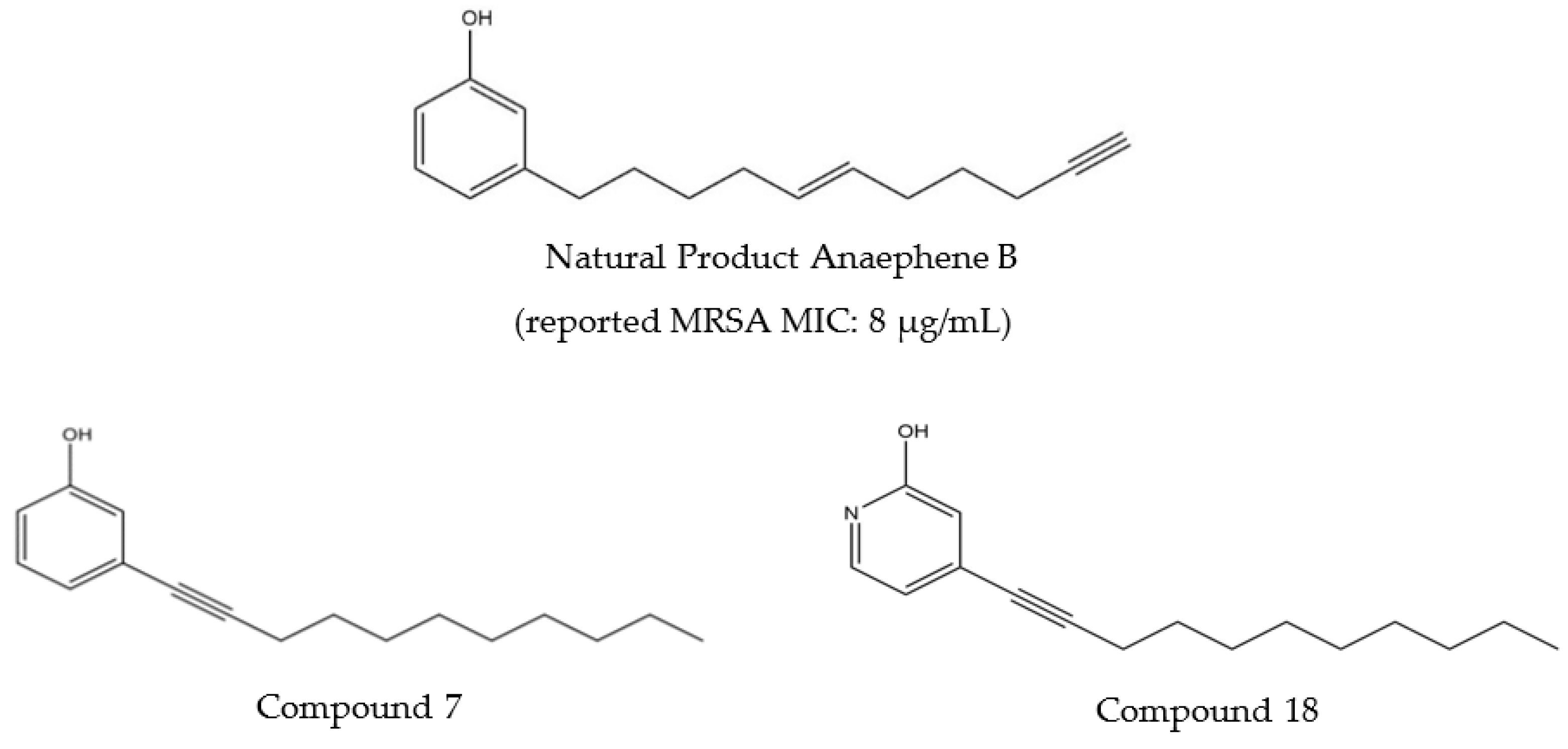
Potent Inhibitory Activity of Natural Product Anaephene B and Analogues against Leishmania tarentolae In Vitro
Abstract
1. Introduction
2. Results and Discussion
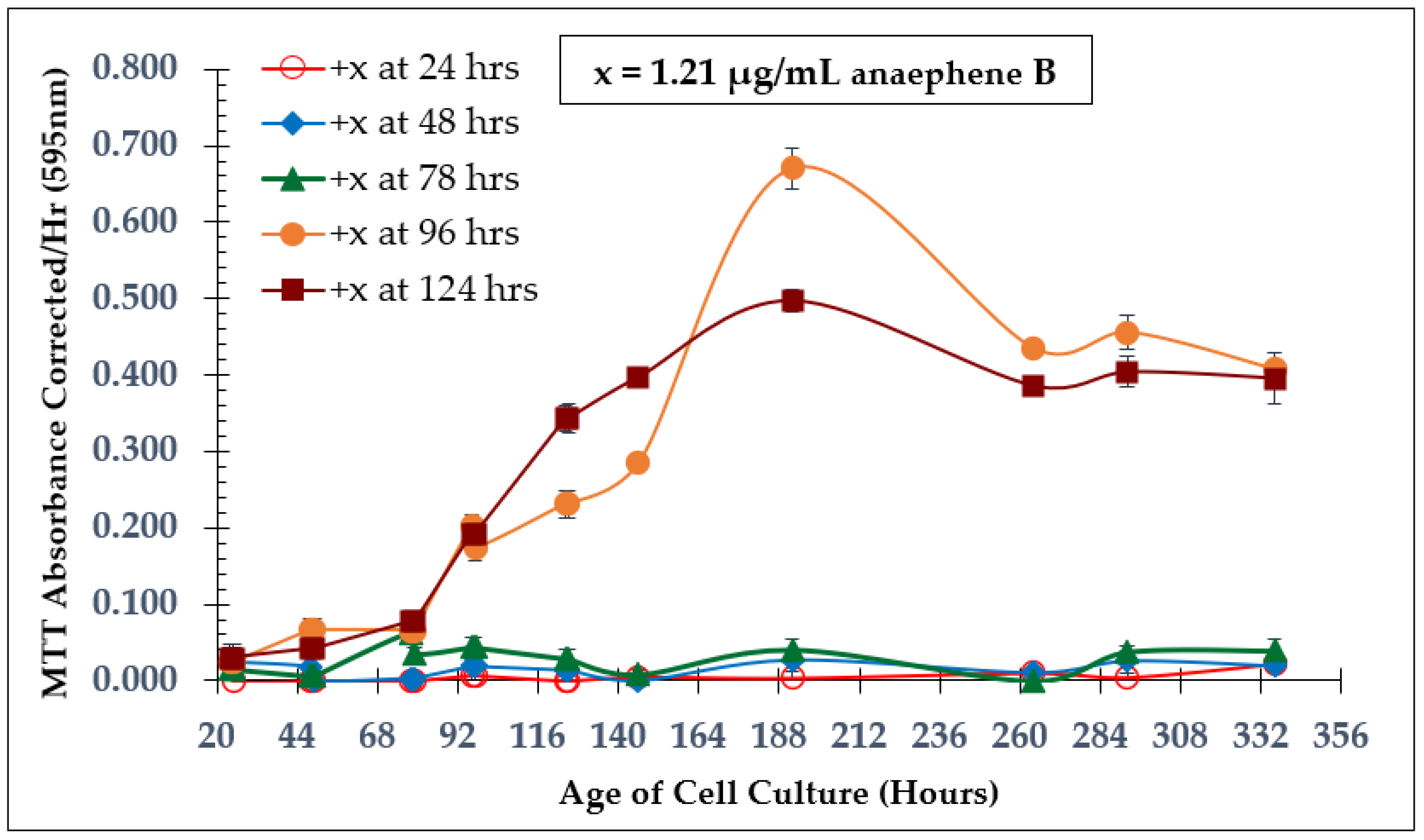
2.1. Time of Addition Study and SAP Assay with Natural Product Anaephene B
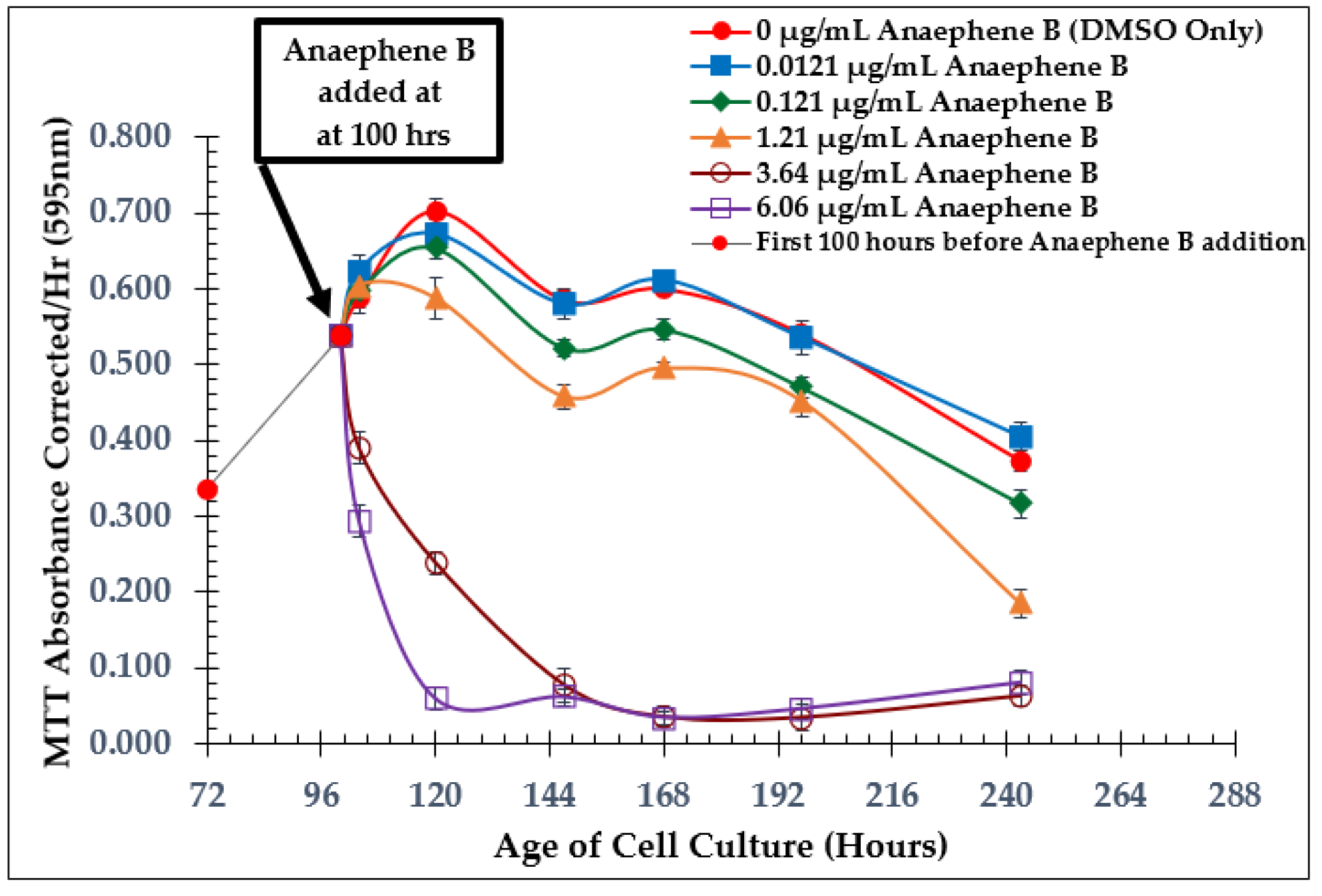
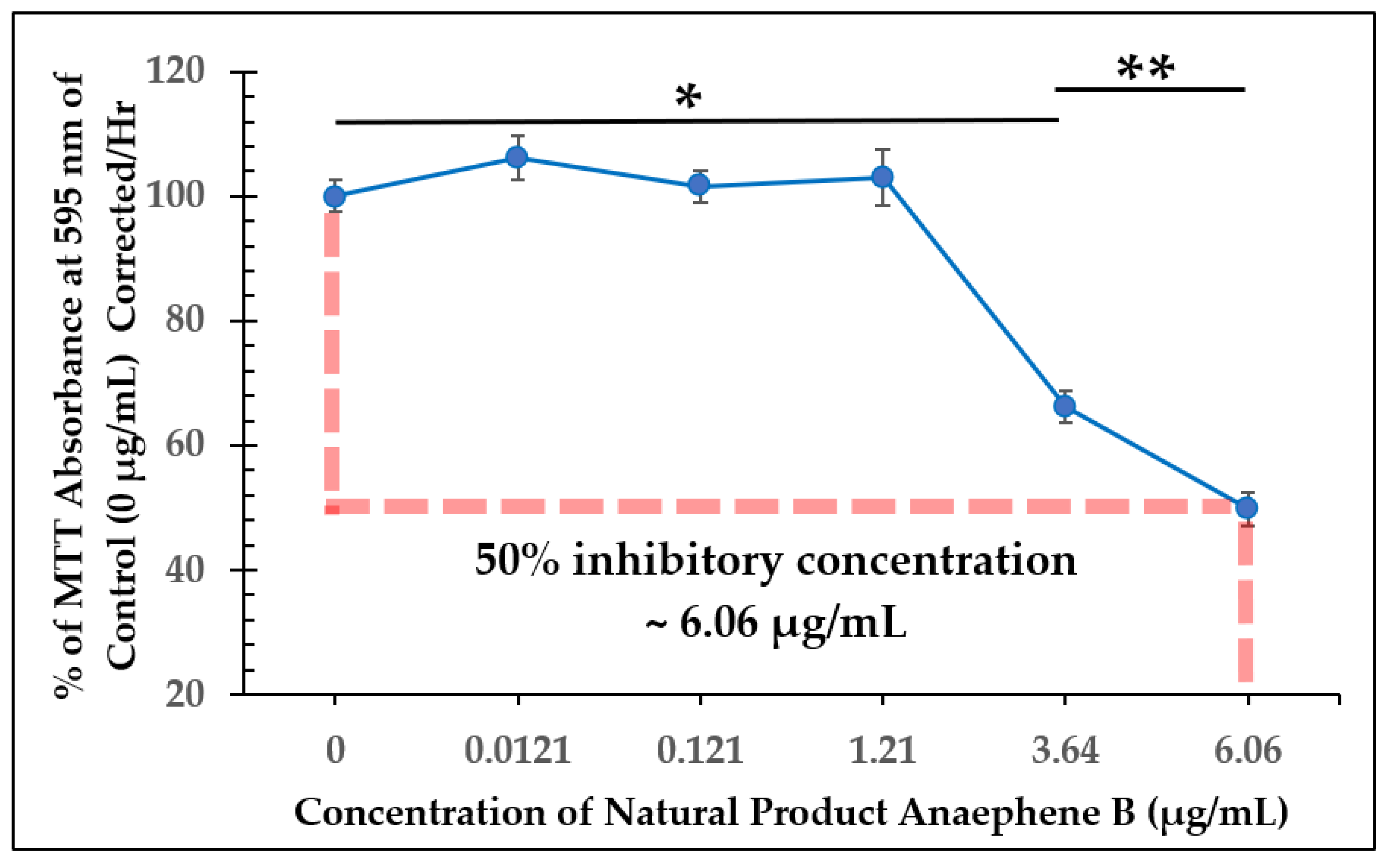
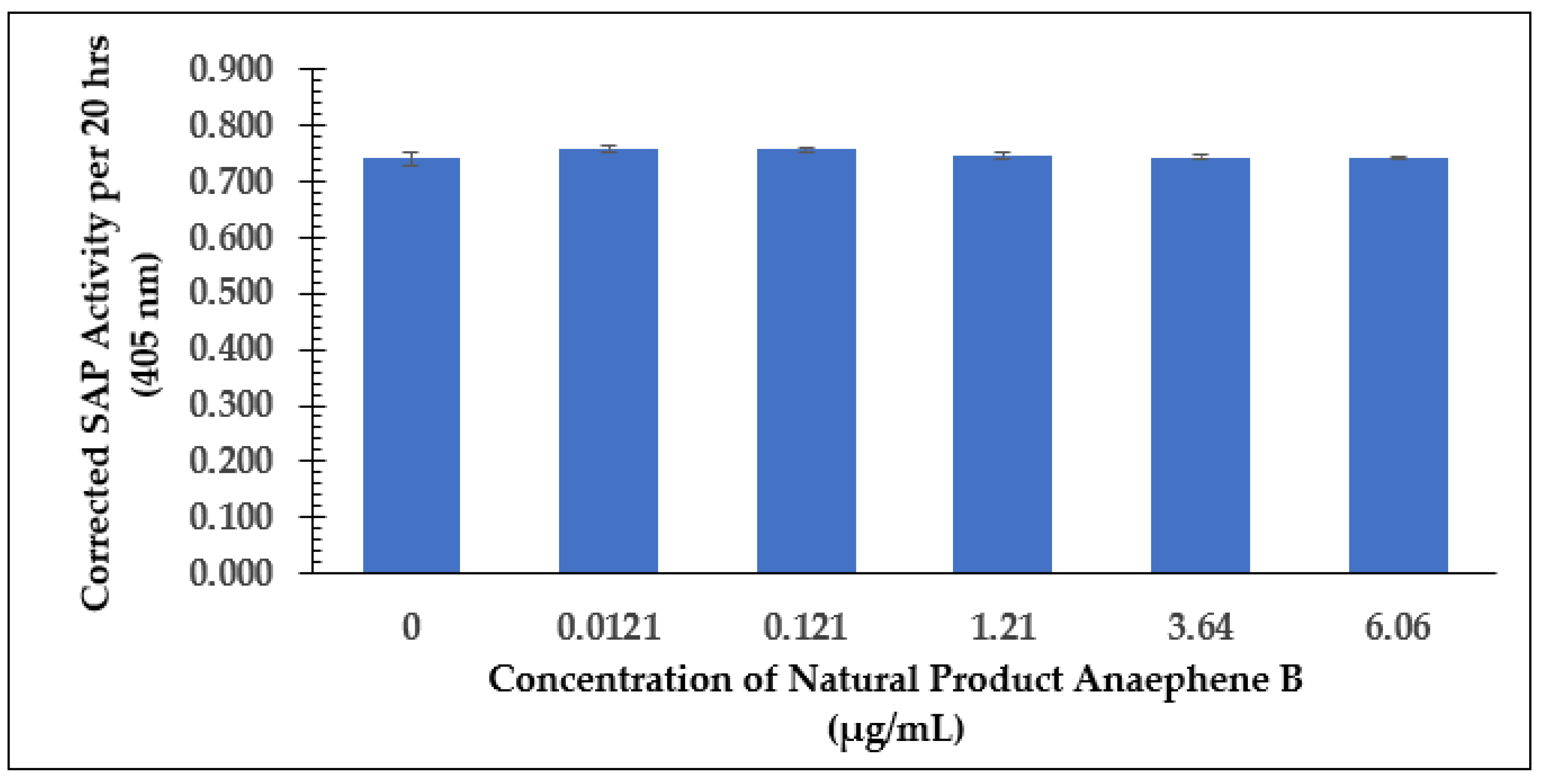
2.2. Dose-Dependent Effect Studies and SAP Assays
3. Materials, Methods, and Procedures
3.1. Cell Cultures of L. tarentolae
3.2. MTT Assay for Cell Viability
3.3. Examining Dose-Dependent Effect of Test Compounds
3.4. Examining SAP Activity from L. tarentolae
3.5. Data Collection and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vannier-Santos, M.A.; Martiny, A.; De Souza, W. Cell biology of Leishmania spp.: Invading and evading. Curr. Pharm. Des. 2002, 8, 297–318. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). About Leishmaniasis. Available online: https//www.cdc.gov/parasites/leishmaniasis/gen_info/faqs.html (accessed on 17 October 2020).
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Li, E.W.; Hamaker, C.; Katinas, J.M.; Jones, M.A. Structural characterization of naphthalene sulfonamides and a sulfonate ester and their in vitro efficacy against Leishmania tarentolae promastigotes. New J. Chem. 2021, 45, 4791–4801. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Parasites-Leishmaniasis. U.S. Department of Health & Human Services. Available online: www.cdc.gov/parasites/leishmaniasis/index.html (accessed on 14 February 2020).
- Croft, S.L.; Sundar, S.; Fairlamb, A.J. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, S.; Vanaerschot, M.; Brunker, K.; Imamura, H.; Müller, S.; Khanal, B.; Rijal, S.; Dujardin, J.-C.; Coombs, G.H. Molecular mechanisms of drug resistance in natural Leishmania populations vary with genetic background. PLoS Negl. Trop. Dis. 2012, 6, e1514. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant Products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Brumley, D.; Spencer, K.A.; Gunasekera, S.P.; Sauvage, T.; Biggs, J.; Paul, V.J.; Luesch, H. Isolation and Characterization of Anaephenes A−C, Alkylphenols from a Filamentous Cyanobacterium (Hormoscilla sp., Oscillatoriales). J. Nat. Prod. 2018, 81, 2716–2721. [Google Scholar] [CrossRef] [PubMed]
- Kukla, D.L.; Canchola, J.; Rosenthal, J.D.; Mills, J.J. Synthesis of the Cyanobacterial antibiotics Anaephene A and B. J. Nat. Products. 2020, 83, 2036–2040. [Google Scholar] [CrossRef] [PubMed]
- Kukla, D.L.; Canchola, J.; Rosenthal, J.D.; Mills, J.J. Design, synthesis, and structure-activity relationship studies of the anaephene antibiotics. Chem. Biol. Drug Des. 2021, 98, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.M.; Munoz, D.L.; Cedeno, D.L.; Velez, I.D.; Jones, M.A.; Robledo, S.M. Leishmania tarentolae: Utility as an in vitro model for screening of antileishmanial agents. Exp. Parasitol. 2010, 126, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, J.B.; Peters, S.J.; Cedeño, D.L.; Constantino, M.H.; Edwards, K.; Kamowski, E.M.; Passini, J.C.; Butkus, B.E.; Young, A.M.; Lash, T.D.; et al. Carbaporphyrin ketals as potential agents for a new photodynamic therapy treatment of leishmaniasis. Bioorganic Med. Chem. 2008, 16, 7033–7038. [Google Scholar] [CrossRef] [PubMed]
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Da Silva-Lopez, R.E.; Pinto Coelho, M.G.; De Simone, S.G. Characterization of an extracellular serine protease of Leishmania (Leishmania) amazonensis. Parasitology 2005, 131, 85–96. [Google Scholar] [CrossRef]
- Grew, R.H.; Saha, A.K.; Das, S.; Remaley, A.T. Biochemistry of the Leishmania species. Microbiol. Rev. 1988, 52, 412–432. [Google Scholar]
- Vannier-Santos, M.A.; Martiny, A.; Meyer-Fernandes, J.R. Leishmanial protein kinase C modulates host cell infection via secreted acid phosphatase. Eur. J. Cell Biol. 1995, 67, 112–119. [Google Scholar] [PubMed]
- Mendez, R.S.; Dorsey, B.M.; McLauchlan, C.C.; Beio, M.; Turner, T.; Nguyen, V.H.; Su, A.; Beynon, W.; Friesen, J.F.; Jones, M.A. Vanadium complexes are in vitro inhibitors of Leishmania secreted acid phosphatases. Int. J. Chem. 2014, 6, 35–49. [Google Scholar] [CrossRef]

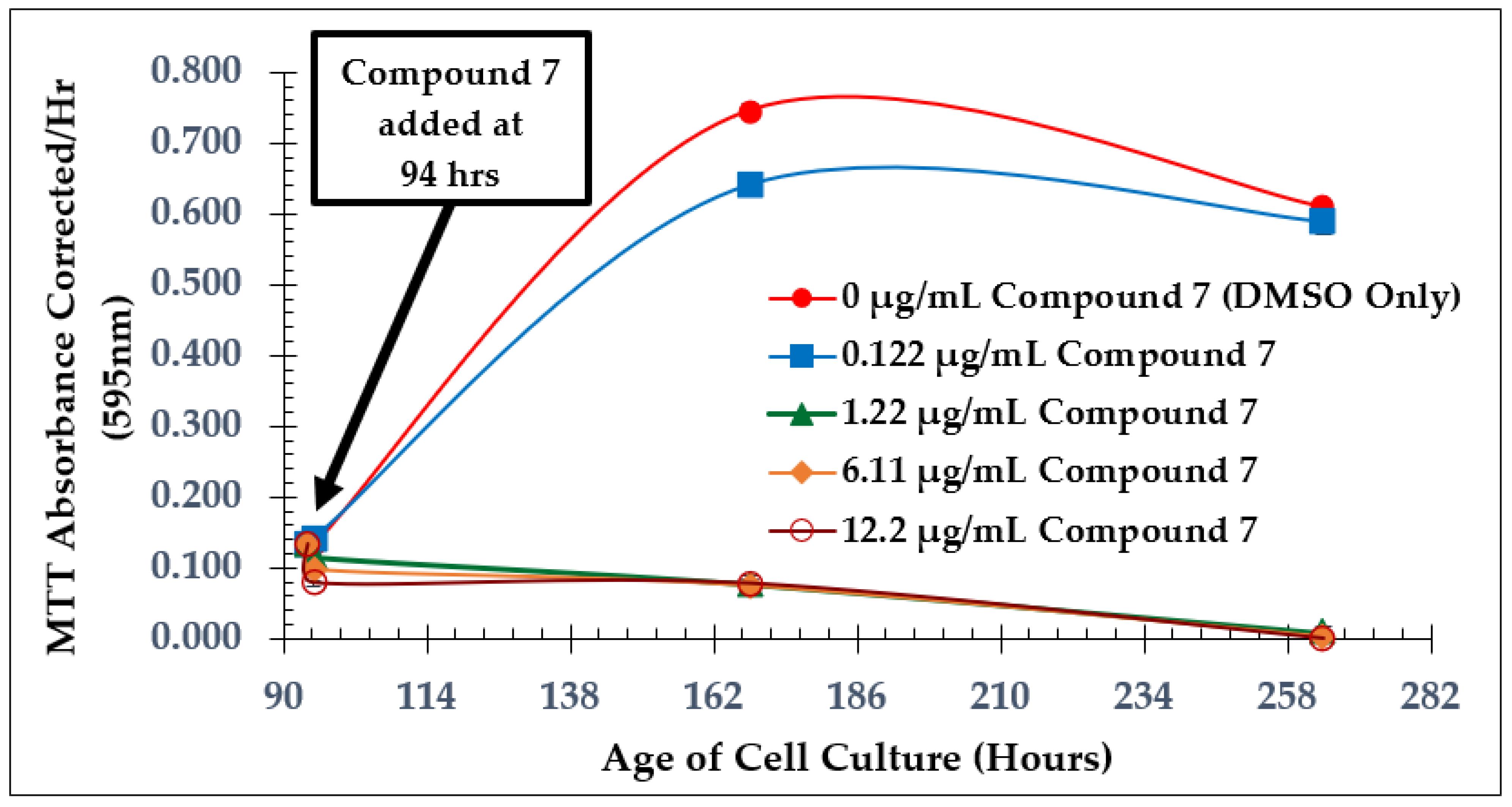
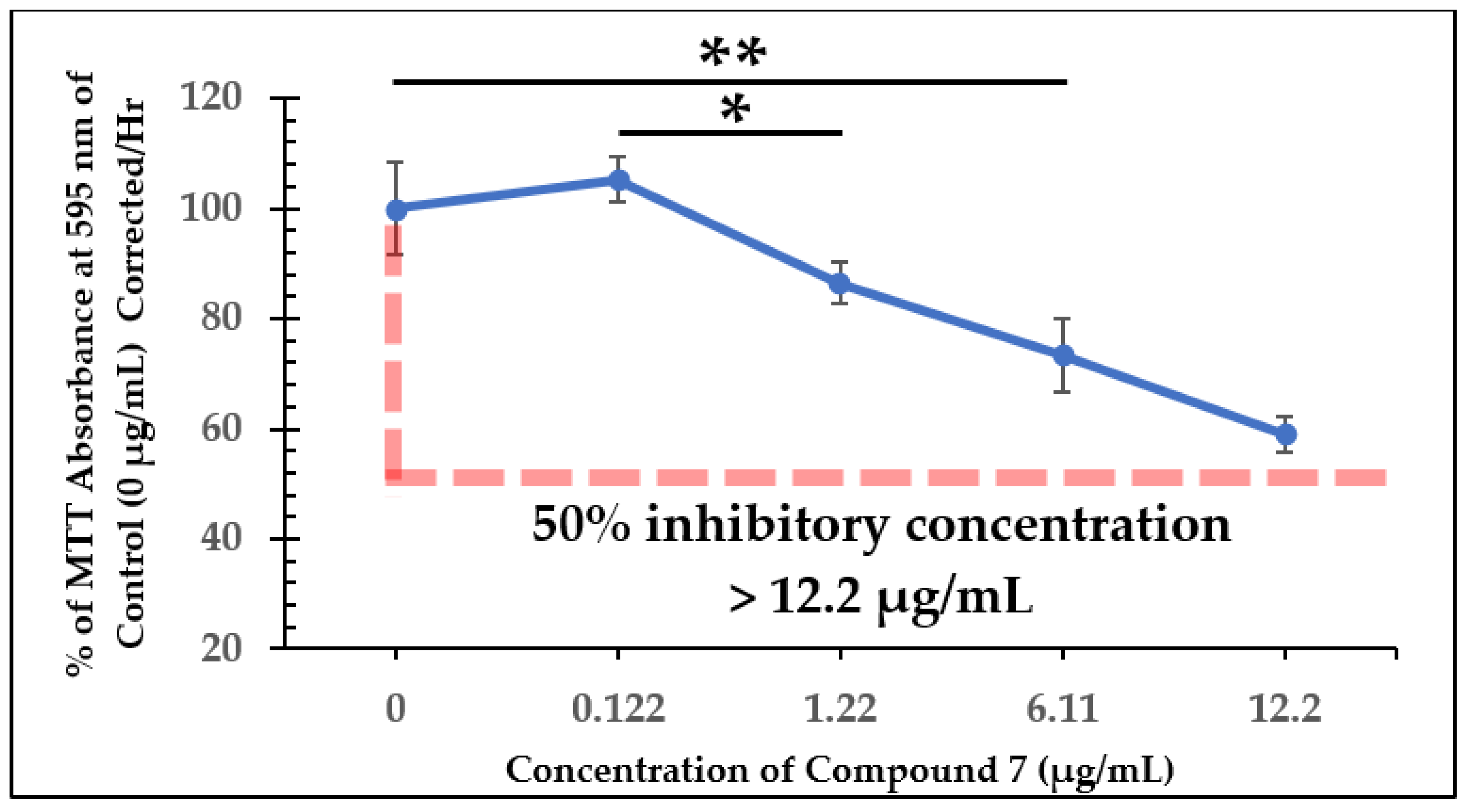
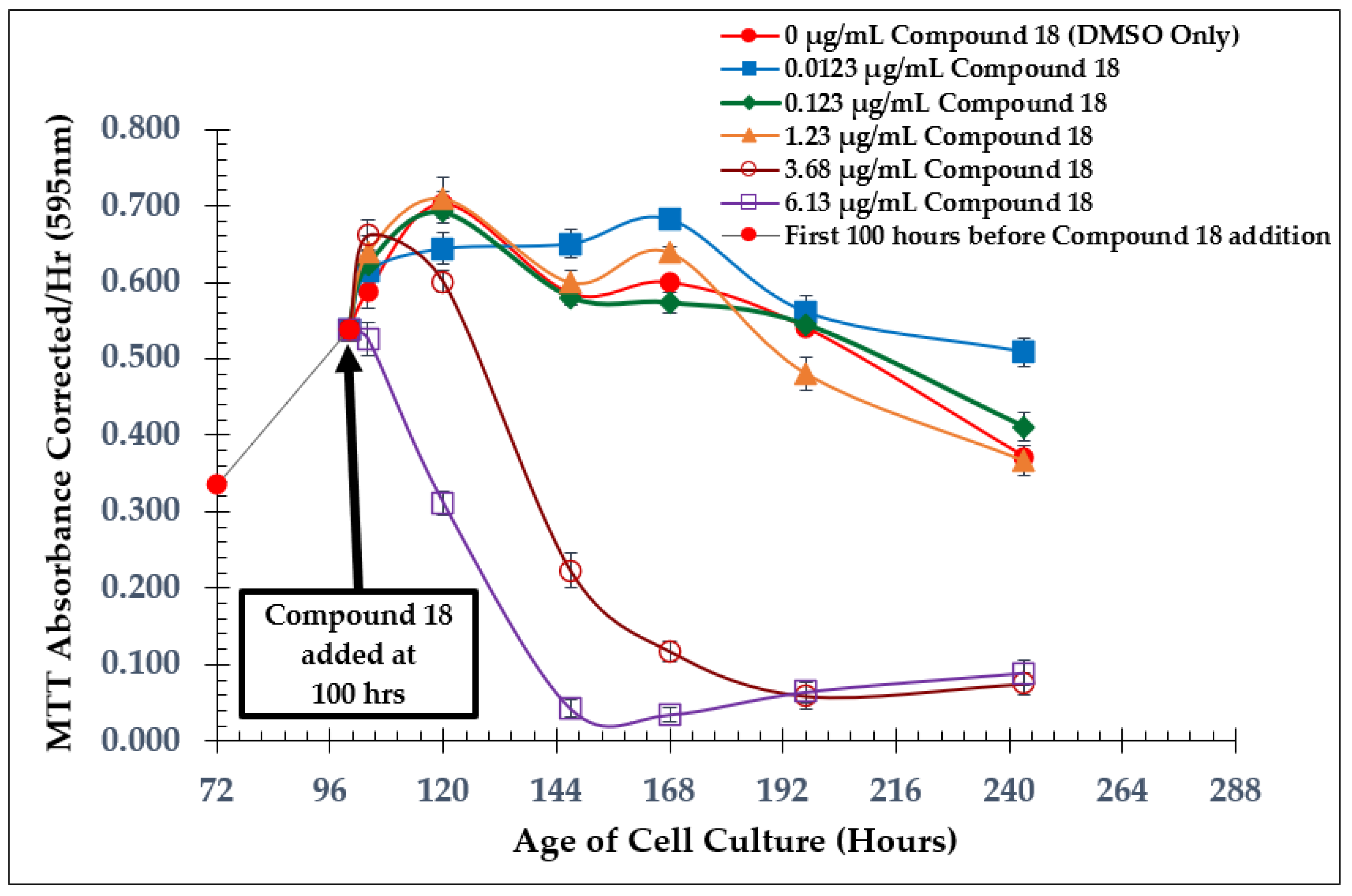
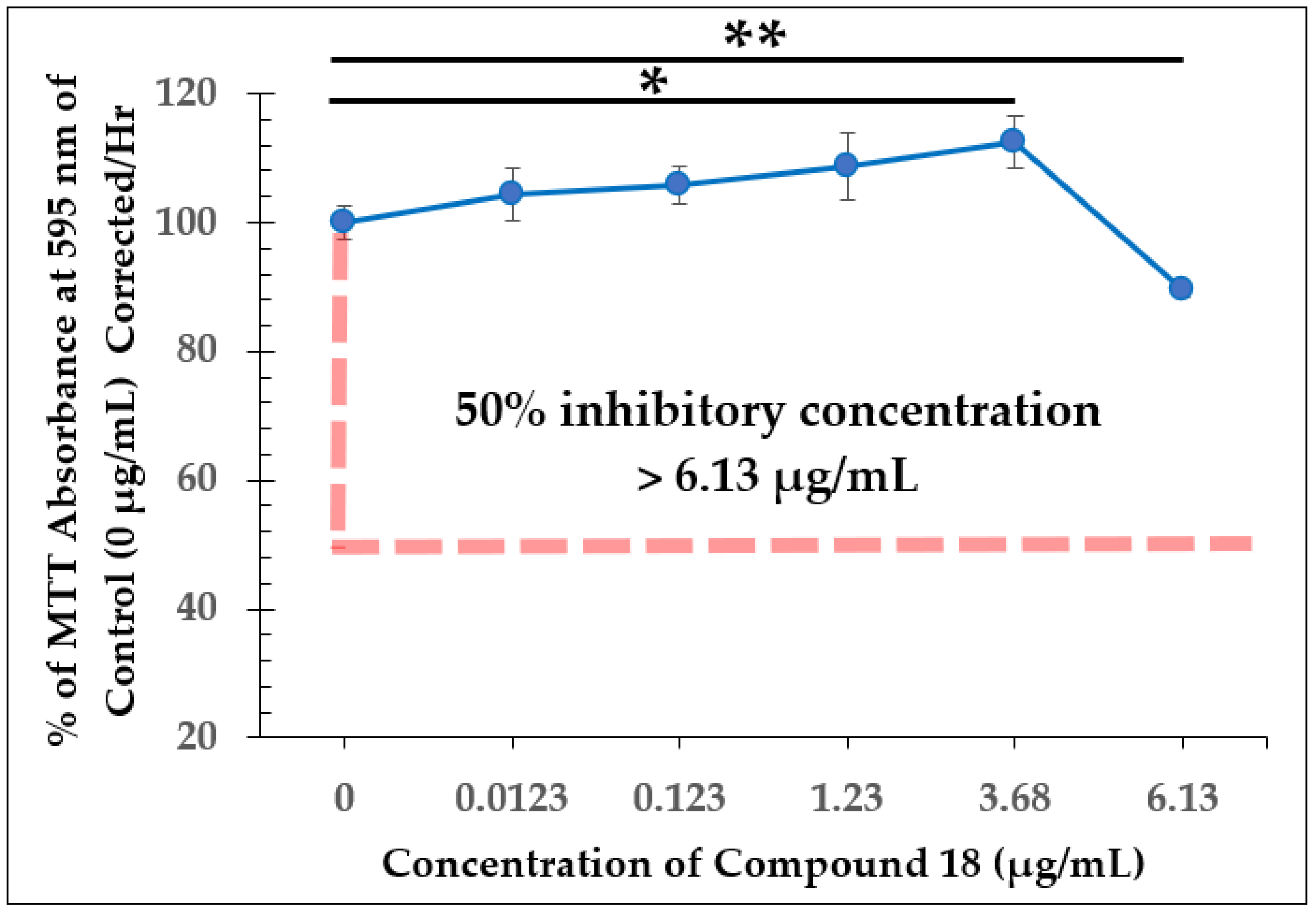

| Test Compound | Minimum Inhibitory Concentration (MIC) |
|---|---|
| Natural Product (Anaephene B) | ~3.64 µg/mL |
| Compound 7 | ~1.22 µg/mL |
| Compound 18 | ~3.68 µg/mL |
| Test Compound | 50% Inhibitory Concentration (IC50) |
|---|---|
| Natural Product (Anaephene B) | ~6.06 µg/mL |
| Compound 7 | >12.2 µg/mL |
| Compound 18 | >6.13 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaman, S.M.; Jones, M.A. Potent Inhibitory Activity of Natural Product Anaephene B and Analogues against Leishmania tarentolae In Vitro. Molecules 2023, 28, 946. https://doi.org/10.3390/molecules28030946
Zaman SM, Jones MA. Potent Inhibitory Activity of Natural Product Anaephene B and Analogues against Leishmania tarentolae In Vitro. Molecules. 2023; 28(3):946. https://doi.org/10.3390/molecules28030946
Chicago/Turabian StyleZaman, Shariq M., and Marjorie A. Jones. 2023. "Potent Inhibitory Activity of Natural Product Anaephene B and Analogues against Leishmania tarentolae In Vitro" Molecules 28, no. 3: 946. https://doi.org/10.3390/molecules28030946
APA StyleZaman, S. M., & Jones, M. A. (2023). Potent Inhibitory Activity of Natural Product Anaephene B and Analogues against Leishmania tarentolae In Vitro. Molecules, 28(3), 946. https://doi.org/10.3390/molecules28030946