Polyaromatic Group Embedded Cd(II)-Coordination Polymers for Microwave-Assisted Solvent-Free Strecker-Type Cyanation of Acetals
Abstract
1. Introduction
2. Results and Discussion
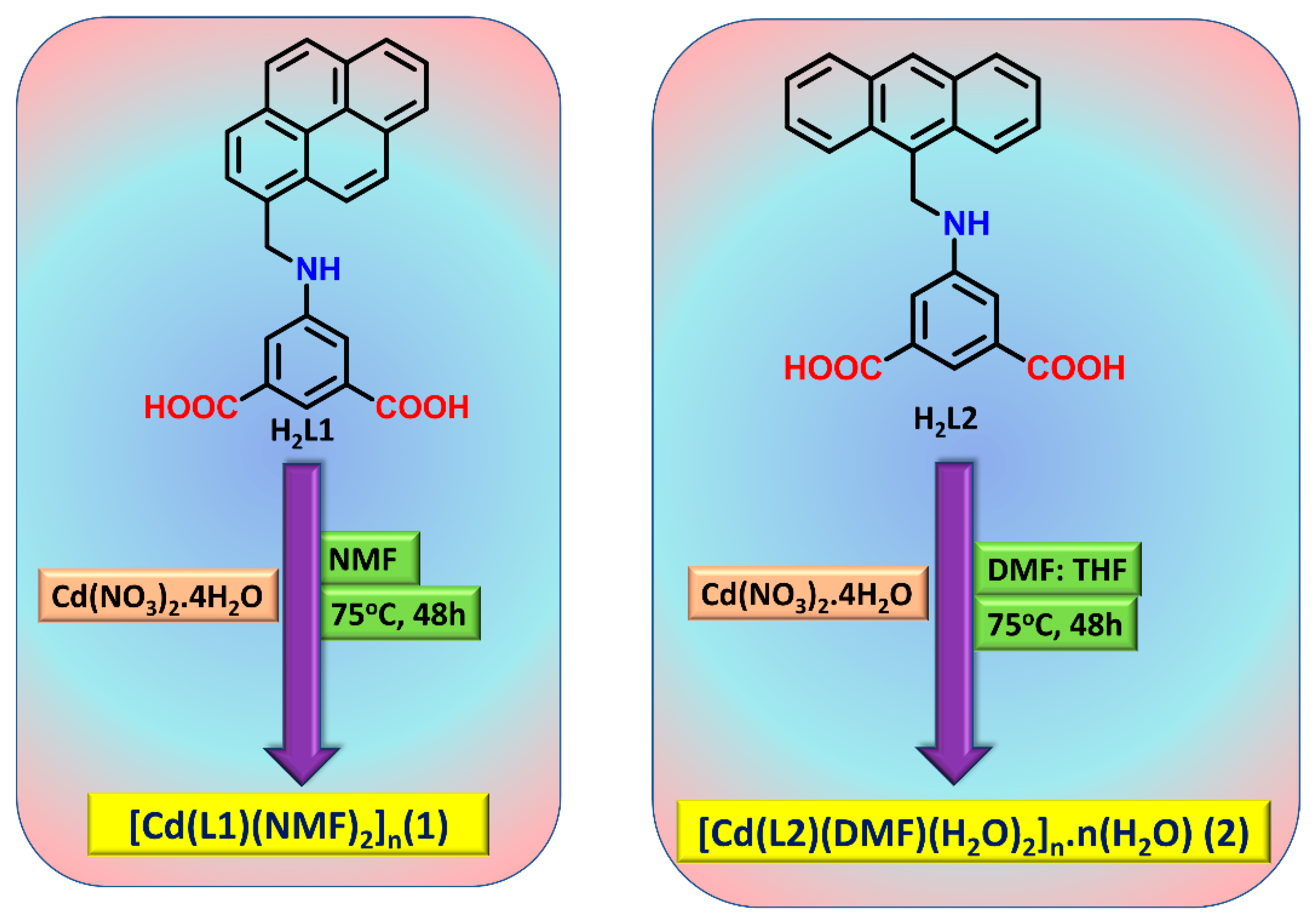
2.1. Synthesis and Characterization
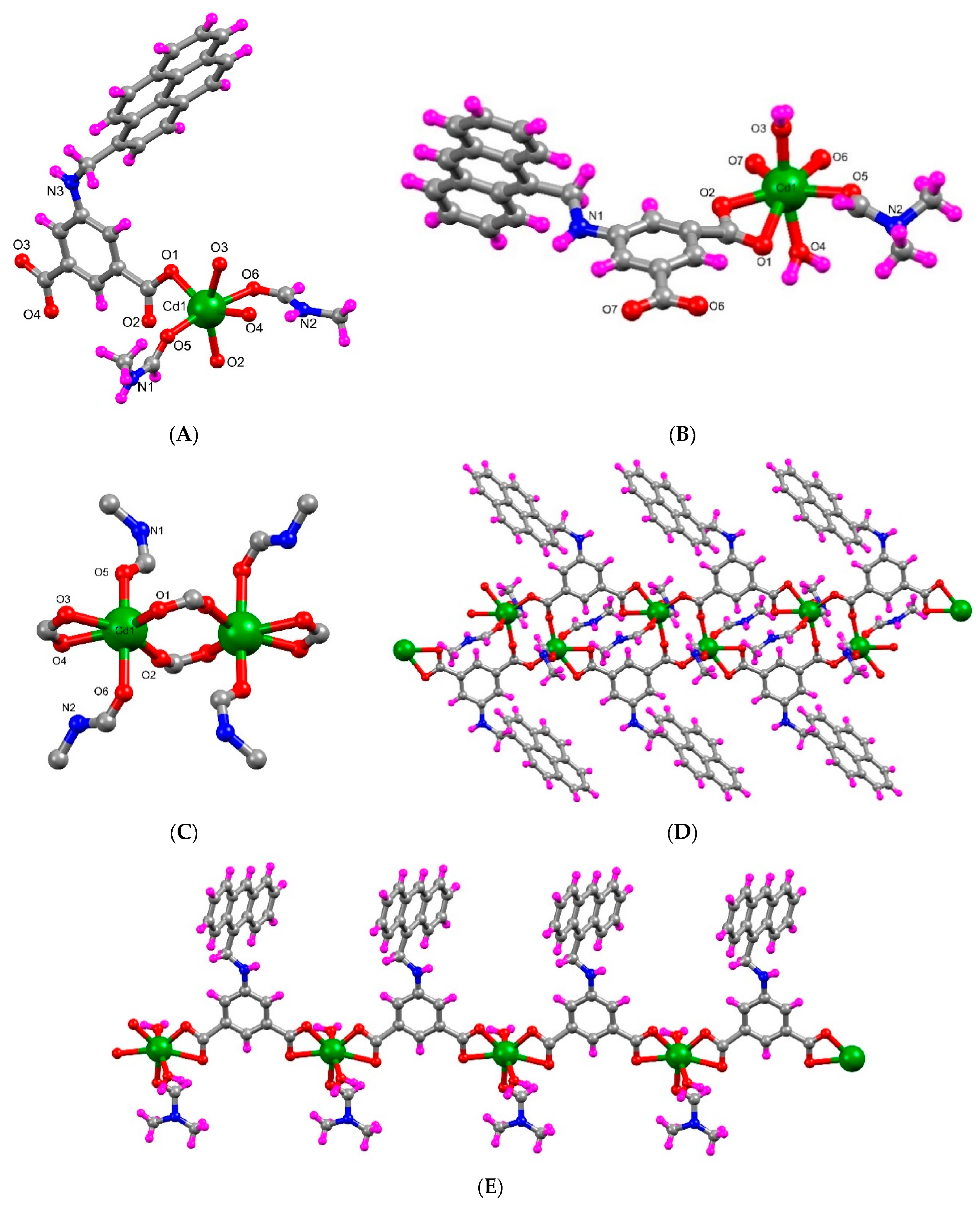
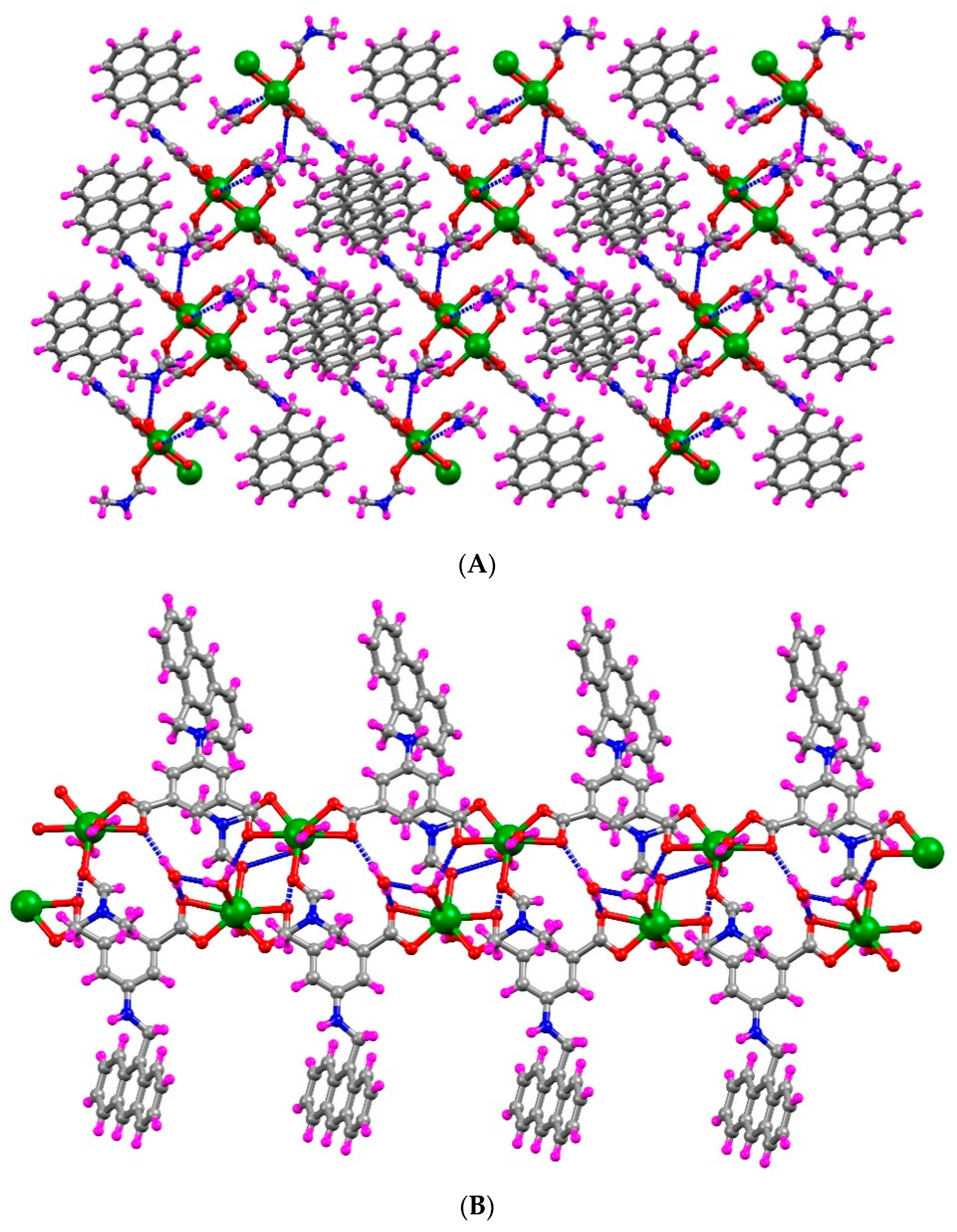
2.2. Crystal Structure Analysis
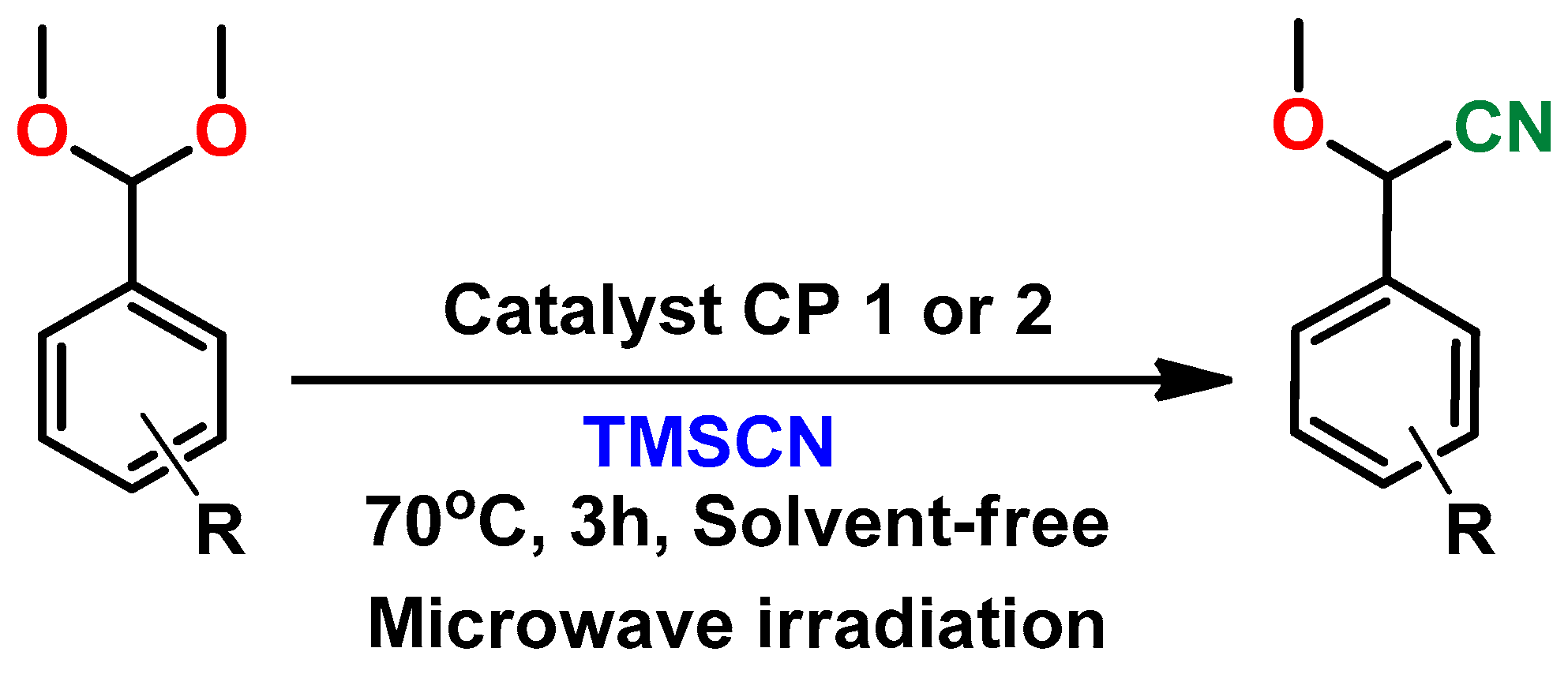
2.3. Catalytic Study
3. Experimental Section
3.1. Synthesis of [Cd(L1)(NMF)2]n (1)
3.2. Synthesis of [Cd(L2)(DMF)(H2O)2]n·n(H2O) (2)
3.3. Procedure for the Microwave Assisted Solvent-Free Cyanation of Acetals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guillerm, V.; Kim, D.; Eubank, J.F.; Luebke, R.; Liu, X.; Adil, K.; Lah, M.S.; Eddaoudi, M. A supermolecular building approach for the design and construction of metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 6141–6172. [Google Scholar] [CrossRef] [PubMed]
- Schoedel, A.; Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Structures of Metal–Organic Frameworks with Rod Secondary Building Units. Chem. Rev. 2016, 116, 12466–12535. [Google Scholar] [CrossRef]
- Karmakar, A.; Pombeiro, A.J.L. Recent advances in amide functionalized metal organic frameworks for heterogeneous catalytic applications. Coord. Chem. Rev. 2019, 395, 86–129. [Google Scholar] [CrossRef]
- Engel, E.R.; Scott, J.L. Advances in the green chemistry of coordination polymer materials. Green Chem. 2020, 22, 3693–3715. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Li, M.; Yin, S.; Lin, M.; Chen, X.; Pan, Y.; Peng, Y.; Sun, J.; Kumar, A.; Liu, J. Current status and prospects of metal–organic frameworks for bone therapy and bone repair. J. Mater. Chem. B 2022, 10, 5105–5128. [Google Scholar] [CrossRef]
- Liu, W.; Yan, Q.; Xia, C.; Wang, X.; Kumar, A.; Wang, Y.; Liu, Y.; Pan, Y.; Liu, J. Recent advances in cell membrane coated metal–organic frameworks (MOFs) for tumor therapy. J. Mater. Chem. B 2021, 9, 4459–4474. [Google Scholar] [CrossRef]
- Ding, Q.; Xu, Z.; Zhou, L.; Rao, C.; Li, W.; Muddassir, M.; Sakiyama, H.; Li, B.; Ouyang, Q.; Liu, J. A multimodal Metal-Organic framework based on unsaturated metal site for enhancing antitumor cytotoxicity through Chemo-Photodynamic therapy. J. Colloid. Interf. Sci. 2022, 621, 180–194. [Google Scholar] [CrossRef]
- Karmakar, A.; Titi, H.M.; Goldberg, I. Coordination Polymers of 5-(2-Amino/Acetamido-4-carboxyphenoxy)-benzene-1,3-dioic Acids with Transition Metal Ions: Synthesis, Structure, and Catalytic Activity. Cryst. Growth Des. 2011, 11, 2621–2636. [Google Scholar] [CrossRef]
- Xue, D.-X.; Wang, Q.; Bai, J. Amide-functionalized metal–organic frameworks: Syntheses, structures and improved gas storage and separation properties. Coord. Chem. Rev. 2019, 378, 2–16. [Google Scholar] [CrossRef]
- Kinik, F.P.; Ortega-Guerrero, A.; Ongari, D.; Ireland, C.P.; Smit, B. Pyrene-based metal organic frameworks: From synthesis to applications. Chem. Soc. Rev. 2021, 50, 3143–3177. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Paul, A.; Santos, I.R.M.; Santos, P.M.R.; Sabatini, E.P.; Gurbanov, A.V.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Highly Efficient Adsorptive Removal of Organic Dyes from Aqueous Solutions Using Polyaromatic Group-Containing Zn(II)-Based Coordination Polymers. Cryst. Growth Des. 2022, 4, 2248–2265. [Google Scholar] [CrossRef]
- Karmakar, A.; Hazra, S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Synthesis, structure and catalytic application of lead(II) complexes in cyanosilylation reactions. Dalton Trans. 2015, 44, 268–280. [Google Scholar] [CrossRef]
- Paul, A.; Karmakar, A.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Amide functionalized metal–organic frameworks for diastereoselective nitroaldol (Henry) reaction in aqueous medium. RSC Adv. 2015, 5, 87400–87410. [Google Scholar] [CrossRef]
- Sotnik, S.A.; Polunin, R.A.; Kiskin, M.A.; Kirillov, A.M.; Dorofeeva, V.N.; Gavrilenko, K.S.; Eremenko, I.L.; Novotortsev, V.M.; Kolotilov, S.V. Heterometallic Coordination Polymers Assembled from Trigonal Trinuclear Fe2Ni-Pivalate Blocks and Polypyridine Spacers: Topological Diversity, Sorption, and Catalytic Properties. Inorg. Chem. 2015, 54, 5169–5181. [Google Scholar] [CrossRef]
- Pramanik, M.; Nandi, M.; Uyama, H.; Bhaumik, A. Organic–inorganic hybrid porous sulfonated zinc phosphonate material: Efficient catalyst for biodiesel synthesis at room temperature. Green Chem. 2012, 14, 2273–2281. [Google Scholar] [CrossRef]
- Karmakar, A.; Soliman, M.M.A.; Rúbio, G.M.D.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Synthesis and catalytic activities of a Zn(II) based metallomacrocycle and a metal–organic framework towards one-pot deacetalization-Knoevenagel tandem reactions under different strategies: A comparative study. Dalton Trans. 2020, 49, 8075–8085. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- Wei, Y.-S.; Zhang, M.; Zou, R.; Xu, Q. Metal–Organic Framework-Based Catalysts with Single Metal Sites. Chem. Rev. 2020, 120, 12089–12174. [Google Scholar] [CrossRef]
- Karmakar, A.; Hazra, S.; Pombeiro, A.J.L. Urea and thiourea based coordination polymers and metal-organic frameworks: Synthesis, structure and applications. Coord. Chem. Rev. 2022, 453, 214314. [Google Scholar] [CrossRef]
- Li, X.; Golz, C.; Alcarazo, M. 5-(Cyano)dibenzothiophenium Triflate: A Sulfur-Based Reagent for Electrophilic Cyanation and Cyanocyclizations. Angew. Chem. Int. Ed. 2019, 58, 9496–9500. [Google Scholar] [CrossRef] [PubMed]
- Nicola, O.; Opatz, T. Heterocycles from α-Aminonitriles. Chem. Eur. J. 2014, 20, 13064–13077. [Google Scholar]
- Saranya, S.; Neetha, M.; Aneeja, T.; Anil kumar, G. Recent Trends in the Iron-Catalyzed Cyanation Reactions. Adv. Synth. Catal. 2020, 362, 4543–4551. [Google Scholar] [CrossRef]
- Kirchmeyer, S.; Mertens, A.; Arvanaghi, M.; Olah, G.A. Synthetic Methods and Reactions; 1141. General Procedure for the Conversion of Acetals and Ketone Acetals into 2-Alkoxyalkanenitriles using Cyanotritnethylsilane. Synthesis 1983, 1983, 498–500. [Google Scholar] [CrossRef]
- Li, H.; Pan, H.; Meng, X.; Zhang, X. Unique chemoselective Strecker-type reaction of acetals with TMSCN catalyzed by MgI2 etherate. Syn. Commun. 2020, 50, 684–691. [Google Scholar]
- Tinant, B.; Wu, S.; Declercq, J.P.; Van Meerssche, M.; Masamba, W.; De Mesmaeker, A.; Viehe, H.G. Captodative substitution and cyclopropane geometry. Part 5. X-ray Structure of Five New Compounds and Asymmetry Parameters of the Substituents. J. Chem. Soc. Perkin Trans. 1988, 2, 1045–1052. [Google Scholar]
- Komatsu, N.; Uda, M.; Suzuki, H.; Takahashi, T.; Domae, T.; Wada, M. Bismuth bromide as an efficient and versatile catalyst for the cyanation and allylation of carbonyl compounds and acetals with organosilicon reagents. Tetrahedron Lett. 1997, 38, 7215–7218. [Google Scholar] [CrossRef]
- Karmakar, A.; Paul, A.; Santos, P.M.R.; Santos, I.R.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Design and construction of polyaromatic group containing Cd(ii)-based coordination polymers for solvent-free Strecker-type cyanation of acetals. New J. Chem. 2022, 46, 10201–10212. [Google Scholar] [CrossRef]
- Kokel, A.; Schäfer, C.; Török, B. Application of microwave-assisted heterogeneous catalysis in sustainable synthesis design. Green Chem. 2017, 19, 3729–3751. [Google Scholar] [CrossRef]
- Karmakar, A.; Martins, L.M.D.R.S.; Hazra, S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Metal-organic frameworks with pyridyl-based isophthalic acid and their catalytic applications in microwave assisted peroxidative oxidation of alcohols and Henry reaction. Cryst. Growth Des. 2016, 16, 1837–1849. [Google Scholar] [CrossRef]
- Rathi, A.K.; Gawande, M.B.; Zboril, R.; Varma, R.S. Microwave-assisted synthesis—Catalytic applications in aqueous media. Coord. Chem. Rev. 2015, 291, 68–94. [Google Scholar] [CrossRef]
- Sánchez, V.; Dafinov, A.; Salagre, P.; Llorca, J.; Cesteros, Y. Microwave-Assisted Furfural Production Using Hectorites and Fluorohectorites as Catalysts. Catalysts 2019, 9, 706. [Google Scholar] [CrossRef]
- Karmakar, A.; Paul, A.; Sabatini, E.P.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Pyrene Carboxylate Ligand Based Coordination Polymers for Microwave-Assisted Solvent-Free Cyanosilylation of Aldehydes. Molecules 2021, 26, 1101. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.M.; Lee, S.W. A 1D palladium coordination polymer and its catalytic activity in microwave-assisted Sonogashira reactions. Polyhedron 2021, 202, 115229. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed.; Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Kotani, S.; Sakamoto, M.; Osakama, K.; Nakajima, M. A Sterically Congested α-Cyanoamine as a Cyanating Reagent: Cyanation of Acetals and Orthoesters. Eur. J. Org. Chem. 2015, 6606–6609. [Google Scholar] [CrossRef]
- Lempers, H.E.B.; Sheldon, R.A. The Stability of Chromium in CrAPO-5, CrAPO-11, and CrS-1 during Liquid Phase Oxidations. J. Catal. 1998, 175, 62–69. [Google Scholar] [CrossRef]
- Ito, Y.; Kato, H.; Imai, H.; Saegusa, T. A novel conjugate hydrocyanation with titanium tetrachloride-tert-butyl isocyanide. J. Am. Chem. Soc. 1982, 104, 6449–6450. [Google Scholar] [CrossRef]
- Miura, T.; Masaki, Y. Carbon–carbon bond formation and reduction of aldehydes, ketones and acetals with silylated nucleophiles catalysed by tetracyanoethylene. J. Chem. Soc. Perkin Trans. 1995, 17, 2155–2158. [Google Scholar] [CrossRef]
- Fujioka, H.; Goto, A.; Otake, K.; Kubo, O.; Yahata, K.; Sawama, Y.; Maegawa, T. Remarkable effect of phosphine on the reactivity of O,P-acetal—Efficient substitution reaction of O.,P.-acetal. Chem. Commun. 2010, 46, 3976–3978. [Google Scholar] [CrossRef] [PubMed]
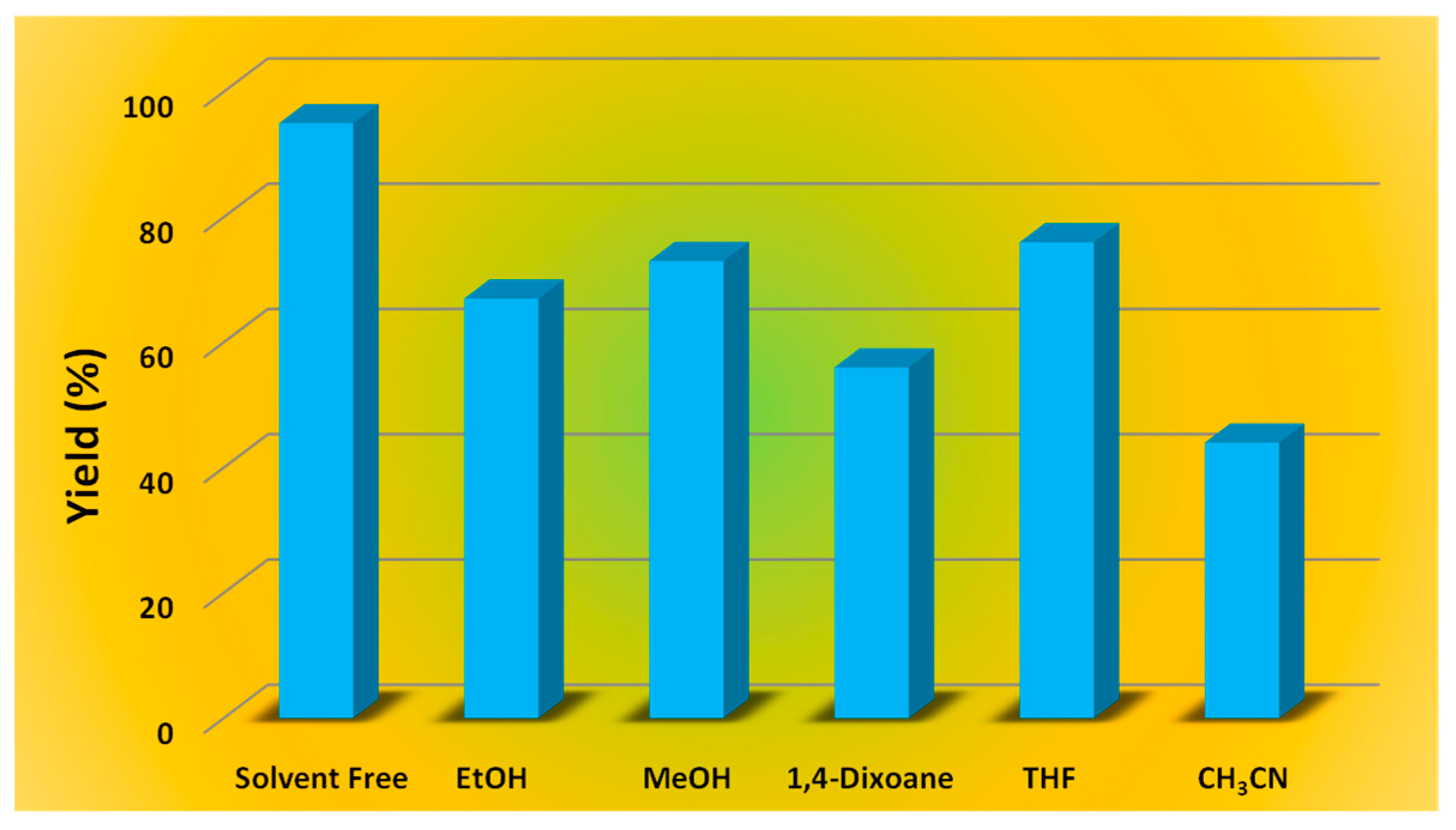
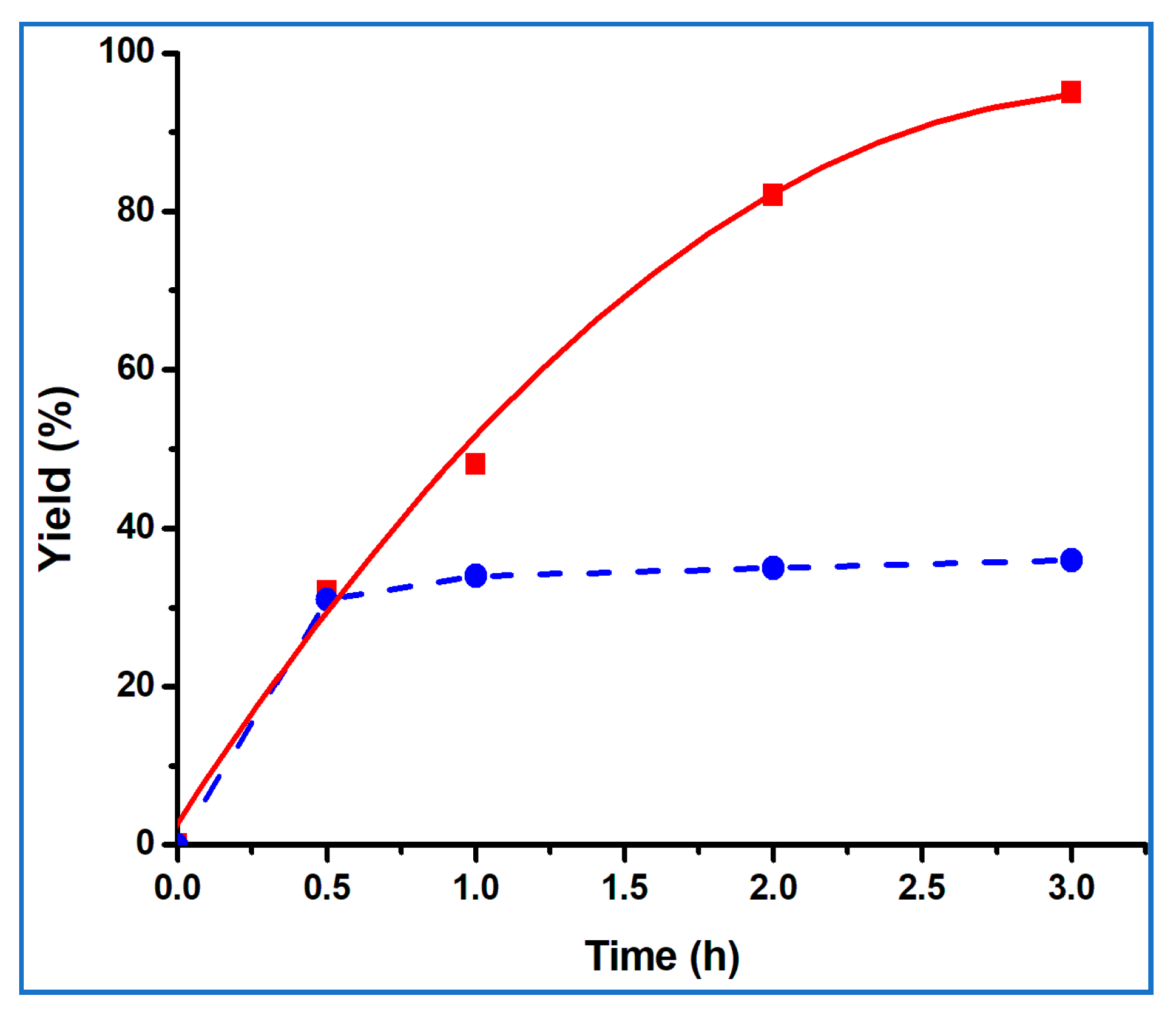
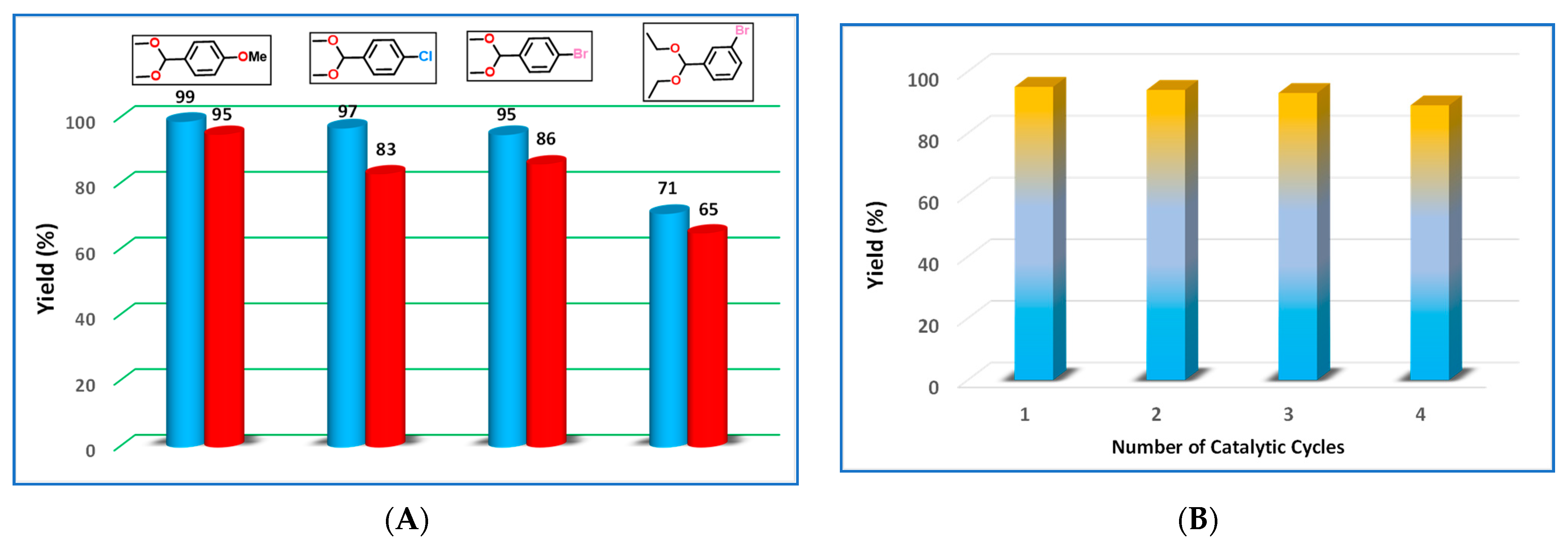

| Entry | Catalyst | Catalyst Amount (mol%) | Temp (°C) | Microwave Frequency (W) | Solvent | Time (h) | Yield (%) b |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 70 | 15 | No solvent | 3 | 95 |
| 2 | 2 | 1 | 70 | 15 | No solvent | 3 | 84 |
| 3 | 1 | 1 | 70 | 15 | No solvent | 0.5 | 32 |
| 4 | 1 | 1 | 70 | 15 | No solvent | 1 | 48 |
| 5 | 1 | 1 | 70 | 15 | No solvent | 2 | 82 |
| 6 | 1 | 1 | 70 | 15 | No solvent | 3 | 95 |
| 7 c | 1 | 1 | 70 | - | No solvent | 0.5 | 25 |
| 8 c | 1 | 1 | 70 | - | No solvent | 1 | 39 |
| 9 c | 1 | 1 | 70 | - | No solvent | 2 | 72 |
| 10 c | 1 | 1 | 70 | - | No solvent | 3 | 84 |
| 11 | 1 | 1 | 70 | 15 | EtOH | 3 | 67 |
| 12 | 1 | 1 | 70 | 15 | MeOH | 3 | 73 |
| 13 | 1 | 1 | 70 | 15 | 1,4-Dioxane | 3 | 56 |
| 14 | 1 | 1 | 70 | 15 | THF | 3 | 76 |
| 15 | 1 | 1 | 70 | 15 | CH3CN | 3 | 44 |
| 16 | 1 | 1 | 25 | 15 | No solvent | 3 | 30 |
| 17 | 1 | 1 | 40 | 15 | No solvent | 3 | 77 |
| 18 | 1 | 1 | 90 | 15 | No solvent | 3 | 91 |
| 19 | 1 | 0.5 | 70 | 15 | No solvent | 3 | 71 |
| 20 | 1 | 2 | 70 | 15 | No solvent | 3 | 94 |
| 21 | 1 | 3 | 70 | 15 | No solvent | 3 | 95 |
| 22 | Cd(NO3)2.4H2O | 1 | 70 | 15 | Solvent free | 3 | 43 |
| 23 | Blank | - | 70 | 15 | Solvent free | 3 | 5 |
| 24 | H2L1 | - | 70 | 15 | Solvent free | 3 | 7 |
| 25 | H2L2 | - | 70 | 15 | Solvent free | 3 | 10 |
| 26 d | 1 | 1 | 70 | 15 | No solvent | 3 | >99 |
| 27 d | 2 | 1 | 70 | 15 | No solvent | 3 | 95 |
| 28 e | 1 | 1 | 70 | 15 | No solvent | 3 | 97 |
| 29 e | 2 | 1 | 70 | 15 | No solvent | 3 | 83 |
| 30 f | 1 | 1 | 70 | 15 | No solvent | 3 | 95 |
| 31 f | 2 | 1 | 70 | 15 | No solvent | 3 | 86 |
| 32 g | 1 | 1 | 70 | 15 | No solvent | 3 | 71 |
| 33 g | 2 | 1 | 70 | 15 | No solvent | 3 | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karmakar, A.; Paul, A.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Polyaromatic Group Embedded Cd(II)-Coordination Polymers for Microwave-Assisted Solvent-Free Strecker-Type Cyanation of Acetals. Molecules 2023, 28, 945. https://doi.org/10.3390/molecules28030945
Karmakar A, Paul A, Guedes da Silva MFC, Pombeiro AJL. Polyaromatic Group Embedded Cd(II)-Coordination Polymers for Microwave-Assisted Solvent-Free Strecker-Type Cyanation of Acetals. Molecules. 2023; 28(3):945. https://doi.org/10.3390/molecules28030945
Chicago/Turabian StyleKarmakar, Anirban, Anup Paul, Maria Fátima C. Guedes da Silva, and Armando J. L. Pombeiro. 2023. "Polyaromatic Group Embedded Cd(II)-Coordination Polymers for Microwave-Assisted Solvent-Free Strecker-Type Cyanation of Acetals" Molecules 28, no. 3: 945. https://doi.org/10.3390/molecules28030945
APA StyleKarmakar, A., Paul, A., Guedes da Silva, M. F. C., & Pombeiro, A. J. L. (2023). Polyaromatic Group Embedded Cd(II)-Coordination Polymers for Microwave-Assisted Solvent-Free Strecker-Type Cyanation of Acetals. Molecules, 28(3), 945. https://doi.org/10.3390/molecules28030945












