Abstract
Despite their remarkable biosynthetic potential, Bacillus subtilis have been widely overlooked. However, their capability to withstand harsh conditions (extreme temperature, Ultraviolet (UV) and γ-radiation, and dehydration) and the promiscuous metabolites they synthesize have created increased commercial interest in them as a therapeutic agent, a food preservative, and a plant-pathogen control agent. Nevertheless, the commercial-scale availability of these metabolites is constrained due to challenges in their accessibility via synthesis and low fermentation yields. In the context of this rising in interest, we comprehensively visualized the antimicrobial peptides produced by B. subtilis and highlighted their prospective applications in various industries. Moreover, we proposed and classified these metabolites produced by the B. subtilis group based on their biosynthetic pathways and chemical structures. The biosynthetic pathway, bioactivity, and chemical structure are discussed in detail for each class. We believe that this review will spark a renewed interest in the often disregarded B. subtilis and its remarkable biosynthetic capabilities.
1. Introduction
The Bacillus subtilis (B. subtilis) group is a ubiquitous Gram-positive bacteria with a remarkable adaptability potential that enables it to survive in highly diverse environments [1]. It is non-pathogenic and presents incredible genetic diversity even within closely related strains [2]. The B. subtilis group consists of four species, including Bacillus subtilis, Bacillus pumilus, Bacillus licheniformis, and Bacillus amyloliquefaciens, discovered several decades ago. Over time, numerous new species and subspecies have been described based on molecular evolution, physiology, and chemotaxonomic characterization [3]. B. subtilis is a model organism used to investigate cell motility, biofilm formation, protein secretion, cell division, secondary metabolites biosynthesis, adherence to plant root and fungal hyphae, cytoplasm altercation via intracellular nanotubes, and kin-recognition [4]. In biotechnological industries, B. subtilis is a popular workhorse used for the biosynthesis of a broad range natural products, from enzymes to purified bioactive compounds [5,6]. Its natural competence to genetic engineering and well-described gene expression system makes it attractive on many occasions [6]. Moreover, recently, it also earned attention as a biocontrol agent in agronomy by antagonizing phytopathogens and promoting plant growth [7].
The impressive skill set of B. subtilis for producing diverse bioactive metabolites was recognized in the last decade. It has been demonstrated that ~5% of a wild-type B. subtilis genome is exclusively devoted to the synthesis of bioactive compounds [8]. For a long time, it was considered for the production only of cyclic peptides such as iturins, surfactins, and fengycins [9,10]. Nonetheless, due to the discovery of numerous antimicrobial exhibiting linear lipopeptides, PKs, and volatile metabolites, it has gained a high commercial interest. The versatile bioactive metabolites produced by the B. subtilis group may be classified based on several criteria, including their biosynthetic pathways, function, structure, source, physicochemical properties, molecular targets, or bonding patterns [11]. Herein, we classified the bioactive metabolites from B. subtilis based on their molecular structures and biosynthetic pathways (Figure 1). In the current review, the bioactive metabolites produced by B. subtilis are classified into five classes, including non-ribosomal peptides (NRPs), polyketides (PKs), ribosomal peptides (RPs), and hybrid and volatile metabolites. These five classes are further categorized into various subclasses and described in detail.
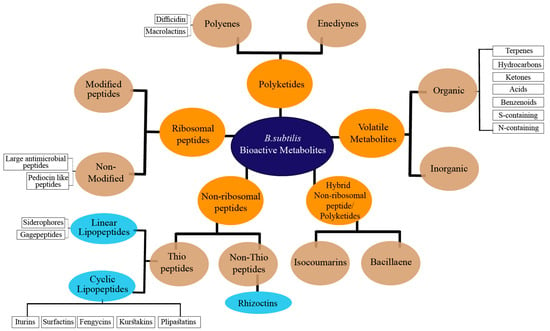
Figure 1.
Classification of bioactive metabolites produced by B. subtilis complex.
Additionally, we comprehensively summarized all the reported bioactive metabolites produced by the B. subtilis group (Supplementary Material, Table S1–S5). Previous reports only focused on a single subclass or class, such as lipopeptides, lantibiotics, macrolides, and volatile metabolites [12,13,14,15]. Currently, in a time of drug resistance and high demand for eco-friendly pest control strategies, B. subtilis and its bioactive compounds could be the right choice. Therefore, we expect that this review will increase commercial interest in utilizing B. subtilis origin antimicrobial metabolites and biosurfactants in various industries.
2. Non-Ribosomal Peptides (NRPs)
NRPs are molecular assembly machines that use multi-modular enzyme complexes rather than a DNA template to construct a protein [16]. A module is a part of the non-ribosomal peptide synthetases (NRPS) enzymes that assimilates the amino acid into a peptide backbone of a specific kind. Further, each module may be divided into three domains, adenylation (A), thiolation (T) or peptidyl carrier protein (PCP), as well as a condensation (C) domain that catalyzes the individual steps of NRP synthesis [17] (Figure 2). The synthesis of the primary product might be post-synthetically modified to obtain its mature form via glycosylation, methylation, hydroxylation, acylation, heterocyclic ring formation, and halogenation [18,19]. NRPs exhibit vast structural diversity and may be found as linear, branched, and cyclic structures. So far (January 2022), the Norine (Non-ribosomal peptide database) contains 1740 peptides, including information about their biosynthesis, structures, and evolution [20].
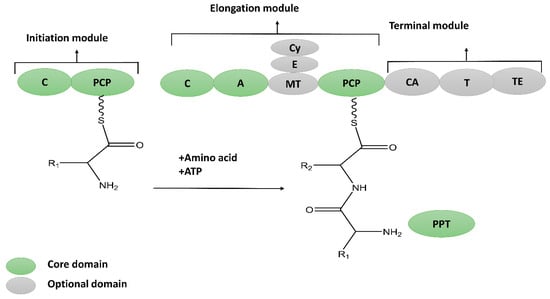
Figure 2.
Biosynthesis of non-ribosomal peptides (NRPs), consisting of core and auxiliary domain. Core domain encoding NRP includes A; Adenylation, PCP; peptidyl carrier domain, C; condensation ans TE; thioesterase domain. Auxiliary or optional domains responsible for cyclization (Cy), N-methylation (MT) and epimerization (E).
The NRPs may be synthesized via a multi-enzyme thio-template mechanism or without the multi-enzyme [21]. The NRPs synthesized through a thio-template are usually 2–50 amino acids and some other moieties such as a fatty acid chain, whereas the NRPs synthesized without a multi-enzyme template are comparatively smaller [22]. Based on biosynthetic pathways, NRPs can be divided into thio-template and non-thiotemplate NRPs.
2.1. Thio-Template NRPs
The thio-template NRPs can be classified as cyclic lipopeptides and non-cyclic or linear lipopeptides based on their chemical structures (Supplementary Material Table S1).
2.1.1. Cyclic Lipopeptides
Thio-template non-ribosomal cyclic lipopeptides were first isolated during the 1950s–1960s from Bacillus spp. The cyclic lipopeptides are primarily synthesized via the sequential addition of residues, either in an iterative or a non-iterative manner. The lipopeptide synthesis pathways are very flexible; therefore, the synthesized peptides are incredibly diverse in nature. The cyclic lipopeptides produced by B. subtilis can be classified into four classes [23].
- Surfactins: Surfactin was first isolated in 1968 from the culture supernatant of B. subtilis, which exhibited an excellent biosurfactant activity [24]. Subsequently, surfactin was demonstrated to be an antitumor, antibacterial, anticoagulant, and hypocholesterolemic agent [25] as shown in Figure 3. A typical structure of a surfactin is shown in Supplementary Material Figure S1.
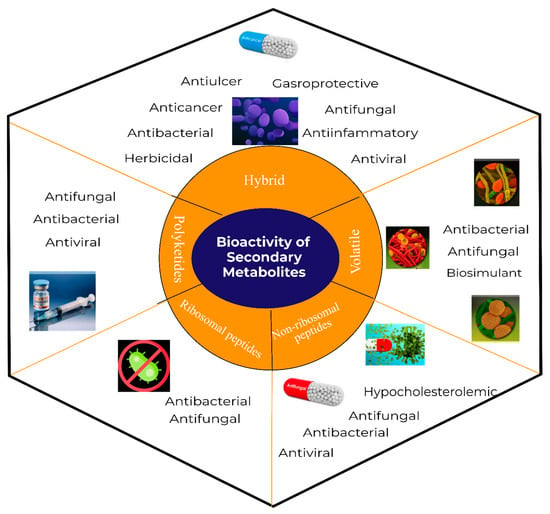 Figure 3. Potential application of secondary metabolites synthesized by Bacillus subtilis.
Figure 3. Potential application of secondary metabolites synthesized by Bacillus subtilis. - Iturins: In 1949, Walton and Woodruff isolated the first antifungal iturin from B. subtilis. Later on, in 1950, a second similar compound iturin was reported, whose name was derived from Ituri (the name of the place in Congo where the soil sample was collected) [26]. The exact structure of iturin was elucidated to be a cyclic hepatolipopeptide attached to the alkyl chain (Figure S1). Iturins are known to display potent antifungal activity and could be used as an active ingredient in several phytopathogen control products. The closely related cyclic lipopeptides could be classified as iturin: bacillomycin L [27], mycosubtilin [28], bacillomycin D, bacillomycin F [29], mojavensin A [30], and subtulene A [31].
- Fengycins: In 1986, Japanese and German scientists simultaneously discovered fengycin from B. subtilis [32]. Initially, it was determined that fengycin inhibits the growth of filamentous fungi and is ineffective against non-filamentous fungi and bacteria. Later on, however, its antiviral [10], antibacterial [33], and anticancer properties were reported [34]. It also exhibited a plant growth-promoting property, which is desirable in the agriculture industry.
- Kurstakins: Kurstakin is a lipo-heptapeptide exhibiting antifungal activity produced by several B. subtilis strains. Kurstakins cannot be recovered from the culture supernatant but are found in association with the producing cells [35]. Nevertheless, the co-infection study conducted with the producing and non-producing strains demonstrated that it is extracellular [36]. This contradiction suggests that kurstakin is an extracellular metabolite having a high affinity to the cell membrane. This affinity is probably due to the presence of histidine, which gives a positive charge to kurstakin and allows its electrostatic interaction with the phospholipid of a membrane.
- Plipastatins: Plipastatin was first reported from B. cereus as an antiphospholipase, before being identified in B. subtilis [37]. Plipastatins are closely related to fengycin. The alteration happens from the inversion of two stereocenters, offering a distinct 3D structure to plipastatin’s backbone. Notably, these apparent small structural differences result in a loss of antifungal activity [38].
2.1.2. Linear Lipopeptides
Linear lipopeptides have been produced by several B. subtilis strains. They can be classified into the following two subclasses
- a.
- Gageopeptides
Recently, several linear lipopeptides have been reported and characterized from B. Subtilis. For instance, Gageostatin was reported from a marine-derived B. subtilis [39]. Gageostatin consists of 3-beta hydroxyl fatty acid attached to heptapeptide (Figure S2). It is composed of the same residues as reported for surfactin. However, differences were found in their structures and molecular masses. Gageostatins were found in linear form with exclusively L-leucine, while surfactins are cyclic lipopeptides with L and D-leucine.
Interestingly, it was reported that a mixture of the two metabolites (gageostatin A and gageostatin B) was more effective and appeared to work synergistically against bacteria and fungi. However, the future application of gageostatins as a drug candidate may be limited due to their broad cytotoxicity [39]. Gageotetrins A–C and gageopeptides A–D are linear antimicrobial peptides comparable to gageostatins. Unlike gageostatins, they exhibit no cytotoxicity and strongly antagonize bacteria and fungi [40]. They are probably synthesized via a hybrid biosynthesis pathway, and gageotetrin A is a potential precursor for gageotetrin B. Gageotetrins were more recently isolated from B. subtilis strain 109GGC020 and displayed promising antibacterial and antifungal activities in a time-dependent manner [40].
- b.
- Siderophores
Siderophores are small molecules having a high affinity toward ferric iron. Besides iron scavenging, they are also used to form stable complexes with environmentally important metals. Based on their chemical moiety, siderophore can be categorized into three types, i.e., hydroxamate, catecholate, and carboxylate siderophores. Most of the siderophores produced by bacteria are catecholate, and few are carboxylate and hydroxamate [41]. Bacillibactin is a well-known catecholate siderophore produced by different B. subtilis strains that exhibits strong antibacterial properties and moderate cytotoxicity [42]. The chemical structures of representative siderophores are shown in Figure S2. Bacillus spp. are widely studied for the synthesis of bioactive metabolites. However, their siderophore-producing capabilities have not yet been much explored. Previously, a siderophore-producing Bacillus spp. was reported that enhanced the bioremediation of metals and increased plant growth. B. subtilis strain CAS15 was isolated from the rhizosphere and identified as producing siderophore, and it also inhibits the growth of phytopathogens [43]. Similarly, the siderophore-mediated bioaccumulation of cadmium (Cd) has been reported in B. subtilis and demonstrated as a substitute bioremediation strategy [44]. In recent years, the biological control of phytopathogens has been the subject of research, because it helps in limiting the use of hazardous chemically synthesized pesticides. The siderophore-producing bacteria provide a promising alternative disease management strategy, as they are able to enhance crop yields and, at the same time, protect plants from pathogens.
2.2. Non-Thio-Template NRPs
B. subtilis is capable of synthesizing antimicrobial NRPs via non-thio-template mechanism. Rhizocticins are non-thio-template peptides that consist of an arginine linked with L-2-amino-5-phosphono-3-cis-pentanoic acid. Sometimes they are supplemented with leucine, isoleucine, and valine [45]. Besides rhizocticins, B. subtilis can also synthesize dipeptide NRPs such as bacilysin (tetain) and chlorotetain (Supplementary Material Figure S2). Regardless of their simple composition (L-alanine link with L-anticapsine), they exhibit strong antibacterial and antifungal activity [46]. The antibacterial activity is mediated by the L-anticapsine, which inhibits the synthesis of glucosamine-6 phosphate. Its inhibition stops the synthesis of peptidoglycan, which is a major constituent of the bacterial cell wall. For antifungal activity, it has been suggested that anticapsine can suppress the biosynthesis of chitin and fungal cell membrane mannoproteins [47]. Tetain and chlorotetain are shown to inhibit the growth of Aspergillus fumigatus and Candida albicans [48]. Mycobacillin and bacitracin are non-thio-template polypeptides produced by B. subtilis. Mycobacillin inhibit the growth of Aspergillus niger by altering its cell membrane [49]. The biosynthesis of mycobacillin is unique. The NRPS complex catalyzed and divided it into three parts, i.e., A, B, and C, but it does not use the thio-template mechanism [50]. Each part of the complex comprises a single polypeptide enzyme that catalyzes the polymerization of pentapeptide A, nonapeptide B, and tridecapeptide C. Bacitracin is a heptapeptide that widely antagonizes Gram-positive bacteria by inhibiting the biosynthesis of petidoglycan [51].
3. Ribosomal Peptides (RPs)
Ribosomal peptides (RPs), also known as ribosomally synthesized and post-translationally modified peptides (RiPPs), are derived from a relatively short precursor peptide and are matured through post-translational modification [52]. Several enzymes are involved in these modifications; thus, structurally diverse peptides are generated (Figure 4). In order to classify the RiPPs produced from B. subtilis, several classifications have been proposed, and the classification based on the biosynthetic pathway or chemical structure is reasonable, as reported for bacteriocin produced by Enterococcus spp. and Streptococcus spp. [13]. Consequently, the known B. subtilis producing RiPPs could be classified into three main classes and several subclasses (Supplementary Material Table S2).
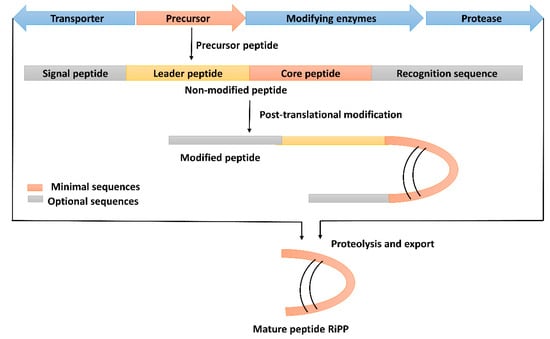
Figure 4.
Schematic representation of biosynthesis of ribosomal peptides (RPs). The short precursor peptides are converted into core peptides, and then the core peptide is matured via post-translational modifications.
3.1. Class I—RiPPs
Class I consists of biologically active short peptides that are ribosomally synthesized and undergo post-translation modification (PTM), resulting in a unique structure and properties. According to PTM differences, class I can be divided into several subclasses.
3.1.1. Lanthipeptides
Class 1 lanthipeptides consist of highly diverse post-translationally modified peptides that characteristically contain cross-link thioether between non-proteinogenic lanthionine and 3-methyllanthionine [53]. Lanthipeptides are produced as precursor peptides and consist of a leader and a core peptide. The precursor peptide is post-translationally modified and cross-linked with thioether, and, subsequently, the leader peptide is removed, and a mature lanthipeptide is released [54]. Lanthipeptides exhibit promising antimicrobial activity, and, indeed, a lanthipeptide “nisin” is commercially used in the food industries. Nisin prevents the synthesis of peptidoglycan transglycosylation and forms a membrane-spanning pore [55]. Several gene clusters are associated with the biosynthesis and maturation of class I lanthipeptides, as identified in the B. subtilis genome using computational tools. Recently, a more robust computational tool has been designed to identify the lanthipeptide gene cluster from genomic data [56]. However, their specific products are yet not purified from the producer strains.
3.1.2. Lasso Peptides
Lasso peptides are a relatively newly characterized class of RiPPs composed of short-chain peptides comprising an N-terminal macrolactam by which the C-terminal is linked [57]. The N-terminal “ring” is formed by 7 to 9 amino acids that are linked by an isopeptide bond between the N-terminal amino group of the first residue and the carboxylate chain of glutamate or aspartate residue [54]. The first residue of lasso peptides, cysteine or glycine, is highly conserved. Therefore, bioinformatics approaches are recommended for the discovery of new lasso peptides [58]. However, few lasso peptides have been discovered with alanine or serine as their first amino acid [59]. Lasso peptides’ gene clusters have been identified from the B. subtilis genome, but the specific product is yet not fully characterized. Lasso peptides are important antimicrobial peptides, and their biosynthesis involves the synthesis of a precursor A-peptide via A-protein. The precursor is post-translationally modified via B-protein and C-protein. B-protein is an ATP-dependent lasso protease that removes the leader peptide, and C-protein is an ATP-dependent synthetase (lasso cyclase) that catalyzes the synthesis of the macrolactam ring between the N-terminal of an amino group and the side chain of glutamate or aspartate in the peptide. Due to the specific threaded loop topology, the peptides resemble “lassos”, hence the term “lasso peptides” [54].
3.1.3. Sactipeptides
Sectippetides are biologically active peptides with exceptional cross-links between the sulfur of cysteine and the alpha carbon of other residues catalyzed by S-adenosylmethionine (SAM) [60]. The post-translational link of a thiol group to the alpha carbon is unusual in RiPPs and is responsible for the antimicrobial activity of sactipeptides [61,62]. Genome mining can efficiently detect the unique SAM enzyme whose coding genes are co-localized with the sactipeptides biosynthesis gene cluster [63]. Several sactipeptides have been identified from Bacillus spp., such as subtilosin A, a sactipeptide produced by the synthesis of B. subtilis 168 from the predecessor by the proteolytic cleavage of the leader peptide and the cyclization of the N and C-terminals via covalent linkage [64,65]. Further, modification of threonine, cysteine, and phenylalanine occurs, and the mature peptide is exported via ABC transporters [13]. Sactipeptides are reported to be active against Bacillus spp. Listeria monocytogen, Gardnerella vaginalis, and Enterococcus faecalis by making pores in their cell membrane [65,66,67].
3.1.4. Linear Azole-Containing Peptides (LAPs)
The linear azole-containing peptides (LAPs) are heterocyclic peptides derived from the threonine, cysteine, and serine of a short precursor peptide [68]. They consist of four obligatory modules: a precursor peptide that is also known as peptide A, a heterotrimeric enzyme complex (dehydrogenase B), and cyclodehydratase C and D. Plantazolicin is a LAP produced by B. amyloliquefaciens. Its biosynthesis initiates with the formation of azoline heterocycles via the C and D complex from threonine/serine and cysteine, followed by dehydrogenation by B leading to the synthesis of the corresponding azole [69,70]. Regardless of the low amino acid identity of the BCD complex between LAP loci, numerous reports revealed that BCD genes could be associated with various LAP biosynthesis pathways by converting the precursor peptide into the active RiPP [70,71]. Therefore, these genes are used in genome mining [72]. Sonorensin was initially reported from a marine-derived Bacillus sp. that effectively antagonizes the growth of both Gram-positive and negative bacteria. Further, it was proposed that growth inhibition occurred due to the increased cell membrane permeability. The sonorensin-coated low-density polyethylene film efficiently controls the food spoilage of Gram-positive bacteria such as S.aureus and L. monocytogenes [73]. The bio-preservative property of the sonorensin-coated film in meat and vegetables demonstrates its potential application in the food industries.
3.1.5. Thiopeptides
Thiopeptides are an emerging group of antibiotics and include more than 100 compounds. Thiopeptides not only exhibit antibacterial activity but also possess broad bioactivities such as anticancer, antiplasmodial, and anti-immunosuppressive activities [74]. Few completely characterized thiopeptides have been reported from the B. subtilis group. Recently, only one thiopeptide was purified and identified among 80 thiopeptide biosynthetic gene clusters detected via genome mining approaches [75].
3.1.6. Cyclic (Head-to-Tail) Peptides
The head-to-tail cyclic peptides are relatively long peptides with 35–70 amino acid residues. The peculiar features of cyclic peptides are not only their large size but also the modifying enzymes associated with their cyclization. Due to their macrocyclization, these peptides are relatively resistant to higher temperatures, pH changes, and several proteases [76]. These peptides are distinguished from lanthipeptides in that they do not contain lanthionine, methyl lanthionine, and hydrated amino acid residues [77]. Amylocyclicin was reported as a new head-to-tail cyclized peptide from B. amyloliquefaciens, which is derived from 112 amino acid residues precursor encoded by the acnA gene [78]. The cyclization of amylocyclicin occurs between leucine one and tryptophan 64. Amylocyclicin inhibits the growth of Gram-positive bacteria, including B. subtilis [78]. Recently, another novel cyclized peptide, “pumilarin”, was detected via genome mining in the B. pumilus genome and was reported to have a cyclized structure and exhibit a wide range of bioactivities [79]. Pumilarin was found to be post-translationally modified so that its N and C-terminals are linked via a peptide bond. The pumilarin biosynthetic gene cluster comprises the pumA, pumB, pumC, pumC1, and pumD genes [79]. However, the exact biosynthetic pathway for pumilarin is yet to be elucidated.
3.2. Class II Peptides
Class II metabolites are heat stable (121 °C), short peptides of less than 10 kDa, and are usually not post-translationally modified [80,81]. Previously, lactic acid bacteria were reported to be the main producer of RiPPs due to their long history of safe use in food [82]. In 1960, nisin was approved as a safe food additive and used in more than 50 countries as an antibacterial agent against Gram-positive bacteria [83]. Nevertheless, the quest for new antimicrobial agents rapidly stretched to other RiPP producing bacterial species. Bacillus species have become increasingly attractive due to being “generally recognized as safe (GRAS)” and having a broader antimicrobial spectrum. [21,84,85].
The non-modified RiPPs produced by the B. subtilis group can be further divided into three subclasses based on the conserved amino acid motifs near N-terminus.
3.2.1. Pediocin-like Peptides
The pediocin-like peptides inhibit numerous clinically relevant pathogens and have a conserved YGNGVXC motif. Despite their great potential as antibacterial agents, the problems associated with their commercial-scale production limit their industrial application. So far, coagulin is the single complete characterized pediocine-like peptide reported from B. coagulans [86]. Coaguline was first reported in 1998, and the complete amino acid sequence was reported in 2000 [80].
3.2.2. Other Non-Modified Peptides
The non-modified peptides have a conserved motif of DWTXWSXL at the N-terminus. In 2001, a hydrophobic and thermotolerant antimicrobial peptide, lichenin, was purified and characterized from B. licheniformis culture [87]. Recently, the biosynthetic gene clusters encoding the class IIb non-modified peptide BhIA were reported from the B. subtilis, B. lechniformis, B. pumilus, and B. amyloliquefaciens genomes via genome mining approaches. The structural analysis revealed significant similarities with holins produced by Geobacillus spp [88]. Holins are phage-encoded peptides involved in the disruption of bacterial cell membranes [89]. However, the function of each biosynthetic coding module remains unknown. The holin-like BhIA metabolite exhibited antibacterial activity against pathogenic Gram-positive bacteria such as Micrococcus luteus and multi-drug resistant S. aureus [90]. The peptide BhIA is composed of 70 amino acids with a transmembrane domain at the N-terminus. Several hydrophilic amino acid residues at the N-terminal and specific membrane topology distinguish BhIA from holin [90]. Aureocin A53 is another new member of the non-modified peptides whose biosynthetic gene cluster was detected in the B. pumilus genome. Aureocin antagonizes the growth of L. monocytogenes by disturbing the cell membrane and inhibits the synthesis of DNA, protein, and polysaccharides simultaneously [91]. LCI was initially isolated from B. subtilis strain A014 and exhibits promising bioactivity against the plant pathogen Xanthomonas campestris [92]. X. campestris causes leaf blight disease in rice, which is a severe threat to rice production and causes significant losses in the rice field annually. LCI is a beta-structured antibacterial peptide comprising 47 amino acid residues. It also carries a hydrophobic core consisting of valine, tryptophan, and tyrosine [93]. LCI is a cationic peptide causing short-lived channels in the target bacterial cell membrane [93].
3.2.3. Large Antimicrobial Peptides
The large antimicrobial peptides are relatively larger and include bioactive metabolites having a size of more than 10 kDa. Their biosynthetic gene cluster consists of an immunity gene and a structural gene. Several Bacillus species, such as B. thuringiensis, B. coagulans, and B. cereus, are reported to produce large antimicrobial peptides [88]. However, as yet, none have been reported from the B. subtilis group.
4. Polyketides (PKs)
Polyketides are structurally diverse bioactive metabolites that contain an alternative methylene and carbonyl group [94]. Polyketides are widely used as therapeutic agents to treat numerous diseases [95]. For instance, tetracycline and erythromycin are used as antibacterials [96], amphotericin is used as an antifungal [97], and anthracyclin is used as an antitumor drug [98]. The biosynthesis of PKs is carried out by a multi-domain enzyme, which consists of ketosynthase, acyltransferase, and thioesterase. Its biosynthesis is initiated by loading the acyl CoA on acyl carrier protein (ACP), catalyzed by acyltransferase (AT). The ketosynthase (KS) extends the carbon chain via decarboxylative condensation. The ketoreductase (KR), enoyl reductase (ER), and dehydratase (DH) domain may further modify the beta-keto group to produce diverse PKs (Figure 5). Subsequently, the thioesterase domain terminates the elongation process by cyclizing or hydrolyzing the PK chain from ACP and releasing a mature PK peptide [99].
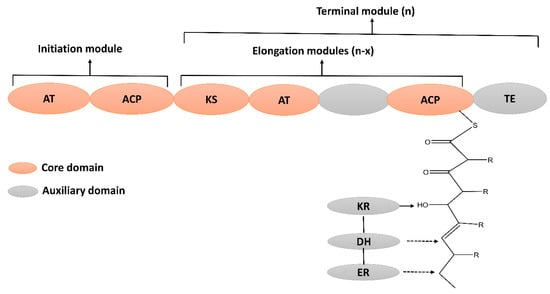
Figure 5.
Schematic representation of enzyme domains involved in the biosynthesis of Polyketide (PKs). Core and auxillary (optional) domains are color coded. Core domains: AT; Acyltransferse, ACP; Acyle carrier protein, KS; Ketosynthase and TE; Thioesterase. Auxiliary domains: KR; Ketoreductase, DH; dehydrase, and ER; Enoyl acyle reductase.
PKs can be classified into three subclasses based on the structural organization of the functional module, i.e., type I PKs, type II PKs, and type III PKs. The type I PKs consist of a large multi-functional enzyme complex carrying several modules bonded covalently and linearly arranged. Type II PKs are multi-enzyme complexes that consist of individual monofunctional enzymes combined during the biosynthesis of ketides. In contrast, type III PKs are chalcone synthase (CHS) like polyketide synthetases, which activate the CoA thioesters directly without the ACP domain [100]. Apart from the structural organization of the functional domain, PKs can be categorized as iterative and non-iterative depending on the number of ketosynthase involved in the biosynthesis of PKs. Bacteria used non-iterative type I polyketide synthase (PKS) enzymes to produce polyketides, and this consensus linearity is employed to identify PKs via genome mining approaches [101]. Besides these differences, due to the great diversity, some other alteration has also been observed. As some times PKs biosynthesis pathways mixed by combining different types of PKSs or even can be associated with NRPSs or fatty acid synthetases to produce a hybrid peptide such as compactin, bacillaene, and fusarin C. bacillaene was previously classified as a PKs-polyene [22]. But, here, based on biosynthetic pathways classified as hybrid PKS/NRPS and will discuss later in hybrid metabolites. PKs can be divided into several classes based on typical structure and carbon skeleton [102]. However, the PKs produced by B. subtilis can be divided into two major types, i.e., Polyenes and enediynes (Supplementary Material Table S3).
4.1. Polyenes
- a.
- Difficidin
Difficidins are unsaturated macrocyclic polyene synthesized by the type 1 PKS (Supplementary Material Figure S3A). Oxydifficidin is an oxidative form of difficidin having an additional hydroxyl group at position 5 [103]. It is encoded by the diff operon that has 14 open reading frames. Several KR, DH, and ER domains are missing within the diff operon and deviate from the colinearity rule. Moreover, the function of diffJ and diffK are unknown, and their activities do not appear in the final product [104]. Difficidin has broad-spectrum antibacterial activity and inhibits the biosynthesis of protein in E. coli [103,105]. Difficidin is produced by B. amyloliquefaciens ATCC strain numbers 39,320 and 39,374 (classified initially as B. subtilis) [103]. B. amyloliquefaciens strain FZB42 showed biocontrol activity against Xanthomonas oryzae by producing difficidin. Scan electron microscopy results showed that difficidin inhibits the growth of a phytopathogen by rupturing the targeted bacterial cell wall. Further, the biocontrol experiments revealed that difficidin caused the downregulation of genes associated with Xanthomonas cell wall synthesis, cell proliferation and virulence [106]. Collectively these studies discuss the future prospective of the strains as a biological control agent against plant pathogens.
- b.
- Aurantinin
The antibacterial exhibiting polyketide aurantinin A and B were isolated initially from Bacillus aurantinus [107]. Recently, along with these two aurantinins, C and D were reisolated in combination with the genome mining approach [108]. The structure of aurantinin and its analogues are very unusual, as they have 5, 6, 7, and 8-membered rings with a highly diverse tail (Supplementary Material Figure S3B). Nevertheless, the absolute structure is yet not elucidated, leaving numerous questions about conformation unanswered. Aurantinin B, C and D exhibit promising antibacterial activity against Clostridium sporogenes and S. aureus. However, they did not show any cytotoxicity against human epithelial colorectal adenocarcinoma and cellosaurus cell lines. The antibacterial mechanism was examined and revealed that aurantinin B-D disrupts bacterial cell membranes causes breakage of cytoplasm and leads to cell death [108]. Owing to their safety profile and discriminatory activity against G-negative bacteria highlights its scope for commercial applications.
Besides that, four antibacterial metabolites have recently been isolated from marine B. subtilis that exhibit antagonistic activity against G-negative food pathogens [109]. Their biosynthetic pathway was speculated to be synthesized via the PKS. Their cytotoxicity, antifungal activity, and complete structure are still unclear.
- c.
- Macrolactins
Macrolactins and their derivatives (7-O-succinyl or 7-O-malonyl) are synthesized via a type 1 PKS. Macrolactins inhibit the growth of bacteria and have been isolated from various species such as Bacillus sp. strain AH1591-1 and B. amyloliquefeciens strain FBZ42 [110,111]. Macrolactins usually consist of 24 lactone rings and three diene moieties in the carbon skeleton. Its biosynthetic gene cluster mln composed of nine operons and 11 KS domains with acetate and malonate is the only used building block. Like dif gene cluster organization, mln appears in an occasional splitting of the modules. The second module is split between mlnB and mlnC, and a similar arrangement can be observed for modules number 5, 7, 8, and 10. A comparison of catalytic domain organization revealed that the ER domain is missing in module number 2, while two DH domains are missing in modules 7 and 10 [110].
The B. subtilis group mostly produces biosynthetic derivatives of macrolactin A (Supplementary Material Figure S3C). For instance, 7-O succinylmacrolactin A showed antibacterial activity against S. aureus with mild cytotoxicity [112,113]. It also exhibits anticancer activity via the CYP2P9 and P13K pathways [114,115,116]. Conveniently, 7-O succinylmacrolactin A also shows anti-inflammatory activity via the same pathways [112]. Compared to macrolactine A, 7-O succinylmacrolactin A might be a better candidate as a lead due to its superior stability and pharmacokinetics [115,117]. The compound 7-O-malonylmacrolactin A exhibits similar anti-inflammatory activity to macrolactin A and 7-O succinylmacrolactin A; however, due to its activity against MRSA, it gained more attention [112]. Bacillomycin D and 7-O malonylmacrolactin A produced by the B. subtilis group have been employed as biocontrol agents against phytopathogenic bacteria and fungi with bioorganic fertilizers [118,119]. Therefore, the coproduction of various bioactive metabolites might be a productive approach for wide-ranging biological control, highlighting the value of B. subtilis.
Moreover, aromatic and unsaturated macrolactin have been reported with 7-O-6′-(2″-acetylphenyl)-5′-hydroxyhexanoate-macrolactin A, demonstrating a fine example of esterification of two PKS products [113]. The compound 7-O-6′-(2″-acetylphenyl)-5′-hydroxyhexanoate-macrolactin A was isolated from a seaweed associated B. subtilis with good antibacterial activity against Gram-positive bacteria [113]. However, its structure is yet to be elucidated. Conversely, 7-O-2′E-butenoylmacrolactin A was extracted from sea-sediment-derived B. subtilis and showed moderate antifungal activity against Colletotrichum gloeosporioides and Pestalotiopsis theae [116]. The compound 7-O-methyl-5′-hydroxy-3′-heptenoate macrolactin A isolated from the algal-associated B. subtilis strain MTCC 10,403 displayed moderate antibacterial activity [120], but the investigator further did not report bioactivity and structure-related information.
Macrolactin B is 7-O-β-glycosylate was first isolated in 1989 and was reisolated from marine B. subtilis [121,122]. Macrolactin B exhibit potent antifungal activity; however, unlike macrolactin A, it is not cytotoxic, indicating the structure-activity relationship of macrolactins. Macrolactin W is the only example of macrolactin which is both 7-O glycosylated and esterified. Its antimicrobial property is similar to macrolactin A and B, though it has no cytotoxic activity [122,123]. The cytotoxic, antiviral, and anti-inflammatory activity is observed for the parent metabolite macrolactin A and as yet not determined for its derivatives. It is anticipated that these compounds are hydrolyzed in vivo and release the active metabolite macrolactin A.
4.2. Enediynes
To date, the PK enediyne is the most cytotoxic natural product, and its application as an anticancer drug has been clinically demonstrated [124]. Due to the substantial cytotoxicity of enediyne, its application is minimal. However, its application in various antibody-drug conjugates and polymer-based drug delivery systems has had great success [125,126]. Enediynes are commonly produced by Streptomyces spp. However, recently reported from Bacillus sp. via the genome mining approach [127]. The complete biosynthetic machinery and structure yet remain to be elucidated.
5. Hybrid Metabolites
Hybrid metabolites are the products of biosynthetic pathways that comprise both (NRPS/PKS) types of modular enzymes. Questions relating to the synthesis of hybrid products are of great present-day interest, as their answer concerns genetic engineering efforts. Both depend on thio-template for acyl chain elongation and monomers triggering. Presently, the factors of molecular events that offer a hybrid pathway to accommodate various assembly line chemical moieties functionally are yet not completely elucidated. However, based on the current knowledge, the hybrid metabolites produced by B. subtilis can be classified as bacillaene and isocoumarins (Supplementary Material Table S4).
- a.
- Bacillaene
Bacillaene has a linear structure (Supplementary Material Figure S3D) and was first reported from B. subtilis strains 55,422 and 3610 [128]. It is encoded by a hybrid PKS-NRPS biosynthetic gene cluster known as bacillaene PksX synthase (Figure 6). The pksX mega gene cluster in B. subtilis 168 genome consisted of 5 open reading frames named pksJ, pksL, pksM, pksN, and pksR [104,129]. The first two adenylation domains of pksJ incorporate glycin and α-hydroxy-isocaproic acid. The third adenylation domain (pksN) is responsible for the incorporation of alanine. While the three open reading frames pksC, baeD, and baeE encode for three separate AT domains are responsible for incorporating malonyl-CoA [130]. Bacillaene inhibits the growth of various bacteria and fungi such as Myxococcus xanthus, and Trichoderma spp. [131,132]. Bacillaene selectively inhibits the biosynthesis of protein in bacteria, indicating a selective inhibition of other strains in their habitat [128].
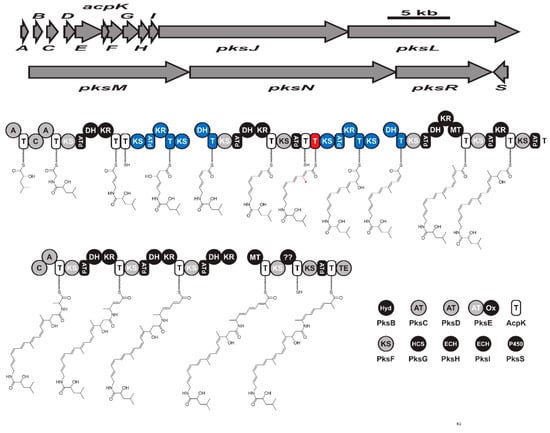
Figure 6.
The biosynthetic gene cluster (pksX) and biosynthesis of hybrid bacillaene. Key: KS (ketosynthase), AT (acyltransferase), T (thiolation), DH (dehydratase), KR (ketoreductase), MT (methyltransferase), A (adenylation), C (condensation), ATd (AT-docking), Hyd (Zn-dependent hydrolase), Ox (flavin mononucleotide-dependent oxidase), HCF (HMG-CoA synthase), ECH (enoyl-CoA hydratase), and TE: (thioesterase).
- b.
- Isocoumarins
Isocoumarins form a large, diverse class of biologically active metabolites with more than 200 metabolites [133]. However, fewer are reported from B. subtilis. It has been reported that B. subtilis specifically produce isopropyl-8-hydroxy-3,4-dihydroisocoumarins with an active side chain or functionalized amino acid [134]. Amicoumacin A-C (Supplementary Material Figure S3E,F) and amicoumacin F are dihydroisocoumarins initially reported from B. pumilus [135,136,137]. Later, they were reported from B. subtilis and determined its strong antibacterial activity against the gastric pathogen Helicobacter pylori [138]. Biosynthesis of these bioactive metabolites has been recently reported from B. subtilis 1779 via a genome mining approach [139]. The BGC encoding amicoumacin was predicted to be 47.4 kb in size and consists of 12 open reading frames. Further, the investigator expressed the biosynthetic gene cluster in a heterologous host and obtained amicoumacin A–C. Based on these findings, they predicted the biosynthetic pathway for amicoumacin to be synthesized via hybrid PKS-NRPS modular enzymes [139]. The biosynthetic gene cluster consists of eight modules that synthesize two discrete pre-amicoumcin molecules. The unique dihydroisocoumarins core is likely to be synthesized via removing the AmiJ-M megasynthase complex to produce an oxygenated PK chain that reorganizes into a cyclic dihydroisocoumarin [139]. Amicoumacin exhibit promising anti-inflammatory and antibacterial activity and has gained increased interest as a pro-drug candidate. Specifically, amicoumacin A is more attractive due to its potent anticancer activity and antibacterial activity against MRSA, with MIC less than 1 µg/mL [136,137]. The antibacterial mechanism of amicoumacin was recently shown to be bind on the ribosome and inhibit protein biosynthesis [140,141]. Amicoumacin A and C exhibit antibacterial activity, while amicoumacin B is non-antibacterial. Interestingly, the hydroxy group in amicoumacin A and C is responsible for their antibacterial activity as the phosphate ester-containing amicoumacin B lacks antibacterial activity [142].
Bacilosarcin A (3-oxa-6,9-diazabicyclo [3.3.1]nonane ring) and B (rare 2-hydroxymorpholine moiety) with unique heterocyclic core structures were isolated from the marine-derived B. subtilis strain TP-B0611 [143]. Later on, their complete chemical structures and structure-activity relationships were demonstrated [144]. Unlike amicoumacin A and B, bacilosarcin A and B displayed marginal anti-herbicidal activity. The low anti-herbicidal activity reflects the side chain functional group and highlights the importance of the side chain for bioactivity. This hypothesis is further supported by the non-antibacterial activity of bacilosarcin C, which has –COOH (carboxylic) instead of –NH2 (amide) group [145].
The piperazin-containing damxungmacin A (dihydroisocoumarin) has an unusual heterocyclic ring that displays weak antibacterial activity and cytotoxicity [146]. Unlike other dihydroisocoumarin, damxungmacin exhibits weak bioactivity, low yield, and a complex molecular structure that offers distinctive bioprocessing techniques development. Similarly, damxungmacin B was isolated and showed no bioactivity against tested strains.
Hetiamacin A has shown promising antibacterial activity against oxacillin resistant S. aureus, Streptococcus pneumonia, and S. epidermidis with MIC less than 2 µm/mL [147,148]. The small differences in their structure and vast differences in their biological activity make them an attractive area of research to investigate the structure-activity relationship. Similar to amicoumacins, hetiamacin B has shown potent antibacterial activity; however, the structure configuration and biosynthesis of hetiamacin is still unknown. Hetiamacin C and D were also isolated from the same bacillus strain, but due to the low yield, their biological activity was not determined [149].
The instantaneous application of amicoumacin in agriculture and human medicine may not be appropriate. However, their strong anti-MRSA activity appears an attractive starting point for drug development. To date, their mode of action is yet not fully elucidated, therefore, compelling further investigation. Whereas some isocoumarins discussed above have already been synthesized, and the paths could be further extended towards sustainable and economical production, amicoumacin derivatives demand further studies to demonstrate the structure-activity relationship of the metabolites.
6. Volatile Metabolites
Volatile metabolites enhance the efficacy of several secondary metabolites produced by bacteria in a specific habitat. The increased vapor pressure facilitates the low molecular biologically active metabolites to move over a longer distance and act on the target organism in soil [150,151]. B. subtilis emit highly diverse volatile secondary metabolites, including terpenes, nitrogen, and sulfur-containing compounds, benzenoids, and hydrocarbons [152]. These metabolites are primarily considered cell signaling molecules mediating inter and intracellular interaction [153]; however, they also displayed antifungal and antibacterial activity [154,155]. For instance, a volatile dimethyl disulfide, promote the growth and survival of plants along with antimicrobial activity [156]. B. subtilis releases many volatile metabolites with an average of 14 metabolites per strain [12]. These volatile metabolites are involved in the biogeochemical cycles and bioconversion of the food chain and numerous metabolic activities such as nitrification and nitrogen mineralization. B. subtilis producing volatile metabolites may be inorganic or organic volatiles (Supplementary Material Table S5).
6.1. Volatile Inorganic Metabolites
The volatile inorganic metabolites are a by-product of the primary metabolites. They are usually nitrogen and sulfur-containing compounds such as ammonia (NH3), hydrogen sulfide (H2S), and hydrogen cyanide (HCN) (Supplementary Material Figure S4). Nitrogen-containing compounds are mainly emitted in the top sediment layer by denitrifying B. subtilis strains. The B. subtilis group also produces nitric oxide, which induces a systemic acquired resistance in plants against Ralstonia solanacearum [157]. In an oxygen-deficient environment, bacteria emit various volatile inorganic metabolites such as hydrogen sulfide and hydrogen. These compounds act as a precursor for amino acid, antimicrobial metabolites synthesis, or act as electron acceptors. B. subtilis produce hydrogen sulfide from sulfate hydrolysis or a by-product of L-cysteine and L-methionine catabolism [158,159]. It is reported that hydrogen sulfide inhibits the growth of phytopathogens, such as Penicillium italicum and Aspergillus niger [160]. Indeed it is also demonstrated that it provides self-protection to producer organisms against antibiotics [161]. Hydrogen cyanide produced from amino acid (glycine) catabolism potentially inhibits the growth of plant pathogens such as Agrobacterium tumefaciens crown and Meloidogyne incognita juveniles [162].
6.2. Volatile Organic Metabolites
Volatile organic metabolites are low molecular weight molecules having a lipophilic moiety, a low boiling point, and high vapor pressure. These characteristics offer fast evaporation and distant distribution in a complex matter like soil [163]. Their production by soil bacteria and distribution in the soil is strongly influenced by the availability of nutrients and oxygen, pH, temperature, soil humidity, architecture, and texture [151,164]. Volatile organic metabolites are mainly a product of the glycolysis and citric acid cycle [163,165]. However, they can also be synthesized via different pathways, such as fermentation, amino acid degradation, terpenes biosynthesis, sulfur reduction, and heterotrophic carbon degradation [166]. So far, 1860 microbial volatile organic metabolites emitted by 944 various microbial species are listed in the mVOC (microbial volatile organic compounds) database [167]. It is reported that about 70% of the Bacillus volatile organic compounds (bVOCs) in the mVOC database are either fatty acid or nitrogen-containing metabolites. A few representatives of the volatile organic metabolites are shown in Supplementary Material Figure S4. The volatile organic metabolites emitted by the B. subtilis group can be classified into seven subclasses.
6.2.1. Terpenes and Terpenoids
Terpenoids, also known as isoprenoids, are widely produced by all living organisms [168]. The end product of the deoxy xylulose phosphate pathway, isopentenyl pyrophosphate (IPP), and their isomer dimethylallyl pyrophosphate (DMAPP) usually act as precursors for terpenoid biosynthesis [158]. However, terpenoids may also be synthesized from isoprene [169]. As previously shown, the isoprene produced by B. subtilis is not synthesized via the deoxy-xylulose phosphate pathway. Isoprenoid plays a vital role in physiological functions such as membrane fluidity, electron transport, light-harvesting, and cell signaling [170]. The involvement of terpenoids in cell signaling is particularly important, as it is associated with some mutualistic, antagonistic, and multitrophic interactions [171]. Isoprenoids may potentially be used as a flavor, nutraceuticals, fragrance, and therapeutic agent in malaria and cancer treatment [172]. However, due to ecological constraints, their natural yields are often insufficient. The vast structural diversity of terpenoids led to the discovery of up to 40,000 compounds by a few groups engaged in pharmaceutical industries [173]. Terpenes are mostly known for their antibacterial, antifungal, antinematode, and insecticidal activities [174,175]. Terpenoids (sesquiterpenes) were previously isolated from B. subtilis KSM 6–10 culture supernatant. Additionally, the authors revealed its enzymatic biosynthesis from a cyclic 30 carbon precursor by squalene cyclases (sqhC) [176]. Besides that, monoterpenes and isoprene isolated from B. subtilis strains were shown to inhibit the growth of nematode and cyanobacteria [175,177]. The mode of action of terpenoids is yet not fully elucidated; however, it could be linked to their lipophilic nature, which enables them to disrupt the integrity of the cell membrane [178]. They may be further classified into three subclasses. (i) Monoterpenes (ii) Isoprene and (iii) Sesquiterpenes [163].
6.2.2. Nitrogen-Containing Metabolites
Nitrogen-containing metabolites can be distinguished based on their degree of cyclization. To date, three groups of non-cyclic (amine, amide, and imines) and five groups of cyclic compounds (pyrazines, azole, pyridines, pyrimidines, and pyridazines) have been detected. Pyrazines are broadly represented among mVOCs and are categorized into two subclasses: higher alkylated and lower alkylated pyrazines [158]. These metabolites are characterized by a strong odor, and most of the B. subtilis strains isolated from rhizosphere and fermented products have been considered as pyrazine producers [179,180]. The compound 2,5-Dimethyl pyrazine was isolated from B. subtilis strain, exhibiting strong antifungal activity and inhibiting P. chlamydospora [181]. Pyrazine produced by B. subtilis strains also inhibits the growth of bacteria such as E. coli, S. aureus, and P. valgaris [182]. The B. subtilis strains are also able to produce other biologically active terpenoids such as 1H-imidazole, 1-ethyl inhibits the growth of phytopathogens [163,183].
The complete biosynthetic pathways for nitrogen-containing volatile organic metabolites remain unknown. However, it is proposed that two major paths may be followed, i.e., the terpenoids such as pyrazines are synthesized from non-enzymatic amination [158] or synthesized from the intermediate produced during amino acid catabolism [184].
6.2.3. Sulfur-Containing Metabolites
Sulfur-containing volatile organic metabolites are synthesized from two primary sources, i.e. organic or inorganic [158]. These metabolites often originate from the catabolism of amino acids, such as methionine, and sometimes from cysteine. However, the inorganic sulfate and sulfite may also act as a precursor for sulfur metabolites. The B. subtilis group produces numerous antifungal and antinematicidal sulfur metabolites such as S-methyl butanethioate, S-methyl thioacetate, 2-methyl disulfide, and 3-methyl trisulfide. Among these, dimethyl disulfide also exhibits antibacterial activity [185,186,187,188]. It is known that dimethyl sulfide disrupts bacterial cell communication by decreasing the sum of acyl-homoserine lactone [189].
6.2.4. Benzenoids
Benzenoids are a diverse subclass often linked with sulfur or/and nitrogen. Most of the benzenoids produced by the B. subtilis group displayed antifungal activity, while some inhibited the growth of bacteria and nematodes. Their antimicrobial mode of action is partially characterized. Nevertheless, the fungal and bacterial cell disruption is documented after exposure to bVOCs.
The B. subtilis strain CF-3 was evaluated for their volatile organic metabolites potential. The investigator identified a plethora of volatile compounds based on solid headspace microextraction. Among them, benzothiazole exhibiting strong antimicrobial activity against fruit fungal pathogens [190]. The volatile organic compounds produced by B. amyloliqueficiens were evaluated for their effect on the growth and pathogenicity of tomato bacterial pathogen R. solanacearum. The results showed that the strain emits several volatile organic compounds, including 1, 3 dimethoxy benzenes, inhibiting 62–85% growth of the tomato pathogen [191].
Similarly, benzoxazole, benzothiazole, and benzyl acetone were isolated from B. subtilis ZD01, strongly inhibited the growth of plant pathogen Alterneria solani. Moreover, the qRT-PCR results indicate that these volatile organic metabolites down-regulate the expression of fungal virulence genes i.e., slt2 and sod [192]. Thus, the strain ZD01 provides a potential application as a biocontrol agent against early blight disease.
6.2.5. Ketones
The biosynthesis of ketones is usually the result of fatty acid decarboxylation. Acetoin and its oxidative form butanedion are synthesized during the anaerobic fermentation of pyruvate. The two pyruvate molecules are condensed and converted to acetolactate by the acetolactate synthase enzyme. The acetolactate is further decarboxylated to form acetoin. Ketones are mainly known to inhibit the growth of plant pathogenic fungi. However, its antibacterial activity is yet not reported. Two ketones metabolites, i.e., 2-decanone, and 2-nonanone, displayed 100% growth inhibition of F. oxysporum [193]. In contrast, pentadecanone and 2-tetradecanone displayed bulky peaks on GCs but did not exhibit significant antifungal activity [193].
Recently, five ketones metabolites were collected from B. velezensis culture, and their antifungal activity was evaluated. The ketone metabolite 6-methyl 5-hepten 2-nonadecanone was revealed to inhibit 85.59% of the growth of A. solani, while 2-tetradecanone and 2-nonadecanone only showed mild antifungal activity [194]. The investigators further demonstrated that the antifungal activity of volatile metabolites might not be correlated with the number of carbon atoms in the metabolite [193,194]. In addition to its antimicrobial activity, ketone VOCs may also perform some other important functions. For example, acetone and butanone have been associated with plant growth and stress tolerance and induce systemic resistance [195].
6.2.6. Hydrocarbon Metabolites
Hydrocarbon metabolites include alkane, alkene, and alkyne metabolites usually derived from fatty acid degradation via elongation decarboxylation or head-to-head condensation. Hydrocarbons are the most stable volatile organic metabolites and tend to remain in their original architecture over a long period of time. They may be used as a biomarker to estimate the age of primeval bacteria [196]. The B. subtilis group secretes various types of hydrocarbons such as nonane, tridecane, tetradecane, 2-methylpropane, and cyclohexane. Hydrocarbons like alkane, alkene, nonane, and decane are gaining particular interest due to their use as antimicrobial agents and fossil fuels. Nonane and 8-methyl heptadecane were isolated from B. velezensis and exhibited antifungal activity against several fungal pathogens [197]. Likewise, 1 3-butadiene inhibits the growth of phytopathogen and, additionally, negatively influences the chemotoxicity of the fungal pathogens [198].
6.2.7. Organic Acids
Volatile organic acids are less abundantly produced as compared to benzenoids and ketones by B. subtilis group. However, several strains have been reported to emit beneficial organic acids. For instance, acetic acid and oleic acid emitted by the B. subtilis group are used as flavoring and preservative agents in food industries.
Acetic acid and 2-methyl propionic acid were isolated from B. amyloliquefaciens and were investigated for their antifungal activities. The authors find that these organic acids significantly inhibit the growth of F. oxysporum and M. perniciosa [199]. The endophytic B. subtilis strain DZSY21 was identified as inhibiting the growth of the maize leaf spot pathogen Curvalaria lunata, [154]. The growth inhibition towards C. lunata was observed in a time-dependent manner, i.e., the inhibition started on the 3rd day and reached 36.2% on day seven. The conjoint analysis of antagonistic activity and GC-MS identified that volatile organic metabolites, including isopentyl acetate and 2-methyl butyric acid, are responsible for antifungal activity [154].
6.2.8. Other Volatile Organic Metabolites
Besides the classes of volatile organic metabolites, the B. subtilis group also produces alkanes, alkenes, aldehydes, esters, and furans. Recently, Marco kai summarized that the B. subtilis group emits 15% ketones, 14% nitrogen-containing metabolites, hydrocarbons, aromatic metabolites, 11% benzenoid and alcohols, 7% organic acids, 6.5% each aldehyde and esters, and 3% sulfur-containing metabolites [12]. The alkenes (1H-indene, 1-methylene) and Furan, 2-pentyl were previously isolated from B. subtilis and B. pumilus strains and displayed significant antifungal activity against soil-born plant pathogenic fungi. However, the sclerotium and mycelial plugs grew normally in a fresh medium after treatment with the antifungal volatiles for one week, hindering the fungicidal activity of the volatiles [200]. This non-anti fungicidal effect may be related to its solubility in water, where the metabolites are adsorbed in an agar medium to perform their function. Aldehyde (nonanal) emitted by B. subtilis strain JA potentially inhibits the growth of the fruit and vegetable pathogen Botrytis cinerea [201]. Esters are recently reported to have antifungal and plant growth-promoting properties. Isopentyle acetate produced by B. subtilis strain DZSY21 was shown to inhibit the growth of plant pathogen Culvularia lunata [154]. The underlying molecular mechanism by which these volatiles impede the growth of fungi is still poorly understood. However, the antifungal activity of volatile metabolites could be related to the cell disruption phenomenon. It induces membrane permeability in fungal spores and decreases the transport of potassium ions into the cell [202]. In order to compensate for this imbalance, the proton efflux pump is activated to increase the flow of hydrogen ions into the cell and maintain the net charge on both sides of the membrane. This probably induces a rapid change in pH inside the cell, and disturbs cell physiology leads to death. Several studies highlighted the ability of volatile metabolites to interrupt pH gradients between extracellular and intracellular medium [203,204,205,206].
7. Miscellaneous Metabolites
There are few bioactive metabolites produced by B. subtilis that do not fit into any class. For instance, bacilysocin is neither synthesized via NRPS nor PKS. Instead, it is a phospholipid antibiotic that accumulates within the B. subtilis cell and presents a unique example of a modified phospholipid. Bacilysocin’s structure is composed of a central glycerol linked with glyceryl phosphate and an anteiso-fatty acid tail. The putative biosynthetic pathway for the bacilysocin initiate is from the conversion of phosphatidic acid to phosphatidylglycerol and then lysophospholipase (YtpA) convert phosphatidylglycerol to bacilysocin. The antimicrobial activity of bacilysocin is limited to Gram-positive bacteria and a few fungal strains, including S. aureus, Candida pseudotropicalis and S. cerevisiae. To date, the biological role, absolute biosynthetic pathway, mode of action, and the specific activity of bacilysocin is unclear.
B. subtilis strain 84R5 produces aminosaccharide 3,3′-neotrehalosadiamine, which exhibits marginal activity against K. pneumoniea and S. aureus [207]. The biosynthetic pathway and mode of action of aminosacharide 3,3-neotrehalosadiamine are yet not elucidated; however, a putative aminotransferase, associated with its biosynthesis has been purified and characterized thoroughly [208].
8. Conclusions and Future Prospective
Ubiquitous bacteria like B. subtilis are usually disregarded and their biosynthetic potential is underestimated; however, they produce promising bioactive metabolites that have earned much attention recent years. Herein, we review in-depth all of the bioactive metabolites produced by the B. subtilis group and described their potential applications in various industries. B. subtilis synthesize a plethora of biologically active metabolites exhibiting broad-spectrum antimicrobial, anticancer, anti-inflammatory, and antinematicidal activities. The bioactive metabolites produced by B. subtilis are also associated with the promotion of plant growth and induce systemic resistance in plants. This great versatility is intensifying the commercial interest in B. subtilis strains, considering their antimicrobial activity against plant and food-borne pathogens and their long history of safe use in food. The current review also proposed a classification system for the bioactive metabolites synthesized by B. Subtilis group. This classification system will assist in establishing robust practices for the characterization and the discovery of new bioactive metabolites. Undeniably, the majority of the reported biologically active metabolites are partially purified and may contain a mixture of metabolites, or the biosynthetic pathways have been identified via genome mining approaches. Therefore, the bioactivity of individual metabolites and the confirmation of particular pathways via gene knockout is required. Further, the concentrations of the partially purified metabolites are often unknown, and it is also rarely reported that the bioactive metabolites within the mixture displayed a synergistic effect. The newly isolated bioactive metabolites need proper identification, characterization, and classification in these cases.
Collectively, the biologically active metabolites produced by B. subtilis present a resilient foundation for developing this species to be utilized in the agronomy, food, and pharmaceutical industries. Nonetheless, the isolation and purification of these metabolites presents a primary challenge, and, also, the isolated yields and their direct applications may not be viable.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28030927/s1, Figure S1: Showing various confirmations of surfactin and iturin cyclic lipopeptides; S2: Showing molecular structure of gageopeptide (gageostatin A, B and C) (A) and siderophore (bacilysin, chlorotetain, and bacillibactin) linear lipopeptide (B and C); S3: Chemical structure of polyketides (PKs), difficidine (A), aurantinin (B) and macrolactin (C) and Hybrid NRPs/PKs metabolites bacillaene (D) and amicoumacin A and B (E) and amicoumacin C (F); S4: Representing structure of volatile bioactive metabolites produced by B. subtilis group. Table S1: NRPs from B. subtilis group.; S2: Ribosomal peptides (RPs) from B. subtilis group; S3: PolyKetides (PKs) from B. subtilis group; S4: Hybrid (NRP/PK) metabolites from B. subtilis group; S5: Volatile metabolites from B. subtilis group. References [209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265] are cited in the supplementary materials.
Author Contributions
S.I. and F.B. designed and wrote the original draft of the manuscript. A.A.R., M.A., B.R.A.S., A.A. (Abdulsalam Alawfi), A.A. (Amer Alshengeti) and T.S.: designing and editing the manuscript, validated and generated tables data. A.K.: design, generated figures, and editing the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Earl, A.M.; Losick, R.; Kolter, R. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 2008, 16, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Maughan, H.; Van der Auwera, G. Bacillus taxonomy in the genomic era finds phenotypes to be essential though often misleading. Infect. Genet. Evol. 2011, 11, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Blom, J.; Klenk, H.-P.; Borriss, R. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis Form an “Operational Group B. amyloliquefaciens” within the B. subtilis Species Complex. Front. Microbiol. 2017, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Á.T. Bacillus subtilis. Trends Microbiol. 2019, 27, 724–725. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.Y.; Araki, M.; Okai, N.; Wakai, S.; Hasunuma, T.; Kondo, A. Development of bio-based fine chemical production through synthetic bioengineering. Microb. Cell Fact. 2014, 13. [Google Scholar] [CrossRef]
- Drejer, E.; Hakvåg, S.; Irla, M.; Brautaset, T. Genetic Tools and Techniques for Recombinant Expression in Thermophilic Bacillaceae. Microorganisms 2018, 6, 42. [Google Scholar] [CrossRef]
- Iqbal, S.; Ullah, N.; Janjua, H.A. In Vitro Evaluation and Genome Mining of Bacillus subtilis Strain RS10 Reveals Its Biocontrol and Plant Growth-Promoting Potential. Agriculture 2021, 11, 1273. [Google Scholar] [CrossRef]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef]
- Cochrane, S.A.; Findlay, B.; Bakhtiary, A.; Acedo, J.Z.; Rodriguez-Lopez, E.M.; Mercier, P.; Vederas, J.C. Antimicrobial lipopeptide tridecaptin A1selectively binds to Gram-negative lipid II. Proc. Natl. Acad. Sci. USA 2016, 113, 11561–11566. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Wang, T.; Liang, Y.; Wu, M.; Chen, Z.; Lin, J.; Yang, L. Natural products from Bacillus subtilis with antimicrobial properties. Chinese J. Chem. Eng. 2015, 23, 744–754. [Google Scholar] [CrossRef]
- Kai, M. Diversity and Distribution of Volatile Secondary Metabolites Throughout Bacillus subtilis Isolates. Front. Microbiol. 2020, 11, 559. [Google Scholar] [CrossRef]
- Abriouel, H.; Franz, C.M.; Omar, N.B.; Gálvez, A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 2011, 35, 201–232. [Google Scholar] [CrossRef]
- Bernat, P.; Paraszkiewicz, K.; Siewiera, P.; Moryl, M.; Płaza, G.; Chojniak, J. Lipid composition in a strain of Bacillus subtilis, a producer of iturin A lipopeptides that are active against uropathogenic bacteria. World J. Microbiol. Biotechnol. 2016, 32, 157. [Google Scholar] [CrossRef]
- Barbosa, J.; Caetano, T.; Mendo, S. Class I and Class II Lanthipeptides Produced by Bacillus spp. J. Nat. Prod. 2015, 78, 2850–2866. [Google Scholar] [CrossRef]
- Bushley, K.E.; Turgeon, B.G. Phylogenomics reveals subfamilies of fungal nonribosomal peptide synthetases and their evolutionary relationships. BMC Evol. Biol. 2010, 10, 1–23. [Google Scholar] [CrossRef]
- Drake, E.J.; Miller, B.R.; Shi, C.; Tarrasch, J.T.; Sundlov, J.A.; Leigh Allen, C.; Skiniotis, G.; Aldrich, C.C.; Gulick, A.M. Structures of two distinct conformations of holo-non-ribosomal peptide synthetases. Nature 2016, 529, 235–238. [Google Scholar] [CrossRef]
- Walsh, C.T.; Chen, H.; Keating, T.A.; Hubbard, B.K.; Losey, H.C.; Luo, L.; Marshall, C.G.; Miller, D.A.; Patel, H.M. Tailoring enzymes that modify nonribosomal peptides during and after chain elongation on NRPS assembly lines. Curr. Opin. Chem. Biol. 2001, 5, 525–534. [Google Scholar] [CrossRef]
- Reimer, J.M.; Aloise, M.N.; Harrison, P.M.; Martin Schmeing, T. Synthetic cycle of the initiation module of a formylating nonribosomal peptide synthetase. Nature 2016, 529, 239–242. [Google Scholar] [CrossRef]
- Flissi, A.; Ricart, E.; Campart, C.; Chevalier, M.; Dufresne, Y.; Michalik, J.; Jacques, P.; Flahaut, C.; Lisacek, F.; Leclère, V.; et al. Norine: Update of the nonribosomal peptide resource. Nucleic Acids Res. 2020, 48, D465–D469. [Google Scholar] [CrossRef]
- Sumi, C.D.; Yang, B.W.; Yeo, I.C.; Hahm, Y.T. Antimicrobial peptides of the genus Bacillus: A new era for antibiotics. Can. J. Microbiol. 2015, 61, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Soberón-chávez, G.; Abdel-mawgoud, A.M.; Hausmann, R.; Le, F. Biosurfactants: From Genes to Applications (Microbiology Monographs, 20); Springer: Berlin, Germany, 2010. [Google Scholar] [CrossRef]
- Arima, K.; Kakinuma, A.; Tamura, G. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: Isolation, characterization and its inhibition of fibrin clot formation. Biochem. Biophys. Res. Commun. 1968, 31, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-H.; Liao, Z.-Y.; Wang, C.-L.; Yang, W.-Y.; Lu, M.-F. Evaluation of a lipopeptide biosurfactant from Bacillus natto TK-1 as a potential source of anti-adhesive, antimicrobial and antitumor activities. Brazilian J. Microbiol. 2009, 40, 373–379. [Google Scholar] [CrossRef]
- Delcambe, L. Iturine, new antibiotic produced by Bacillus subtilis. C. R. Seances Soc. Biol. Fil. 1950, 144, 1431–1434. [Google Scholar]
- Besson, F.; Peypoux, F.; Michel, G.; Delcambe, L. Structure de la bacillomycine L, antibiotique de Bacillus subtils. Eur. J. Biochem. 1977, 77, 61–67. [Google Scholar] [CrossRef]
- Peypoux, F.; Pommier, M.T.; Michel, G.; Marion, D.; Ptak, M.; Das, B.C. Revised structure of mycosubtilin, a peptidolipid antibiotic from Bacillus subtilis. J. Antibiot. 1986, 39, 636–641. [Google Scholar] [CrossRef]
- Mhammedi, A.; Peypoux, F.; Besson, F.; Michel, G. Bacillomycin f, a new antibiotic of iturin group: Isolation and characterization. J. Antibiot. 1982, 35, 306–311. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; Hu, J.; Wang, S. Isolation and characterization of a new iturinic lipopeptide, mojavensin A produced by a marine-derived bacterium Bacillus mojavensis B0621A. J. Antibiot. 2012, 65, 317–322. [Google Scholar] [CrossRef]
- Thasana, N.; Prapagdee, B.; Rangkadilok, N.; Sallabhan, R.; Aye, S.L.; Ruchirawat, S.; Loprasert, S. Bacillus subtilis SSE4 produces subtulene A, a new lipopeptide antibiotic possessing an unusual C15 unsaturated β-amino acid. FEBS Lett. 2010, 584, 3209–3214. [Google Scholar] [CrossRef]
- Vanittanakom, N.; Loeffler, W.; Koch, U.; Jung, G. Fengycin--a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J. Antibiot. 1986, 39, 888–901. [Google Scholar] [CrossRef]
- Zhao, H.; Shao, D.; Jiang, C.; Shi, J.; Li, Q.; Huang, Q.; Rajoka, M.S.R.; Yang, H.; Jin, M. Biological activity of lipopeptides from Bacillus. Appl. Microbiol. Biotechnol. 2017, 101, 5951–5960. [Google Scholar] [CrossRef]
- Yin, H.; Guo, C.; Wang, Y.; Liu, D.; Lv, Y.; Lv, F.; Lu, Z. Fengycin inhibits the growth of the human lung cancer cell line 95D through reactive oxygen species production and mitochondria-dependent apoptosis. Anticancer. Drugs 2013, 24, 587–598. [Google Scholar] [CrossRef]
- Price, N.P.J.; Rooney, A.P.; Swezey, J.L.; Perry, E.; Cohan, F.M. Mass spectrometric analysis of lipopeptides from Bacillus strains isolated from diverse geographical locations. FEMS Microbiol. Lett. 2007, 271, 83–89. [Google Scholar] [CrossRef]
- Dubois, T.; Faegri, K.; Perchat, S.; Lemy, C.; Buisson, C.; Nielsen-LeRoux, C.; Gohar, M.; Jacques, P.; Ramarao, N.; Kolstø, A.B.; et al. Necrotrophism is a Quorum-sensing-regulated lifestyle in Bacillus thuringiensis. PLoS Pathog. 2012, 8. [Google Scholar] [CrossRef]
- Volpon, L.; Besson, F.; Lancelin, J.M. NMR structure of antibiotics plipastatins A and B from Bacillus subtilis inhibitors of phospholipase A2. FEBS Lett. 2000, 485, 76–80. [Google Scholar] [CrossRef]
- Umezawa, H.; Aoyagi, T.; Nishikiori, T.; Okuyama, A.; Yamagishi, Y.; Hamada, M.; Takeuchi, T. Plipastatins: New inhibitors of phospholipase A2, produced by Bacillus cereus BMG302-fF67. I. Taxonomy, production, isolation and preliminary characterization. J. Antibiot. 1986, 39, 737–744. [Google Scholar] [CrossRef]
- Tareq, F.S.; Lee, M.A.; Lee, H.S.; Lee, J.S.; Lee, Y.J.; Shin, H.J. Gageostatins A-C, antimicrobial linear lipopeptides from a marine Bacillus subtilis. Mar. Drugs 2014, 12, 871–885. [Google Scholar] [CrossRef]
- Chakraborty, M.; Mahmud, N.U.; Gupta, D.R.; Tareq, F.S.; Shin, H.J.; Islam, T. Inhibitory Effects of Linear Lipopeptides From a Marine Bacillus subtilis on the Wheat Blast Fungus Magnaporthe oryzae Triticum. Front. Microbiol. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, F.; Yang, X.; Jin, J.; Dong, X.; Zeng, K.-W.; Liu, D.; Zhang, Y.; Ma, M.; Yang, D. Bacillibactin and Bacillomycin Analogues with Cytotoxicities against Human Cancer Cell Lines from Marine Bacillus sp. PKU-MA00093 and PKU-MA00092. Mar. Drugs 2018, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ai, C.; Xin, L.; Zhou, G. The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 2011, 47, 138–145. [Google Scholar] [CrossRef]
- Khan, A.; Gupta, A.; Singh, P.; Mishra, A.K.; Ranjan, R.K.; Srivastava, A. Siderophore-assisted cadmium hyperaccumulation in Bacillus subtilis. Int. Microbiol. 2020, 23, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Kugler, M.; Loeffler, W.; Rapp, C.; Kern, A.; Jung, G. Rhizocticin A, an antifungal phosphono-oligopeptide of Bacillus subtilis ATCC 6633: Biological properties. Arch. Microbiol. 1990, 153, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Mahlstedt, S.A.; Walsh, C.T. Investigation of anticapsin biosynthesis reveals a four-enzyme pathway to tetrahydrotyrosine in Bacillus subtilis. Biochemistry 2010, 49, 912–923. [Google Scholar] [CrossRef]
- Milewski, S.; Chmara, H.; Borowski, E. Antibiotic tetaine--a selective inhibitor of chitin and mannoprotein biosynthesis in Candida albicans. Arch. Microbiol. 1986, 145, 234–240. [Google Scholar] [CrossRef]
- Rapp, C.; Jung, G.; Katzer, W.; Loeffler, W. Chlorotetain from Bacillus subtilis, an Antifungal Dipeptide with an Unusual Chlorine-containing Amino Acid. Angew. Chemie Int. Ed. English 1988, 27, 1733–1734. [Google Scholar] [CrossRef]
- Majumdar, S.K.; Bose, S.K. Mycobacillin, a New Antifungal Antibiotic produced by B. subtilis. Nature 1958, 181, 134–135. [Google Scholar] [CrossRef]
- Zuber, P.; Nakano, M.M.; Marahiel, M.A. Peptide Antibiotics. Bacillus subtilis and Other Gram-Positive Bacteria. Biochemistry, Physiology, and Molecular Genetics; ASM Press: Washington, DC, USA, 1993; pp. 897–916. [Google Scholar]
- Johnson, B.A.; Anker, H.; Meleney, F.L. Bacitracin: A new antibiotic produced by a member of the B. subtilis group. Science 1945, 102, 376–377. [Google Scholar] [CrossRef]
- Oman, T.J.; van der Donk, W.A. Follow the leader: The use of leader peptides to guide natural product biosynthesis. Nat. Chem. Biol. 2010, 6, 9–18. [Google Scholar] [CrossRef]
- Knerr, P.J.; van der Donk, W.A. Discovery, biosynthesis, and engineering of lantipeptides. Annu. Rev. Biochem. 2012, 81, 479–505. [Google Scholar] [CrossRef]
- Harwood, C.R.; Mouillon, J.-M.; Pohl, S.; Arnau, J. Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiol. Rev. 2018, 42, 721–738. [Google Scholar] [CrossRef]
- Wiedemann, I.; Breukink, E.; van Kraaij, C.; Kuipers, O.P.; Bierbaum, G.; de Kruijff, B.; Sahl, H.G. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 2001, 276, 1772–1779. [Google Scholar] [CrossRef]
- Walsh, C.J.; Guinane, C.M.; O’ Toole, P.W.; Cotter, P.D. A Profile Hidden Markov Model to investigate the distribution and frequency of LanB-encoding lantibiotic modification genes in the human oral and gut microbiome. PeerJ 2017, 5, e3254. [Google Scholar] [CrossRef]
- Weber, W.; Fischli, W.; Hochuli, E.; Kupfer, E.; Weibel, E.K. Anantin--a peptide antagonist of the atrial natriuretic factor (ANF). I. Producing organism, fermentation, isolation and biological activity. J. Antibiot. 1991, 44, 164–171. [Google Scholar] [CrossRef]
- Tietz, J.I.; Schwalen, C.J.; Patel, P.S.; Maxson, T.; Blair, P.M.; Tai, H.-C.; Zakai, U.I.; Mitchell, D.A. A new genome-mining tool redefines the lasso peptide biosynthetic landscape. Nat. Chem. Biol. 2017, 13, 470–478. [Google Scholar] [CrossRef]
- Koos, J.D.; Link, A.J. Heterologous and in Vitro Reconstitution of Fuscanodin, a Lasso Peptide from Thermobifida fusca. J. Am. Chem. Soc. 2019, 141, 928–935. [Google Scholar] [CrossRef]
- Yang, X.; van der Donk, W.A. Ribosomally synthesized and post-translationally modified peptide natural products: New insights into the role of leader and core peptides during biosynthesis. Chemistry 2013, 19, 7662–7677. [Google Scholar] [CrossRef]
- Flühe, L.; Knappe, T.A.; Gattner, M.J.; Schäfer, A.; Burghaus, O.; Linne, U.; Marahiel, M.A. The radical SAM enzyme AlbA catalyzes thioether bond formation in subtilosin A. Nat. Chem. Biol. 2012, 8, 350–357. [Google Scholar] [CrossRef]
- Flühe, L.; Marahiel, M.A. Radical S-adenosylmethionine enzyme catalyzed thioether bond formation in sactipeptide biosynthesis. Curr. Opin. Chem. Biol. 2013, 17, 605–612. [Google Scholar] [CrossRef]
- Murphy, K.; O’Sullivan, O.; Rea, M.C.; Cotter, P.D.; Ross, R.P.; Hill, C. Genome Mining for Radical SAM Protein Determinants Reveals Multiple Sactibiotic-Like Gene Clusters. PLoS ONE 2011, 6, e20852. [Google Scholar] [CrossRef] [PubMed]
- Kawulka, K.; Sprules, T.; McKay, R.T.; Mercier, P.; Diaper, C.M.; Zuber, P.; Vederas, J.C. Structure of subtilosin A, an antimicrobial peptide from Bacillus subtilis with unusual posttranslational modifications linking cysteine sulfurs to alpha-carbons of phenylalanine and threonine. J. Am. Chem. Soc. 2003, 125, 4726–4727. [Google Scholar] [CrossRef] [PubMed]
- Sutyak, K.E.; Wirawan, R.E.; Aroutcheva, A.A.; Chikindas, M.L. Isolation of the Bacillus subtilis antimicrobial peptide subtilosin from the dairy product-derived Bacillus amyloliquefaciens. J. Appl. Microbiol. 2008, 104, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Noll, K.S.; Sinko, P.J.; Chikindas, M.L. Elucidation of the Molecular Mechanisms of Action of the Natural Antimicrobial Peptide Subtilosin Against the Bacterial Vaginosis-associated Pathogen Gardnerella vaginalis. Probiotics Antimicrob. Proteins 2011, 3, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Geng, H.; Miyyapuram, V.R.; Sit, C.S.; Vederas, J.C.; Nakano, M.M. Isolation of a variant of subtilosin A with hemolytic activity. J. Bacteriol. 2009, 191, 5690–5696. [Google Scholar] [CrossRef]
- Arnison, P.G.; Bibb, M.J.; Bierbaum, G.; Bowers, A.A.; Bugni, T.S.; Bulaj, G.; Camarero, J.A.; Campopiano, D.J.; Challis, G.L.; Clardy, J.; et al. Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 2013, 30, 108–160. [Google Scholar] [CrossRef]
- Scholz, R.; Molohon, K.J.; Nachtigall, J.; Vater, J.; Markley, A.L.; Süssmuth, R.D.; Mitchell, D.A.; Borriss, R. Plantazolicin, a Novel Microcin B17/Streptolysin S-Like Natural Product from Bacillus amyloliquefaciens FZB42. J. Bacteriol. 2011, 193, 215–224. [Google Scholar] [CrossRef]
- Molohon, K.J.; Melby, J.O.; Lee, J.; Evans, B.S.; Dunbar, K.L.; Bumpus, S.B.; Kelleher, N.L.; Mitchell, D.A. Structure determination and interception of biosynthetic intermediates for the plantazolicin class of highly discriminating antibiotics. ACS Chem. Biol. 2011, 6, 1307–1313. [Google Scholar] [CrossRef]
- Lee, S.W.; Mitchell, D.A.; Markley, A.L.; Hensler, M.E.; Gonzalez, D.; Wohlrab, A.; Dorrestein, P.C.; Nizet, V.; Dixon, J.E. Discovery of a widely distributed toxin biosynthetic gene cluster. Proc. Natl. Acad. Sci. USA 2008, 105, 5879–5884. [Google Scholar] [CrossRef]
- Velásquez, J.E.; van der Donk, W.A. Genome mining for ribosomally synthesized natural products. Curr. Opin. Chem. Biol. 2011, 15, 11–21. [Google Scholar] [CrossRef]
- Chopra, L.; Singh, G.; Kumar Jena, K.; Sahoo, D.K. Sonorensin: A new bacteriocin with potential of an anti-biofilm agent and a food biopreservative. Sci. Rep. 2015, 5, 13412. [Google Scholar] [CrossRef]
- Just-Baringo, X.; Albericio, F.; Álvarez, M. Thiopeptide antibiotics: Retrospective and recent advances. Mar. Drugs 2014, 12, 317–351. [Google Scholar] [CrossRef]
- Grubbs, K.J.; Bleich, R.M.; Santa Maria, K.C.; Allen, S.E.; Farag, S.; Team, A.; Shank, E.A.; Bowers, A.A. Large-Scale Bioinformatics Analysis of Bacillus Genomes Uncovers Conserved Roles of Natural Products in Bacterial Physiology. mSystems 2017, 2, e00040-17. [Google Scholar] [CrossRef]
- Sánchez-Hidalgo, M.; Montalbán-López, M.; Cebrián, R.; Valdivia, E.; Martínez-Bueno, M.; Maqueda, M. AS-48 bacteriocin: Close to perfection. Cell. Mol. Life Sci. 2011, 68, 2845–2857. [Google Scholar] [CrossRef]
- González, C.; Langdon, G.M.; Bruix, M.; Gálvez, A.; Valdivia, E.; Maqueda, M.; Rico, M. Bacteriocin AS-48, a microbial cyclic polypeptide structurally and functionally related to mammalian NK-lysin. Proc. Natl. Acad. Sci. USA 2000, 97, 11221–11226. [Google Scholar] [CrossRef]
- Scholz, R.; Vater, J.; Budiharjo, A.; Wang, Z.; He, Y.; Dietel, K.; Schwecke, T.; Herfort, S.; Lasch, P.; Borriss, R. Amylocyclicin, a Novel Circular Bacteriocin Produced by <span class="named-content genus-species" id="named-content-1">Bacillus amyloliquefaciens</span> FZB42. J. Bacteriol. 2014, 196, 1842–1852. [Google Scholar] [CrossRef]
- van Heel, A.J.; Montalban-Lopez, M.; Oliveau, Q.; Kuipers, O.P. Genome-guided identification of novel head-to-tail cyclized antimicrobial peptides, exemplified by the discovery of pumilarin. Microb. Genom. 2017, 3, e000134. [Google Scholar] [CrossRef]
- Le Marrec, C.; Hyronimus, B.; Bressollier, P.; Verneuil, B.; Urdaci, M.C. Biochemical and genetic characterization of coagulin, a new antilisterial bacteriocin in the pediocin family of bacteriocins, produced by Bacillus coagulans I(4). Appl. Environ. Microbiol. 2000, 66, 5213–5220. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Ross, R.P.; Hill, C. Potential of bacteriocin-producing lactic acid bacteria for improvements in food safety and quality. Biochimie 2002, 84, 593–604. [Google Scholar] [CrossRef]
- Klaenhammer, T.R. Bacteriocins of lactic acid bacteria. Biochimie 1988, 70, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.B.; Bjørnvad, M.E.; Rasmussen, M.D.; Petersen, J.N. Cytotoxic Potential of Industrial Strains of Bacillus sp. Regul. Toxicol. Pharmacol. 2002, 36, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.A.; Wertz, J.E. Bacteriocins: Evolution, ecology, and application. Annu. Rev. Microbiol. 2002, 56, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Hyronimus, B.; Le Marrec, C.; Urdaci, M.C. Coagulin, a bacteriocin-like inhibitory substance produced by Bacillus coagulans I4. J. Appl. Microbiol. 1998, 85, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, P.; Kaushik, J.K.; Grover, S.; Batish, V.K. Purification and characterization of a bacteriocin-like compound (Lichenin) produced anaerobically by Bacillus licheniformis isolated from water buffalo. J. Appl. Microbiol. 2001, 91, 636–645. [Google Scholar] [CrossRef]
- Zhao, X.; Kuipers, O.P. Identification and classification of known and putative antimicrobial compounds produced by a wide variety of Bacillales species. BMC Genomics 2016, 17, 882. [Google Scholar] [CrossRef]
- Ziedaite, G.; Daugelavicius, R.; Bamford, J.K.H.; Bamford, D.H. The Holin protein of bacteriophage PRD1 forms a pore for small-molecule and endolysin translocation. J. Bacteriol. 2005, 187, 5397–5405. [Google Scholar] [CrossRef]
- Aunpad, R.; Panbangred, W. Evidence for two putative holin-like peptides encoding genes of Bacillus pumilus strain WAPB4. Curr. Microbiol. 2012, 64, 343–348. [Google Scholar] [CrossRef]
- Netz, D.J.A.; Bastos, M. do C. de F.; Sahl, H.-G. Mode of action of the antimicrobial peptide aureocin A53 from Staphylococcus aureus. Appl. Environ. Microbiol. 2002, 68, 5274–5280. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Pan, N.; Chen, Z. Purification and partial characterization of an antibacterial protein LCIII. Chin. J. Biotechnol. 1992, 8, 187–193. [Google Scholar]
- Gong, W.; Wang, J.; Chen, Z.; Xia, B.; Lu, G. Solution structure of LCI, a novel antimicrobial peptide from Bacillus subtilis. Biochemistry 2011, 50, 3621–3627. [Google Scholar] [CrossRef]
- McDaniel, R.; Ebert-Khosla, S.; Hopwood, D.A.; Khosla, C. Engineered biosynthesis of novel polyketides. Science 1993, 262, 1546–1550. [Google Scholar] [CrossRef]
- Gomes, E.S.; Schuch, V.; de Macedo Lemos, E.G. Biotechnology of polyketides: New breath of life for the novel antibiotic genetic pathways discovery through metagenomics. Braz. J. Microbiol. 2014, 44, 1007–1034. [Google Scholar] [CrossRef]
- Fang, L.; Guell, M.; Church, G.M.; Pfeifer, B.A. Heterologous erythromycin production across strain and plasmid construction. Biotechnol. Prog. 2018, 34, 271–276. [Google Scholar] [CrossRef]
- Klaus, M.; Grininger, M. Engineering strategies for rational polyketide synthase design. Nat. Prod. Rep. 2018, 35, 1070–1081. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. Engl. 2009, 48, 4688–4716. [Google Scholar] [CrossRef]
- Chen, H.; Du, L. Iterative polyketide biosynthesis by modular polyketide synthases in bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 541–557. [Google Scholar] [CrossRef]
- Aleti, G.; Sessitsch, A.; Brader, G. Genome mining: Prediction of lipopeptides and polyketides from Bacillus and related Firmicutes. Comput. Struct. Biotechnol. J. 2015, 13, 192–203. [Google Scholar] [CrossRef]
- Eustáquio, A.S.; McGlinchey, R.P.; Liu, Y.; Hazzard, C.; Beer, L.L.; Florova, G.; Alhamadsheh, M.M.; Lechner, A.; Kale, A.J.; Kobayashi, Y.; et al. Biosynthesis of the salinosporamide A polyketide synthase substrate chloroethylmalonyl-coenzyme A from S-adenosyl-L-methionine. Proc. Natl. Acad. Sci. USA 2009, 106, 12295–12300. [Google Scholar] [CrossRef]
- Zimmerman, S.B.; Schwartz, C.D.; Monaghan, R.L.; Pelak, B.A.; Weissberger, B.; Gilfillan, E.C.; Mochales, S.; Hernandez, S.; Currie, S.A.; Tejera, E. Difficidin and oxydifficidin: Novel broad spectrum antibacterial antibiotics produced by Bacillus subtilis. I. Production, taxonomy and antibacterial activity. J. Antibiot. 1987, 40, 1677–1681. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-H.; Vater, J.; Piel, J.; Franke, P.; Scholz, R.; Schneider, K.; Koumoutsi, A.; Hitzeroth, G.; Grammel, N.; Strittmatter, A.W.; et al. Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB 42. J. Bacteriol. 2006, 188, 4024–4036. [Google Scholar] [CrossRef] [PubMed]
- Zweerink, M.M.; Edison, A. Difficidin and oxydifficidin: Novel broad spectrum antibacterial antibiotics produced by Bacillus subtilis. III. Mode of action of difficidin. J. Antibiot. 1987, 40, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, H.; Chen, L.; Yu, X.; Borriss, R.; Gao, X. Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Sci. Rep. 2015, 5, 12975. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, A.; Konda, Y.; Hatano, A.; Harigaya, Y.; Onda, M.; Omura, S. Structure and biosynthesis of novel antibiotics, aurantinins A and B produced by Bacillus aurantinus. J. Org. Chem. 1988, 53, 2660–2661. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, X.; Cao, M.; Wang, C.; Zhang, C.; Lu, Z.; Lu, F. Genomics-Inspired Discovery of Three Antibacterial Active Metabolites, Aurantinins B, C, and D from Compost-Associated Bacillus subtilis fmb60. J. Agric. Food Chem. 2016, 64, 8811–8820. [Google Scholar] [CrossRef]
- Chakraborty, K.; Thilakan, B.; Chakraborty, R.D.; Raola, V.K.; Joy, M. O-heterocyclic derivatives with antibacterial properties from marine bacterium Bacillus subtilis associated with seaweed, Sargassum myriocystum. Appl. Microbiol. Biotechnol. 2017, 101, 569–583. [Google Scholar] [CrossRef]
- Schneider, K.; Chen, X.-H.; Vater, J.; Franke, P.; Nicholson, G.; Borriss, R.; Süssmuth, R.D. Macrolactin is the polyketide biosynthesis product of the pks2 cluster of Bacillus amyloliquefaciens FZB42. J. Nat. Prod. 2007, 70, 1417–1423. [Google Scholar] [CrossRef]
- Zheng, C.-J.; Lee, S.; Lee, C.-H.; Kim, W.-G. Macrolactins O-R, glycosylated 24-membered lactones from Bacillus sp. AH159-1. J. Nat. Prod. 2007, 70, 1632–1635. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, Y.-X.; Tang, X.-X.; Liu, X.-X.; Yi, Z.-W.; Fang, M.-J.; Wu, Z.; Jiang, F.-Q.; Qiu, Y.-K. Macrolactins from Marine-Derived Bacillus subtilis B5 Bacteria as Inhibitors of Inducible Nitric Oxide and Cytokines Expression. Mar. Drugs 2016, 14, 195. [Google Scholar] [CrossRef]
- Simpson, J.S.; Garson, M.J.; Blunt, J.W.; Munro, M.H.G.; Hooper, J.N.A. Mycalamides C and D, Cytotoxic Compounds from the Marine Sponge Stylinos n. Species. J. Nat. Prod. 2000, 63, 704–706. [Google Scholar] [CrossRef]
- Regmi, S.C.; Park, S.Y.; Kim, S.J.; Banskota, S.; Shah, S.; Kim, D.-H.; Kim, J.-A. The Anti-Tumor Activity of Succinyl Macrolactin A Is Mediated through the β-Catenin Destruction Complex via the Suppression of Tankyrase and PI3K/Akt. PLoS ONE 2015, 10, e0141753. [Google Scholar] [CrossRef]
- Bae, S.H.; Kwon, M.J.; Park, J.B.; Kim, D.; Kim, D.-H.; Kang, J.-S.; Kim, C.-G.; Oh, E.; Bae, S.K. Metabolic drug-drug interaction potential of macrolactin A and 7-O-succinyl macrolactin A assessed by evaluating cytochrome P450 inhibition and induction and UDP-glucuronosyltransferase inhibition in vitro. Antimicrob. Agents Chemother. 2014, 58, 5036–5046. [Google Scholar] [CrossRef]
- Li, W.; Tang, X.-X.X.; Yan, X.; Wu, Z.; Yi, Z.-W.W.; Fang, M.-J.J.; Su, X.; Qiu, Y.-K.K. A new macrolactin antibiotic from deep sea-derived bacteria Bacillus subtilis B5. Nat. Prod. Res. 2016, 30, 2777–2782. [Google Scholar] [CrossRef]
- Noh, K.; Kim, D.H.; Shin, B.S.; Yun, H.; Kim, E.; Kang, W. Simultaneous determination of 7-O-succinyl macrolactin A and its metabolite macrolactin A in rat plasma using liquid chromatography coupled to tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 98, 85–89. [Google Scholar] [CrossRef]
- Xu, W.-M.; Han, F.-F.; He, M.; Hu, D.-Y.; He, J.; Yang, S.; Song, B.-A. Inhibition of Tobacco Bacterial Wilt with Sulfone Derivatives Containing an 1,3,4-Oxadiazole Moiety. J. Agric. Food Chem. 2012, 60, 1036–1041. [Google Scholar] [CrossRef]
- Liu, L.; Sun, C.; Liu, S.; Chai, R.; Huang, W.; Liu, X.; Tang, C.; Zhang, Y. Bioorganic fertilizer enhances soil suppressive capacity against bacterial wilt of tomato. PLoS ONE 2015, 10, e0121304. [Google Scholar] [CrossRef]
- Chakraborty, K.; Thilakan, B.; Raola, V.K. Polyketide family of novel antibacterial 7-O-methyl-5’-hydroxy-3’-heptenoate-macrolactin from seaweed-associated Bacillus subtilis MTCC 10403. J. Agric. Food Chem. 2014, 62, 12194–12208. [Google Scholar] [CrossRef]
- Gustafson, K.; Roman, M.; Fenical, W. The macrolactins, a novel class of antiviral and cytotoxic macrolides from a deep-sea marine bacterium. J. Am. Chem. Soc. 1989, 111, 7519–7524. [Google Scholar] [CrossRef]
- Tareq, F.S.; Kim, J.H.; Lee, M.A.; Lee, H.-S.; Lee, J.-S.; Lee, Y.-J.; Shin, H.J. Antimicrobial gageomacrolactins characterized from the fermentation of the marine-derived bacterium Bacillus subtilis under optimum growth conditions. J. Agric. Food Chem. 2013, 61, 3428–3434. [Google Scholar] [CrossRef]
- Mondol, M.A.M.; Shin, H.J.; Islam, M.T. Diversity of secondary metabolites from marine Bacillus species: Chemistry and biological activity. Mar. Drugs 2013, 11, 2846–2872. [Google Scholar] [CrossRef] [PubMed]
- Galm, U.; Hager, M.H.; Van Lanen, S.G.; Ju, J.; Thorson, J.S.; Shen, B. Antitumor antibiotics: Bleomycin, enediynes, and mitomycin. Chem. Rev. 2005, 105, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Hamann, P.R.; Hinman, L.M.; Hollander, I.; Beyer, C.F.; Lindh, D.; Holcomb, R.; Hallett, W.; Tsou, H.-R.; Upeslacis, J.; Shochat, D.; et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug. Chem. 2002, 13, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Hindra; Yan, X.; Huang, T.; Ge, H.; Yang, D.; Teng, Q.; Rudolf, J.D.; Lohman, J.R. Enediynes: Exploration of microbial genomics to discover new anticancer drug leads. Bioorg. Med. Chem. Lett. 2015, 25, 9–15. [Google Scholar] [CrossRef]
- Patel, P.S.; Huang, S.; Fisher, S.; Pirnik, D.; Aklonis, C.; Dean, L.; Meyers, E.; Fernandes, P.; Mayerl, F. Bacillaene, a novel inhibitor of procaryotic protein synthesis produced by Bacillus subtilis: Production, taxonomy, isolation, physico-chemical characterization and biological activity. J. Antibiot. 1995, 48, 997–1003. [Google Scholar] [CrossRef]
- Butcher, R.A.; Schroeder, F.C.; Fischbach, M.A.; Straight, P.D.; Kolter, R.; Walsh, C.T.; Clardy, J. The identification of bacillaene, the product of the PksX megacomplex in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2007, 104, 1506–1509. [Google Scholar] [CrossRef]
- Calderone, C.T.; Kowtoniuk, W.E.; Kelleher, N.L.; Walsh, C.T.; Dorrestein, P.C. Convergence of isoprene and polyketide biosynthetic machinery: Isoprenyl-S-carrier proteins in the pksX pathway of Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2006, 103, 8977–8982. [Google Scholar] [CrossRef]
- Müller, S.; Strack, S.N.; Hoefler, B.C.; Straight, P.D.; Kearns, D.B.; Kirby, J.R. Bacillaene and sporulation protect Bacillus subtilis from predation by Myxococcus xanthus. Appl. Environ. Microbiol. 2014, 80, 5603–5610. [Google Scholar] [CrossRef]
- Um, S.; Fraimout, A.; Sapountzis, P.; Oh, D.-C.; Poulsen, M. The fungus-growing termite Macrotermes natalensis harbors bacillaene-producing Bacillus sp. that inhibit potentially antagonistic fungi. Sci. Rep. 2013, 3, 3250. [Google Scholar] [CrossRef]
- Saeed, A. Isocoumarins, miraculous natural products blessed with diverse pharmacological activities. Eur. J. Med. Chem. 2016, 116, 290–317. [Google Scholar] [CrossRef]
- Kaspar, F.; Neubauer, P.; Gimpel, M. Bioactive Secondary Metabolites from Bacillus subtilis: A Comprehensive Review. J. Nat. Prod. 2019, 82, 2038–2053. [Google Scholar] [CrossRef]
- Shimojima, Y.; Hayashi, H.; Ooka, T.; Shibukawa, M.; Iitaka, Y. (Studies on AI-77s, microbial products with pharmacological activity) structures and the chemical nature of AI-77s. Tetrahedron Lett. 1982, 23, 5435–5438. [Google Scholar] [CrossRef]
- Itoh, J.; Shomura, T.; Omoto, S.; Miyado, S.; Yuda, Y.; Shibata, U.; Inouye, S. Isolation, Physicochemical Properties and Biological Activities of Amicoumacins Produced by Bacillus pumilus. Agric. Biol. Chem. 1982, 46, 1255–1259. [Google Scholar] [CrossRef]
- Itoh, J.; Omoto, S.; Shomura, T.; Nishizawa, N.; Miyado, S.; Yuda, Y.; Shibata, U.; Inouye, S. Amicoumacin-A, a new antibiotic with strong antiinflammatory and antiulcer activity. J. Antibiot. 1981, 34, 611–613. [Google Scholar] [CrossRef]
- Pinchuk, I.V.; Bressollier, P.; Verneuil, B.; Fenet, B.; Sorokulova, I.B.; Mégraud, F.; Urdaci, M.C. In vitro anti-Helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob. Agents Chemother. 2001, 45, 3156–3161. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Yamanaka, K.; Xu, Y.; Zhang, W.; Vlamakis, H.; Kolter, R.; Moore, B.S.; Qian, P.-Y. Directed natural product biosynthesis gene cluster capture and expression in the model bacterium Bacillus subtilis. Sci. Rep. 2015, 5, 9383. [Google Scholar] [CrossRef]
- Polikanov, Y.S.; Osterman, I.A.; Szal, T.; Tashlitsky, V.N.; Serebryakova, M.V.; Kusochek, P.; Bulkley, D.; Malanicheva, I.A.; Efimenko, T.A.; Efremenkova, O.V.; et al. Amicoumacin A Inhibits Translation by Stabilizing mRNA Interaction with the Ribosome. Mol. Cell 2014, 56, 531–540. [Google Scholar] [CrossRef]
- Lama, A.; Pané-Farré, J.; Chon, T.; Wiersma, A.M.; Sit, C.S.; Vederas, J.C.; Hecker, M.; Nakano, M.M. Response of methicillin-resistant Staphylococcus aureus to amicoumacin A. PLoS ONE 2012, 7, e34037. [Google Scholar] [CrossRef]
- Hashimoto, M.; Taguchi, T.; Nishida, S.; Ueno, K.; Koizumi, K.; Aburada, M.; Ichinose, K. Isolation of 8′-Phosphate Ester Derivatives of Amicoumacins: Structure-activity Relationship of Hydroxy Amino Acid Moiety. J. Antibiot. 2007, 60, 752–756. [Google Scholar] [CrossRef]
- Azumi, M.; Ogawa, K.; Fujita, T.; Takeshita, M.; Yoshida, R.; Furumai, T.; Igarashi, Y. Bacilosarcins A and B, novel bioactive isocoumarins with unusual heterocyclic cores from the marine-derived bacterium Bacillus subtilis. Tetrahedron 2008, 64, 6420–6425. [Google Scholar] [CrossRef]
- Kurasawa, K.; Kuwahara, S.; Enomoto, M. Synthesis of bacilosarcins B and C. Tetrahedron Lett. 2016, 57, 4997–4999. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Liu, L.; Han, Z.; Lai, P.Y.; Guo, X.; Zhang, X.; Lin, W.; Qian, P.Y. Five new amicoumacins isolated from a marine-derived Bacterium Bacillus subtilis. Mar. Drugs 2012, 10, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.L.; Sun, C.H.; Hu, X.X.; You, X.F.; Wang, M.; Liu, S.W. Damxungmacin A and B, two new amicoumacins with rare heterocyclic cores isolated from Bacillus subtilis XZ-7. Molecules 2016, 21, 1601. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-W.; Jin, J.; Chen, C.; Liu, J.-M.; Li, J.-Y.; Wang, F.-F.; Jiang, Z.-K.; Hu, J.-H.; Gao, Z.-X.; Yao, F.; et al. PJS, a novel isocoumarin with hexahydropyrimidine ring from Bacillus subtilis PJS. J. Antibiot. 2013, 66, 281–284. [Google Scholar] [CrossRef]
- Wu, G.; Liu, S.; Wang, T.; Jiang, Z.; Lv, K.; Wang, Y.; Sun, C. Total Synthesis of Originally Proposed and Revised Structure of Hetiamacin A. Org. Lett. 2018, 20, 3566–3569. [Google Scholar] [CrossRef]
- Liu, S.; Han, X.; Jiang, Z.; Wu, G.; Hu, X.; You, X.; Jiang, J.; Zhang, Y.; Sun, C. Hetiamacin B–D, new members of amicoumacin group antibiotics isolated from Bacillus subtilis PJS. J. Antibiot. 2016, 69, 769–772. [Google Scholar] [CrossRef]
- Schulz-Bohm, K.; Gerards, S.; Hundscheid, M.; Melenhorst, J.; de Boer, W.; Garbeva, P. Calling from distance: Attraction of soil bacteria by plant root volatiles. ISME J. 2018, 12, 1252–1262. [Google Scholar] [CrossRef]
- Effmert, U.; Kalderás, J.; Warnke, R.; Piechulla, B. Volatile mediated interactions between bacteria and fungi in the soil. J. Chem. Ecol. 2012, 38, 665–703. [Google Scholar] [CrossRef]
- Lemfack, M.C.; Nickel, J.; Dunkel, M.; Preissner, R.; Piechulla, B. mVOC: A database of microbial volatiles. Nucleic Acids Res. 2014, 42, D744–D748. [Google Scholar] [CrossRef]
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial Volatiles: Small Molecules with an Important Role in Intra- and Inter-Kingdom Interactions. Front. Microbiol. 2017, 8, 2484. [Google Scholar] [CrossRef]
- Xie, S.; Liu, J.; Gu, S.; Chen, X.; Jiang, H.; Ding, T. Antifungal activity of volatile compounds produced by endophytic Bacillus subtilis DZSY21 against Curvularia lunata. Ann. Microbiol. 2020, 70, 2. [Google Scholar] [CrossRef]
- Xie, S.; Zang, H.; Wu, H.; Uddin Rajer, F.; Gao, X. Antibacterial effects of volatiles produced by Bacillus strain D13 against Xanthomonas oryzae pv. oryzae. Mol. Plant Pathol. 2018, 19, 49–58. [Google Scholar] [CrossRef]
- Meldau, D.G.; Meldau, S.; Hoang, L.H.; Underberg, S.; Wünsche, H.; Baldwin, I.T. Dimethyl disulfide produced by the naturally associated bacterium Bacillus sp B55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition. Plant Cell 2013, 25, 2731–2747. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Tosa, Y.; Nakayashiki, H.; Mayama, S. Nitric oxide-overproducing transformants of Pseudomonas fluorescens with enhanced biocontrol of tomato bacterial wilt. J. Gen. Plant Pathol. 2005, 71, 33–38. [Google Scholar] [CrossRef]
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar] [CrossRef]
- Even, S.; Burguière, P.; Auger, S.; Soutourina, O.; Danchin, A.; Martin-Verstraete, I. Global Control of Cysteine Metabolism by CymR in Bacillus subtilis. J. Bacteriol. 2006, 188, 2184–2197. [Google Scholar] [CrossRef]
- Fu, L.-H.; Hu, K.-D.; Hu, L.-Y.; Li, Y.-H.; Hu, L.-B.; Yan, H.; Liu, Y.-S.; Zhang, H. An Antifungal Role of Hydrogen Sulfide on the Postharvest Pathogens Aspergillus niger and Penicillium italicum. PLoS ONE 2014, 9, e104206. [Google Scholar] [CrossRef]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A universal defense against antibiotics in bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef]
- Abd El-Rahman, A.F.; Shaheen, H.A.; Abd El-Aziz, R.M.; Ibrahim, D.S.S. Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne incognita. Egypt J. Biol. Pest Control 2019, 29, 41. [Google Scholar] [CrossRef]
- Schmidt, R.; Cordovez, V.; de Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile affairs in microbial interactions. ISME J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Insam, H.; Seewald, M.S.A. Volatile organic compounds (VOCs) in soils. Biol. Fertil. Soils 2010, 46, 199–213. [Google Scholar] [CrossRef]
- Korpi, A.; Järnberg, J.; Pasanen, A.-L. Microbial volatile organic compounds. Crit. Rev. Toxicol. 2009, 39, 139–193. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Asensio, D.; Tholl, D.; Wenke, K.; Rosenkranz, M.; Piechulla, B.; Schnitzler, J.P. Biogenic volatile emissions from the soil. Plant. Cell Environ. 2014, 37, 1866–1891. [Google Scholar] [CrossRef] [PubMed]
- Lemfack, M.C.; Gohlke, B.-O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. mVOC 2.0: A database of microbial volatiles. Nucleic Acids Res. 2018, 46, D1261–D1265. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Julsing, M.K.; Rijpkema, M.; Woerdenbag, H.J.; Quax, W.J.; Kayser, O. Functional analysis of genes involved in the biosynthesis of isoprene in Bacillus subtilis. Appl. Microbiol. Biotechnol. 2007, 75, 1377–1384. [Google Scholar] [CrossRef]
- Fisher, A.J.; Rosenstiel, T.N.; Shirk, M.C.; Fall, R. Nonradioactive Assay for Cellular Dimethylallyl Diphosphate. Anal. Biochem. 2001, 292, 272–279. [Google Scholar] [CrossRef]
- Shrivastava, G.; Ownley, B.H.; Augé, R.M.; Toler, H.; Dee, M.; Vu, A.; Chen, F. Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis 2015, 65, 65–74. [Google Scholar] [CrossRef]
- Withers, S.T.; Gottlieb, S.S.; Lieu, B.; Newman, J.D.; Keasling, J.D. Identification of Isopentenol Biosynthetic Genes from Bacillus subtilis by a Screening Method Based on Isoprenoid Precursor Toxicity. Appl. Environ. Microbiol. 2007, 73, 6277–6283. [Google Scholar] [CrossRef]
- Caputi, L.; Aprea, E. Use of Terpenoids as Natural Flavouring Compounds in Food Industry. Recent Pat. Food. Nutr. Agric. 2011, 3, 9–16. [Google Scholar] [CrossRef]
- Justicia, J.; Oltra, J.E.; Barrero, A.F.; Guadaño, A.; González-Coloma, A.; Cuerva, J.M. Total Synthesis of 3-Hydroxydrimanes Mediated by Titanocene(III)–Evaluation of Their Antifeedant Activity. European J. Org. Chem. 2005, 2005, 712–718. [Google Scholar] [CrossRef]
- Gu, Y.-Q.; Mo, M.-H.; Zhou, J.-P.; Zou, C.-S.; Zhang, K.-Q. Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol. Biochem. 2007, 39, 2567–2575. [Google Scholar] [CrossRef]
- Bosak, T.; Losick, R.M.; Pearson, A. A polycyclic terpenoid that alleviates oxidative stress. Proc. Natl. Acad. Sci. 2008, 105, 6725–6729. [Google Scholar] [CrossRef]
- Wright, S.J.L.; Thompson, R.J. Bacillus volatiles antagonize cyanobacteria. FEMS Microbiol. Lett. 1985, 30, 263–267. [Google Scholar] [CrossRef]
- Inoue, Y.; Shiraishi, A.; Hada, T.; Hirose, K.; Hamashima, H.; Shimada, J. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol. Lett. 2004, 237, 325–331. [Google Scholar] [CrossRef]
- Larroche, C.; Besson, I.; Gros, J.-B. High pyrazine production by Bacillus subtilis in solid substrate fermentation on ground soybeans. Process Biochem. 1999, 34, 667–674. [Google Scholar] [CrossRef]
- Leejeerajumnean, A.; Duckham, S.C.; Owens, J.D.; Ames, J.M. Volatile compounds in Bacillus-fermented soybeans. J. Sci. Food Agric. 2001, 81, 525–529. [Google Scholar] [CrossRef]
- Haidar, R.; Roudet, J.; Bonnard, O.; Dufour, M.C.; Corio-Costet, M.F.; Fert, M.; Gautier, T.; Deschamps, A.; Fermaud, M. Screening and modes of action of antagonistic bacteria to control the fungal pathogen Phaeomoniella chlamydospora involved in grapevine trunk diseases. Microbiol. Res. 2016, 192, 172–184. [Google Scholar] [CrossRef]
- Coutts, R.T.; Pitkethly, W.N.; Wibberley, D.G. Antibacterial Activity of Some Quinolines Containing a Cyclic Hydroxamic Acid Group. J. Pharm. Sci. 1965, 54, 792–795. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Ng, T.B.; Zhang, J.; Zhou, M.; Song, F.; Lu, F.; Liu, Y. Bacisubin, an antifungal protein with ribonuclease and hemagglutinating activities from Bacillus subtilis strain B-916. Peptides 2007, 28, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-F.; Xu, Y.; Fan, W.-L. High-yield fermentative preparation of tetramethylpyrazine by Bacillus sp. using an endogenous precursor approach. J. Ind. Microbiol. Biotechnol. 2010, 37, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Mannaa, M.; Kim, K.D. Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus and Penicillium spp. predominant in stored rice grains: Study II. Mycobiology 2018, 46, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Velivelli, S.L.S.; Kromann, P.; Lojan, P.; Rojas, M.; Franco, J.; Suarez, J.P.; Prestwich, B.D. Identification of mVOCs from Andean Rhizobacteria and Field Evaluation of Bacterial and Mycorrhizal Inoculants on Growth of Potato in its Center of Origin. Microb. Ecol. 2015, 69, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Haustein, M.; Molina, F.; Petri, A.; Scholz, B.; Piechulla, B. Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 2009, 81, 1001–1012. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control 2017, 105, 27–39. [Google Scholar] [CrossRef]
- Chernin, L.; Toklikishvili, N.; Ovadis, M.; Kim, S.; Ben-Ari, J.; Khmel, I.; Vainstein, A. Quorum-sensing quenching by rhizobacterial volatiles. Environ. Microbiol. Rep. 2011, 3, 698–704. [Google Scholar] [CrossRef]
- Gao, H.; Li, P.; Xu, X.; Zeng, Q.; Guan, W. Research on Volatile Organic Compounds From Bacillus subtilis CF-3: Biocontrol Effects on Fruit Fungal Pathogens and Dynamic Changes During Fermentation. Front. Microbiol. 2018, 9, 456. [Google Scholar] [CrossRef]
- Raza, W.; Wang, J.; Wu, Y.; Ling, N.; Wei, Z.; Huang, Q.; Shen, Q. Effects of volatile organic compounds produced by Bacillus amyloliquefaciens on the growth and virulence traits of tomato bacterial wilt pathogen Ralstonia solanacearum. Appl. Microbiol. Biotechnol. 2016, 100, 7639–7650. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, S.; Yang, Y.; Zhang, J.; Zhao, D.; Pan, Y.; Fan, S.; Yang, Z.; Zhu, J. Antifungal Effects of Volatiles Produced by Bacillus subtilis Against Alternaria solani in Potato. Front. Microbiol. 2020, 11, 1196. [Google Scholar] [CrossRef]
- Yuan, J.; Raza, W.; Shen, Q.; Huang, Q. Antifungal Activity of Bacillus amyloliquefaciens NJN-6 Volatile Compounds against <span class="named-content genus-species" id="named-. Appl. Environ. Microbiol. 2012, 78, 5942–5944. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, S.; Zhao, D.; Zhang, J.; Pan, Y.; Yang, Y.; Yang, Z.; Zhu, J.; Zhao, Y.; Li, R. Inhibitory effects of non-volatiles lipopeptides and volatiles ketones metabolites secreted by Bacillus velezensis C16 against Alternaria solani. Biol. Control 2021, 152, 104421. [Google Scholar] [CrossRef]
- Ryu, C.-M. Bacterial volatiles as airborne signals for plants and bacteria. In Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 53–61. ISBN 978-3-319-08575-3. [Google Scholar]
- Summons, R.E.; Jahnke, L.L.; Hope, J.M.; Logan, G.A. 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature 1999, 400, 554–557. [Google Scholar] [CrossRef]
- Calvo, H.; Mendiara, I.; Arias, E.; Gracia, A.P.; Blanco, D.; Venturini, M.E. Antifungal activity of the volatile organic compounds produced by Bacillus velezensis strains against postharvest fungal pathogens. Postharvest Biol. Technol. 2020, 166, 111208. [Google Scholar] [CrossRef]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Niu, Y.; Huo, R.; Gao, X. Bacillus volatiles adversely affect the physiology and ultra-structure of Ralstonia solanacearum and induce systemic resistance in tobacco against bacterial wilt. Sci. Rep. 2017, 7, 40481. [Google Scholar] [CrossRef]
- Chaves-López, C.; Serio, A.; Gianotti, A.; Sacchetti, G.; Ndagijimana, M.; Ciccarone, C.; Stellarini, A.; Corsetti, A.; Paparella, A. Diversity of food-borne Bacillus volatile compounds and influence on fungal growth. J. Appl. Microbiol. 2015, 119, 487–499. [Google Scholar] [CrossRef]
- Liu, W.; Mu, W.; Zhu, B.; Du, Y.; Liu, F. Antagonistic Activities of Volatiles from Four Strains of Bacillus spp. and Paenibacillus spp. Against Soil-Borne Plant Pathogens. Agric. Sci. China 2008, 7, 1104–1114. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, X.; Wang, J.; Wu, L.; Zheng, Z.; Yu, Z. Antagonistic effects of volatiles generated by Bacillus subtilis on spore germination and hyphal growth of the plant pathogen, Botrytis cinerea. Biotechnol. Lett. 2008, 30, 919–923. [Google Scholar] [CrossRef]
- Plumridge, A.; Hesse, S.J.A.; Watson, A.J.; Lowe, K.C.; Stratford, M.; Archer, D.B. The weak acid preservative sorbic acid inhibits conidial germination and mycelial growth of Aspergillus niger through intracellular acidification. Appl. Environ. Microbiol. 2004, 70, 3506–3511. [Google Scholar] [CrossRef]
- Kunova, A.; Pizzatti, C.; Cortesi, P. Impact of tricyclazole and azoxystrobin on growth, sporulation and secondary infection of the rice blast fungus, Magnaporthe oryzae. Pest Manag. Sci. 2013, 69, 278–284. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Kaddes, A.; Fauconnier, M.-L.; Sassi, K.; Nasraoui, B.; Jijakli, M.H. Antifungal Properties of Two Volatile Organic Compounds on Barley Pathogens and Introduction to Their Mechanism of Action. Int. J. Environ. Res. Public Health 2019, 16, 2866. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Mishra, P. Dimorphism-associated changes in plasma membrane H+-ATPase activity of Candida albicans. Arch. Microbiol. 1991, 156, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Inaoka, T.; Takahashi, K.; Yada, H.; Yoshida, M.; Ochi, K. RNA Polymerase Mutation Activates the Production of a Dormant Antibiotic 3,3′-Neotrehalosadiamine via an Autoinduction Mechanism in Bacillus subtilis. J. Biol. Chem. 2004, 279, 3885–3892. [Google Scholar] [CrossRef] [PubMed]
- van Straaten, K.E.; Langill, D.M.; Palmer, D.R.J.; Sanders, D.A.R. Purification, crystallization and preliminary X-ray analysis of NtdA, a putative pyridoxal phosphate-dependent aminotransferase from Bacillus subtilis. Acta Crystallogr. Sect. F 2009, 65, 426–429. [Google Scholar] [CrossRef]
- Zhao, P.; Quan, C.; Wang, Y.; Wang, J.; Fan, S. Bacillus amyloliquefaciens Q-426 as a potential biocontrol agent against Fusarium oxysporum f. sp. spinaciae. J. Basic Microbiol. 2014, 54, 448–456. [Google Scholar] [CrossRef]
- Cawoy, H.; Debois, D.; Franzil, L.; De Pauw, E.; Thonart, P.; Ongena, M. Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb. Biotechnol. 2015, 8, 281–295. [Google Scholar] [CrossRef]
- Wise, C.; Falardeau, J.; Hagberg, I.; Avis, T.J. Cellular Lipid Composition Affects Sensitivity of Plant Pathogens to Fengycin, an Antifungal Compound Produced by Bacillus subtilis Strain CU12. Phytopathology 2014, 104, 1036–1041. [Google Scholar] [CrossRef]
- Li, L.; Ma, M.; Huang, R.; Qu, Q.; Li, G.; Zhou, J.; Zhang, K.; Lu, K.; Niu, X.; Luo, J. Induction of Chlamydospore Formation in Fusarium by Cyclic Lipopeptide Antibiotics from Bacillus subtilis C2. J. Chem. Ecol. 2012, 38, 966–974. [Google Scholar] [CrossRef]
- Malfanova, N.; Franzil, L.; Lugtenberg, B.; Chebotar, V.; Ongena, M. Cyclic lipopeptide profile of the plant-beneficial endophytic bacterium Bacillus subtilis HC8. Arch. Microbiol. 2012, 194, 893–899. [Google Scholar] [CrossRef]
- Gao, L.; Han, J.; Liu, H.; Qu, X.; Lu, Z.; Bie, X. Plipastatin and surfactin coproduction by Bacillus subtilis pB2-L and their effects on microorganisms. Antonie van Leeuwenhoek 2017, 110, 1007–1018. [Google Scholar] [CrossRef]
- Romero, D.; de Vicente, A.; Rakotoaly, R.H.; Dufour, S.E.; Veening, J.-W.; Arrebola, E.; Cazorla, F.M.; Kuipers, O.P.; Paquot, M.; Pérez-García, A. The Iturin and Fengycin Families of Lipopeptides Are Key Factors in Antagonism of Bacillus subtilis Toward Podosphaera fusca. Mol. Plant-Microbe Interact. 2007, 20, 430–440. [Google Scholar] [CrossRef]
- Raubitschek, F.; Dostrovsky, A. An Antibiotic Active against Dermatophytes, Derived from Bacillus Subtilis. Dermatology 1950, 100, 45–49. [Google Scholar] [CrossRef]
- Moyne, A.-L.; Shelby, R.; Cleveland, T.E.; Tuzun, S. Bacillomycin D: An iturin with antifungal activity against Aspergillus flavus. J. Appl. Microbiol. 2001, 90, 622–629. [Google Scholar] [CrossRef]
- Lee, S.-C.; Kim, S.-H.; Park, I.-H.; Chung, S.-Y.; Choi, Y.-L. Isolation and structural analysis of bamylocin A, novel lipopeptide from Bacillus amyloliquefaciens LP03 having antagonistic and crude oil-emulsifying activity. Arch. Microbiol. 2007, 188, 307–312. [Google Scholar] [CrossRef]
- Thimon, L.; Peyoux, F.; Maget-Dana, R.; Michel, G. Surface-active properties of antifungal lipopeptides produced by Bacillus subtilis. J. Am. Oil Chem. Soc. 1992, 69, 92–93. [Google Scholar] [CrossRef]
- Besson, F.; Peypoux, F.; Michel, G.; Delcambe, L. Characterization of iturin A in antibiotics from various strains of Bacillus subtilis. J. Antibiot. 1976, 29, 1043–1049. [Google Scholar] [CrossRef]
- Dunlap, C.A.; Schisler, D.A.; Price, N.P.; Vaughn, S.F. Cyclic lipopeptide profile of three Bacillus subtilis strains; antagonists of Fusarium head blight. J. Microbiol. 2011, 49, 603. [Google Scholar] [CrossRef]
- Leclère, V.; Béchet, M.; Adam, A.; Guez, J.-S.; Wathelet, B.; Ongena, M.; Thonart, P.; Gancel, F.; Chollet-Imbert, M.; Jacques, P. Mycosubtilin Overproduction by Bacillus subtilis BBG100 Enhances the Organism’s Antagonistic and Biocontrol Activities. Appl. Environ. Microbiol. 2005, 71, 4577–4584. [Google Scholar] [CrossRef]
- Lee, D.W.; Kim, B.S. Antimicrobial Cyclic Peptides for Plant Disease Control. Plant Pathol. J. 2015, 31, 1–11. [Google Scholar] [CrossRef]
- Hsieh, F.-C.; Lin, T.-C.; Meng, M.; Kao, S.-S. Comparing Methods for Identifying Bacillus Strains Capable of Producing the Antifungal Lipopeptide Iturin A. Curr. Microbiol. 2008, 56, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jenny, K.; Käppeli, O.; Fiechter, A. Biosurfactants from Bacillus licheniformis: Structural analysis and characterization. Appl. Microbiol. Biotechnol. 1991, 36, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; Timmis, K.N.; Wray, V.; Fredrickson, H.L. Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BAS50. Appl. Environ. Microbiol. 1995, 61, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Liu, X.; Zhou, X.; Guo, J.; Truong, J.; Wang, X.; Zhou, H.; Li, X.; Chen, Z. Unusual biosynthesis and structure of locillomycins from Bacillus subtilis 916. Appl. Environ. Microbiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Zhu, F.; Du, P.; Yang, X.; Qiu, D.; Yu, Z.; Chen, J.; Zhao, X. Lipopeptide induces apoptosis in fungal cells by a mitochondria-dependent pathway. Peptides 2010, 31, 1978–1986. [Google Scholar] [CrossRef]
- Naruse, N.; Tenmyo, O.; Kobaru, S.; Kamei, H.; Miyaki, T.; Konishi, M.; Oki, T. Pumilacidin, a complex of new antiviral antibiotics. J. Antibiot. 1990, 43, 267–280. [Google Scholar] [CrossRef]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus subtilis against Infection of Arabidopsis Roots by Pseudomonas syringae Is Facilitated by Biofilm Formation and Surfactin Production. Plant Physiol. 2004, 134, 307–319. [Google Scholar] [CrossRef]
- Preecha, C.; Sadowsky, M.J.; Prathuangwong, S. Lipopeptide surfactin produced by Bacillus amyloliquefaciens KPS46 is required for biocontrol efficacy against Xanthomonas axonopodis pv. Glycines. Kasetsart J. Nat. Sci. 2010, 44, 84–99. [Google Scholar]
- Dimkić, I.; Živković, S.; Berić, T.; Ivanović, Ž.; Gavrilović, V.; Stanković, S.; Fira, D. Characterization and evaluation of two Bacillus strains, SS-12.6 and SS-13.1, as potential agents for the control of phytopathogenic bacteria and fungi. Biol. Control. 2013, 65, 312–321. [Google Scholar] [CrossRef]
- Romano, A.; Vitullo, D.; Senatore, M.; Lima, G.; Lanzotti, V. Antifungal Cyclic Lipopeptides from Bacillus amyloliquefaciens Strain BO5A. J. Nat. Prod. 2013, 76, 2019–2025. [Google Scholar] [CrossRef]
- Sabaté, D.C.; Audisio, M.C. Inhibitory activity of surfactin, produced by different Bacillus subtilis subsp. subtilis strains, against Listeria monocytogenes sensitive and bacteriocin-resistant strains. Microbiol. Res. 2013, 168, 125–129. [Google Scholar] [CrossRef]
- Sharma, S.; Pandey, L.M. Production of biosurfactant by Bacillus subtilis RSL-2 isolated from sludge and biosurfactant mediated degradation of oil. Bioresour. Technol. 2020, 307, 123261. [Google Scholar] [CrossRef]
- Wu, L.; Wu, H.; Chen, L.; Xie, S.; Zang, H.; Borriss, R.; Gao, X. Bacilysin from Bacillus amyloliquefaciens FZB42 has Specific Bactericidal Activity against Harmful Algal Bloom Species. Appl. Environ. Microbiol. 2014. [Google Scholar] [CrossRef]
- Zuber, P.; Nakano, M.M.; Marahiel, M.A. Peptide Antibiotics. In Bacillus subtilis and Other Gram-Positive Bacteria; American Society of Microbiology: New York, NY, USA, 1993. [Google Scholar]
- Hussein, A.; AL-Janabi, S. Identification of bacitracin produced by local isolate of Bacillus licheniformis. Afr. J. Biotechnol. 2006, 5. [Google Scholar]
- Zheng, M.; Shi, J.; Shi, J.; Wang, Q.; Li, Y. Antimicrobial effects of volatiles produced by two antagonistic Bacillus strains on the anthracnose pathogen in postharvest mangos. Biol. Control. 2013, 65, 200–206. [Google Scholar] [CrossRef]
- Raza, W.; Ling, N.; Yang, L.; Huang, Q.; Shen, Q. Response of tomato wilt pathogen Ralstonia solanacearum to the volatile organic compounds produced by a biocontrol strain Bacillus amyloliquefaciens SQR-9. Sci. Rep. 2016, 6, 24856. [Google Scholar] [CrossRef]
- Fuchs, S.W.; Jaskolla, T.W.; Bochmann, S.; Kötter, P.; Wichelhaus, T.; Karas, M.; Stein, T.; Entian, K.-D. Entianin, a Novel Subtilin-Like Lantibiotic from Bacillus subtilis DSM 15029T with High Antimicrobial Activity. Appl. Environ. Microbiol. 2011. [Google Scholar] [CrossRef]
- Stein, T.; Borchert, S.; Conrad, B.; Feesche, J.; Hofemeister, B.; Hofemeister, J.; Entian, K.-D. Two different lantibiotic-like peptides originate from the ericin gene cluster of Bacillus subtilis A1/3. J Bacteriol 2002, 184, 1703–1711. [Google Scholar] [CrossRef]
- Agrios, G.N. 1-INTRODUCTION TO PLANT PATHOLOGY. In Plant Pathology, 3rd ed.; Agrios, G.N., Ed.; Academic Press: New York, NY, USA, 1988; pp. 3–39. [Google Scholar]
- Heinzmann, S.; Entian, K.-D.; Stein, T. Engineering Bacillus subtilis ATCC 6633 for improved production of the lantibiotic subtilin. Appl. Microbiol. Biotechnol. 2006, 69, 532–536. [Google Scholar] [CrossRef]
- Brötz, H.; Bierbaum, G.; Markus, A.; Molitor, E.; Sahl, H.G. Mode of action of the lantibiotic mersacidin: Inhibition of peptidoglycan biosynthesis via a novel mechanism? Antimicrob. Agents Chemother. 1995, 39, 714–719. [Google Scholar] [CrossRef]
- Paik, S.H.; Chakicherla, A.; Hansen, J.N. Identification and Characterization of the Structural and Transporter Genes for, and the Chemical and Biological Properties of, Sublancin 168, a Novel Lantibiotic Produced by Bacillus subtilis 168. J. Biol. Chem. 1998, 273, 23134–23142. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Cotter, P.D.; Hill, C.; Ross, R.P. Identification of a Novel Two-Peptide Lantibiotic, Lichenicidin, following Rational Genome Mining for LanM Proteins. Appl. Environ. Microbiol. 2009, 75, 5451–5460. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, C.E.; An, F.Y.; Dholpe, V.; Ramamoorthy, A.; Lopatin, D.E.; Lantz, M.S. The spectrum of antimicrobial activity of the bacteriocin subtilosin A. J. Antimicrob. Chemother. 2007, 59, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Hammami, I.; Jaouadi, B.; Bacha, A.B.; Rebai, A.; Bejar, S.; Nesme, X.; Rhouma, A. Bacillus subtilis bacteriocin Bac 14B with a broad inhibitory spectrum: Purification, amino acid sequence analysis, and physicochemical characterization. Biotechnol. Bioprocess Eng. 2012, 17, 41–49. [Google Scholar] [CrossRef]
- Wong, J.H.; Hao, J.; Cao, Z.; Qiao, M.; Xu, H.; Bai, Y.; Ng, T.B. An antifungal protein from Bacillus amyloliquefaciens. J. Appl. Microbiol. 2008, 105, 1888–1898. [Google Scholar] [CrossRef]
- Phelan, R.W.; Barret, M.; Cotter, P.D.; O’Connor, P.M.; Chen, R.; Morrissey, J.P.; Dobson, A.D.; O’Gara, F.; Barbosa, T.M. Subtilomycin: A new lantibiotic from Bacillus subtilis strain MMA7 isolated from the marine sponge Haliclona simulans. Mar. Drugs 2013, 11, 1878–1898. [Google Scholar] [CrossRef]
- Arguelles Arias, A.; Ongena, M.; Devreese, B.; Terrak, M.; Joris, B.; Fickers, P. Characterization of amylolysin, a novel lantibiotic from Bacillus amyloliquefaciens GA1. PLoS ONE 2013, 8, e83037. [Google Scholar] [CrossRef]
- Chakraborty, K.; Thilakan, B.; Kizhakkekalam, V. Antibacterial aryl-crowned polyketide from Bacillus subtilis associated with seaweed Anthophycus longifolius. J. Appl. Microbiol. 2018, 124, 108–125. [Google Scholar] [CrossRef]
- Romero-Tabarez, M.; Jansen, R.; Sylla, M.; Lünsdorf, H.; Häußler, S.; Santosa, D.A.; Timmis, K.N.; Molinari, G. 7-O-malonyl macrolactin A, a new macrolactin antibiotic from Bacillus subtilis active against methicillin-resistant Staphylococcus aureus, Vancomycin-resistant enterococci, and a small-colony variant of Burkholderia cepacia. Antimicrob. Agents Chemother. 2006, 50, 1701–1709. [Google Scholar] [CrossRef]
- Arbsuwan, N.; Payoungkiattikun, W.; Sirithorn, P.; Daduang, S.; Jangpromma, N.; Dhiravisit, A.; Hahm, Y.T.; Neubert, L.K.; Klaynongsruang, S. Purification and characterization of macrolactins and amicoumacins from Bacillus licheniformis BFP011: A new source of food antimicrobial substances. CyTA-J. Food 2018, 16, 50–60. [Google Scholar] [CrossRef]
- Mondol, M.M.; Ju, L.Y.; Seok, L.J.; Jae, S.H. Macrolactin Antibiotics from a Marine-derived Bacterium Bacillus sp. 102CH635-3. In Proceedings of the Fall International Convention of the Pharmaceutical Society of Korea, Incheon, Korea, 7–9 November 2011. [Google Scholar]
- Liu, C.; Lu, J.; Lu, L.; Liu, Y.; Wang, F.; Xiao, M. Isolation, structural characterization and immunological activity of an exopolysaccharide produced by Bacillus licheniformis 8-37-0-1. Bioresour. Technol. 2010, 101, 5528–5533. [Google Scholar] [CrossRef]
- Yang, S.; Du, G.; Chen, J.; Kang, Z. Characterization and application of endogenous phase-dependent promoters in Bacillus subtilis. Appl. Microbiol. Biotechnol. 2017, 101, 4151–4161. [Google Scholar] [CrossRef]
- Okazaki, W.; Akiba, T.; Horikoshi, K.; Akahoshi, R. Production and properties of two types of xylanases from alkalophilic thermophilic Bacillus spp. Appl. Microbiol. Biotechnol. 1984, 19, 335–340. [Google Scholar] [CrossRef]
- Miwa, H.; Ahmed, I.; Yokota, A.; Fujiwara, T. Lysinibacillus parviboronicapiens sp. nov., a low-boron-containing bacterium isolated from soil. Int. J. Syst. Evol. Microbiol. 2009, 59, 1427–1432. [Google Scholar] [CrossRef]
- Tang, Q.; Yin, K.; Qian, H.; Zhao, Y.; Wang, W.; Chou, S.-H.; Fu, Y.; He, J. Cyclic di-GMP contributes to adaption and virulence of Bacillus thuringiensis through a riboswitch-regulated collagen adhesion protein. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Canedo, V.; Benavides, J.; Golmirzaie, A.; Cisneros, F.; Ghislain, M.; Lagnaoui, A. Assessing Bt-transformed potatoes for potato tuber moth, Phthorimaea operculella (Zeller), management. CIP Program Rep. 1997, 1989, 161–170. [Google Scholar]
- Iqbal, S.; Vollmers, J.; Janjua, H.A. Genome mining and comparative genome analysis revealed niche-specific genome expansion in antibacterial bacillus pumilus strain SF-4. Genes 2021, 12, 1060. [Google Scholar] [CrossRef]
- Gotor-Vila, A.; Teixidó, N.; Di Francesco, A.; Usall, J.; Ugolini, L.; Torres, R.; Mari, M. Antifungal effect of volatile organic compounds produced by Bacillus amyloliquefaciens CPA-8 against fruit pathogen decays of cherry. Food Microbiol. 2017, 64, 219–225. [Google Scholar] [CrossRef]
- Giorgio, A.; De Stradis, A.; Lo Cantore, P.; Iacobellis, N.S. Biocide effects of volatile organic compounds produced by potential biocontrol rhizobacteria on Sclerotinia sclerotiorum. Front. Microbiol. 2015, 6, 1056. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).