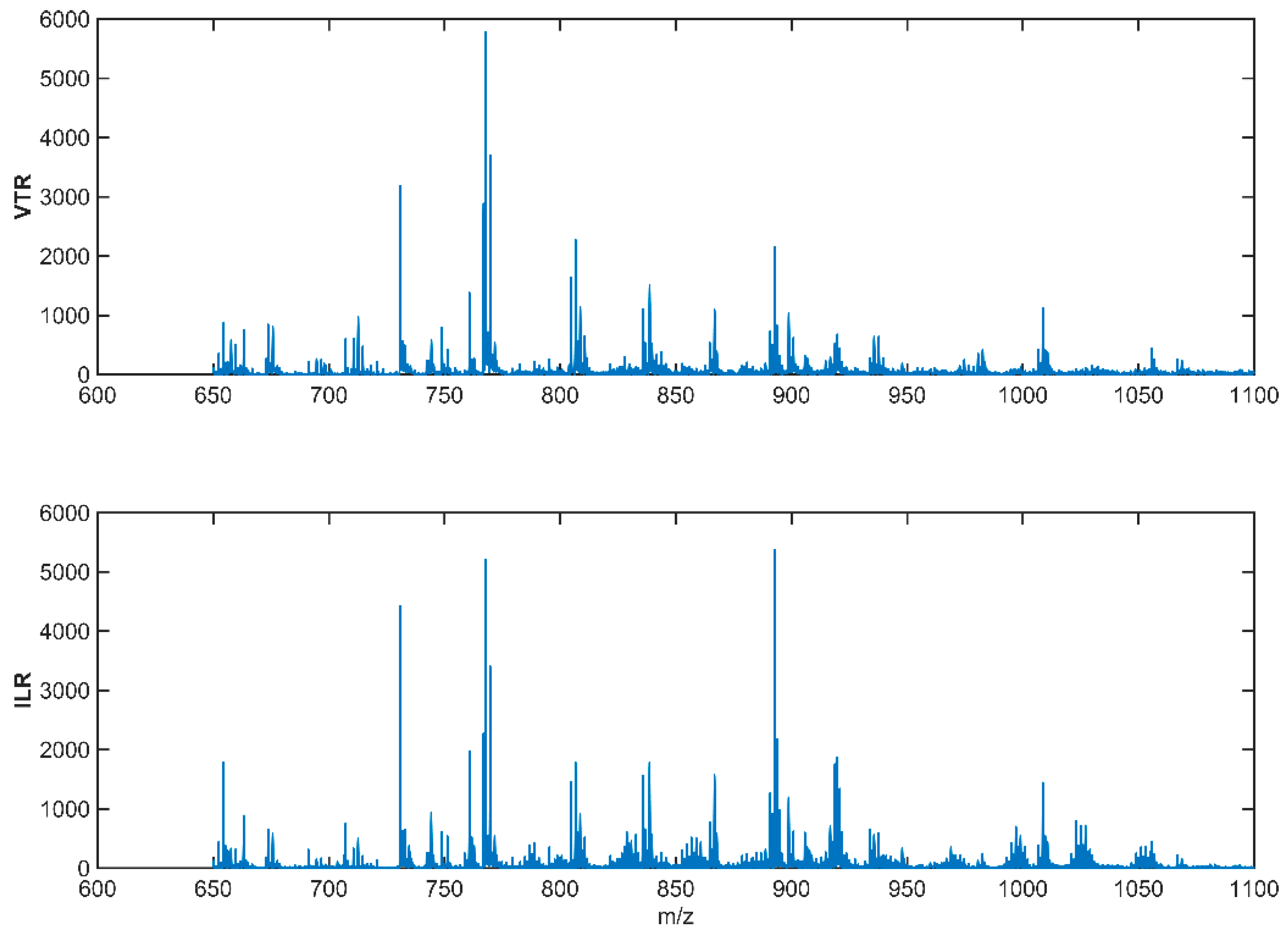
2.3. Lipidomic Analysis of the Milk Samples
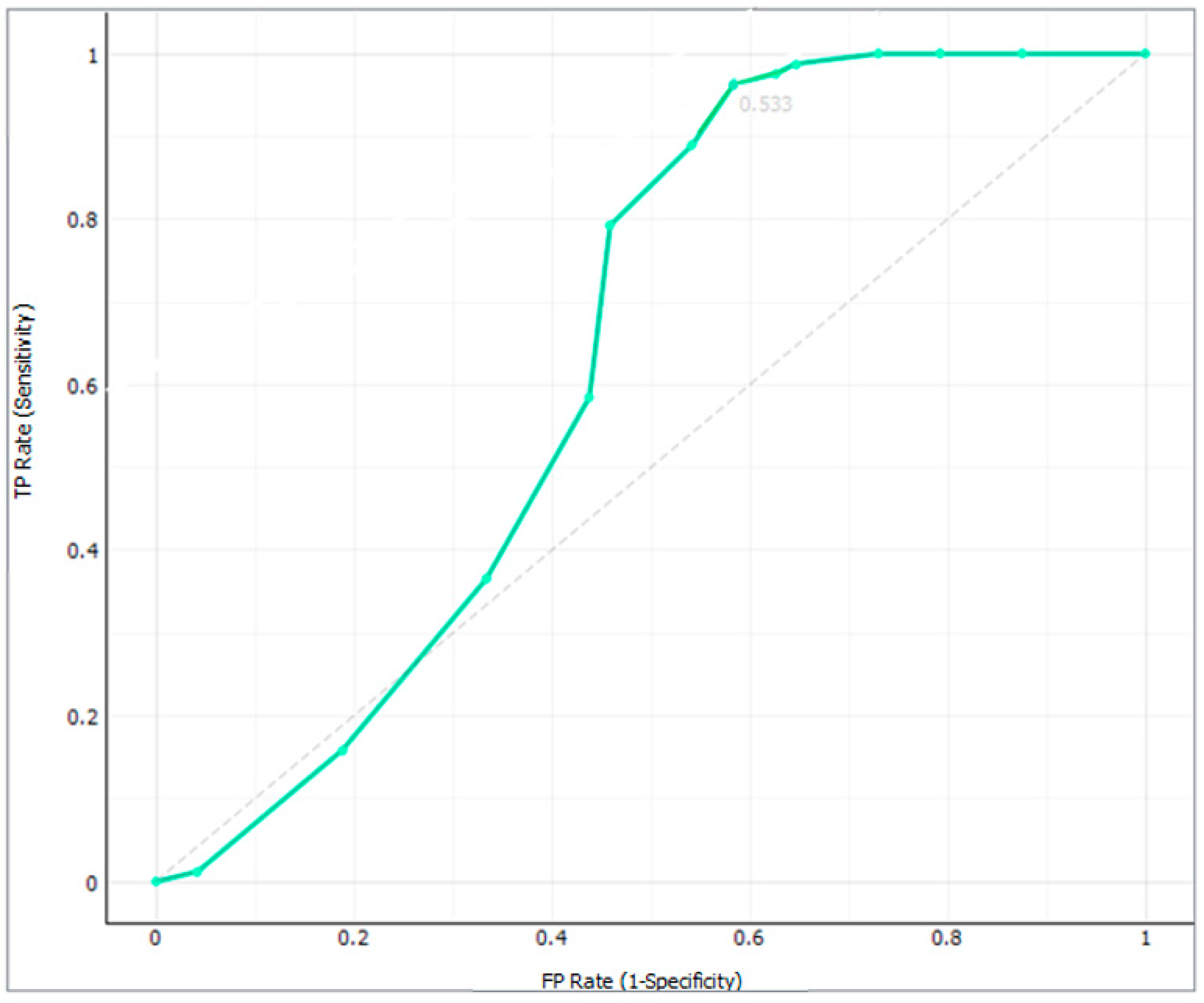
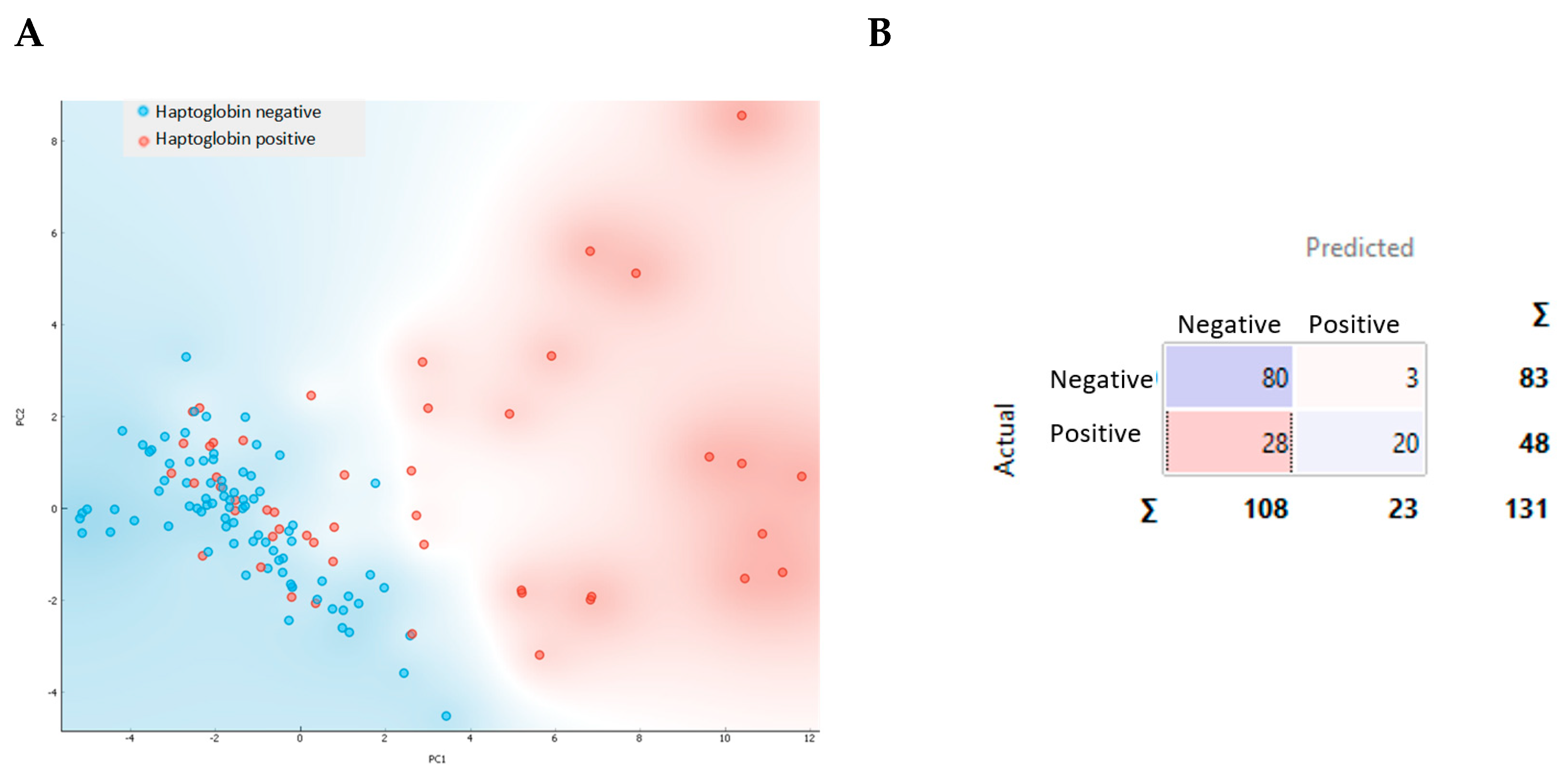
2.4. Classification of the Milk Samples Based on Their Haptoglobin Concentration and Lipid Biomarkers
3. Discussion
4. Materials and Methods
4.1. Samples
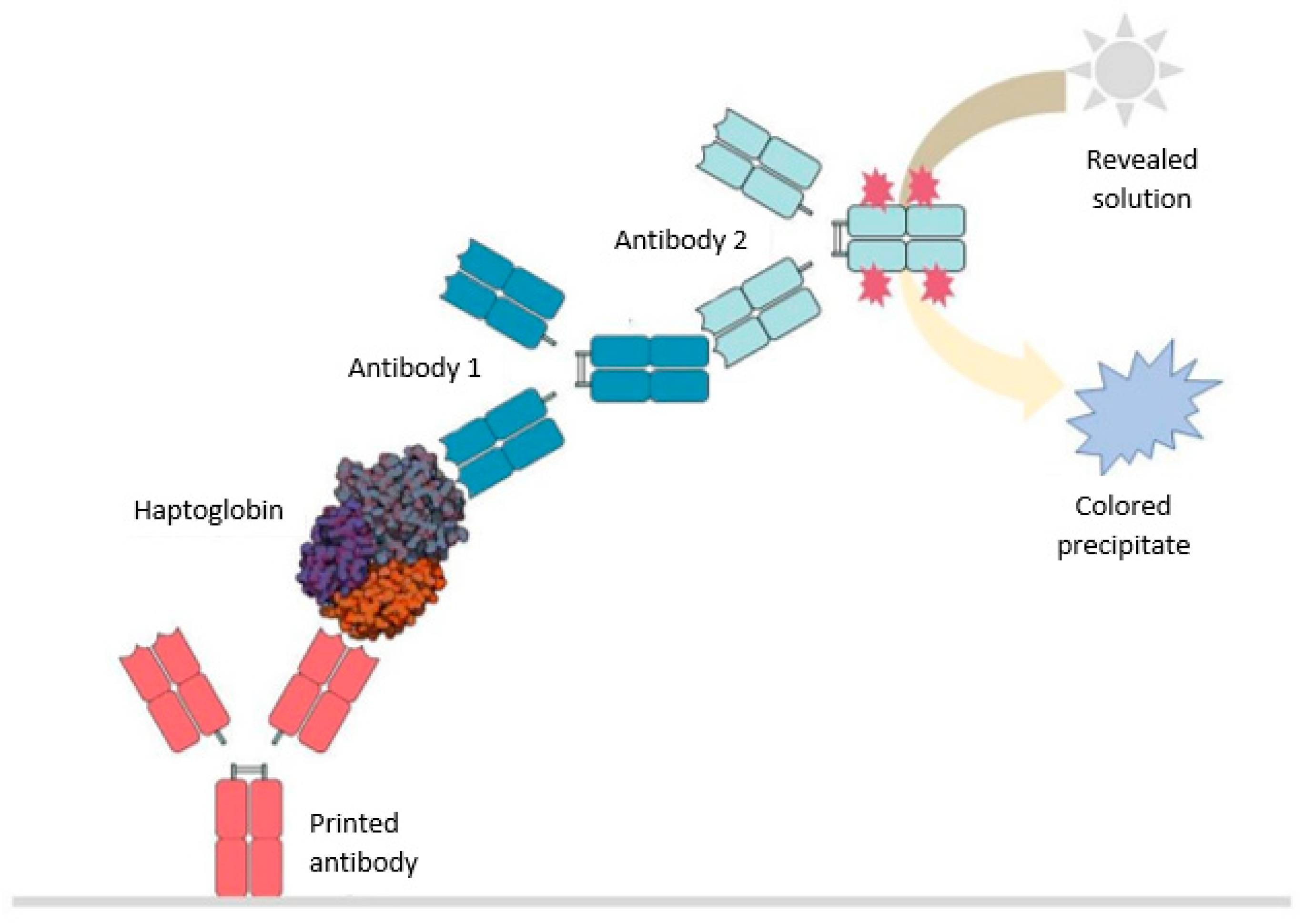
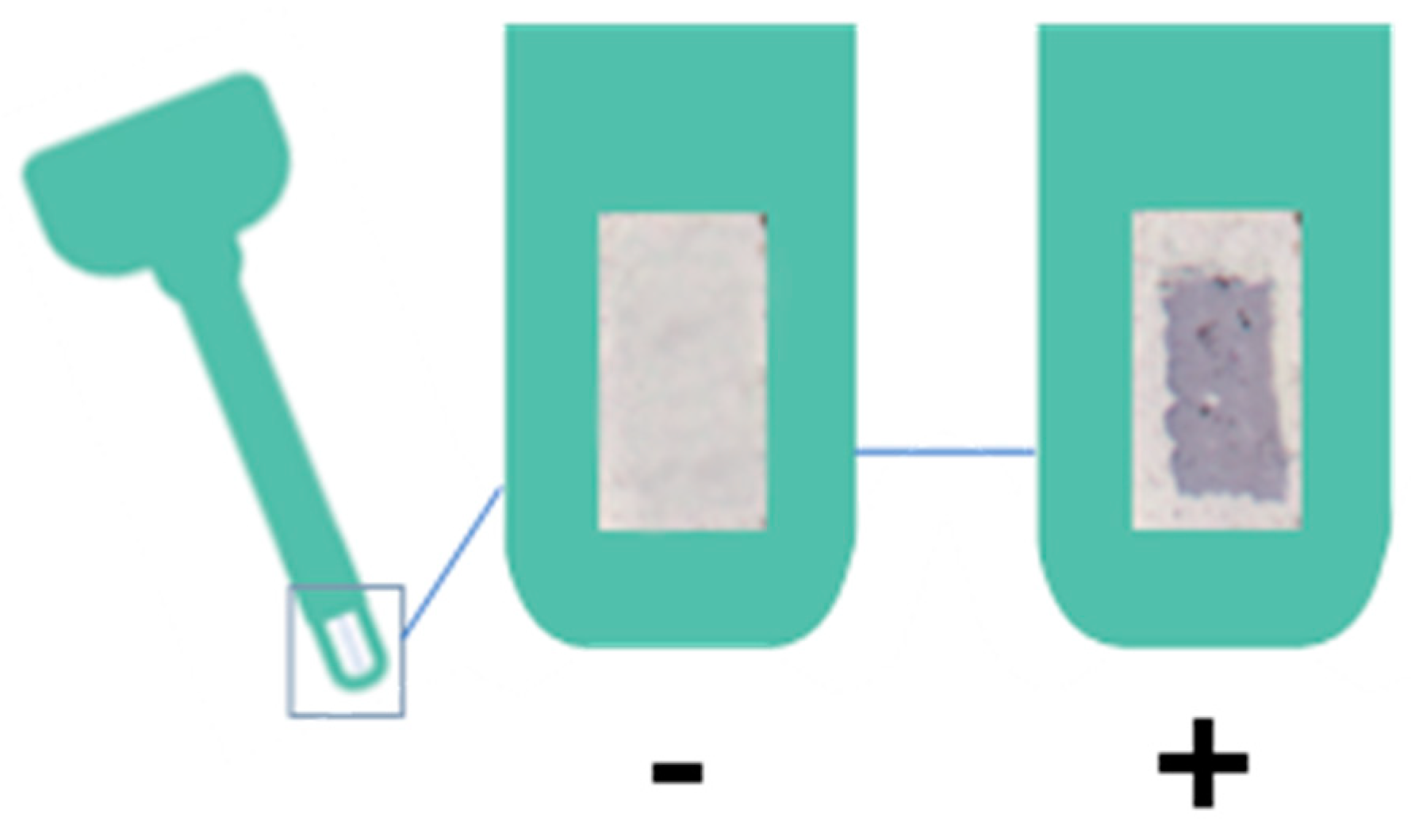
4.2. Haptokit
4.3. MALDI-TOF Mass Spectrometry
4.4. Spectrum Processing
4.5. Statistical Analyses
4.6. Lipid Assignment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Milk Sample | Haptoglobin Concentration (µg/mL) |
|---|---|
| 1 green | 14.71 |
| 2 green | 2.19 |
| 3 green | 89.2 |
| 1 blue | 1.81 |
| 2 blue | 0.37 |
| 3 blue | 56.4 |
| 1 red | 252.0 |
| 2 red | 225.6 |
| 3 red | 48.2 |
| Control | 2.86 |
| Animal Type | Farm’s Name | Samples | Results of the Haptokit Test | Prevalence of Positive Results in Each Animal Group |
|---|---|---|---|---|
| Healthy cows | Marcilla farm | 1 | − | |
| 2 | + | |||
| 3 | − | |||
| 4 | + | |||
| 5 | − | |||
| Valtierra farm | 6 | − | ||
| 7 | − | |||
| 8 | − | |||
| 9 | − | |||
| 10 | − | |||
| Murchante farm | 11 | − | 24% | |
| 12 | − | |||
| 13 | − | |||
| 14 | − | |||
| 15 | + | |||
| Fitero farm | 16 | - | ||
| 17 | − | |||
| 18 | − | |||
| 19 | + | |||
| 20 | + | |||
| Ilarregi farm | 21 | - | ||
| 22 | + | |||
| 23 | − | |||
| 24 | − | |||
| 25 | − | |||
| Pathological cows | Marcilla farm | 1 | + | 50% |
| 2 | − | |||
| 3 | − | |||
| Valtierra farm | 4 | + | ||
| Murchante farm | 5 | + | ||
| 6 | − | |||
| Fitero farm | 7 | + | ||
| 8 | − | |||
| 9 | + | |||
| 10 | + | |||
| Ilarregi farm | 11 | − | ||
| 12 | + | |||
| 13 | − | |||
| 14 | − |
| Farm’s Name | Reference | Healthy Animal Samples | Pathological Animal Samples | Tank’s Samples | Nº of Cattle | More Recent Welfare Quality® Qualification | Feed | Observations |
|---|---|---|---|---|---|---|---|---|
| Alfajarín | ALF | - | - | 1 | 70 | GOOD | - | Full tank of two milking batches. |
| Montañana | MNT | - | - | 1 | 50 | ENOUGH | - | Full tank of two milking batches. |
| Miralbueno | MRB | - | - | 1 | 60 | GOOD | - | Full tank of two milking batches. |
| Movera | MVR | - | - | 1 | 120 | GOOD | - | Full tank of two milking batches. |
| Marcilla | MAR | 5 | 3 | 1 | 90 | ENOUGH | 12 kg feed + 10 kg alfalfa silo + 12 kg ray grass silo + 4 kg fescue silo + 4 kg pea silo + 0.6 kg straw | Samples collected during afternoon milking. Temperatures above 40 °C. |
| Valtierra | VTR | 5 | 1 | 1 | 105 | GOOD | 11 kg feed + 10 kg silo ray grass + 13 kg silo corn + 10 kg pulp beetroot + 1 kg straw + 4 kg alfalfa silo | Samples collected during afternoon milking from a single batch. Heat and storm during the visit. |
| Murchante | MCH | 5 | 2 | 1 | 260 | ENOUGH | - | Samples taken during morning milking with the tank full from two milking runs. Sick animals have their own stables. |
| Fitero | FTR | 5 | 4 | 1 | 90 | GOOD | 12 kg feed + 10 kg ray grass silo + 26 kg corn silo | Tank sample from two milking batches, collected on an afternoon with temperatures above 40 °C. Farm with mastitis problems, active epidemic, and with numerous affected animals throughout the summer. |
| Ilarregi | ILR | 5 | 4 | 1 | 30 | GOOD | 10 kg feed + 15 kg silo local grass + sheepherding at discretion | Animals with freedom and sheepherding on hills, which has caused them to be subject to different accidents. Above all, limps and back injuries due to the interaction between them. Sampling during the morning and with the tank full from milking. |
| m/z | Lipid # | Issomer I # | Issomer II # | Issomer III # | Issomer IIII # | Bartlett p Value | Mean Comparison Method | p Value | Sig | Relative Increase |
|---|---|---|---|---|---|---|---|---|---|---|
| 673.4122 | NM | 0.9043 | T-test (Parametric) | 0.0000 | **** | −0.340 | ||||
| 677.3009 | NM | 0.5619 | t-test (Parametric) | 0.0006 | *** | −0.230 | ||||
| 722.3078 | NM | 0.0382 | Wilcoxon (Non-parametric) | 0.0056 | ** | −0.239 | ||||
| 728.5111 | NM | 0.0016 | Wilcoxon (Non-parametric) | 0.0175 | * | −0.188 | ||||
| 764.5491 | NM | 0.0607 | t-test (Parametric) | 0.0095 | ** | −0.176 | ||||
| 769.51 | NM | 0.0359 | Wilcoxon (Non-parametric) | 0.0003 | *** | −0.361 | ||||
| 781.4299 | NM | 0.0000 | Wilcoxon (Non-parametric) | 0.0234 | * | −0.193 | ||||
| 792.5595 | NM | 0.2846 | t-test (Parametric) | 0.0214 | * | −0.145 | ||||
| 804.4105 | PE-P 32:2 | 14:0p/18:2 | 16:1p/16:1 | 0.0000 | Wilcoxon (Non-parametric) | 0.0002 | *** | −0.350 | ||
| 806.3951 | PE-P32:1 | 14:0p/18:1 | 16:0p/16:1 | 0.0000 | Wilcoxon (Non-parametric) | 0.0002 | *** | −0.426 | ||
| 807.422 | NM | 0.3173 | t-test (Parametric) | 0.0053 | ** | −0.192 | ||||
| 808.4068 | PC 28:1 | 14:0/14:1 | 10:0/18:1 | 10:0/18:1 | 16:1/12:0 | 0.0000 | Wilcoxon (Non-parametric) | 0.0002 | *** | −0.328 |
| 809.46 | SM 32:0 | d16:0/16:0 | d18:0/14:0 | 0.2844 | t-test (Parametric) | 0.0016 | ** | −0.177 | ||
| 810.405 | PC 28:0 | 16:0/12:0 | 14:0/14:0 | 0.2296 | t-test (Parametric) | 0.0001 | **** | −0.275 | ||
| 821.4216 | SM 33:1 | d17:1/16:0 | d18:1/15:0 | 0.7195 | t-test (Parametric) | 0.0360 | * | −0.098 | ||
| 835.4581 | SM 34:1 | d16:1/18:0 | d18:1/16:0 | 0.0001 | Wilcoxon (Non-parametric) | 0.0002 | *** | 0.423 | ||
| 836.469 | NM | 0.0000 | Wilcoxon (Non-parametric) | 0.0008 | *** | 0.286 | ||||
| 837.4799 | SM 34:0 | d18:0/16:0 | d16:0/18:0 | 0.0001 | Wilcoxon (Non-parametric) | 0.0146 | * | 0.170 | ||
| 838.43 | PC 30:0 | 16:0/14:0 | 0.9789 | t-test (Parametric) | 0.0010 | ** | −0.250 | |||
| 839.4421 | NM | 0.9406 | t-test (Parametric) | 0.0000 | **** | −0.293 | ||||
| 841.4498 | NM | 0.1850 | t-test (Parametric) | 0.0000 | **** | −0.253 | ||||
| 843.4841 | NM | 0.4795 | t-test (Parametric) | 0.0008 | *** | −0.218 | ||||
| 845.4523 | NM | 0.8644 | t-test (Parametric) | 0.0316 | * | −0.118 | ||||
| 851.4923 | NM | 0.0000 | Wilcoxon (Non-parametric) | 0.0482 | * | −0.169 | ||||
| 865.4501 | NM | 0.7561 | t-test (Parametric) | 0.0103 | * | −0.098 | ||||
| 876.4757 | PC-P 34:1 | 16:0p/18:1 | 0.0000 | Wilcoxon (Non-parametric) | 0.0000 | **** | 0.803 | |||
| 877.4849 | NM | 0.3720 | t-test (Parametric) | 0.0001 | **** | 0.280 | ||||
| 878.4929 | NM | 0.0000 | Wilcoxon (Non-parametric) | 0.0000 | **** | 0.430 | ||||
| 879.478 | NM | 0.4485 | t-test (Parametric) | 0.0253 | * | 0.121 | ||||
| 890.4792 | PE 37:2 | 19:1/18:1 | 0.0000 | Wilcoxon (Non-parametric) | 0.0004 | *** | 0.729 | |||
| 891.4703 | NM | 0.0008 | Wilcoxon (Non-parametric) | 0.0033 | ** | 0.328 | ||||
| 892.4964 | NM | 0.7175 | t-test (Parametric) | 0.0023 | ** | 0.263 | ||||
| 893.4884 | NM | 0.6588 | t-test (Parametric) | 0.0178 | * | 0.188 | ||||
| 902.4965 | PC-P 36:2 | 18:0p/18:2 | 0.0000 | Wilcoxon (Non-parametric) | 0.0001 | **** | 0.433 | |||
| 904.5208 | PC-P 36:1 | 18:0p/18:1 | 0.0000 | Wilcoxon (Non-parametric) | 0.0000 | **** | 1.357 | |||
| 906.503 | PC 35:1 | 17:0/18:1 | 17:1/18:0 | 19:1/16:0 | 0.2284 | t-test (Parametric) | 0.0000 | **** | 0.213 | |
| 908.5696 | NM | 0.6146 | t-test (Parametric) | 0.0415 | * | −0.099 | ||||
| 911.596 | NM | 0.0654 | t-test (Parametric) | 0.0337 | * | −0.179 | ||||
| 916.4961 | PC 36:3 | 18:1/18:2 | 18:0/18:3 | 16:0/20:3 | 0.0000 | Wilcoxon (Non-parametric) | 0.0001 | **** | 0.530 | |
| 917.5023 | NM | 0.0007 | Wilcoxon (Non-parametric) | 0.0002 | *** | 0.344 | ||||
| 918.5086 | PC 36:2 | 18:1/18:1 | 18:0/18:2 | 0.0000 | Wilcoxon (Non-parametric) | 0.0000 | **** | 1.284 | ||
| 919.5429 | SM 40:1 | d16:1/24:0 | d17:1/23:0 | d18:1/22:0 | 0.0009 | Wilcoxon (Non-parametric) | 0.0003 | *** | 0.396 | |
| 920.5241 | PC 36:1 | 18:0/18:1 | 0.0000 | Wilcoxon (Non-parametric) | 0.0001 | *** | 0.599 | |||
| 921.5324 | NM | 0.0184 | Wilcoxon (Non-parametric) | 0.0009 | *** | 0.299 | ||||
| 922.5318 | NM | 0.4771 | t-test (Parametric) | 0.0058 | ** | 0.142 | ||||
| 923.5115 | NM | 0.0000 | Wilcoxon (Non-parametric) | 0.0000 | **** | −0.412 | ||||
| 925.504 | NM | 0.2278 | t-test (Parametric) | 0.0082 | ** | −0.153 | ||||
| 945.6063 | SM 42:2 | d18:1/24:1 | 0.0125 | Wilcoxon (Non-parametric) | 0.0000 | **** | 0.380 | |||
| 946.5717 | NM | 0.0000 | Wilcoxon (Non-parametric) | 0.0002 | *** | 0.585 | ||||
| 957.558 | NM | 0.0010 | Wilcoxon (Non-parametric) | 0.0199 | * | −0.197 | ||||
| 961.587 | NM | 0.2321 | t-test (Parametric) | 0.0140 | * | −0.171 | ||||
| 962.5731 | NM | 0.0021 | Wilcoxon (Non-parametric) | 0.0307 | * | −0.170 | ||||
| 979.516 | NM | 0.2131 | t-test (Parametric) | 0.0287 | * | −0.167 | ||||
| 980.3565 | NM | 0.8849 | t-test (Parametric) | 0.0005 | *** | −0.239 | ||||
| 982.3834 | NM | 0.4531 | t-test (Parametric) | 0.0001 | **** | −0.277 | ||||
| 1006.3298 | NM | 0.4139 | t-test (Parametric) | 0.0000 | **** | −0.265 | ||||
| 1009.4115 | NM | 0.9389 | t-test (Parametric) | 0.0017 | ** | −0.189 | ||||
| 1010.3477 | NM | 0.7787 | t-test (Parametric) | 0.0066 | ** | −0.165 | ||||
| 1070.4714 | NM | 0.0026 | Wilcoxon (Non-parametric) | 0.0278 | * | −0.196 | ||||
| 1093.3747 | NM | 0.2474 | t-test (Parametric) | 0.0072 | ** | −0.225 | ||||
| 1119.465 | NM | 0.3233 | t-test (Parametric) | 0.0350 | * | −0.272 | ||||
| 1128.4544 | NM | 0.0882 | t-test (Parametric) | 0.0488 | * | −0.177 | ||||
| 1130.4393 | NM | 0.3415 | t-test (Parametric) | 0.0058 | ** | −0.273 | ||||
| 1138.4385 | NM | 0.0190 | Wilcoxon (Non-parametric) | 0.0063 | ** | −0.249 | ||||
| 1147.4911 | NM | 0.0128 | Wilcoxon (Non-parametric) | 0.0116 | * | −0.235 | ||||
| 1151.5643 | NM | 0.0000 | Wilcoxon (Non-parametric) | 0.0052 | ** | −0.466 | ||||
| 1175.9367 | NM | 0.0342 | Wilcoxon (Non-parametric) | 0.0296 | * | −0.202 | ||||
| 1178.7439 | NM | 0.0071 | Wilcoxon (Non-parametric) | 0.0151 | * | −0.270 | ||||
| 1189.9013 | NM | 0.0055 | Wilcoxon (Non-parametric) | 0.0370 | * | −0.296 | ||||
| 1206.6016 | NM | 0.3981 | t-test (Parametric) | 0.0360 | * | −0.259 | ||||
| 1261.6735 | NM | 0.0000 | Wilcoxon (Non-parametric) | 0.0060 | ** | −0.377 | ||||
| 1384.1603 | NM | 0.0000 | Wilcoxon (Non-parametric) | 0.0002 | *** | −0.622 |

References
- Dirección General de Producciones y Mercados Agrarios. Subdirección General de Producciones Ganaderas y Cinegéticas Informe de Coyuntura del Sector Vacuno de Leche; Madrid Spain. Available online: https://cpage.mpr.gob.es/ (accessed on 7 July 2022).
- Pilarczyk, R.; Wójcik, J.; Sablik, P.; Czerniak, P. Fatty Acid Profile and Health Lipid Indices in the Raw Milk of Simmental and Holstein-Friesian Cows from an Organic Farm. South Afr. J. Anim. Sci. 2015, 45, 30–38. [Google Scholar] [CrossRef]
- Pereira, P.C. Milk Nutritional Composition and Its Role in Human Health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Lindmark Månsson, H. Fatty Acids in Bovine Milk Fat. SNF Swed. Nutr. Found. 2008, 52, 1821. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Rochfort, S.; Cocks, B. Milk Lipidomics: What We Know and What We Don’t. Prog. Lipid Res. 2018, 71, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine Milk in Human Nutrition—A Review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.G. The Composition of Bovine Milk Lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- Vyssotski, M.; Bloor, S.J.; Lagutin, K.; Wong, H.; Williams, D.B.G. Efficient Separation and Analysis of Triacylglycerols: Quantitation of β-Palmitate (OPO) in Oils and Infant Formulas. J. Agric. Food Chem. 2015, 63, 5985–5992. [Google Scholar] [CrossRef]
- Smiddy, M.A.; Huppertz, T.; van Ruth, S.M. Triacylglycerol and Melting Profiles of Milk Fat from Several Species. Int. Dairy J. 2012, 24, 64–69. [Google Scholar] [CrossRef]
- Zou, X.; Huang, J.; Jin, Q.; Guo, Z.; Liu, Y.; Cheong, L.; Xu, X.; Wang, X. Lipid Composition Analysis of Milk Fats from Different Mammalian Species: Potential for Use as Human Milk Fat Substitutes. J. Agric. Food Chem. 2013, 61, 7070–7080. [Google Scholar] [CrossRef]
- Narine, S.S.; Marangoni, A.G. Relating Structure of Fat Crystal Networks to Mechanical Properties: A Review. Food Res. Int. 1999, 32, 227–248. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A.; Mansbridge, R.M.; Doreau, M. Ruminant Milk Fat Plasticity: Nutritional Control of Saturated, Polyunsaturated, Trans and Conjugated Fatty Acids. Anim. Res. 2000, 49, 181–205. [Google Scholar] [CrossRef]
- Giannuzzi, D.; Toscano, A.; Pegolo, S.; Gallo, L.; Tagliapietra, F.; Mele, M.; Minuti, A.; Trevisi, E.; Marsan, P.A.; Schiavon, S.; et al. Associations between Milk Fatty Acid Profile and Body Condition Score, Ultrasound Hepatic Measurements and Blood Metabolites in Holstein Cows. Animals 2022, 12, 1202. [Google Scholar] [CrossRef] [PubMed]
- Contarini, G.; Povolo, M. Phospholipids in Milk Fat: Composition, Biological and Technological Significance, and Analytical Strategies. Int. J. Mol. Sci. 2013, 14, 2808. [Google Scholar] [CrossRef] [PubMed]
- Rombaut, R.; Dewettinck, K. Properties, Analysis and Purification of Milk Polar Lipids. Int. Dairy J. 2006, 16, 1362–1373. [Google Scholar] [CrossRef]
- Liu, Z.; Ezernieks, V.; Wang, J.; Wanni Arachchillage, N.; Garner, J.B.; Wales, W.J.; Cocks, B.G.; Rochfort, S. Heat Stress in Dairy Cattle Alters Lipid Composition of Milk. Sci. Rep. 2017, 7, 961. [Google Scholar] [CrossRef]
- Manimaran, A.; Kumaresan, A.; Jeyakumar, S.; Mohanty, T.K.; Sejian, V.; Kumar, N.; Sreela, L.; Arul Prakash, M.; Mooventhan, P.; Anantharaj, A.; et al. Potential of Acute Phase Proteins as Predictor of Postpartum Uterine Infections during Transition Period and Its Regulatory Mechanism in Dairy Cattle. Vet. World 2016, 9, 91–100. [Google Scholar] [CrossRef]
- Alonso-Fauste, I.; Andrés, M.; Iturralde, M.; Lampreave, F.; Gallart, J.; Álava, M.A. Proteomic Characterization by 2-DE in Bovine Serum and Whey from Healthy and Mastitis Affected Farm Animals. J. Proteom. 2012, 75, 3015–3030. [Google Scholar] [CrossRef]
- Bagga, A.; Randhawa, S.S.; Sharma, S.; Bansal, B.K. Acute Phase Response in Lame Crossbred Dairy Cattle. Vet. World 2016, 9, 1204. [Google Scholar] [CrossRef]
- Huzzey, J.M.; Duffield, T.F.; LeBlanc, S.J.; Veira, D.M.; Weary, D.M.; von Keyserlingk, M.A.G. Short Communication: Haptoglobin as an Early Indicator of Metritis. J. Dairy Sci. 2009, 92, 621–625. [Google Scholar] [CrossRef]
- Åkerstedt, M.; Björck, L.; Persson Waller, K.; Sternesjö, Å. Biosensor Assay for Determination of Haptoglobin in Bovine Milk. J. Dairy Res. 2006, 73, 299–305. [Google Scholar] [CrossRef]
- Grönlund, U.; Hallén Sandgren, C.; Persson Waller, K. Haptoglobin and Serum Amyloid A in Milk from Dairy Cows with Chronic Sub-Clinical Mastitis. Vet. Res. 2005, 36, 191–198. [Google Scholar] [CrossRef]
- Hiss, S.; Mueller, U.; Neu-Zahren, A.; Sauerwein, H. Haptoglobin and Lactate Dehydrogenase Measurements in Milk for the Identification of Subclinically Diseased Udder Quarters. Vet. Med. (Praha) 2007, 52, 245–252. [Google Scholar] [CrossRef]
- Heck, J.M.L.; van valenberg, H.J.F.; Dijkstra, J.; van Hooijdonk, A.C.M. Seasonal Variation in the Dutch Bovine Raw Milk Composition. J. Dairy Sci. 2009, 92, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, R.; Ponce-Ceballo, P. Effect of Silvopastoral Production Systems on Composition of Milk from Cattle. Livest. Res. Rural Dev. 2004, 16, 43. [Google Scholar]
- Nantapo, C.T.W.; Muchenje, V.; Hugo, A. Atherogenicity Index and Health-Related Fatty Acids in Different Stages of Lactation from Friesian, Jersey and Friesian×Jersey Cross Cow Milk under a Pasture-Based Dairy System. Food Chem. 2014, 146, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Oltenacu, P.A.; Broom, D.M. The Impact of Genetic Selection for Increased Milk Yield on the Welfare of Dairy Cows. Anim. Welf. 2010, 19, 39–49. [Google Scholar] [CrossRef]
- Ruegg, P.L. Management of Mastitis on Organic and Conventional Dairy Farms. J. Anim. Sci. 2009, 87, 43–55. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef]
- Heikkilä, A.M.; Liski, E.; Pyörälä, S.; Taponen, S. Pathogen-Specific Production Losses in Bovine Mastitis. J. Dairy Sci. 2018, 101, 9493–9504. [Google Scholar] [CrossRef]
- Krueger, A.; Cruickshank, J.; Trevisi, E.; Bionaz, M. Systems for Evaluation of Welfare on Dairy Farms. J. Dairy Res. 2020, 87, 13–19. [Google Scholar] [CrossRef]
- Blokhuis, H.J. International Cooperation in Animal Welfare: The Welfare Quality® Project. Acta Vet. Scand. 2008, 50, S10. [Google Scholar] [CrossRef]
- de Vries, M.; Bokkers, E.A.M.; van Schaik, G.; Botreau, R.; Engel, B.; Dijkstra, T.; de Boer, I.J.M. Evaluating Results of the Welfare Quality Multi-Criteria Evaluation Model for Classification of Dairy Cattle Welfare at the Herd Level. J. Dairy Sci. 2013, 96, 6264–6273. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, C.; Pryce, J.; Rochfort, S. Comprehensive Characterization of Bovine Milk Lipids: Phospholipids, Sphingolipids, Glycolipids, and Ceramides. J. Agric. Food Chem. 2020, 68, 6726–6738. [Google Scholar] [CrossRef] [PubMed]
- Arena, P.; Rigano, F.; Guarnaccia, P.; Dugo, P.; Mondello, L.; Trovato, E. Elucidation of the Lipid Composition of Hemp (Cannabis Sativa L.) Products by Means of Gas Chromatography and Ultra-High Performance Liquid Chromatography Coupled to Mass Spectrometry Detection. Molecules 2022, 27, 3358. [Google Scholar] [CrossRef]
- Huijps, K.; Lam, T.J.G.M.; Hogeveen, H. Costs of Mastitis: Facts and Perception. J. Dairy Res. 2008, 75, 113–120. [Google Scholar] [CrossRef]
- De Vliegher, S.; Fox, L.K.; Piepers, S.; McDougall, S.; Barkema, H.W. Invited Review: Mastitis in Dairy Heifers: Nature of the Disease, Potential Impact, Prevention, and Control. J. Dairy Sci. 2012, 95, 1025–1040. [Google Scholar] [CrossRef]
- Sinha, M.K.; Thombare, N.N.; Mondal, B. Subclinical Mastitis in Dairy Animals: Incidence, Economics, and Predisposing Factors. Sci. World J. 2014, 2014, 523984. [Google Scholar] [CrossRef]
- Wani, S.A.; Haq, U.; Parray, O.R.; Ul, Q.; Nazir, A.; Mushtaq, M.; Bhat, R.A.; Parrah, J.U.; Chakraborty, S.; Dhama, K.; et al. A Brief Analysis of Economic Losses Due to Mastitis in Dairy Cattle. Indian Vet. J. 2022, 90, 27–31. [Google Scholar]
- Halasa, T.; Huijps, K.; Østerås, O.; Hogeveen, H. Economic Effects of Bovine Mastitis and Mastitis Management: A Review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef]
- Fernández, R.; Garate, J.; Tolentino-Cortez, T.; Herraiz, A.; Lombardero, L.; Ducrocq, F.; Rodríguez-Puertas, R.; Trifilieff, P.; Astigarraga, E.; Barreda-Gómez, G.; et al. Microarray and Mass Spectrometry-Based Methodology for Lipid Profiling of Tissues and Cell Cultures. Anal. Chem. 2019, 91, 15967–15973. [Google Scholar] [CrossRef]
- Ducrocq, F.; Walle, R.; Contini, A.; Oummadi, A.; Caraballo, B.; van der Veldt, S.; Boyer, M.-L.; Aby, F.; Tolentino-Cortez, T.; Helbling, J.C.; et al. Causal Link between N-3 Polyunsaturated Fatty Acid Deficiency and Motivation Deficits. Cell Metab. 2020, 31, 755–772.e7. [Google Scholar] [CrossRef] [PubMed]
- Perez-Valle, A.; Abad-García, B.; Fresnedo, O.; Barreda-Gómez, G.; Aspichueta, P.; Asumendi, A.; Astigarraga, E.; Fernández, J.A.; Boyano, M.D.; Ochoa, B. A UHPLC-Mass Spectrometry View of Human Melanocytic Cells Uncovers Potential Lipid Biomarkers of Melanoma. Int. J. Mol. Sci. 2021, 22, 12061. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Moate, P.; Cocks, B.; Rochfort, S. Comprehensive Polar Lipid Identification and Quantification in Milk by Liquid Chromatography-Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed Life Sci. 2015, 978–979, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.M.; Sejian, V.; Wallage, A.L.; Steel, C.C.; Mader, T.L.; Lees, J.C.; Gaughan, J.B. The Impact of Heat Load on Cattle. Animals 2019, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Herbut, P.; Angrecka, S.; Walczak, J. Environmental Parameters to Assessing of Heat Stress in Dairy Cattle-a Review. Int. J. Biometeorol. 2018, 62, 2089–2097. [Google Scholar] [CrossRef]
- Mader, T.L.; Griffin, D. Management of Cattle Exposed to Adverse Environmental Conditions. Vet. Clin. N. Am. Food Anim. Pract. 2015, 31, 247–258. [Google Scholar] [CrossRef]
- Belasco, E.J.; Cheng, Y.; Schroeder, T.C. The Impact of Extreme Weather on Cattle Feeding Profits. J. Agric. Resour. Econ. 2015, 40, 285–305. [Google Scholar]
- Kim, W.S.; Lee, J.S.; Jeon, S.W.; Peng, D.Q.; Kim, Y.S.; Bae, M.H.; Jo, Y.H.; Lee, H.G. Correlation between Blood, Physiological and Behavioral Parameters in Beef Calves under Heat Stress. Asian-Australas J. Anim. Sci. 2018, 31, 919. [Google Scholar] [CrossRef]
- Penev, T.; Naydenova, N.; Dimov, D.; Marinov, I. Influence of Heat Stress and Physiological Indicators Related to It on Health Lipid Indices in Milk of Holstein-Friesian Cows. J. Oleo Sci. 2021, 70, 745–755. [Google Scholar] [CrossRef]
- Dauria, B.D.; Sigdel, A.; Petrini, J.; Bóscollo, P.P.; Pilonetto, F.; Salvian, M.; Rezende, F.M.; Pedrosa, V.B.; Bittar, C.M.M.; Machado, P.F.; et al. Genetic Effects of Heat Stress on Milk Fatty Acids in Brazilian Holstein Cattle. J. Dairy Sci. 2022, 105, 3296–3305. [Google Scholar] [CrossRef]
- Krishnamoorthy, P.; Goudar, A.L.; Suresh, K.P.; Roy, P. Global and Countrywide Prevalence of Subclinical and Clinical Mastitis in Dairy Cattle and Buffaloes by Systematic Review and Meta-Analysis. Res. Vet. Sci. 2021, 136, 561–586. [Google Scholar] [CrossRef] [PubMed]
- Hiitiö, H.; Vakkamäki, J.; Simojoki, H.; Autio, T.; Junnila, J.; Pelkonen, S.; Pyörälä, S. Prevalence of Subclinical Mastitis in Finnish Dairy Cows: Changes during Recent Decades and Impact of Cow and Herd Factors. Acta Vet. Scand. 2017, 59, 22. [Google Scholar] [CrossRef] [PubMed]
- Iriondo, A.; Tainta, M.; Saldias, J.; Arriba, M.; Ochoa, B.; Goñi, F.M.; Martinez-Lage, P.; Abad-García, B. Isopropanol Extraction for Cerebrospinal Fluid Lipidomic Profiling Analysis. Talanta 2019, 195, 619–627. [Google Scholar] [CrossRef] [PubMed]
| m/z | Lipid | Isomer I * | Isomer II * | Isomer II * | Correlation |
|---|---|---|---|---|---|
| 902.54 | HexCer d39:1 | d16:1/23:0 | d17:1/22:0 | 0.84 | |
| 903.52 | SM d39:2 | d16:1/23:1 | 0.57 | ||
| 916.48 | PC 36:3 | 18:1/18:2 | 18:0/18:3 | 16:0/20:3 | 0.51 |
| 973.61 | SM d44:2 | d18:1/26:1 | −0.46 | ||
| 706.33 | HexCer d25:1 | NM | −0.48 | ||
| 825.44 | SM t32:0 | NM | −0.48 | ||
| 864.44 | PC 32:1 | 18:1/14:0 | 16:0/16:1 | 17:1/15:0 | −0.55 |
| Location of Farms | Farm Name | Reference Name of the Farm | Results in the Haptokit Test |
|---|---|---|---|
| Aragón | Alfajarín | ALF | + |
| Montañana | MNT | − | |
| Miralbueno | MRB | − | |
| Movera | MVR | − | |
| Navarra | Marcilla | MAR | − |
| Valtierra | VTR | − | |
| Murchante | MCH | − | |
| Fitero | FTR | + | |
| Ilarregi | ILR | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garro-Aguilar, Y.; Fernández, R.; Calero, S.; Noskova, E.; Gulak, M.; de la Fuente, M.; Adell, A.; Simón, E.; Muzquiz, U.; Rodríguez-Piñón, D.; et al. Acute Stress-Induced Changes in the Lipid Composition of Cow’s Milk in Healthy and Pathological Animals. Molecules 2023, 28, 980. https://doi.org/10.3390/molecules28030980
Garro-Aguilar Y, Fernández R, Calero S, Noskova E, Gulak M, de la Fuente M, Adell A, Simón E, Muzquiz U, Rodríguez-Piñón D, et al. Acute Stress-Induced Changes in the Lipid Composition of Cow’s Milk in Healthy and Pathological Animals. Molecules. 2023; 28(3):980. https://doi.org/10.3390/molecules28030980
Chicago/Turabian StyleGarro-Aguilar, Yaiza, Roberto Fernández, Silvia Calero, Ekaterina Noskova, Marina Gulak, Miguel de la Fuente, Albert Adell, Edurne Simón, Urko Muzquiz, Diego Rodríguez-Piñón, and et al. 2023. "Acute Stress-Induced Changes in the Lipid Composition of Cow’s Milk in Healthy and Pathological Animals" Molecules 28, no. 3: 980. https://doi.org/10.3390/molecules28030980
APA StyleGarro-Aguilar, Y., Fernández, R., Calero, S., Noskova, E., Gulak, M., de la Fuente, M., Adell, A., Simón, E., Muzquiz, U., Rodríguez-Piñón, D., Astigarraga, E., & Barreda-Gómez, G. (2023). Acute Stress-Induced Changes in the Lipid Composition of Cow’s Milk in Healthy and Pathological Animals. Molecules, 28(3), 980. https://doi.org/10.3390/molecules28030980







