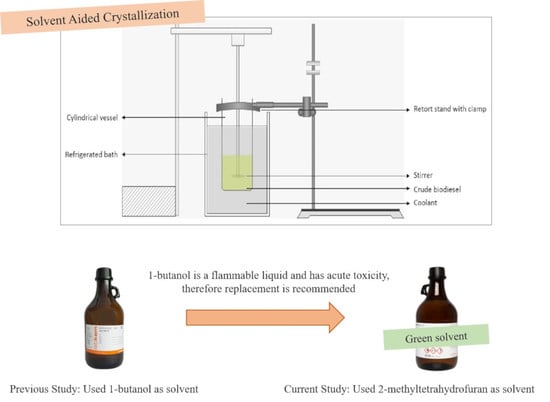
Biodiesel Purification by Solvent-Aided Crystallization Using 2-Methyltetrahydrofuran
Abstract
1. Introduction
2. Results
2.1. Characterization of Crude Biodiesel
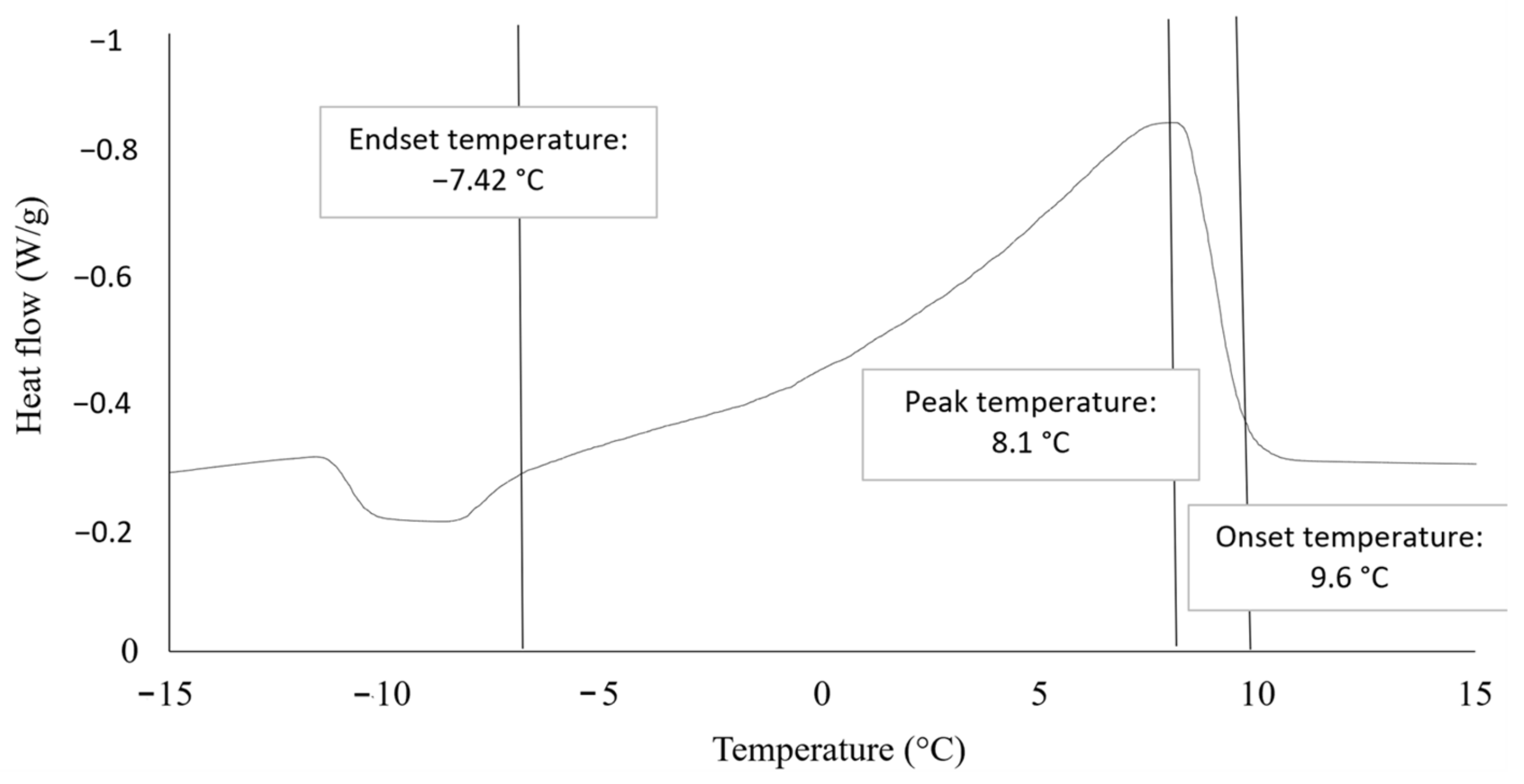
2.1.1. Differential Scanning Calorimetry
2.1.2. Gas Chromatography–Mass Spectroscopy
2.2. Effect of Coolant Temperature in SAC
2.3. Effect of Cooling Time in SAC
2.4. Effect of Stirring Speed in SAC
3. Discussion
3.1. Characterization of Crude Biodiesel
3.1.1. Differential Scanning Calorimetry
3.1.2. Gas Chromatography–Mass Spectroscopy
3.2. Effect of Coolant Temperature in SAC
3.3. Effect of Cooling Time in SAC
3.4. Effect of Stirring Speed in SAC
4. Materials and Methods
4.1. Materials Used
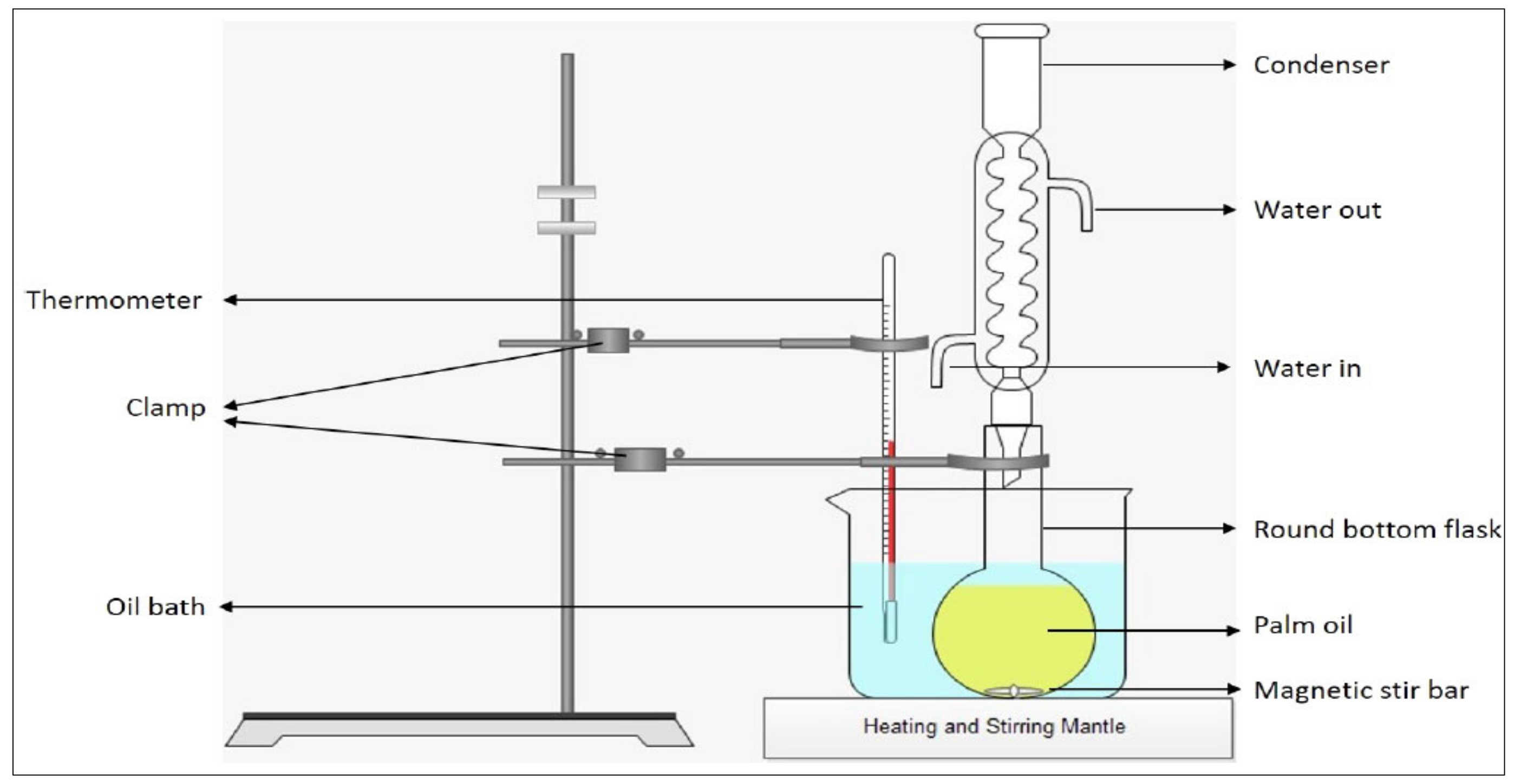
4.2. Transesterification Process
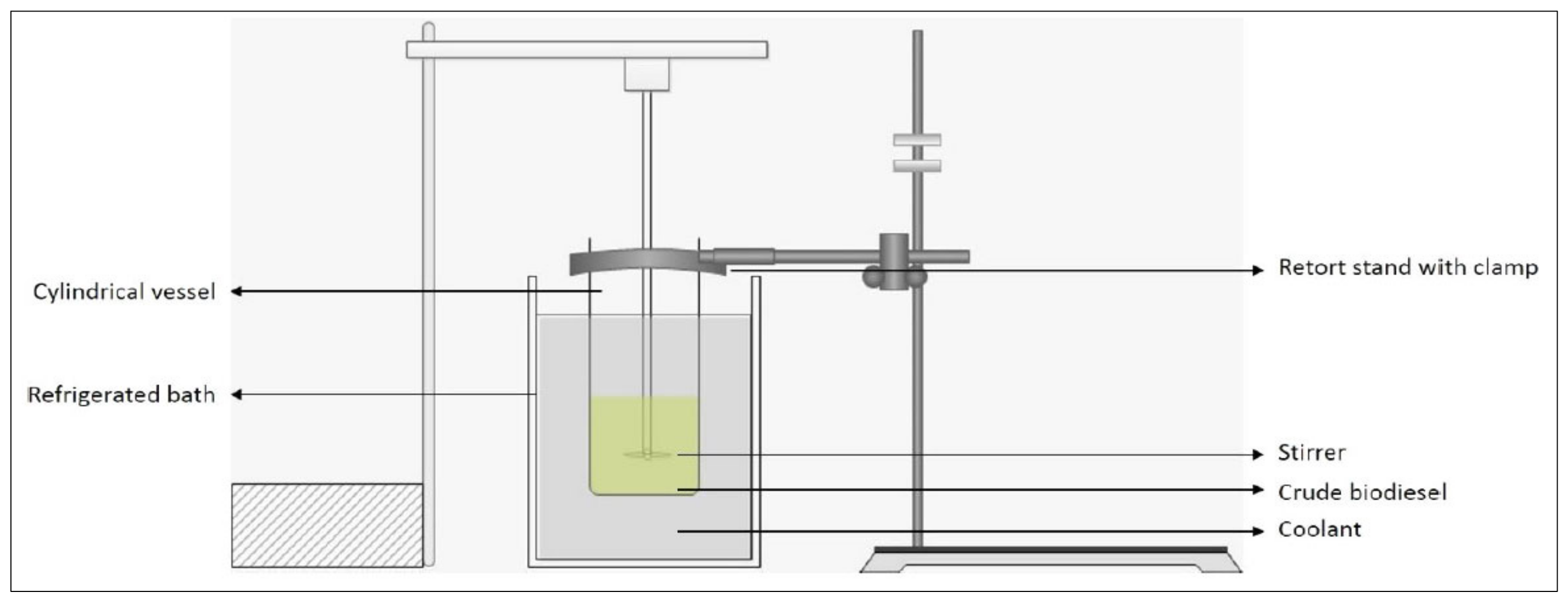
4.3. Solvent-Aided Crystallization
4.4. Characterization of Biodiesel
4.4.1. Differential Scanning Calorimetry
4.4.2. Gas Chromatography–Mass Spectroscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zulqarnain; Yusoff, M.H.M.; Ayoub, M.; Jusoh, N.; Abdullah, A.Z. The Challenges of a Biodiesel Implementation Program in Malaysia. Processes 2020, 8, 1244. [Google Scholar] [CrossRef]
- Lim, S.; Teong, L.K. Recent trends, opportunities and challenges of biodiesel in Malaysia: An overview. Renew. Sustain. Energy Rev. 2010, 14, 938–954. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Ferreira, G.F.; Maciel, M.R.W.; Filho, R.M. Biodiesel purification by column chromatography and liquid-liquid extraction using green solvents. Fuel 2019, 235, 1123–1130. [Google Scholar] [CrossRef]
- Arenas, E.; Villafán-Cáceres, S.; Rodríguez-Mejía, Y.; García-Loyola, J.; Masera, O.; Sandoval, G. Biodiesel Dry Purification Using Unconventional Bioadsorbents. Processes 2021, 9, 194. [Google Scholar] [CrossRef]
- Atadashi, I.; Aroua, M.; Aziz, A.A.; Sulaiman, N. Refining technologies for the purification of crude biodiesel. Appl. Energy 2011, 88, 4239–4251. [Google Scholar] [CrossRef]
- Atadashi, I.; Aroua, M.; Aziz, A.A.; Sulaiman, N. High quality biodiesel obtained through membrane technology. J. Membr. Sci. 2012, 421-422, 154–164. [Google Scholar] [CrossRef]
- Atadashi, I. Purification of crude biodiesel using dry washing and membrane technologies. Alex. Eng. J. 2015, 54, 1265–1272. [Google Scholar] [CrossRef]
- Leonardo, R.; Valle, M. Evaluation of the Volatility Characteristics of Diesel/Biodiesel Blends Using Thermal Analysis Techniques. 2019. Available online: https://www.semanticscholar.org/paper/Evaluation-of-the-volatility-characteristics-of-%2F-Leonardo-Valle/2cad370eb33d87c714eeca3c5759352c053afc40 (accessed on 13 January 2023).
- Goodrum, J. Volatility and boiling points of biodiesel from vegetable oils and tallow. Biomass-Bioenergy 2002, 22, 205–211. [Google Scholar] [CrossRef]
- Eisenbart, F.; Ulrich, J. Solvent-aided layer crystallization—Case study glycerol–water. Chem. Eng. Sci. 2015, 133, 24–29. [Google Scholar] [CrossRef]
- Ahmad, M.; Samsuri, S. Biodiesel Purification via Ultrasonic-Assisted Solvent-Aided Crystallization. Crystals 2021, 11, 212. [Google Scholar] [CrossRef]
- Samsuri, S.; Jian, N.L.; Jusoh, F.W.; Yáñez, E.H.; Yahya, N.Y. Solvent-Aided Crystallization for Biodiesel Purification. Chem. Eng. Technol. 2019, 43, 447–456. [Google Scholar] [CrossRef]
- Watanabe, K. The Toxicological Assessment of Cyclopentyl Methyl Ether (CPME) as a Green Solvent. Molecules 2013, 18, 3183–3194. [Google Scholar] [CrossRef] [PubMed]
- Smoleń, M.; Kędziorek, M.; Grela, K. 2-Methyltetrahydrofuran: Sustainable solvent for ruthenium-catalyzed olefin metathesis. Catal. Commun. 2014, 44, 80–84. [Google Scholar] [CrossRef]
- Choi, J.Y.; Nam, J.; Yun, B.Y.; Kim, Y.U.; Kim, S. Utilization of corn cob, an essential agricultural residue difficult to disposal: Composite board manufactured improved thermal performance using microencapsulated PCM. Ind. Crop. Prod. 2022, 183. [Google Scholar] [CrossRef]
- Redaelli, R.; Berardo, N. Prediction of fibre components in oat hulls by near infrared reflectance spectroscopy. J. Sci. Food Agric. 2007, 87, 580–585. [Google Scholar] [CrossRef]
- Bao, W.-H.; Wang, Z.; Tang, X.; Zhang, Y.-F.; Tan, J.-X.; Zhu, Q.; Cao, Z.; Lin, Y.-W.; He, W.-M. Clean preparation of S-thiocarbamates with in situ generated hydroxide in 2-methyltetrahydrofuran. Chin. Chem. Lett. 2019, 30, 2259–2262. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Letchumanan, A.; Samsuri, S.; Mazli, W.N.A.; Saad, J.M. Parametric study of glycerol and contaminants removal from biodiesel through solvent-aided crystallization. Bioresour. Bioprocess. 2021, 8, 54. [Google Scholar] [CrossRef]
- Samsuri, S.; Amran, N.A.; Zheng, L.J.; Bakri, M.M.M. Effect of coolant temperature and cooling time on fractional crystallization of biodiesel and glycerol. Malays. J. Fundam. Appl. Sci. 2017, 13, 676–679. [Google Scholar] [CrossRef]
- Yahya, N.; Zakaria, Z.Y.; Ali, N.; Jusoh, M. Effect of Coolant Temperature on Progressive Freeze Concentration of Refined, Bleached and Deodorised Palm Oil based on Process Efficiency and Heat Transfer. J. Teknol. 2015, 74. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Bandari, C. Lab-scale catalytic production of biodiesel from waste cooking oil—A review. Biofuels 2017, 11, 409–419. [Google Scholar] [CrossRef]
- Jusoh, M.; Nor, N.N.M.; Zakaria, Z.Y. Progressive Freeze Concentration of Coconut Water. J. Teknol. 2014, 67. [Google Scholar] [CrossRef]
- Wierzbicka-Miernik, A. Fundamentals of the Differential Scanning Calorimetry Application in Materials Science. Available online: http://www.imim.pl/PHD/www.imim-phd.edu.pl/contents/Relevant%20Articles/Fundamentals%20of%20the%20Differential%20Scanning%20Calorimetry%20application%20in%20materials%20science%20A%20Wierzbicka-Miernik.pdf (accessed on 6 November 2022).
- Chauhan, A. GC-MS Technique and its Analytical Applications in Science and Technology. J. Anal. Bioanal. Tech. 2014, 5, 222. [Google Scholar] [CrossRef]
- Sneddon, J.; Masuram, S.; Richert, J.C. Gas Chromatography-Mass Spectrometry-Basic Principles, Instrumentation and Selected Applications for Detection of Organic Compounds. Anal. Lett. 2007, 40, 1003–1012. [Google Scholar] [CrossRef]




| Parameter | Diagram | Observation | ||
|---|---|---|---|---|
| Temperature (°C) | Stirring Speed (rpm) | Cooling Time (min) | ||
| −4 | 140 | 15 |  | The glycerol layer is not completely crystallized. |
| −6 |  | The colour of the biodiesel layer appears to be cloudy. | ||
| −8 |  | |||
| −10 | 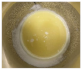 | The biodiesel layer appears to be viscous. Only a little biodiesel is obtained. The glycerol layer appears to be thick. | ||
| −12 |  | |||
| Parameter | Diagram | Observation | ||
|---|---|---|---|---|
| Cooling Time (min) | Stirring Speed (rpm) | Temperature (°C) | ||
| 5 | 140 | −8 |  | Glycerol is crystallized. A thin white layer of glycerol is formed. |
| 10 |  | Glycerol is crystallized. A white layer of glycerol is formed. | ||
| 15 |  | The colour of the biodiesel layer appears to be cloudy. | ||
| 20 |  | The colour of the biodiesel layer appears to be cloudy. The glycerol layer appears to be very thick. | ||
| 25 |  | |||
| Parameter | Diagram | Observation | ||
|---|---|---|---|---|
| Stirring Speed (rpm) | Cooling Time (min) | Temperature (°C) | ||
| 120 | 15 | −8 |  | The colour of the biodiesel layer appears to be cloudy. The glycerol layer appears to be very thick. |
| 130 |  | The pale colour of the biodiesel layer is formed. A thin white layer of glycerol is formed. | ||
| 140 |  | The colour of the biodiesel layer appears to be cloudy. | ||
| 170 | 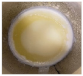 | The biodiesel layer appears to be viscous. The glycerol layer appears to be thick. The colour of the biodiesel layer appears to be cloudy. | ||
| 210 |  | The biodiesel layer appears to be very viscous. Only a little biodiesel is obtained. The glycerol layer appears to be thick. The colour of the biodiesel layer appears to be cloudy | ||
| Systematic Name | Trivial Name | Types of Fatty Acid | Retention Time (min) | Composition of FAME (%) |
|---|---|---|---|---|
| Dodecanoic acid | Lauric | Saturated | 2.143 | 0.41 |
| Methyl tetradecanoate | Myristic | Saturated | 2.959 | 1.6 |
| 9-Hexadecanoic acid | Palmitoleic | Unsaturated | 4.473 | 0.14 |
| Hexadecanoic acid | Palmitic | Saturated | 4.828 | 18.31 |
| Hexadecanoic acid | Palmitic | Saturated | 4.977 | 12.19 |
| Hexadecanoic acid | Palmitic | Saturated | 6.053 | 0.27 |
| 9-Octadecenoic acid | Oleic | Unsaturated | 8.321 | 48.64 |
| 9-Octadecenoic acid | Oleic | Unsaturated | 8.376 | 3.19 |
| 9-Octadecenoic acid | Oleic | Unsaturated | 8.456 | 6.48 |
| Methyl strearate | Stearic | Saturated | 8.692 | 6.86 |
| Eicosanoic acid | Arachidic | Saturated | 14.641 | 0.61 |
| Hexadecanoic acid | Palmitic | Saturated | 20.358 | 0.42 |
| Total Unsaturated Fatty Acid | 58.45 | |||
| Total Saturated Fatty Acid | 40.67 | |||
| Total Fatty Acid | 99.12 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan Osman, W.N.A.; Badrol, N.A.I.; Samsuri, S. Biodiesel Purification by Solvent-Aided Crystallization Using 2-Methyltetrahydrofuran. Molecules 2023, 28, 1512. https://doi.org/10.3390/molecules28031512
Wan Osman WNA, Badrol NAI, Samsuri S. Biodiesel Purification by Solvent-Aided Crystallization Using 2-Methyltetrahydrofuran. Molecules. 2023; 28(3):1512. https://doi.org/10.3390/molecules28031512
Chicago/Turabian StyleWan Osman, Wan Nur Aisyah, Nur Athirah Izzati Badrol, and Shafirah Samsuri. 2023. "Biodiesel Purification by Solvent-Aided Crystallization Using 2-Methyltetrahydrofuran" Molecules 28, no. 3: 1512. https://doi.org/10.3390/molecules28031512
APA StyleWan Osman, W. N. A., Badrol, N. A. I., & Samsuri, S. (2023). Biodiesel Purification by Solvent-Aided Crystallization Using 2-Methyltetrahydrofuran. Molecules, 28(3), 1512. https://doi.org/10.3390/molecules28031512