Improved Antioxidant and Mechanical Properties of Food Packaging Films Based on Chitosan/Deep Eutectic Solvent, Containing Açaí-Filled Microcapsules
Abstract
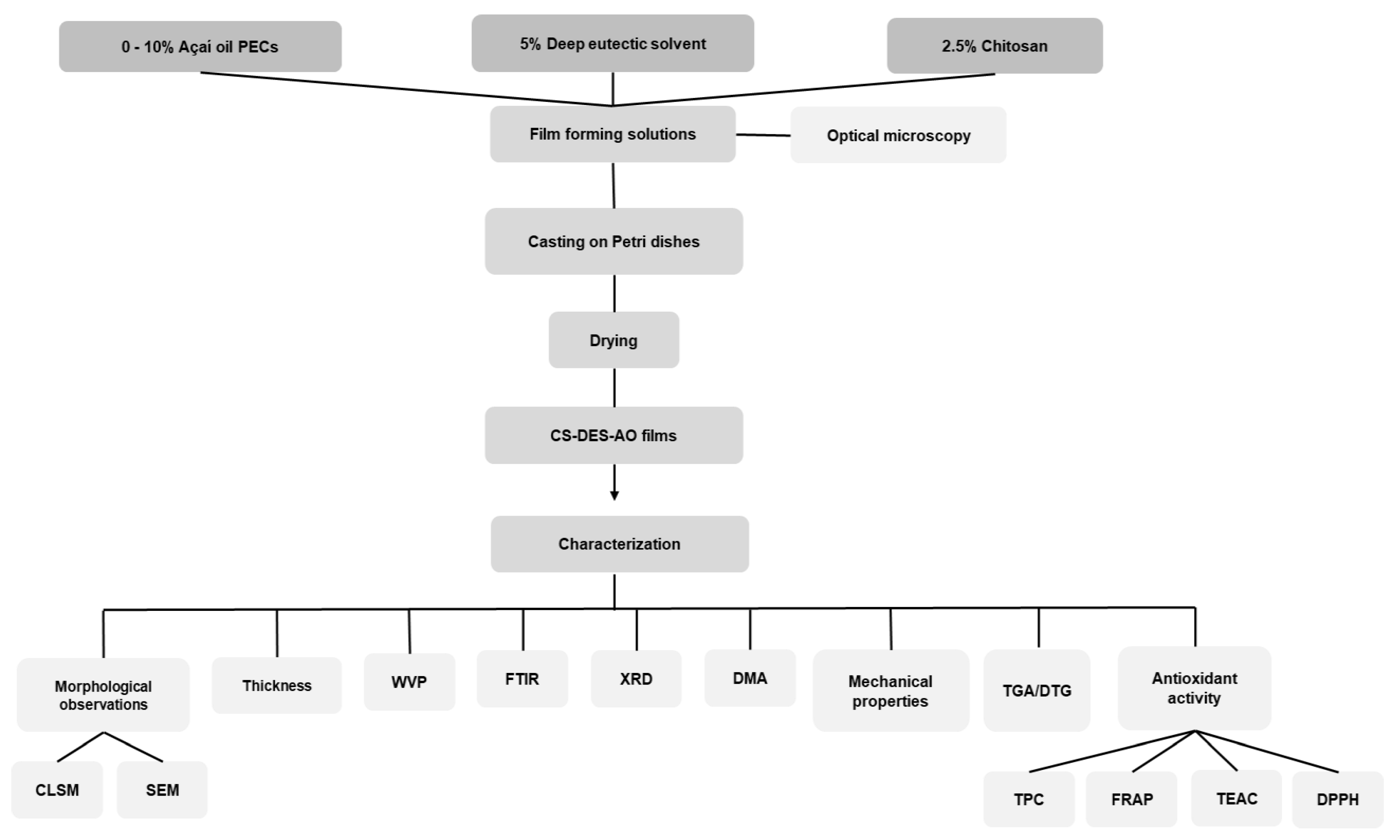
:1. Introduction
2. Results and Discussion
2.1. Morphological Observations
2.2. Thickness and Water Vapor Permeability (WVP) of the Films
2.3. FTIR Spectroscopy
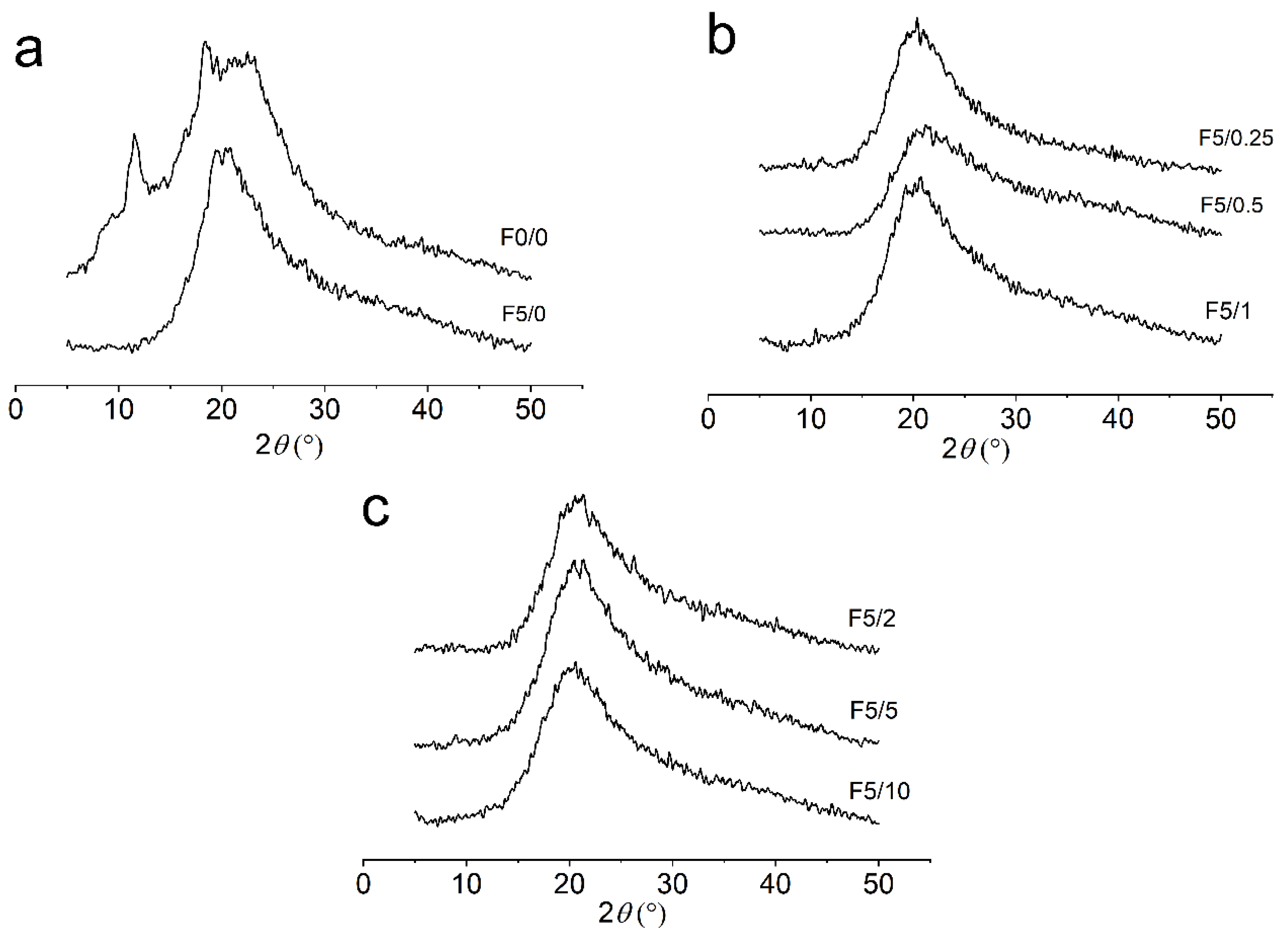
2.4. X-ray Diffraction (XRD)
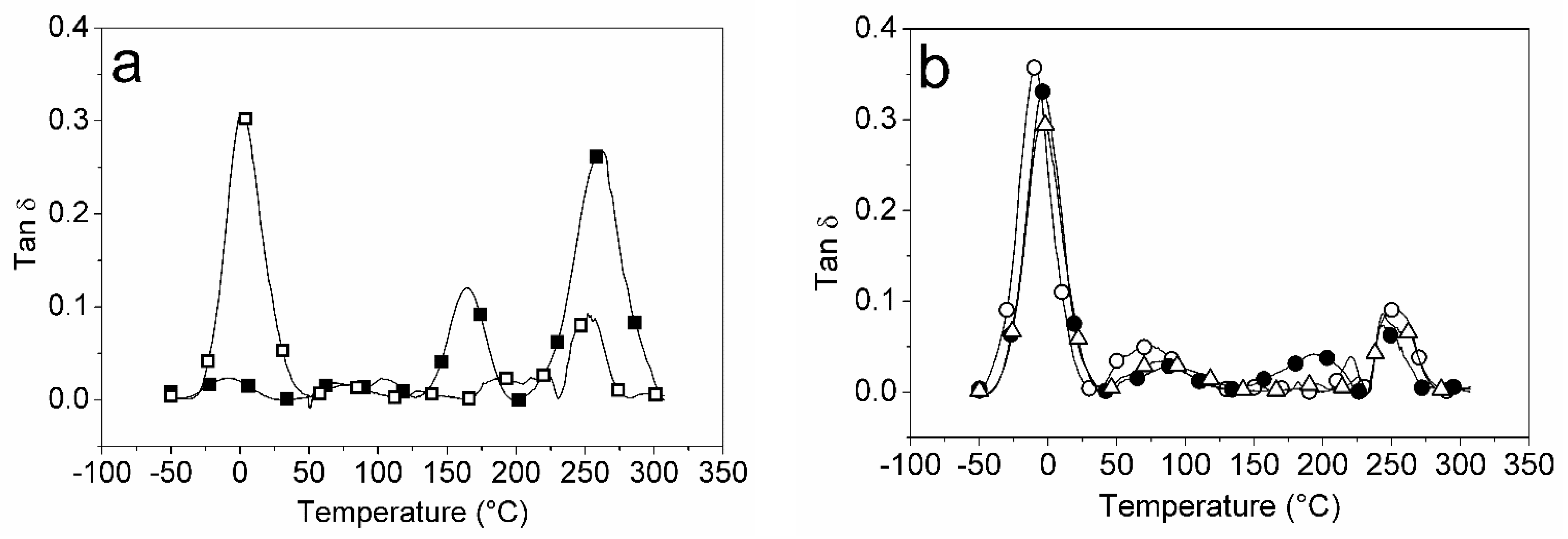
2.5. Mechanical Properties and DMA of the Films
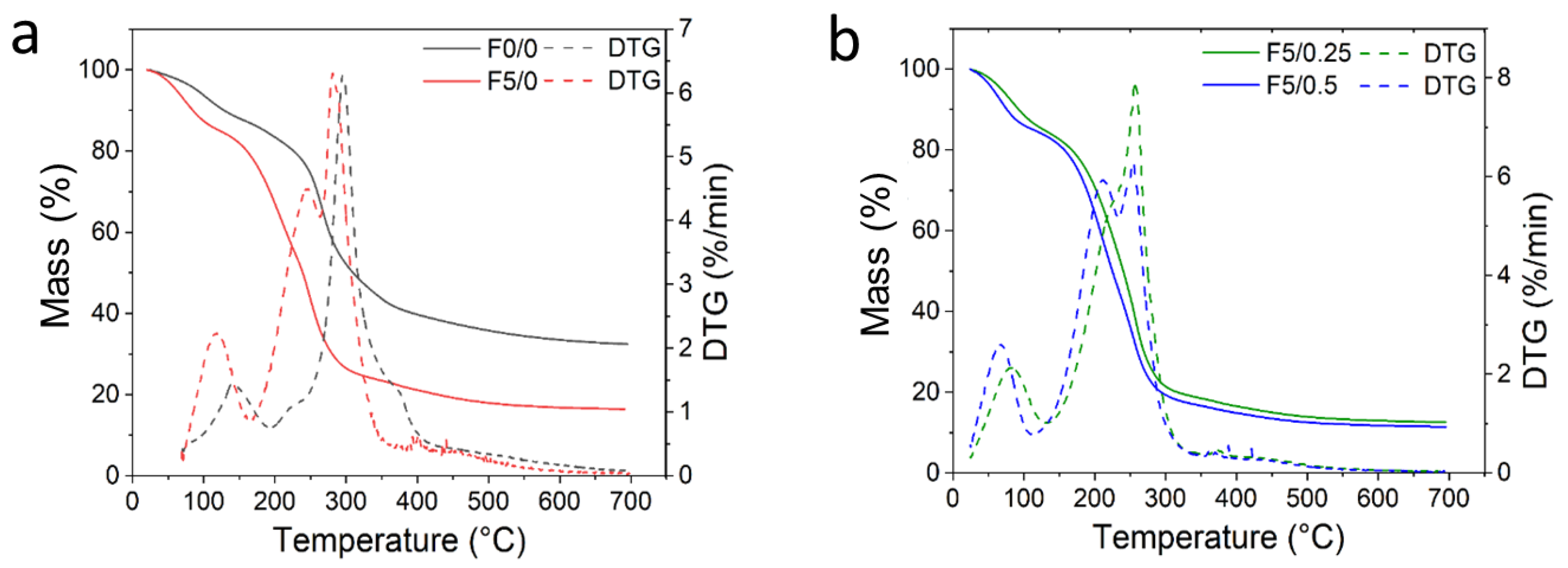
2.6. Thermogravimetric Analysis (TGA) of the Films
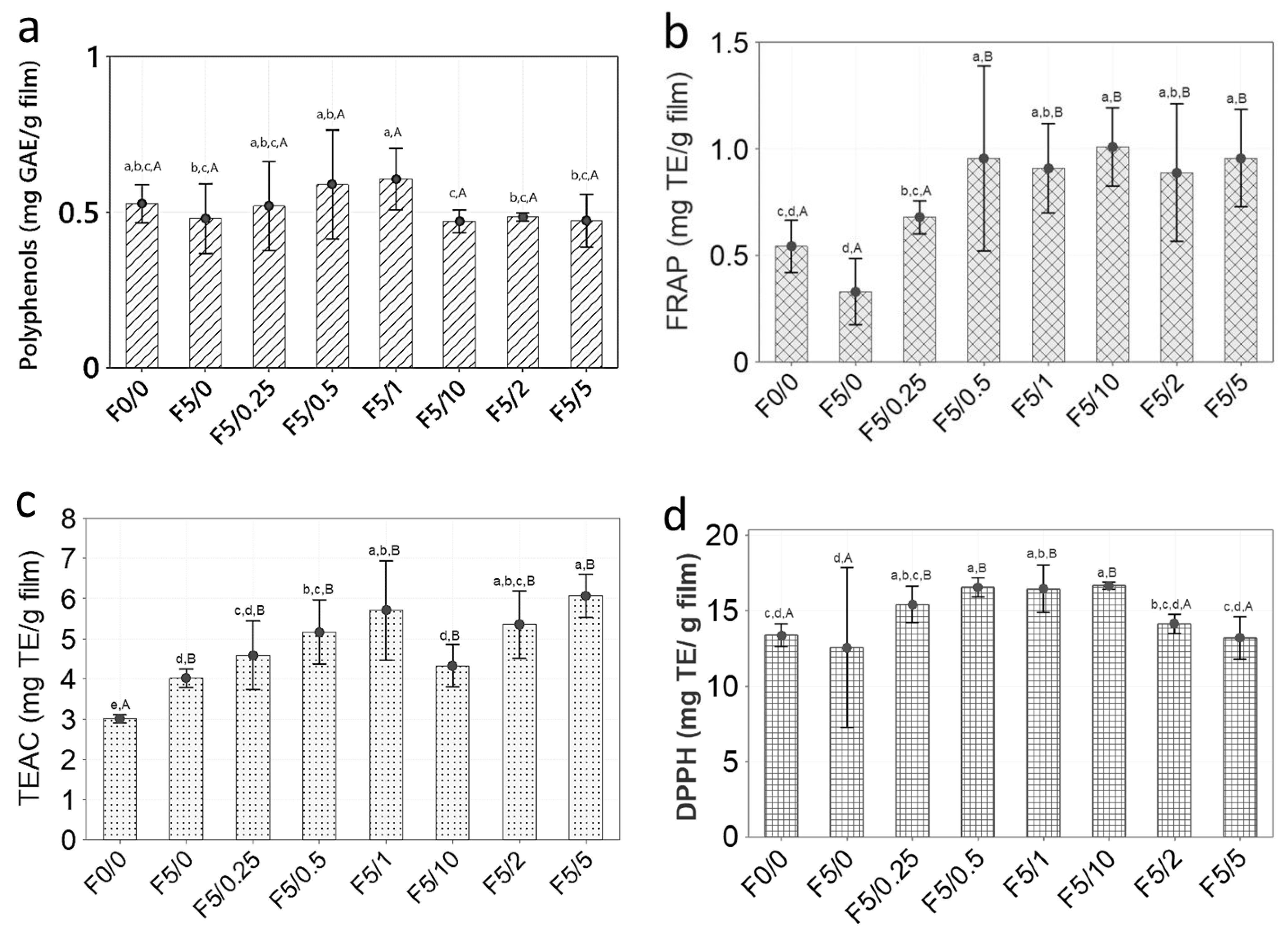
2.7. Antioxidant Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Deep Eutectic Solvent and the CS-Composite Films
3.3. Morphological Observations of the Films
3.4. Thickness and Water Vapor Permeability of the Films
3.5. Fourier Transform Infrared (FTIR) Spectroscopy and X-ray Diffraction (XRD) of the Films
3.6. Mechanical Properties and Dynamic Mechanical Analyses (DMA) of the Films
3.7. Thermogravimetric Analysis (TGA) of the Films
3.8. Antioxidant Properties of Açaí Oil and the Films
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carocho, M.; Heleno, S.; Rodrigues, P.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. A Novel Natural Coating for Food Preservation: Effectiveness on Microbial Growth and Physicochemical Parameters. LWT 2019, 104, 76–83. [Google Scholar] [CrossRef]
- Khorrami, N.K.; Radi, M.; Amiri, S.; McClements, D.J. Fabrication and Characterization of Alginate-Based Films Functionalized with Nanostructured Lipid Carriers. Int. J. Biol. Macromol. 2021, 182, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent Advances in Bioplastics: Application and Biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current Advancements in Chitosan-Based Film Production for Food Technology; A Review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; Jiang, S.; He, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef]
- Wang, J.; Han, X.; Zhang, C.; Liu, K.; Duan, G. Source of Nanocellulose and Its Application in Nanocomposite Packaging Material: A Review. Nanomaterials 2022, 12, 3158. [Google Scholar] [CrossRef]
- da Costa, J.C.M.; Miki, K.S.L.; da Silva Ramos, A.; Teixeira-Costa, B.E. Development of Biodegradable Films Based on Purple Yam Starch/Chitosan for Food Application. Heliyon 2020, 6, e03718. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Natural Polymers Used in Edible Food Packaging—History, Function and Application Trends as a Sustainable Alternative to Synthetic Plastic. Polysaccharides 2021, 3, 32–58. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining Chitin, Chitosan and Their Melanin Complexes from Insects. Int. J. Biol. Macromol. 2021, 167, 1319–1328. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Chitosan as a Valuable Biomolecule from Seafood Industry Waste in the Design of Green Food Packaging. Biomolecules 2021, 11, 1599. [Google Scholar] [CrossRef] [PubMed]
- Rivero, S.; Damonte, L.; Garcia, M.A.; Pinotti, A. An Insight into the Role of Glycerol in Chitosan Films. Food Biophys. 2016, 11, 117–127. [Google Scholar] [CrossRef]
- Galvis-Sánchez, A.C.; Castro, M.C.R.; Biernacki, K.; Gonçalves, M.P.; Souza, H.K.S. Natural Deep Eutectic Solvents as Green Plasticizers for Chitosan Thermoplastic Production with Controlled/Desired Mechanical and Barrier Properties. Food Hydrocoll. 2018, 82, 478–489. [Google Scholar] [CrossRef]
- Bystrzanowska, M.; Tobiszewski, M. Assessment and Design of Greener Deep Eutectic Solvents—A Multicriteria Decision Analysis. J. Mol. Liq. 2021, 321, 114878. [Google Scholar] [CrossRef]
- Gurkan, B.E.; Maginn, E.J.; Pentzer, E.B. Deep Eutectic Solvents: A New Class of Versatile Liquids. J. Phys. Chem. B 2020, 124, 11313–11315. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep Eutectic Solvents for Polysaccharides Processing. A Review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Ilgen, F.; Ott, D.; Kralisch, D.; Reil, C.; Palmberger, A.; König, B. Conversion of Carbohydrates into 5-Hydroxymethylfurfural in Highly Concentrated Low Melting Mixtures. Green Chem. 2009, 11, 1948–1954. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Magalhães, J.M.C.S.; Souza, H.K.S.; Gonçalves, M.P. The Role of Choline Chloride-Based Deep Eutectic Solvent and Curcumin on Chitosan Films Properties. Food Hydrocoll. 2018, 81, 456–466. [Google Scholar] [CrossRef]
- Pereira, P.F.; Andrade, C.T. Optimized PH-Responsive Film Based on a Eutectic Mixture-Plasticized Chitosan. Carbohydr. Polym. 2017, 165, 238–246. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Souza, H.K.S.; Latona, N.; Liu, C.K.; Gonçalves, M.P.; Liu, L. Choline Chloride Based Ionic Liquid Analogues as Tool for the Fabrication of Agar Films with Improved Mechanical Properties. Carbohydr. Polym. 2014, 111, 206–214. [Google Scholar] [CrossRef]
- Kranz, M.; Hofmann, T. Food-Grade Synthesis of Maillard-Type Taste Enhancers Using Natural Deep Eutectic Solvents (NADES). Molecules 2018, 23, 261. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Euring, M.; Ostendorf, K.; Zhang, K. Biobased Materials for Food Packaging. J. Bioresour. Bioprod. 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Nogueira, G.F.; de Oliveira, R.A.; Velasco, J.I.; Fakhouri, F.M. Methods of Incorporating Plant-Derived Bioactive Compounds into Films Made with Agro-Based Polymers for Application as Food Packaging: A Brief Review. Polymers 2020, 12, 2518. [Google Scholar] [CrossRef]
- Lagos, J.B.; Vargas, F.C.; Oliveira, T.G.; da Aparecida Makishi, G.L.; do Amaral Sobral, P.J. Recent Patents on the Application of Bioactive Compounds in Food: A Short Review. Curr. Opin. Food Sci. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Chaudhary, V.; Thakur, N.; Kajla, P.; Thakur, S.; Punia, S. Application of Encapsulation Technology in Edible Films: Carrier of Bioactive Compounds. Front. Sustain. Food Syst. 2021, 5, 734921. [Google Scholar] [CrossRef]
- Eghbal, N.; Choudhary, R. Complex Coacervation: Encapsulation and Controlled Release of Active Agents in Food Systems. LWT—Food Sci. Technol. 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex Coacervation: Principles, Mechanisms and Applications in Microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A Review of Microencapsulation Methods for Food Antioxidants: Principles, Advantages, Drawbacks and Applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.d.C.R.; de Oliveira, M.S.; Araújo, M.E.; Rodrigues, A.M.C.; Botelho, J.R.S.; da Silva Souza Filho, A.P.; Machado, N.T.; Carvalho, R.N. Supercritical CO2 Extraction of Açaí (Euterpe oleracea) Berry Oil: Global Yield, Fatty Acids, Allelopathic Activities, and Determination of Phenolic and Anthocyanins Total Compounds in the Residual Pulp. J. Supercrit. Fluids 2016, 107, 364–369. [Google Scholar] [CrossRef]
- Santos, G.A.; Carvalho, A.A.C.; Oliveira, A.P.; Naozuka, J.; Matta, F.V.; Felipe-Sotelo, M.; Ward, N.I.; Corrêa, N.C.F.; Nomura, C.S. Bioaccessibility of Essential Elements in Açaí (Euterpe oleracea Mart.) Pulp. ACS Food Sci. Technol. 2021, 1, 874–883. [Google Scholar] [CrossRef]
- Matta, F.V.; Xiong, J.; Lila, M.A.; Ward, N.I.; Felipe-Sotelo, M.; Esposito, D. Chemical Composition and Bioactive Properties of Commercial and Non-Commercial Purple and White Açaí Berries. Foods 2020, 9, 1481. [Google Scholar] [CrossRef]
- Rufino, M.d.S.M.; Pérez-Jiménez, J.; Arranz, S.; Alves, R.E.; de Brito, E.S.; Oliveira, M.S.P.; Saura-Calixto, F. Açaí (Euterpe Oleraceae) “BRS Pará”: A Tropical Fruit Source of Antioxidant Dietary Fiber and High Antioxidant Capacity Oil. Food Res. Int. 2011, 44, 2100–2106. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical Composition, Antioxidant Properties, and Thermal Stability of a Phytochemical Enriched Oil from Açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef]
- Yamaguchi, K.K.D.L.; Pereira, L.F.R.; Lamarão, C.V.; Lima, E.S.; da Veiga-Junior, V.F. Amazon Acai: Chemistry and Biological Activities: A Review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef]
- Rabelo, C.A.S.; Taarji, N.; Khalid, N.; Kobayashi, I.; Nakajima, M.; Neves, M.A. Formulation and Characterization of Water-in-Oil Nanoemulsions Loaded with Açaí Berry Anthocyanins: Insights of Degradation Kinetics and Stability Evaluation of Anthocyanins and Nanoemulsions. Food Res. Int. 2018, 106, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.-J.; Tang, C.-H.; Yin, S.-W.; Yin, Y.; Yang, X.-Q.; Wu, L.-Y.; Zhao, Z.-G. Development and Characterization of Novel Chitosan Emulsion Films via Pickering Emulsions Incorporation Approach. Food Hydrocoll. 2016, 52, 253–264. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Silva Pereira, B.C.; Lopes, G.K.; Tristão Andrade, C. Encapsulation and Antioxidant Activity of Assai Pulp Oil (Euterpe oleracea) in Chitosan/Alginate Polyelectrolyte Complexes. Food Hydrocoll. 2020, 109, 106097. [Google Scholar] [CrossRef]
- Dammak, I.; Bittante, A.M.Q.B.; Lourenço, R.V.; do Amaral Sobral, P.J. Properties of Gelatin-Based Films Incorporated with Chitosan-Coated Microparticles Charged with Rutin. Int. J. Biol. Macromol. 2017, 101, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.M.; Yoksan, R. Morphological Characteristics and Barrier Properties of Thermoplastic Starch/Chitosan Blown Film. Carbohydr. Polym. 2016, 150, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Rodrigues, L.C.; Fernandes, E.M.; Gomes, J.M.; Vilas-Boas, Â.; Pirraco, R.P.; Reis, R.L. Approach on Chitosan/Virgin Coconut Oil-Based Emulsion Matrices as a Platform to Design Superabsorbent Materials. Carbohydr. Polym. 2020, 249, 116839. [Google Scholar] [CrossRef] [PubMed]
- Moschakis, T.; Biliaderis, C.G. Biopolymer-Based Coacervates: Structures, Functionality and Applications in Food Products. Curr. Opin. Colloid Interface Sci. 2017, 28, 96–109. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between Alginate and Chitosan Biopolymers Characterized Using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- Sokolova, M.P.; Smirnov, M.A.; Samarov, A.A.; Bobrova, N.V.; Vorobiov, V.K.; Popova, E.N.; Filippova, E.; Geydt, P.; Lahderanta, E.; Toikka, A.M. Plasticizing of Chitosan Films with Deep Eutectic Mixture of Malonic Acid and Choline Chloride. Carbohydr. Polym. 2018, 197, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Rohman, A.; Man, Y.B.C. Fourier Transform Infrared (FTIR) Spectroscopy for Analysis of Extra Virgin Olive Oil Adulterated with Palm Oil. Food Res. Int. 2010, 43, 886–892. [Google Scholar] [CrossRef]
- Delgado-Mellado, N.; Larriba, M.; Navarro, P.; Rigual, V.; Ayuso, M.; García, J.; Rodríguez, F. Thermal Stability of Choline Chloride Deep Eutectic Solvents by TGA/FTIR-ATR Analysis. J. Mol. Liq. 2018, 260, 37–43. [Google Scholar] [CrossRef]
- Moalla, S.; Ammar, I.; Fauconnier, M.-L.; Danthine, S.; Blecker, C.; Besbes, S.; Attia, H. Development and Characterization of Chitosan Films Carrying Artemisia Campestris Antioxidants for Potential Use as Active Food Packaging Materials. Int. J. Biol. Macromol. 2021, 183, 254–266. [Google Scholar] [CrossRef]
- Qiao, C.; Ma, X.; Wang, X.; Liu, L. Structure and Properties of Chitosan Films: Effect of the Type of Solvent Acid. LWT—Food Sci. Technol. 2021, 135, 109984. [Google Scholar] [CrossRef]
- Madian, N.G.; Mohamed, N. Enhancement of the Dynamic Mechanical Properties of Chitosan Thin Films by Crosslinking with Greenly Synthesized Silver Nanoparticles. J. Mater. Res. Technol. 2020, 9, 12970–12975. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and Characterization of Chitosan Film Incorporated with Thinned Young Apple Polyphenols as an Active Packaging Material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Wong, W.Y.; Walvekar, R.; Loh, K.S.; Khalid, M.; Lim, K.L. Effect of Deep Eutectic Solvent in Proton Conduction and Thermal Behaviour of Chitosan-Based Membrane. J. Mol. Liq. 2018, 269, 675–683. [Google Scholar] [CrossRef]
- Escárcega-Galaz, A.A.; Sánchez-Machado, D.I.; López-Cervantes, J.; Sanches-Silva, A.; Madera-Santana, T.J.; Paseiro-Losada, P. Characterization Data of Chitosan-Based Films: Antimicrobial Activity, Thermal Analysis, Elementary Composition, Tensile Strength and Degree Crystallinity. Data Br. 2018, 21, 473–479. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, Y.; Zhang, Y.; Yu, C.; Cao, S. Preparation and Structural Analysis of Chitosan Films with and without Sorbitol. Food Hydrocoll. 2013, 33, 186–191. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of Chitosan Concentration on Mechanical and Barrier Properties of Corn Starch/Chitosan Films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Karbowiak, T.; Debeaufort, F. Bioactive Edible Films for Food Applications:Influence of the Bioactive Compounds on Film Structure and Properties. Crit. Rev. Food Sci. Nutr. 2017, 59, 1137–1153. [Google Scholar] [CrossRef] [PubMed]
- Tuhin, M.O.; Rahman, N.; Haque, M.E.; Khan, R.A.; Dafader, N.C.; Islam, R.; Nurnabi, M.; Tonny, W. Modification of Mechanical and Thermal Property of Chitosan–Starch Blend Films. Radiat. Phys. Chem. 2012, 81, 1659–1668. [Google Scholar] [CrossRef]
- Kamdem, D.P.; Shen, Z.; Nabinejad, O. Development of Biodegradable Composite Chitosan-Based Films Incorporated with Xylan and Carvacrol for Food Packaging Application. Food Packag. Shelf Life 2019, 21, 100344. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro Antibacterial and Antioxidant Properties of Chitosan Edible Films Incorporated with Thymus moroderi or Thymus Piperella Essential Oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical Properties and Antioxidant Activity of an Active Film from Chitosan Incorporated with Green Tea Extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Vitchayakitti, W. Improving Functional Properties of Chitosan Films as Active Food Packaging by Incorporating with Propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- Cuevas-Acuña, D.A.; Ruiz-Cruz, S.; Arias-Moscoso, J.L.; Lopez-Mata, M.A.; Zamudio-Flores, P.B.; Burruel-Ibarra, S.E.; Carmen Santacruz-Ortega, H. Effects of the Addition of Ultrasound-pulsed Gelatin to Chitosan on Physicochemical and Antioxidant Properties of Casting Films. Polym. Int. 2020, 69, 423–428. [Google Scholar] [CrossRef]
- Yang, T.S.; Liu, T.T.; Lin, I.H. Functionalities of Chitosan Conjugated with Stearic Acid and Gallic Acid and Application of the Modified Chitosan in Stabilizing Labile Aroma Compounds in an Oil-in-Water Emulsion. Food Chem. 2017, 228, 541–549. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango Leaf Extract Incorporated Chitosan Antioxidant Film for Active Food Packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef]
- Rui, L.; Xie, M.; Hu, B.; Zhou, L.; Yin, D.; Zeng, X. A Comparative Study on Chitosan/Gelatin Composite Films with Conjugated or Incorporated Gallic Acid. Carbohydr. Polym. 2017, 173, 473–481. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Razavi Rohani, S.M.; Oromiehie, A.R.; Malekinejad, H.; Aliakbarlu, J.; Hadian, M. Characterization of Antioxidant Chitosan Film Incorporated with Zataria multiflora Boiss Essential Oil and Grape Seed Extract. LWT—Food Sci. Technol. 2012, 46, 477–484. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and Characterization of Chitosan-Based Antimicrobial Active Food Packaging Film Incorporated with Apple Peel Polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.P.; Ferreira, W.H.; Gouvêa, R.F.; Andrade, C.T. Effect of Chitosan on the Properties of Electrospun Fibers From Mixed Poly(Vinyl Alcohol)/Chitosan Solutions. Mater. Res. 2017, 20, 984–993. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Hayyan, M.; Alsaadi, M.A.; Akib, S.; Hayyan, A.; Hashim, M.A. Glycerol-Based Deep Eutectic Solvents: Physical Properties. J. Mol. Liq. 2016, 215, 98–103. [Google Scholar] [CrossRef]
- American Society for Testing and Materials, ASTM. Standard Test Methods for Water Vapor Transmission of Materials 1. In ASTM; ASTM International: West Conshohocken, PA, USA, 2016; Volume 14, pp. 1–10. [Google Scholar]
- Ferreira, W.H.; Andrade, C.T. Characterization of Glycerol-Plasticized Starch and Graphene Oxide Extruded Hybrids. Ind. Crop. Prod. 2015, 77, 684–690. [Google Scholar] [CrossRef]
- Jost, V.; Langowski, H.C. Effect of Different Plasticisers on the Mechanical and Barrier Properties of Extruded Cast PHBV Films. Eur. Polym. J. 2015, 68, 302–312. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lotito, S.B.; Frei, B. The Increase in Human Plasma Antioxidant Capacity after Apple Consumption Is Due to the Metabolic Effect of Fructose on Urate, Not Apple-Derived Antioxidant Flavonoids. Free Radic. Biol. Med. 2004, 37, 251–258. [Google Scholar] [CrossRef] [PubMed]

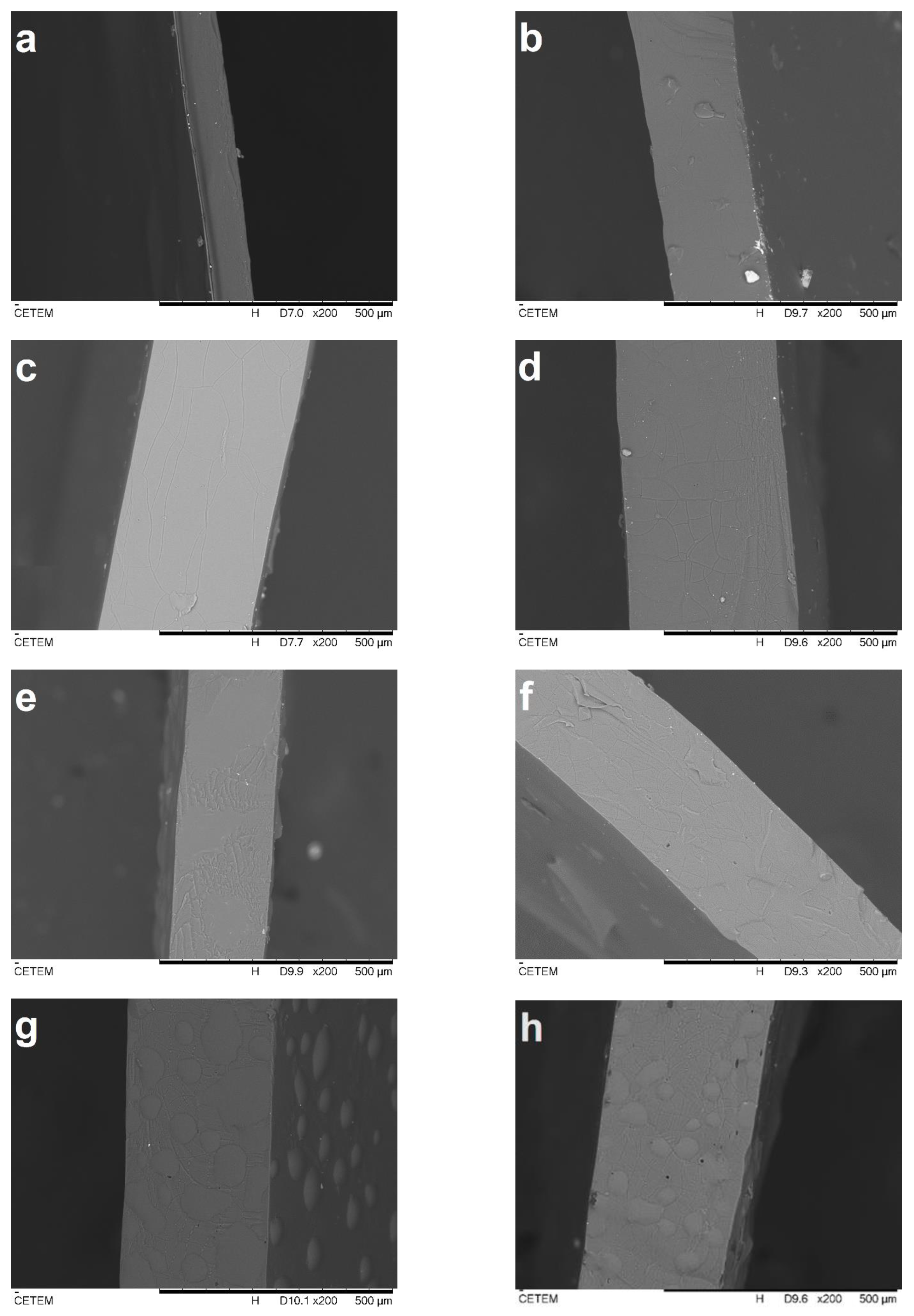
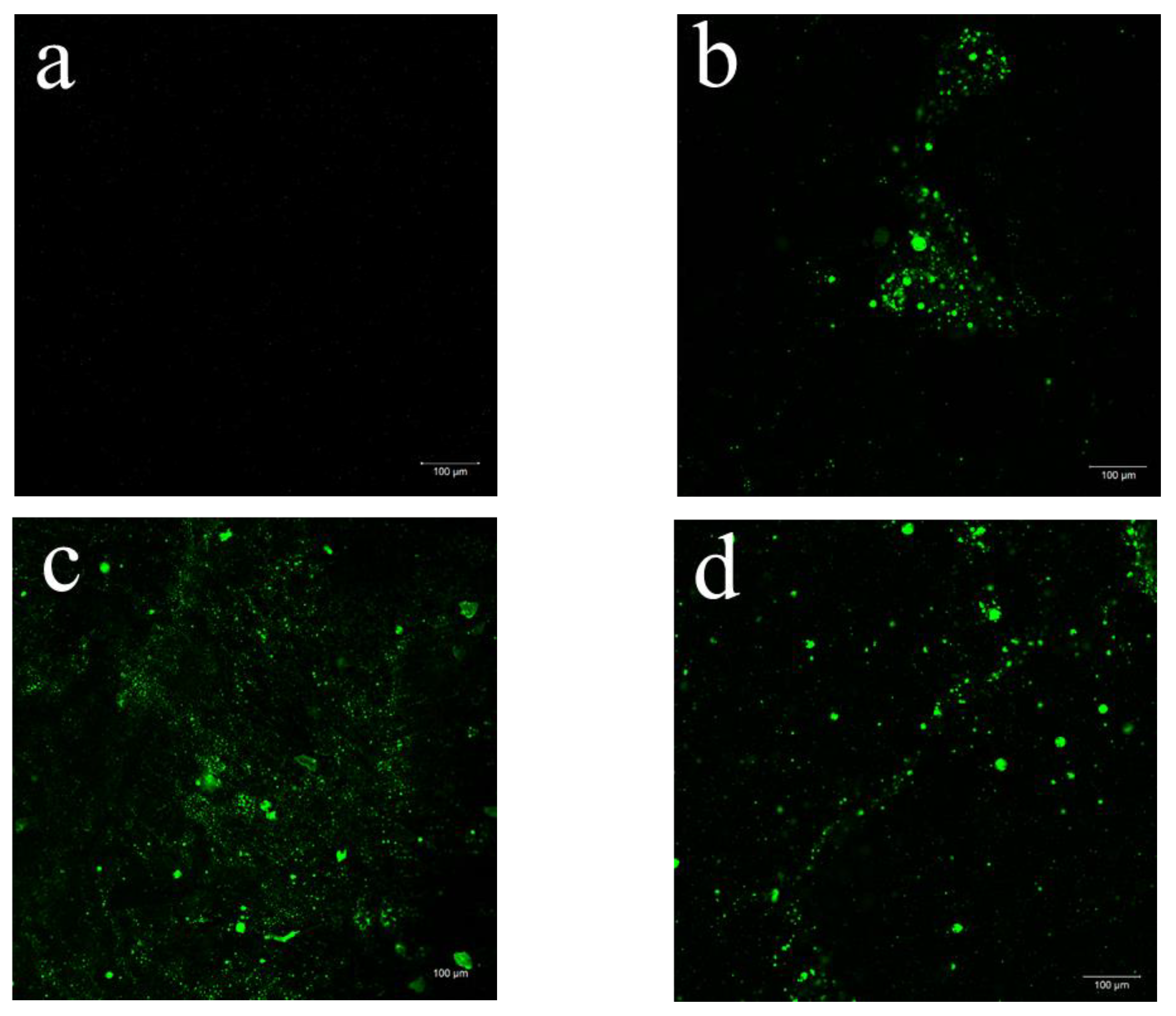
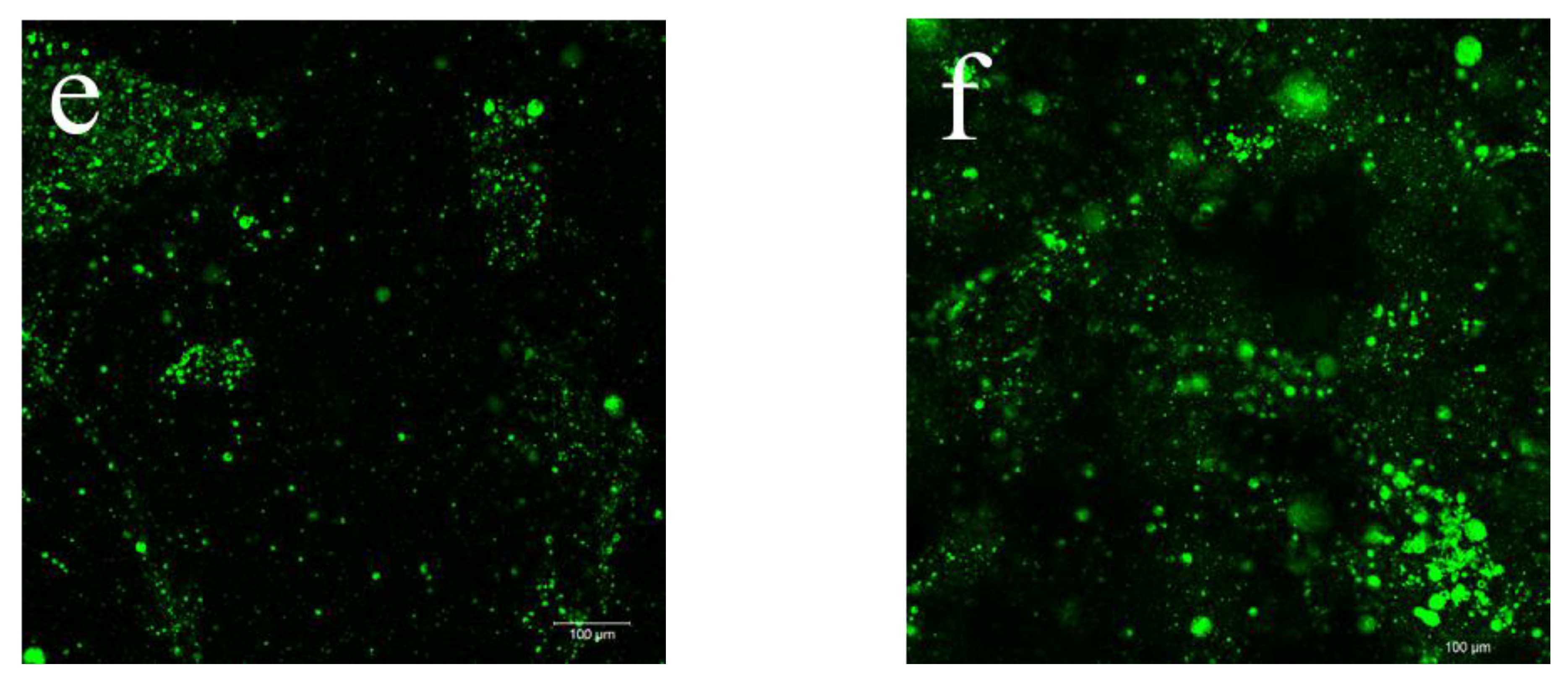
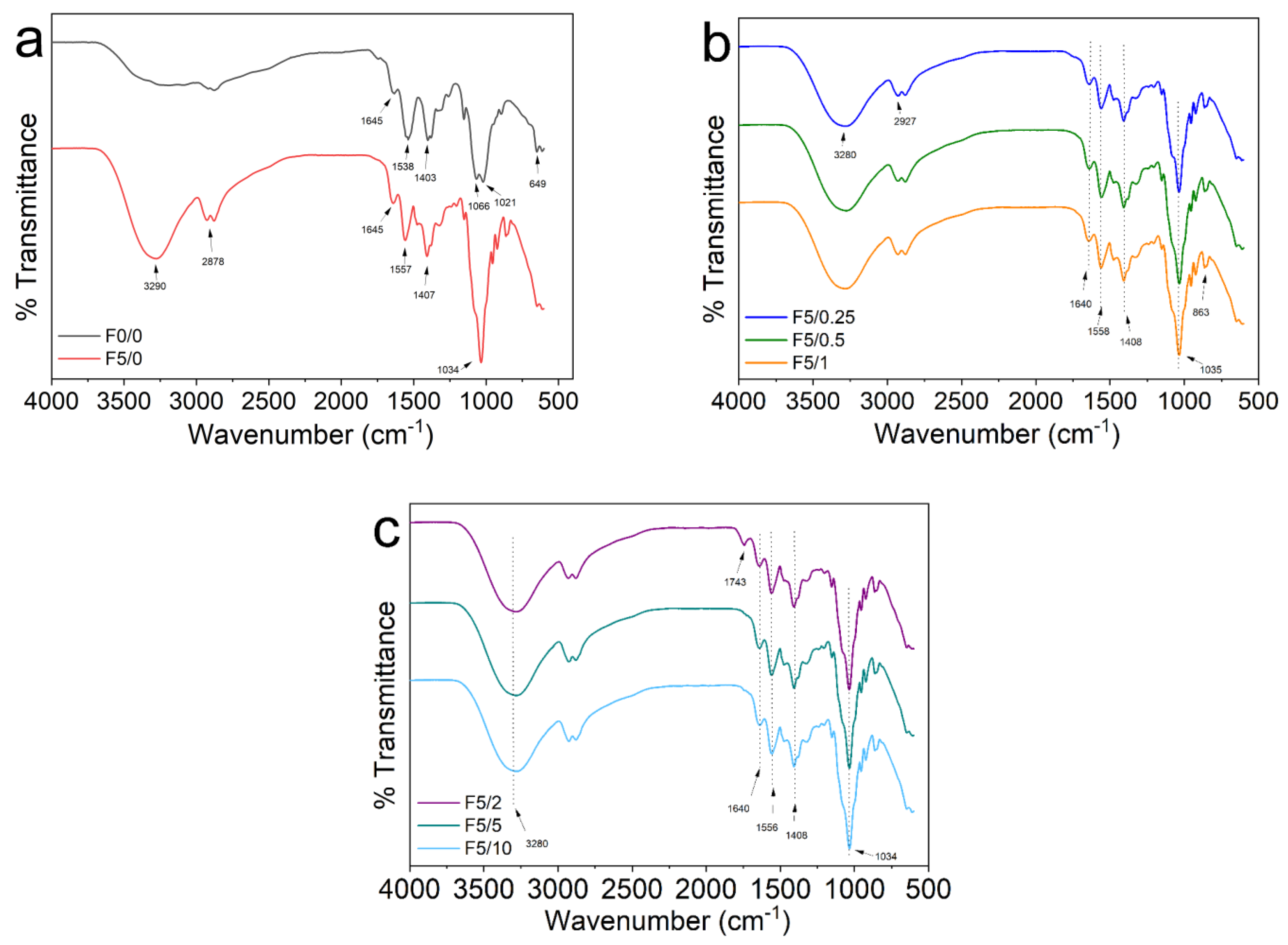



| Sample | DES (%) | Açaí PECs (%) | Thickness (mm) | WVP (g m−1 s−1 Pa−1) |
|---|---|---|---|---|
| F0/0 | 0 | 0 | 0.16 ± 0.02 c | 1.1 × 10−10 ± 1.0 × 10−11 c |
| F5/0 | 5 | 0 | 0.19 ± 0.03 bc | 1.9 × 10−10 ± 9.9 × 10−12 bc |
| F5/0.25 | 5 | 0.25 | 0.36 ± 0.02 a | 3.9 × 10−10 ± 3.2 × 10−11 a |
| F5/0.5 | 5 | 0.50 | 0.34 ± 0.01 a | 3.4 × 10−10 ± 2.3 × 10−11 ab |
| F5/1 | 5 | 1 | 0.33 ± 0.01 a | 3.9 × 10−10 ± 5.3 × 10−11 a |
| F5/2 | 5 | 2 | 0.27 ± 0.05 abc | 4.2 × 10−10 ± 9.9 × 10−11 a |
| F5/5 | 5 | 5 | 0.28 ± 0.04 abc | 2.9 × 10−10 ± 5.0 × 10−12 ab |
| F5/10 | 5 | 10 | 0.29 ± 0.06 ab | 3.4 × 10−10 ± 4.0 × 10−11 ab |
| Sample | (%) | TS (MPa) | εmax (%) | YM (MPa) |
|---|---|---|---|---|
| F0/0 | 7.95 | 11.0 ± 1.5 a | 0.60 ± 0.1 d | 2927 ± 154 a |
| F5/0 | 4.35 | 0.74 ± 0.07 b | 36.4 ± 1.9 c | 1.7 ± 0.1 b |
| F5/0.25 | 4.45 | 1.20 ± 0.04 b | 89.2 ± 3.7 a | 1.7 ± 0.4 b |
| F5/0.5 | 4.13 | 1.26 ± 0.16 b | 99.3 ± 4.5 a | 1.6 ± 0.4 b |
| F5/1 | 3.93 | 1.13 ± 0.24 b | 83.0 ± 9.6 a | 1.7 ± 0.2 b |
| F5/2 | 4.94 | 1.47 ± 0.31 b | 103.6 ± 8.4 a | 1.7 ± 0.2 b |
| F5/5 | 5.23 | 0.63 ± 0.16 b | 52.0 ± 7.1 b | 1.4 ± 0.3 b |
| F5/10 | 5.74 | 0.66 ± 0.08 b | 49.3 ± 2.3 b,c | 1.35 ± 0.06 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira-Costa, B.E.; Ferreira, W.H.; Goycoolea, F.M.; Murray, B.S.; Andrade, C.T. Improved Antioxidant and Mechanical Properties of Food Packaging Films Based on Chitosan/Deep Eutectic Solvent, Containing Açaí-Filled Microcapsules. Molecules 2023, 28, 1507. https://doi.org/10.3390/molecules28031507
Teixeira-Costa BE, Ferreira WH, Goycoolea FM, Murray BS, Andrade CT. Improved Antioxidant and Mechanical Properties of Food Packaging Films Based on Chitosan/Deep Eutectic Solvent, Containing Açaí-Filled Microcapsules. Molecules. 2023; 28(3):1507. https://doi.org/10.3390/molecules28031507
Chicago/Turabian StyleTeixeira-Costa, Barbara E., Willian Hermogenes Ferreira, Francisco M. Goycoolea, Brent S. Murray, and Cristina T. Andrade. 2023. "Improved Antioxidant and Mechanical Properties of Food Packaging Films Based on Chitosan/Deep Eutectic Solvent, Containing Açaí-Filled Microcapsules" Molecules 28, no. 3: 1507. https://doi.org/10.3390/molecules28031507
APA StyleTeixeira-Costa, B. E., Ferreira, W. H., Goycoolea, F. M., Murray, B. S., & Andrade, C. T. (2023). Improved Antioxidant and Mechanical Properties of Food Packaging Films Based on Chitosan/Deep Eutectic Solvent, Containing Açaí-Filled Microcapsules. Molecules, 28(3), 1507. https://doi.org/10.3390/molecules28031507








