Reduced Graphene Oxide-Zinc Sulfide Nanocomposite Decorated with Silver Nanoparticles for Wastewater Treatment by Adsorption, Photocatalysis and Antimicrobial Action
Abstract
1. Introduction
2. Results and Discussion
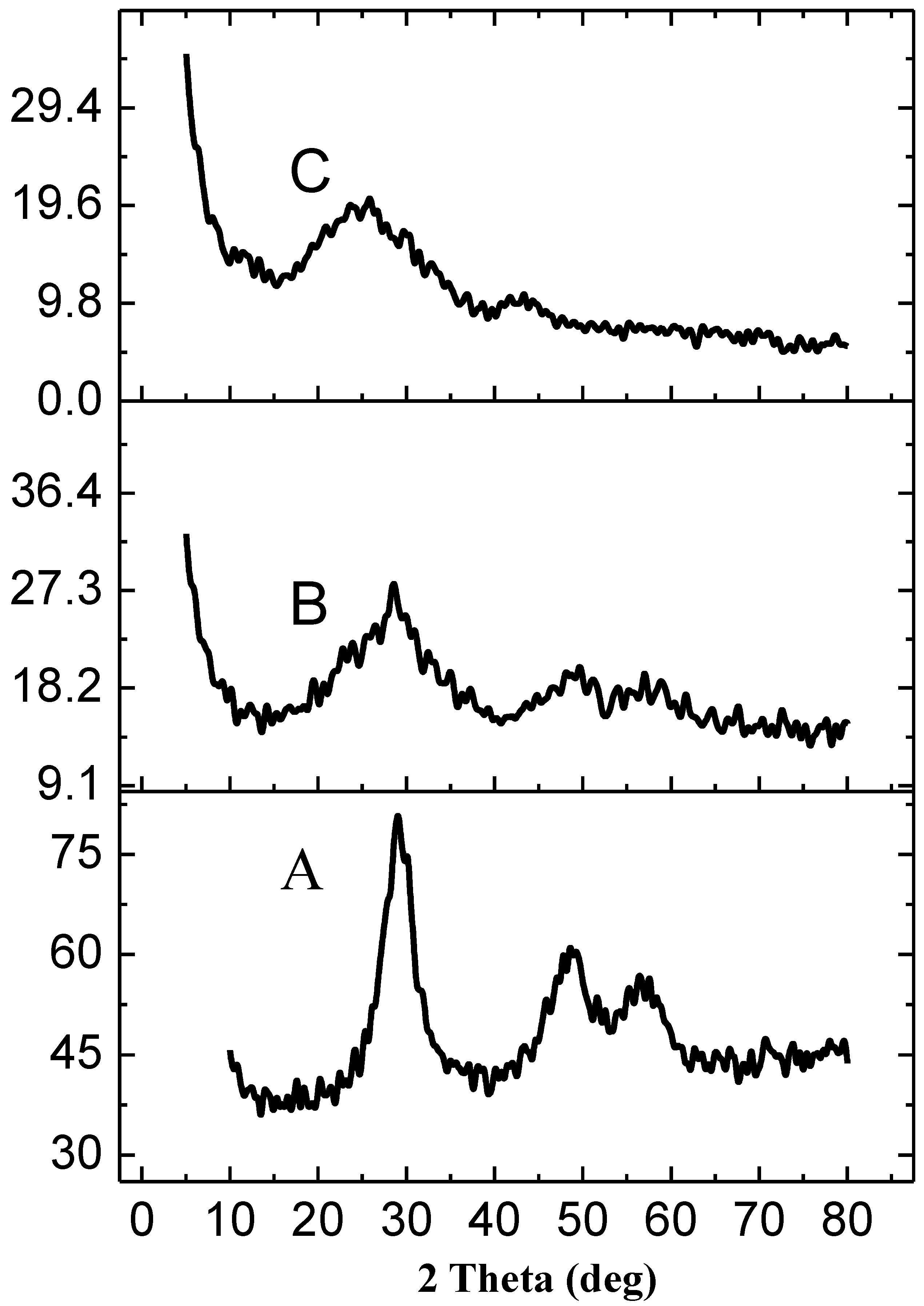
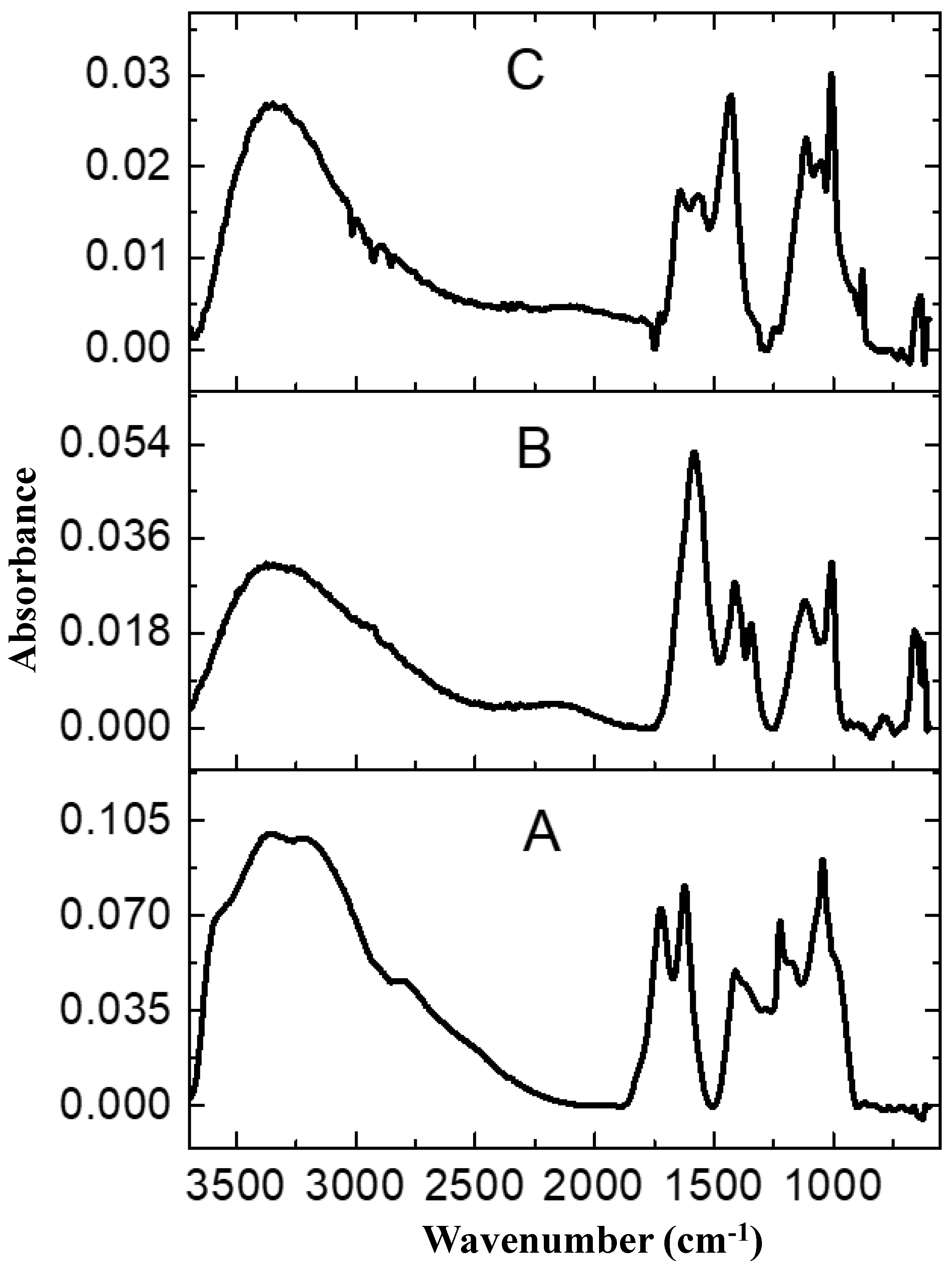
2.1. Characterization
2.2. Nitrogen Gas Adsorption/Desorption Analysis
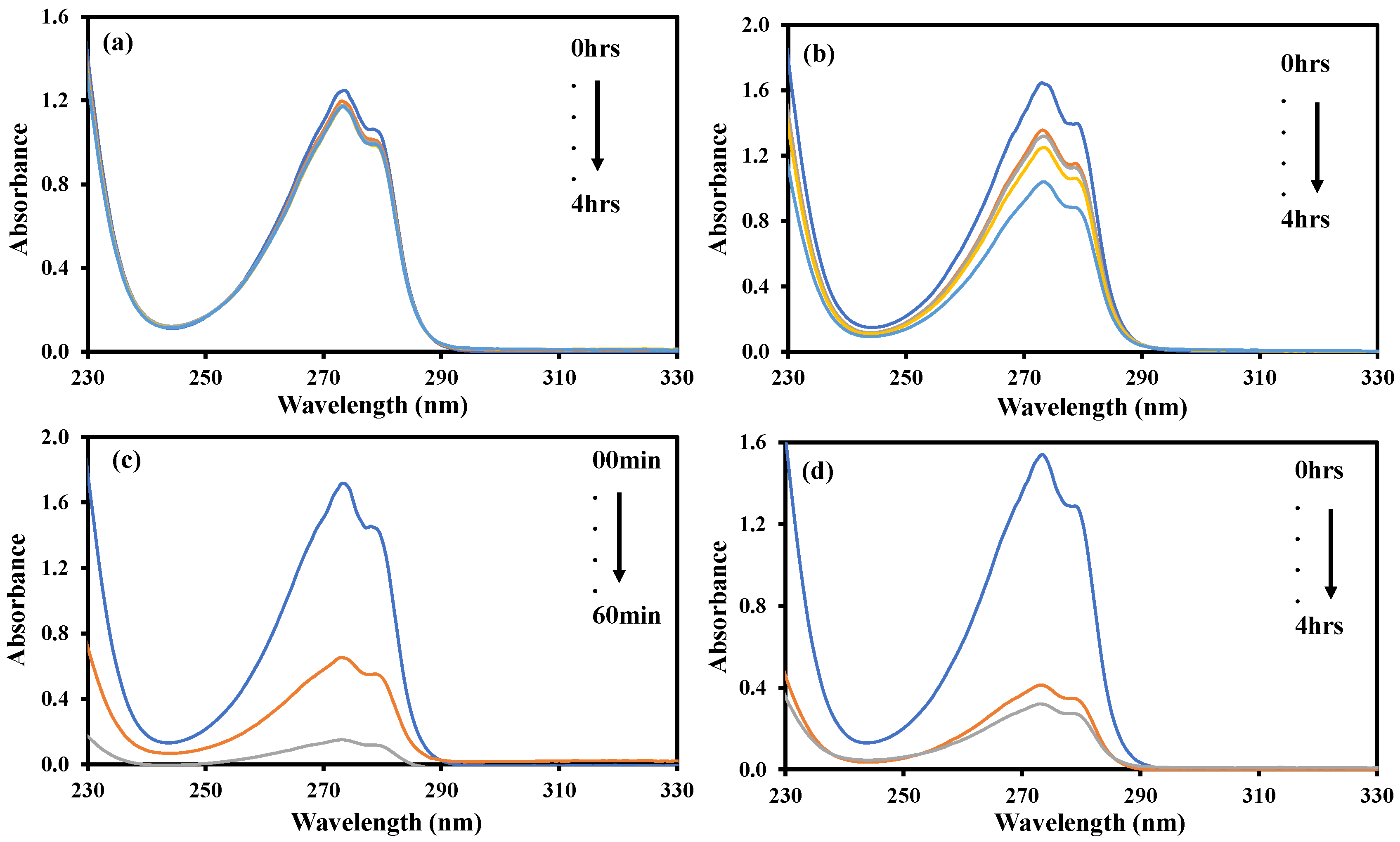
2.3. Adsorption Test
2.4. Effect of Dyes Initial Concentration on Adsorption Rate
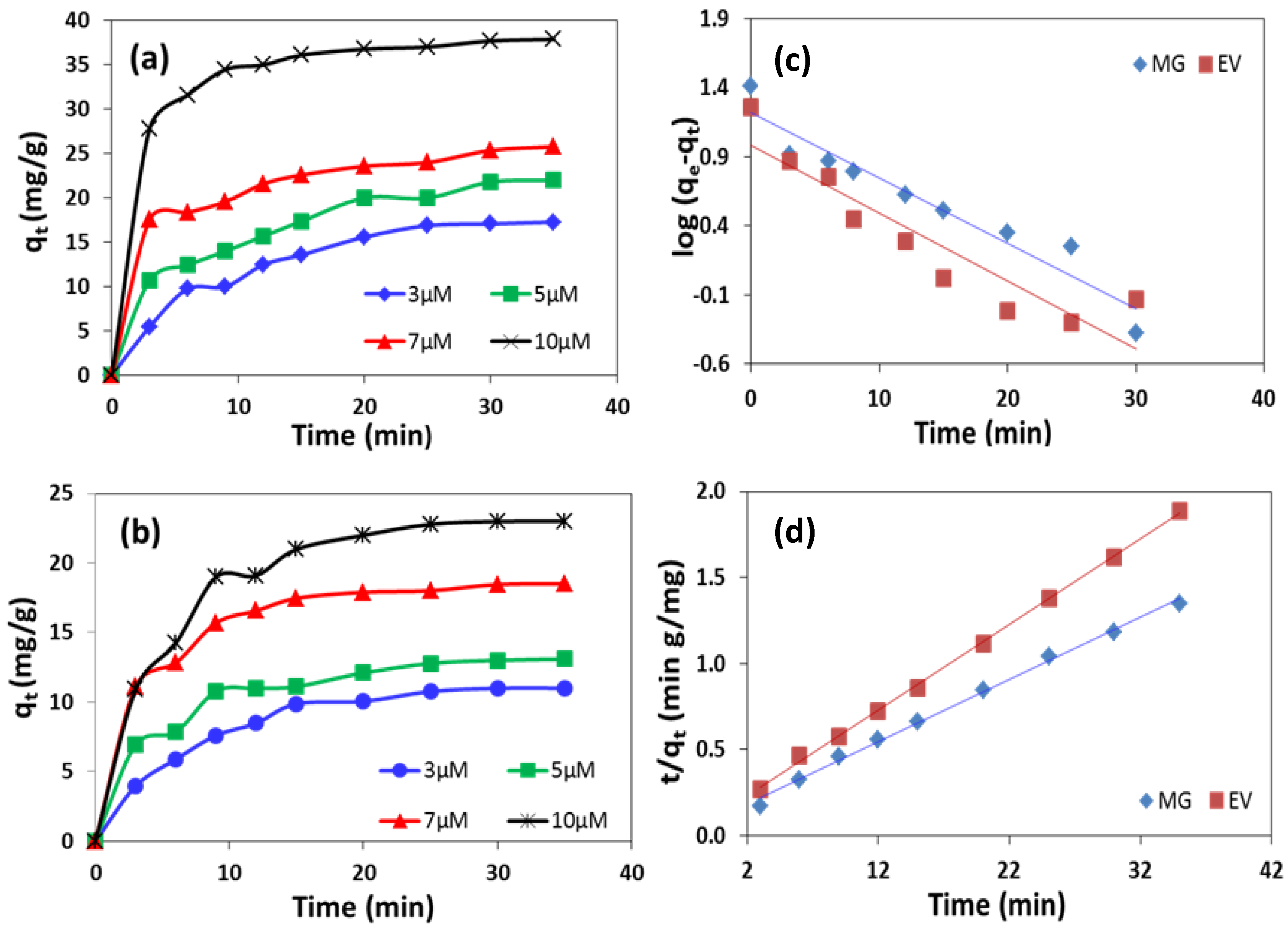
2.5. Adsorption Kinetics
2.6. Pseudo 1st Order Kinetics
2.7. Pseudo 2nd Order Kinetics
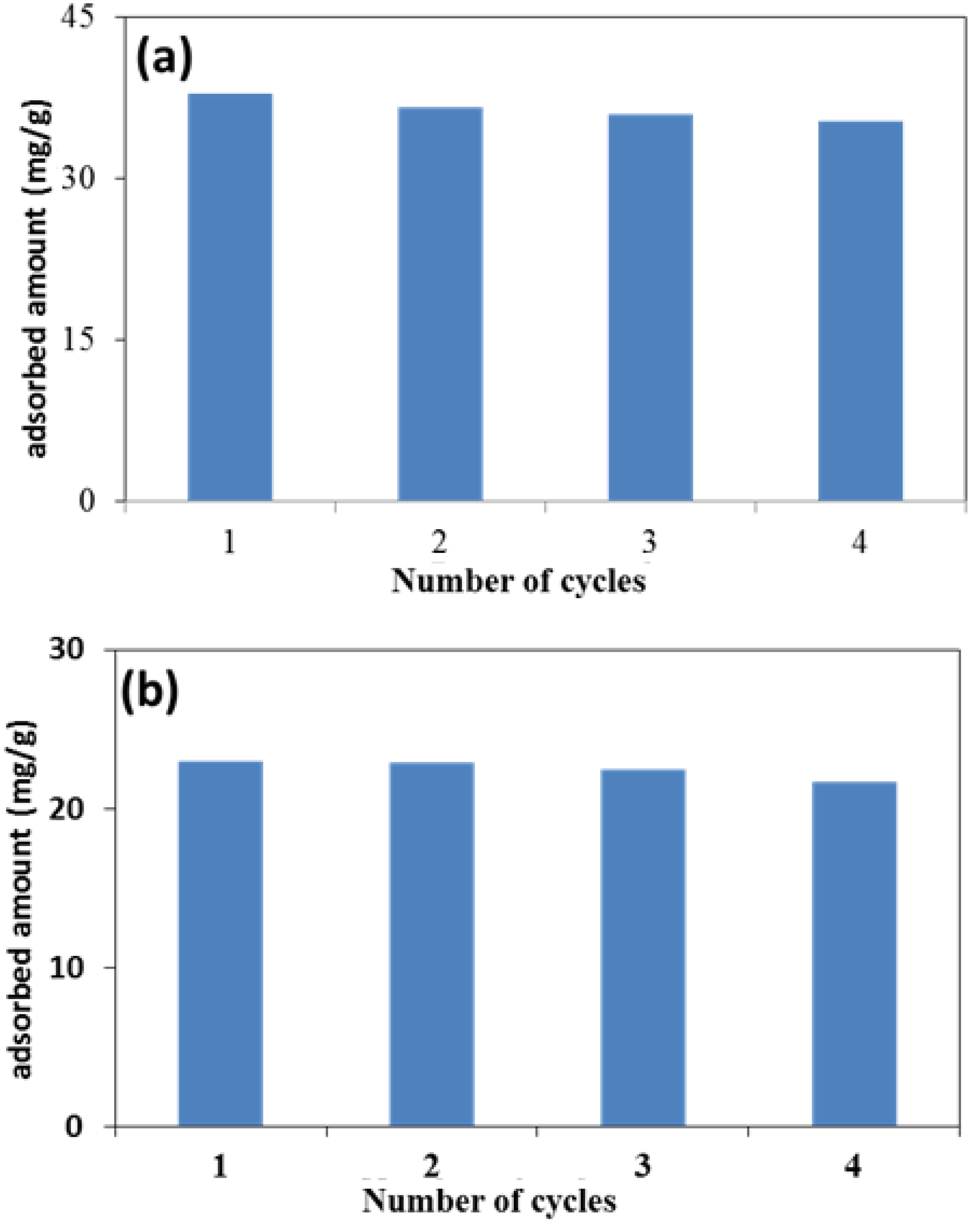
2.8. Reusability of Adsorbent
2.9. Photocatalytic Degradation of Resorcinol
2.10. Effect of Initial pH
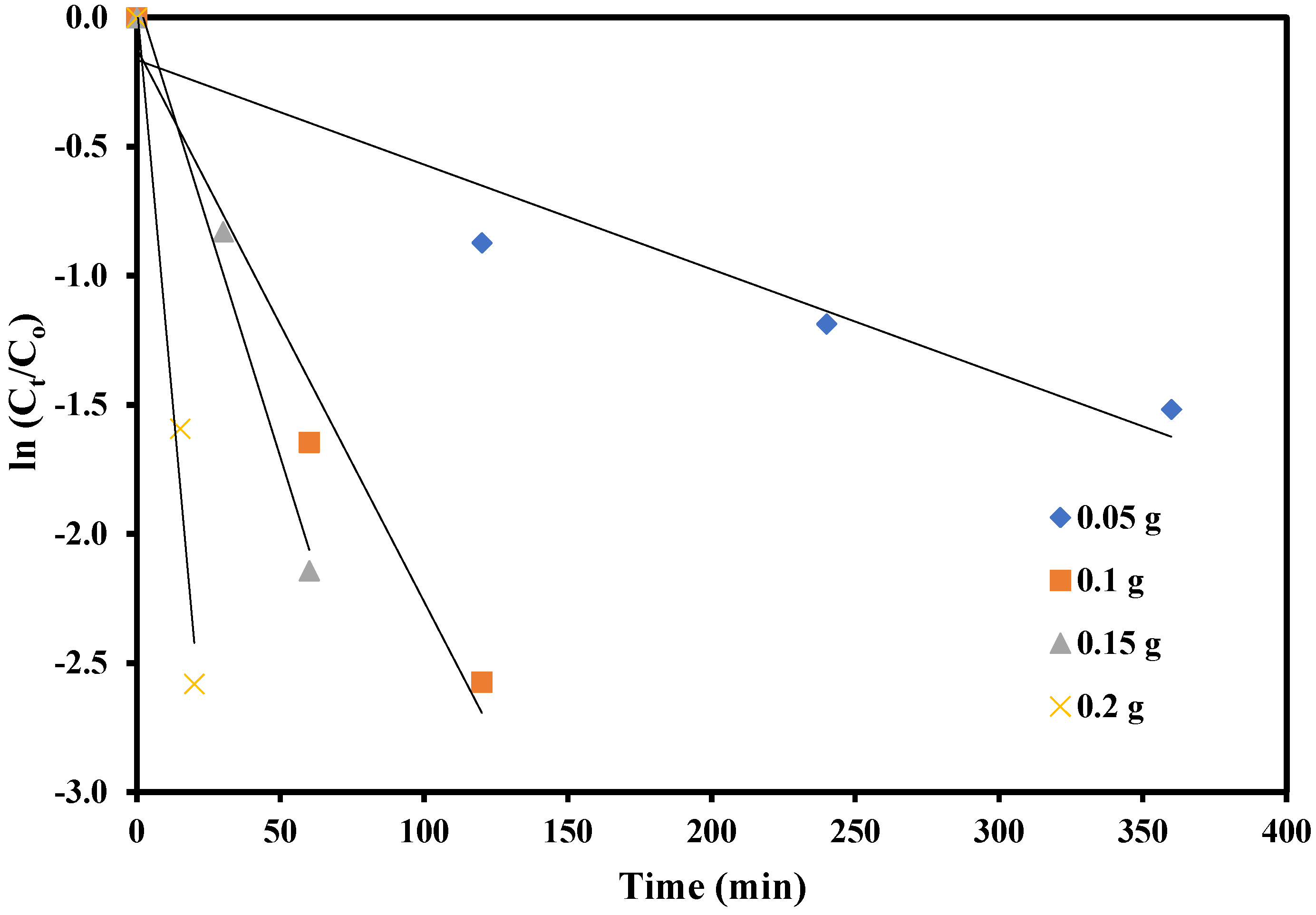
2.11. Effect of Catalyst Dose on Photodegradation
2.12. Degradation Kinetics
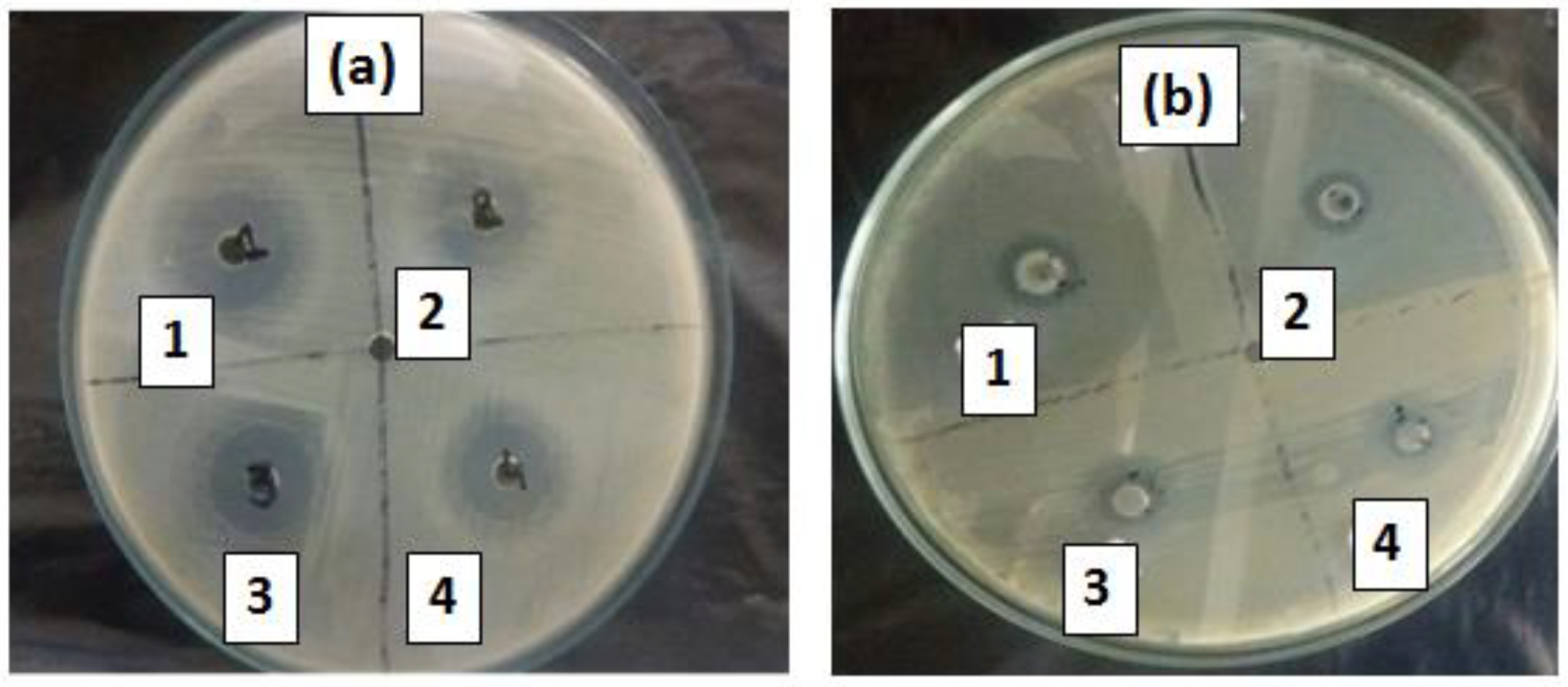
2.13. Antibacterial Application
3. Experimental Part
3.1. Materials
3.2. Graphene Oxide Synthesis
3.3. Reduced Graphene Oxide-ZnS Nanocomposite Synthesis
3.4. Synthesis of SILVER (Ag) Doped rGO-ZnS Nanocomposite
3.5. Adsorption Experiment
3.6. Photo-Degradation Experiment
3.7. Sample Preparation for Antimicrobial Studies
3.8. Characterization Techniques for Prepared Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pe, I.; Staciwa, P.; Sibera, D.; Pe, R.; Sobczuk, K.S.; Kayalar, G.Y.; Narkiewicz, U.; Cormia, R. CO2 Adsorption Study of Potassium—Based Activation of Carbon Spheres. Molecules 2022, 27, 5379. [Google Scholar] [CrossRef]
- Roosta, M.; Ghaedi, M.; Shokri, N.; Daneshfar, A.; Sahraei, R.; Asghari, A. Optimization of the Combined Ultrasonic Assisted/Adsorption Method for the Removal of Malachite Green by Gold Nanoparticles Loaded on Activated Carbon: Experimental Design. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Kennes, C.; Rene, E.R.; Veiga, M.C. Bioprocesses for Air Pollution Control. J. Chem. Technol. Biotechnol. 2009, 84, 1419–1436. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical Review of Scientific Rationale, Environmental Importance and Significance for Pollution Treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Zhao, L.; Mitomo, H. Adsorption of Heavy Metal Ions from Aqueous Solution onto Chitosan Entrapped CM-Cellulose Hydrogels Synthesized by Irradiation. J. Appl. Polym. Sci. 2008, 110, 1388–1395. [Google Scholar] [CrossRef]
- Jang, S.H.; Jeong, Y.G.; Min, B.G.; Lyoo, W.S.; Lee, S.C. Preparation and Lead Ion Removal Property of Hydroxyapatite/Polyacrylamide Composite Hydrogels. J. Hazard. Mater. 2008, 159, 294–299. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, S.; Tian, Z.; Duan, G.; Pan, H.; Yue, Y.; Li, S.; Jian, S.; Yang, W.; Liu, K.; et al. MOFs Meet Wood: Reusable Magnetic Hydrophilic Composites toward Efficient Water Treatment with Super-High Dye Adsorption Capacity at High Dye Concentration. Chem. Eng. J. 2022, 446, 136851. [Google Scholar] [CrossRef]
- Naseem, K.; Begum, R.; Wu, W.; Usman, M.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Adsorptive Removal of Heavy Metal Ions Using Polystyrene-Poly(N-Isopropylmethacrylamide-Acrylic Acid) Core/Shell Gel Particles: Adsorption Isotherms and Kinetic Study. J. Mol. Liq. 2019, 277, 522–531. [Google Scholar] [CrossRef]
- Shehzad, H.; Ahmed, E.; Sharif, A.; Farooqi, Z.H.; Din, M.I.; Begum, R.; Liu, Z.; Zhou, L.; Ouyang, J.; Irfan, A.; et al. Modified Alginate-Chitosan-TiO2 Composites for Adsorptive Removal of Ni(II) Ions from Aqueous Medium. Int. J. Biol. Macromol. 2022, 194, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Naseem, K.; Huma, R.; Shahbaz, A.; Jamal, J.; Ur Rehman, M.Z.; Sharif, A.; Ahmed, E.; Begum, R.; Irfan, A.; Al-Sehemi, A.G.; et al. Extraction of Heavy Metals from Aqueous Medium by Husk Biomass: Adsorption Isotherm, Kinetic and Thermodynamic Study. Zeitschrift Fur Phys. Chemie 2019, 233, 201–223. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Srinivasan, S.; Jeevanantham, S.; Vishnu, M.; Amith, K.V.; Sruthi, R.; Saravanan, R.; Vo, D.V.N. Insights on Synthesis and Applications of Graphene-Based Materials in Wastewater Treatment: A Review. Chemosphere 2022, 298, 134284. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liao, J.; He, B.; Zhang, F.; Zhang, F.; Huang, X.; Zhou, L. One-Step Fabrication of Graphene Oxide Enhanced Magnetic Composite Gel for Highly Efficient Dye Adsorption and Catalysis. ACS Sustain. Chem. Eng. 2015, 3, 1677–1685. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Jiang, X.; Yu, J.; Chen, X. Adsorption and Removal of Malachite Green from Aqueous Solution Using Magnetic β-Cyclodextrin-Graphene Oxide Nanocomposites as Adsorbents. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 466, 166–173. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, H.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Wu, Z.; Li, H. One-Pot Self-Assembly and Photoreduction Synthesis of Silver Nanoparticle-Decorated Reduced Graphene Oxide/MIL-125(Ti) Photocatalyst with Improved Visible Light Photocatalytic Activity. Appl. Organomet. Chem. 2016, 30, 289–296. [Google Scholar] [CrossRef]
- Naushad, M.; ALOthman, Z.A. Separation of Toxic Pb2+ Metal from Aqueous Solution Using Strongly Acidic Cation-Exchange Resin: Analytical Applications for the Removal of Metal Ions from Pharmaceutical Formulation. New Pub Balaban 2015, 53, 2158–2166. [Google Scholar] [CrossRef]
- Naushad, M.; ALOthman, Z.A.; Sharma, G. Inamuddin Kinetics, Isotherm and Thermodynamic Investigations for the Adsorption of Co(II) Ion onto Crystal Violet Modified Amberlite IR-120 Resin. Ionics 2015, 21, 1453–1459. [Google Scholar] [CrossRef]
- Yang, Y.; Liao, H.; Tong, Z.; Wang, C. Porous Ag/Polymer Composite Microspheres for Adsorption and Catalytic Degradation of Organic Dyes in Aqueous Solutions. Compos. Sci. Technol. 2015, 107, 137–144. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural Zeolites as Effective Adsorbents in Water and Wastewater Treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, X.; Tang, Y.; Su, K.; Kong, J. Hierarchically Porous Silicon–Carbon–Nitrogen Hybrid Materials towards Highly Efficient and Selective Adsorption of Organic Dyes. Sci. Rep. 2015, 5, 7910. [Google Scholar] [CrossRef] [PubMed]
- Fathy, R.; Ragab, E.; Ali, K.A. Adsorption of Methylene Blue on A Polymeric Matrix of Poly Lactic Acid/Sodium Alginate/Carbon Nanoparticles (PLA/SA/CNP); Cairo University: Giza, Egypt, 2022. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S.; Hussain, Q.; Ullah, H.; Al-Wabel, M.I.; Ahmad, M.; Kong, J. High-Efficiency Remediation of Cadmium (Cd2+) from Aqueous Solution Using Poultry Manure– and Farmyard Manure–Derived Biochars. Sep. Sci. Technol. 2016, 51, 2307–2317. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Rodgers, P.; Heath, J. Nanoscience and Technology: A Collection of Reviews from Nature Journals, 1st ed.; World Scientific: London, UK, 2009; pp. 1–346. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H.; Khraisheh, M.; Atieh, M.; Al-Ghouti, M. Synthesis of Graphene Oxides Particle of High Oxidation Degree Using a Modified Hummers Method. Ceram. Int. 2020, 46, 23997–24007. [Google Scholar] [CrossRef]
- Bradder, P.; Ling, S.K.; Wang, S.; Liu, S. Dye Adsorption on Layered Graphite Oxide. J. Chem. Eng. Data 2010, 56, 138–141. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Sun, M.; Li, X.; Lu, F.; Qiu, H. Preparation of Novel Magnetic Chitosan/Graphene Oxide Composite as Effective Adsorbents toward Methylene Blue. Bioresour. Technol. 2012, 114, 703–706. [Google Scholar] [CrossRef]
- Zhao, W.; Tang, Y.; Xi, J.; Kong, J. Functionalized Graphene Sheets with Poly(Ionic Liquid)s and High Adsorption Capacity of Anionic Dyes. Appl. Surf. Sci. 2015, 326, 276–284. [Google Scholar] [CrossRef]
- Barman, B.K.; Nanda, K.K. The Dual Role of Zn–Acid Medium for One-Step Rapid Synthesis of M@rGO (M = Au, Pt, Pd and Ag) Hybrid Nanostructures at Room Temperature. Chem. Commun. 2013, 49, 8949–8951. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of Novel Magnetic Chitosan Grafted with Graphene Oxide to Enhance Adsorption Properties for Methyl Blue. J. Hazard. Mater. 2012, 215–216, 272–279. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of Solution-Processed Reduced Graphene Oxide Films as Transparent Conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef]
- Faniyi, I.O.; Fasakin, O.; Olofinjana, B.; Adekunle, A.S.; Oluwasusi, T.V.; Eleruja, M.A.; Ajayi, E.O.B. The Comparative Analyses of Reduced Graphene Oxide (RGO) Prepared via Green, Mild and Chemical Approaches. SN Appl. Sci. 2019, 1, 1181. [Google Scholar] [CrossRef]
- Ossonon, B.D.; Bélanger, D. Synthesis and Characterization of Sulfophenyl-Functionalized Reduced Graphene Oxide Sheets. RSC Adv. 2017, 7, 27224–27234. [Google Scholar] [CrossRef]
- Paranthaman, V.; Sundaramoorthy, K.; Chandra, B.; Muthu, S.P.; Alagarsamy, P.; Perumalsamy, R. Investigation on the Performance of Reduced Graphene Oxide as Counter Electrode in Dye Sensitized Solar Cell Applications. Phys. Status Solidi 2018, 215, 1800298. [Google Scholar] [CrossRef]
- Guerrero-Fajardo, C.A.; Giraldo, L.; Moreno-Piraján, J.C. Graphene Oxide: Study of Pore Size Distribution and Surface Chemistry Using Immersion Calorimetry. Nanomaterials 2020, 10, 1492. [Google Scholar] [CrossRef]
- Li, L.; Fan, L.; Sun, M.; Qiu, H.; Li, X.; Duan, H.; Luo, C. Adsorbent for Chromium Removal Based on Graphene Oxide Functionalized with Magnetic Cyclodextrin–Chitosan. Colloids Surfaces B Biointerfaces 2013, 107, 76–83. [Google Scholar] [CrossRef]
- Haubner, K.; Murawski, J.; Olk, P.; Eng, L.M.; Ziegler, C.; Adolphi, B.; Jaehne, E. The Route to Functional Graphene Oxide. ChemPhysChem 2010, 11, 2131–2139. [Google Scholar] [CrossRef]
- Naseer, F.; Ajmal, M.; Bibi, F.; Farooqi, Z.H.; Siddiq, M. Copper and Cobalt Nanoparticles Containing Poly(Acrylic Acid-Co-Acrylamide) Hydrogel Composites for Rapid Reduction of 4-Nitrophenol and Fast Removal of Malachite Green from Aqueous Medium. Polym. Compos. 2018, 39, 3187–3198. [Google Scholar] [CrossRef]
- Ajmal, M.; Demirci, S.; Siddiq, M.; Aktas, N.; Sahiner, N. Amidoximated Poly(Acrylonitrile) Particles for Environmental Applications: Removal of Heavy Metal Ions, Dyes, and Herbicides from Water with Different Sources. J. Appl. Polym. Sci. 2016, 133, 43032. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhang, C.; Jing, Y. Adsorption of Malachite Green from Aqueous Solution onto Carbon Prepared from Arundo Donax Root. J. Hazard. Mater. 2008, 150, 774–782. [Google Scholar] [CrossRef]
- Tsai, W.T.; Chang, Y.M.; Lai, C.W.; Lo, C.C. Adsorption of Ethyl Violet Dye in Aqueous Solution by Regenerated Spent Bleaching Earth. J. Colloid Interface Sci. 2005, 289, 333–338. [Google Scholar] [CrossRef]
- Jabbari, V.; Veleta, J.M.; Zarei-Chaleshtori, M.; Gardea-Torresdey, J.; Villagrán, D. Green Synthesis of Magnetic MOF@GO and MOF@CNT Hybrid Nanocomposites with High Adsorption Capacity towards Organic Pollutants. Chem. Eng. J. 2016, 304, 774–783. [Google Scholar] [CrossRef]
- Arshad, A.; Iqbal, J.; Siddiq, M.; Mansoor, Q.; Ismail, M.; Mehmood, F.; Ajmal, M.; Abid, Z. Graphene Nanoplatelets Induced Tailoring in Photocatalytic Activity and Antibacterial Characteristics of MgO/Graphene Nanoplatelets Nanocomposites. J. Appl. Phys. 2017, 121, 024901. [Google Scholar] [CrossRef]
- Karaer, H.; Uzun, I. Adsorption of Basic Dyestuffs from Aqueous Solution by Modified Chitosan. New Pub Balaban 2013, 51, 2294–2305. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Acid Modified Local Clay Beads as Effective Low-Cost Adsorbent for Dynamic Adsorption of Methylene Blue. J. Ind. Eng. Chem. 2013, 19, 1153–1161. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, R.D.; Purohit, L.P. Novel ZnO Tetrapod-Reduced Graphene Oxide Nanocomposites for Enhanced Photocatalytic Degradation of Phenolic Compounds and MB Dye. J. Mol. Liq. 2021, 327, 114814. [Google Scholar] [CrossRef]
- Sharma, P.; Hussain, N.; Borah, D.J.; Das, M.R. Kinetics and Adsorption Behavior of the Methyl Blue at the Graphene Oxide/Reduced Graphene Oxide Nanosheet-Water Interface: A Comparative Study. J. Chem. Eng. Data 2013, 58, 3477–3488. [Google Scholar] [CrossRef]
- Ajmal, M.; Demirci, S.; Uzun, Y.; Siddiq, M.; Aktas, N.; Sahiner, N. Introduction of Double Amidoxime Group by Double Post Surface Modification on Poly(Vinylbenzyl Chloride) Beads for Higher Amounts of Organic Dyes, As (V) and Cr (VI) Removal. J. Colloid Interface Sci. 2016, 470, 39–46. [Google Scholar] [CrossRef]
- Naeem, H.; Ajmal, M.; Muntha, S.; Ambreen, J.; Siddiq, M. Synthesis and Characterization of Graphene Oxide Sheets Integrated with Gold Nanoparticles and Their Applications to Adsorptive Removal and Catalytic Reduction of Water Contaminants. RSC Adv. 2018, 8, 3599–3610. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Xiong, Z.; Yong, Y.; Zhao, X.S. Preparation, Characterization and Antibacterial Properties of Silver-Modified Graphene Oxide. J. Mater. Chem. 2011, 21, 3350–3352. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, D.; Qi, P. Synthesis and Characterization of Silver Nanoparticle and Graphene Oxide Nanosheet Composites as a Bactericidal Agent for Water Disinfection. J. Colloid Interface Sci. 2011, 360, 463–470. [Google Scholar] [CrossRef]


| Method | Single Point BET | Multi Point BET | BJH Cumulative Adsorption | BJH Cumulative Desorption | DH Cumulative Adsorption | DH Cumulative Desorption | Langmuir | DFT Cumulative |
|---|---|---|---|---|---|---|---|---|
| Surface Area (m2/g) | 456.1 | 550.3 | 431.6 | 457.2 | 441.9 | 468.3 | 1082 | 291.2 |
| Pore volume (cc/g) | - | - | 0.6898 | 0.6991 | 0.6791 | 0.6888 | - | 0.6892 |
| Pore Radius (Å) | 16.21 | 16.02 | 16.21 | 16.02 | 15.85 |
| Kinetics | Constants | Dyes | |
|---|---|---|---|
| MG | EV | ||
| Pseudo-first order model | k1 (min−1) | 0.108 | 0.11 |
| qe (mg/g) | 16.5 | 9.63 | |
| R2 | 0.9242 | 0.8543 | |
| Pseudo-second order Model | k2 (g/mg min) | 0.011 | 0.019 |
| qe (mg/g) | 27.54 | 20.04 | |
| R2 | 0.9961 | 0.999 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naeem, H.; Tofil, H.M.; Soliman, M.; Hai, A.; Zaidi, S.H.H.; Kizilbash, N.; Alruwaili, D.; Ajmal, M.; Siddiq, M. Reduced Graphene Oxide-Zinc Sulfide Nanocomposite Decorated with Silver Nanoparticles for Wastewater Treatment by Adsorption, Photocatalysis and Antimicrobial Action. Molecules 2023, 28, 926. https://doi.org/10.3390/molecules28030926
Naeem H, Tofil HM, Soliman M, Hai A, Zaidi SHH, Kizilbash N, Alruwaili D, Ajmal M, Siddiq M. Reduced Graphene Oxide-Zinc Sulfide Nanocomposite Decorated with Silver Nanoparticles for Wastewater Treatment by Adsorption, Photocatalysis and Antimicrobial Action. Molecules. 2023; 28(3):926. https://doi.org/10.3390/molecules28030926
Chicago/Turabian StyleNaeem, Hina, Hafiz Muhammad Tofil, Mohamed Soliman, Abdul Hai, Syeda Huma H. Zaidi, Nadeem Kizilbash, Daliyah Alruwaili, Muhammad Ajmal, and Muhammad Siddiq. 2023. "Reduced Graphene Oxide-Zinc Sulfide Nanocomposite Decorated with Silver Nanoparticles for Wastewater Treatment by Adsorption, Photocatalysis and Antimicrobial Action" Molecules 28, no. 3: 926. https://doi.org/10.3390/molecules28030926
APA StyleNaeem, H., Tofil, H. M., Soliman, M., Hai, A., Zaidi, S. H. H., Kizilbash, N., Alruwaili, D., Ajmal, M., & Siddiq, M. (2023). Reduced Graphene Oxide-Zinc Sulfide Nanocomposite Decorated with Silver Nanoparticles for Wastewater Treatment by Adsorption, Photocatalysis and Antimicrobial Action. Molecules, 28(3), 926. https://doi.org/10.3390/molecules28030926







