Characterization and Optimization of Salep Mucilage Bionanocomposite Films Containing Allium jesdianum Boiss. Nanoliposomes for Antibacterial Food Packaging Utilization
Abstract
Highlights
- Salep mucilage and NLP/AEO were used to develop an antibacterial bionanocomposite film.
- A thin-layer hydration method was used to produce antibacterial NLP/AEO.
- The incorporation of AEO and NLP/AEO affected surface morphology, physicochemical, barrier, and mechanical properties of the Salep mucilage bionanocomposite film.
- Encapsulating AEO into NLP decreased the AEO release rate from the Salep mucilage bionanocomposite film.
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Essential Oil Extraction and Characterization
2.3. Nanoliposome Preparation and Characterization
2.3.1. Determination of Size and Zeta Potential
2.3.2. Encapsulation Efficiency
2.3.3. Morphology
2.4. Preparation of Bionanocomposite Films and Characterization
2.4.1. Color Parameters
2.4.2. Physical Properties
Film Thickness
Moisture Content (MC)
Water Solubility (WS)
Transparency
Wettability
2.4.3. Permeability Properties
Water Vapor Permeability (WVP)
Oxygen Permeability (O2P)
2.4.4. Mechanical Properties
2.4.5. Release Properties
2.4.6. Antibacterial Properties
3. Results
3.1. Identification of AEO Components
3.2. Characterization of NLP/AEO
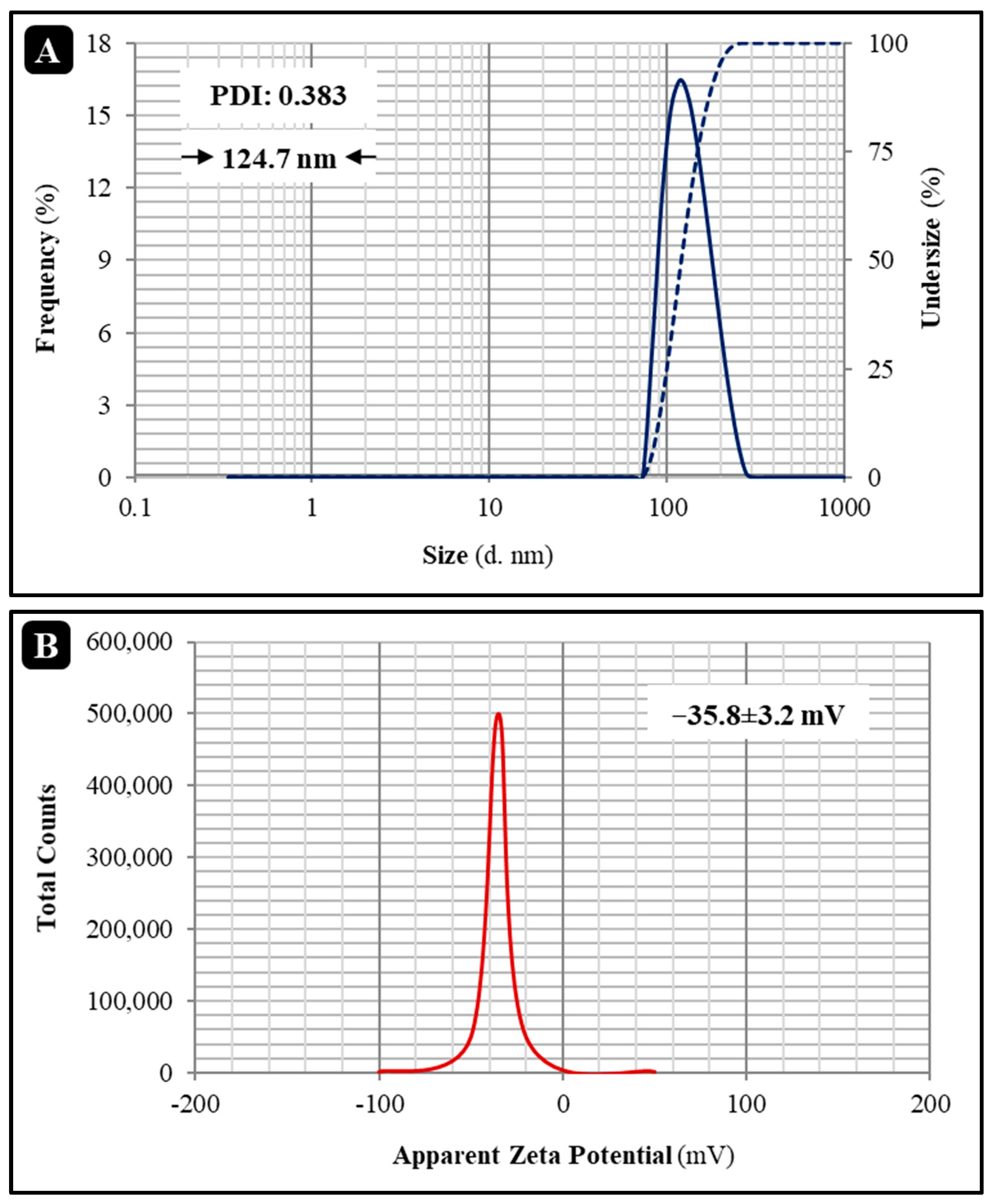
3.2.1. Determination of Size, Zeta Potential, and Encapsulation Efficiency
3.2.2. Morphology
3.3. Performance Analysis of the Film
3.3.1. Color Parameters
3.3.2. Physical Properties
3.3.3. Mechanical Properties
3.3.4. Permeability Properties
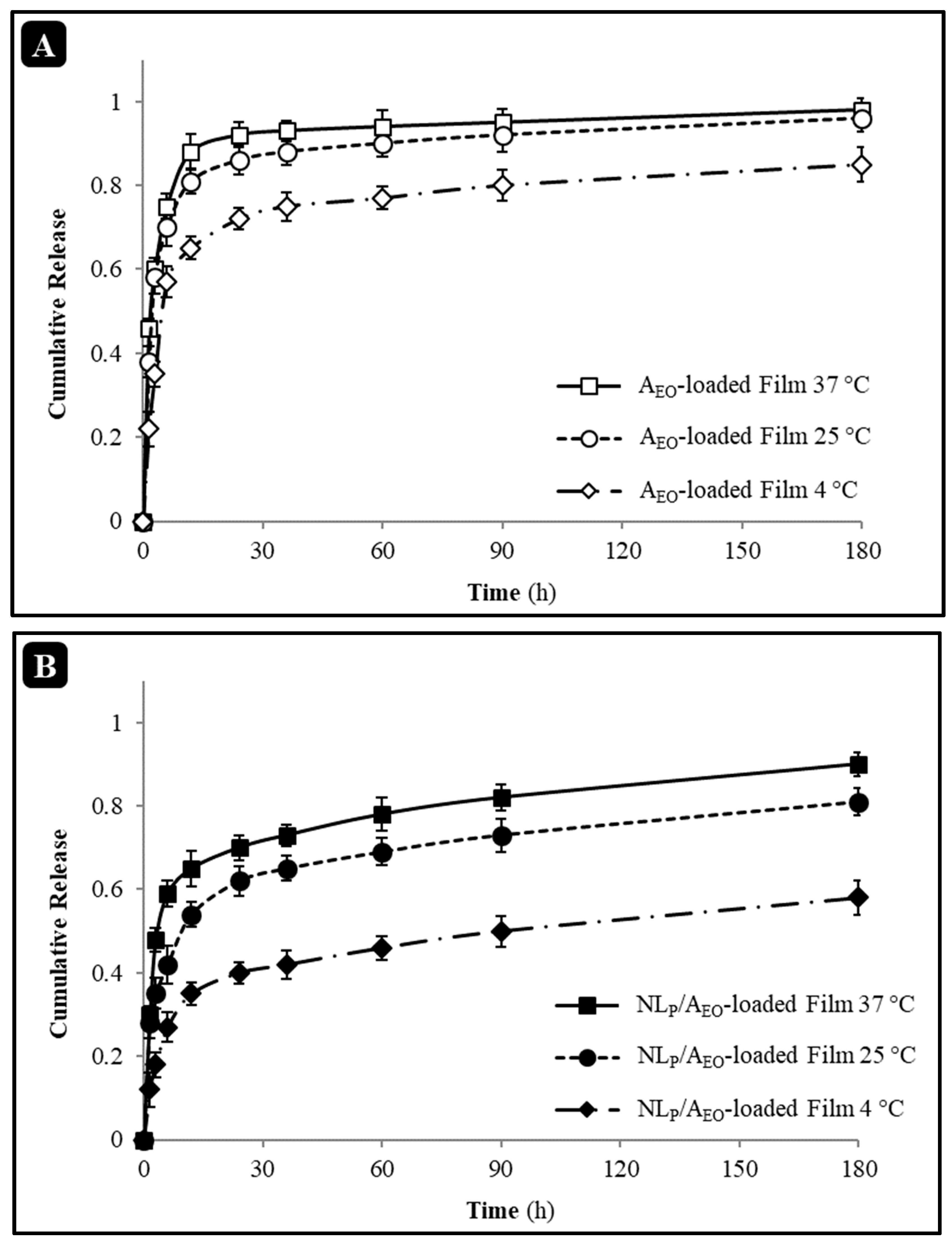
3.3.5. In Vitro Release
3.3.6. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.M.; Cerqueira, M.A.; Rodrigues, C.; Figueira, C.; Teixeira, J. Development and Characterization of Edible Films from Polysaccharides of Different Sources. In Proceedings of the 12nd International Meeting of the Portuguese Carbohydrate Chemistry Group, Aveiro, Portugal, 11–13 September 2017. [Google Scholar]
- Farhoosh, R.; Riazi, A. A compositional study on two current types of salep in Iran and their rheological properties as a function of concentration and temperature. Food Hydrocoll. 2007, 21, 660–666. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Soleyman, R.; Barajee, G.R. Novel nanoporous superabsorbent hydrogel based on poly (acrylic acid) grafted onto salep: Synthesis and swelling behavior. Starch Stärke 2008, 60, 467–475. [Google Scholar] [CrossRef]
- Silvestre, W.; Livinalli, N.; Baldasso, C.; Tessaro, I. Pervaporation in the separation of essential oil components: A review. Trends Food Sci. Technol. 2019, 93, 42–52. [Google Scholar] [CrossRef]
- Gholami, A.; Arabestani, M.R.; Ahmadi, M. Evaluation of antibacterial activity of aqueous and methanol extracts of Allium jesdianum plant on a number of pathogenic bacteria resistant to antibiotics. Pajouhan Sci. J. 2016, 14, 18–26. [Google Scholar] [CrossRef]
- Naeini, A.; Yaraee, R.; Shokri, H. Antifungal and immunomodulatory activity of Allium jesdianum Boiss extracts. J. Herbmed Pharmacol. 2019, 9, 75–80. [Google Scholar] [CrossRef]
- Sohrabinezhad, Z.; Dastan, D.; Asl, S.S.; Nili-Ahmadabadi, A. Allium jesdianum extract improve acetaminophen-induced hepatic failure through inhibition of oxidative/nitrosative stress. J. Pharmacopunct. 2019, 22, 239. [Google Scholar] [CrossRef]
- Yazdanian, E.; Golkar, P.; Vahabi, M.R.; Taghizadeh, M. Elicitation Effects on Some Secondary Metabolites and Antioxidant Activity in Callus Cultures of Allium jesdianum Boiss. & Buhse.: Methyl Jasmonate and Putrescine. Appl. Biochem. Biotechnol. 2022, 194, 601–619. [Google Scholar]
- Nahr, F.K.; Ghanbarzadeh, B.; Hamishehkar, H.; Kafil, H.S.; Hoseini, M.; Moghadam, B.E. Investigation of physicochemical properties of essential oil loaded nanoliposome for enrichment purposes. LWT 2019, 105, 282–289. [Google Scholar] [CrossRef]
- Zamani, P.; Momtazi-Borojeni, A.A.; Nik, M.E.; Oskuee, R.K.; Sahebkar, A. Nanoliposomes as the adjuvant delivery systems in cancer immunotherapy. J. Cell. Physiol. 2018, 233, 5189–5199. [Google Scholar] [CrossRef]
- Lin, L.; Chen, W.; Li, C.; Cui, H. Enhancing stability of Eucalyptus citriodora essential oil by solid nanoliposomes encapsulation. Ind. Crops Prod. 2019, 140, 111615. [Google Scholar] [CrossRef]
- Ekrami, A.; Ghadermazi, M.; Ekrami, M.; Hosseini, M.A.; Emam-Djomeh, Z.; Hamidi-Moghadam, R. Development and evaluation of Zhumeria majdae essential oil-loaded nanoliposome against multidrug-resistant clinical pathogens causing nosocomial infection. J. Drug Deliv. Sci. Technol. 2022, 69, 103148. [Google Scholar] [CrossRef]
- Aguilar-Pérez, K.M.; Medina, D.I.; Narayanan, J.; Parra-Saldívar, R.; Iqbal, H. Synthesis and nano-sized characterization of bioactive oregano essential oil molecule-loaded small unilamellar nanoliposomes with antifungal potentialities. Molecules 2021, 26, 2880. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, M.; Dureja, H. Recent developments in the formulation of nanoliposomal delivery systems. Curr. Nanomater. 2018, 3, 62–74. [Google Scholar] [CrossRef]
- Lopez-Polo, J.; Monasterio, A.; Cantero-López, P.; Osorio, F.A. Combining edible coatings technology and nanoencapsulation for food application: A brief review with an emphasis on nanoliposomes. Food Res. Int. 2021, 145, 110402. [Google Scholar] [CrossRef]
- Homayounpour, P.; Shariatifar, N.; Alizadeh-Sani, M. Development of nanochitosan-based active packaging films containing free and nanoliposome caraway (Carum carvi L.) seed extract. Food Sci. Nutr. 2021, 9, 553–563. [Google Scholar] [CrossRef]
- Chavoshi, F.; Didar, Z.; Vazifedoost, M.; Shahidi Noghabi, M.; Zendehdel, A. Psyllium seed gum films loading Oliveria decumbens essential oil encapsulated in nanoliposomes: Preparation and characterization. J. Food Meas. Charact. 2022, 1–13. [Google Scholar] [CrossRef]
- Ghasempour, Z.; Khodaeivandi, S.; Ahangari, H.; Hamishehkar, H.; Amjadi, S.; Moghaddas Kia, E.; Ehsani, A. Characterization and optimization of Persian gum/whey protein bionanocomposite films containing betanin nanoliposomes for food packaging utilization. J. Polym. Environ. 2022, 30, 2800–2811. [Google Scholar] [CrossRef]
- Ekrami, M.; Emam-Djomeh, Z.; Ghoreishy, S.A.; Najari, Z.; Shakoury, N. Characterization of a high-performance edible film based on Salep mucilage functionalized with pennyroyal (Mentha pulegium). Int. J. Biol. Macromol. 2019, 133, 529–537. [Google Scholar] [CrossRef]
- Arabi, M.H.; Chabok, H.; Mirzapour, A.; Ardestani, M.S.; Saffari, M. Preparation of nanoliposomes containing Rosmarinus officinalis L. essential oil: A comparative study. Biosci. Biotech. Res. Comm 2017, 10, 103–108. [Google Scholar] [CrossRef]
- Ekrami, M.; Emam-Djomeh, Z.; Joolaei-Ahranjani, P.; Mahmoodi, S.; Khaleghi, S. Eco-friendly UV protective bionanocomposite based on Salep-mucilage/flower-like ZnO nanostructures to control photo-oxidation of kilka fish oil. Int. J. Biol. Macromol. 2021, 168, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Ekrami, M.; Emam-Djomeh, Z. Water vapor permeability, optical and mechanical properties of salep-based edible film. J. Food Process. Preserv. 2014, 38, 1812–1820. [Google Scholar] [CrossRef]
- ASTM D 1746; Standard Test Method for Transparency of Plastic Sheeting. American Society for Testing and Materials: Philadelphia, PA, USA, 1997.
- ASTM E96-95; Standard Test Methods for Water Vapor Transmission of Material. American Society for Testing and Materials: Philadelphia, PA, USA, 1995.
- ASTM D3985; Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. American Society for Testing and Materials: Philadelphia, PA, USA, 2010.
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. Standard Designations. American Society for Testing and Materials: Philadelphia, PA, USA, 2001.
- Aziz, S.G.-G.; Almasi, H. Physical characteristics, release properties, and antioxidant and antimicrobial activities of whey protein isolate films incorporated with thyme (Thymus vulgaris L.) extract-loaded nanoliposomes. Food Bioprocess Technol. 2018, 11, 1552–1565. [Google Scholar] [CrossRef]
- Askari, Y. Composition of essential oil of Dorema aucheri Boiss. and Allium jesdianum Boiss. medicinal plants. Int. J. Adv. Biol. Biomed. Res. 2022, 10, 72–83. [Google Scholar]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Al-Sadi, A.M. Biosynthesis of essential oils in aromatic plants: A review. Food Rev. Int. 2016, 32, 117–160. [Google Scholar] [CrossRef]
- Cui, H.; Li, W.; Li, C.; Vittayapadung, S.; Lin, L. Liposome containing cinnamon oil with antibacterial activity against methicillin-resistant Staphylococcus aureus biofilm. Biofouling 2016, 32, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Kalantari, M.; Raji, M.; Fekri, H.S.; Saber, R.; Asnani, G.; Mortazavi, S.; Mozafari, M.; Rasti, B.; Taheriazam, A. Probing nanoliposomes using single particle analytical techniques: Effect of excipients, solvents, phase transition and zeta potential. Heliyon 2018, 4, e01088. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Bouarab, L.; Maherani, B.; Kheirolomoom, A.; Hasan, M.; Aliakbarian, B.; Linder, M.; Arab-Tehrany, E. Influence of lecithin–lipid composition on physico-chemical properties of nanoliposomes loaded with a hydrophobic molecule. Colloids Surf. B Biointerfaces 2014, 115, 197–204. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Ge, S.; Wang, S.; Qin, Z.; Chen, L.; Zheng, Q.; Liu, Q.; Zhang, Q. The preparation, characterization, antimicrobial stability and in vitro release evaluation of fish gelatin films incorporated with cinnamon essential oil nanoliposomes. Food Hydrocoll. 2015, 43, 427–435. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tajik, S.; Shojaee-Aliabadi, S.; Ghasemlou, M.; Moayyed, H.; Khaksar, R.; Noghabi, M.S. Development of new active packaging film made from a soluble soybean polysaccharide incorporated Zataria multiflora Boiss and Mentha pulegium essential oils. Food Chem. 2014, 146, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Jouki, M.; Mortazavi, S.A.; Yazdi, F.T.; Koocheki, A. Characterization of antioxidant–antibacterial quince seed mucilage films containing thyme essential oil. Carbohydr. Polym. 2014, 99, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Hafsa, J.; ali Smach, M.; Khedher, M.R.B.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT-Food Sci. Technol. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Vedove, T.M.; Maniglia, B.C.; Tadini, C.C. Production of sustainable smart packaging based on cassava starch and anthocyanin by an extrusion process. J. Food Eng. 2021, 289, 110274. [Google Scholar] [CrossRef]
- Fathi, N.; Almasi, H.; Pirouzifard, M.K. Sesame protein isolate based bionanocomposite films incorporated with TiO2 nanoparticles: Study on morphological, physical and photocatalytic properties. Polym. Test. 2019, 77, 105919. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Physico-chemical properties, morphology and antioxidant activity of film from fish skin gelatin incorporated with root essential oils. J. Food Eng. 2013, 117, 350–360. [Google Scholar] [CrossRef]
- Hosseini, M.; Razavi, S.; Mousavi, M. Antimicrobial, physical and mechanical properties of chitosan-based films incorporated with thyme, clove and cinnamon essential oils. J. Food Process. Preserv. 2009, 33, 727–743. [Google Scholar] [CrossRef]
- Pérez-Gago, M.B.; Krochta, J.M. Lipid particle size effect on water vapor permeability and mechanical properties of whey protein/beeswax emulsion films. J. Agric. Food Chem. 2001, 49, 996–1002. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Aliheidari, N.; Fahmi, R.; Shojaee-Aliabadi, S.; Keshavarz, B.; Cran, M.J.; Khaksar, R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr. Polym. 2013, 98, 1117–1126. [Google Scholar] [CrossRef]
- Kester, J.J.; Fennema, O. Edible films and coatings: A review. Food Technol. 1986, 40, 47–59. [Google Scholar]
- McHugh, T.H.; Krochta, J.M. Sorbitol-vs glycerol-plasticized whey protein edible films: Integrated oxygen permeability and tensile property evaluation. J. Agric. Food Chem. 1994, 42, 841–845. [Google Scholar] [CrossRef]
- Salari, S.; Salari, R. Nanoliposomal system of rosemary essential oil made by specific human cell phospholipids and evaluation of its anti-cancer properties. Appl. Nanosci. 2019, 9, 2085–2089. [Google Scholar] [CrossRef]
- Karim, N.; Shishir, M.R.I.; Chen, W. Surface decoration of neohesperidin-loaded nanoliposome using chitosan and pectin for improving stability and controlled release. Int. J. Biol. Macromol. 2020, 164, 2903–2914. [Google Scholar] [CrossRef] [PubMed]
- Seyedabadi, M.M.; Rostami, H.; Jafari, S.M.; Fathi, M. Development and characterization of chitosan-coated nanoliposomes for encapsulation of caffeine. Food Biosci. 2021, 40, 100857. [Google Scholar] [CrossRef]
- Savaghebi, D.; Barzegar, M.; Mozafari, M.R. Manufacturing of nanoliposomal extract from Sargassum boveanum algae and investigating its release behavior and antioxidant activity. Food Sci. Nutr. 2020, 8, 299–310. [Google Scholar] [CrossRef]
- Risaliti, L.; Pini, G.; Ascrizzi, R.; Donato, R.; Sacco, C.; Bergonzi, M.C.; Salvatici, M.C.; Bilia, A.R. Artemisia annua essential oil extraction, characterization, and incorporation in nanoliposomes, smart drug delivery systems against Candida species. J. Drug Deliv. Sci. Technol. 2020, 59, 101849. [Google Scholar] [CrossRef]
- Zivanovic, S.; Chi, S.; Draughon, A.F. Antimicrobial activity of chitosan films enriched with essential oils. J. Food Sci. 2005, 70, M45–M51. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Hipwell, M.; Ryan, T.; Cavanagh, H.M. Bioactivity of Backhousia citriodora: Antibacterial and antifungal activity. J. Agric. Food Chem. 2003, 51, 76–81. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Ru, J.; Qian, X.; Wang, Y. Study on antibacterial finishing of cotton fabric with silver nanoparticles stabilized by nanoliposomes. Cellulose 2018, 25, 5443–5454. [Google Scholar] [CrossRef]
- NAJAF NAJAFI, M.; Arianmehr, A.; Sani, A.M. Preparation of Barije (Ferula gummosa) Essential Oil–Loaded Liposomes and Evaluation of Physical and Antibacterial Effect on Escherichia coli O157: H7. J. Food Prot. 2020, 83, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.; Shafaghi Rad, M. Encapsulation of Tribulus terrestris and Valeriana officinalis Extracts in Nanoliposomes and Evaluation of its Antibacterial and Antioxidant Properties. J. Food Biosci. Technol. 2021, 11, 59–68. [Google Scholar]
- Jabraeili, S.; Mirzaei, H.; Anarjan, N.; Javadi, A.; Behnajady, M.A. Nanoliposomal thyme (Thymus vulgaris) essential oil: Effects of formulation parameters. Food Sci. Technol. Int. 2022, 28, 257–272. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef] [PubMed]
- Heckler, C.; Silva, C.M.M.; Cacciatore, F.A.; Daroit, D.J.; da Silva Malheiros, P. Thymol and carvacrol in nanoliposomes: Characterization and a comparison with free counterparts against planktonic and glass-adhered Salmonella. LWT 2020, 127, 109382. [Google Scholar] [CrossRef]



| Compound | RI * | Concentration (%) ** |
|---|---|---|
| Hexenal | 777 | 0.24 |
| 1-Heptanal | 904 | 0.12 |
| Dimethyl trisulfide | 963 | 19.05 |
| Dehydroxy-trans-Linalool oxide | 996 | 0.29 |
| Benzene acetaldehyde | 1039 | 0.41 |
| Linalool | 1086 | 1.28 |
| Dimethyl tetrasulfide | 1110 | 11.84 |
| Trans-propenyl propyl disulfide | 1117 | 2.38 |
| Dipropyl trisulfide | 1129 | 8.11 |
| Methyl 2-propenyl trisulfide | 1140 | 1.85 |
| 1,3,5-Trithiane | 1156 | 0.35 |
| Borneol | 1168 | 2.89 |
| Iso-Verbanol | 1181 | 1.49 |
| Safranal | 1189 | 0.38 |
| Tetradecanoic acid | 1191 | 2.18 |
| n-Decanal | 1196 | 0.73 |
| Neral (Z-Citral) | 1229 | 6.20 |
| Hexadecanoic acid | 1257 | 7.77 |
| Geranyl acetate | 1371 | 1.52 |
| Pentadecanoic acid | 1380 | 2.61 |
| β-Cubenene | 1384 | 0.18 |
| Pentacosane | 1400 | 5.81 |
| β-Caryophyllene | 1433 | 1.23 |
| β-Ionone | 1477 | 0.76 |
| γ-Cadinene | 1515 | 0.29 |
| Caryophyllene oxide | 1572 | 4.33 |
| Epi-α-Muurolol | 1636 | 1.91 |
| α-Cadinol | 1659 | 2.06 |
| n-Heptadecane | 1684 | 0.13 |
| Gereninal (E-Citral) | 1765 | 3.60 |
| (Z,Z)-9,12-Octadecadienoic acid | 1819 | 1.08 |
| Hexacosane | 1848 | 0.93 |
| Nonacosane | 1906 | 0.62 |
| Total identified | 94.62 |
| Samples | L | a | b | ΔE | WI | YI | Transparency (%) |
|---|---|---|---|---|---|---|---|
| Control film | 57.33 ± 3.06 a | 0.51 ± 0.26 c | −0.87 ± 0.42 c | 40.48 ± 3.06 c | 57.32 ± 3.06 a | −2.16 ± 1.03 c | 62.71 ± 1.05 a |
| AEO-loaded Film | 41.00 ± 3.61 c | 10.90 ± 0.46 a | 20.30 ± 1.05 a | 61.31 ± 3.32 a | 36.64 ± 3.32 c | 71.01 ± 5.75 a | 40.32 ± 0.84 c |
| NLP/AEO-loaded Film | 49.67 ± 4.50 b | 3.13 ± 0.61 b | 6.13 ± 0.77 b | 48.63 ± 4.53 b | 49.19 ± 4.53 b | 17.87 ± 3.83 b | 56.01 ± 0.67 b |
| Samples | Thickness (µm) | Contact Angle (°) | Moisture Content (%) | Water Solubility (%) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|---|
| Control film | 86.90 ± 2.19 b | 68.39 ± 3.42 c | 12.89 ± 0.12 a | 50.71 ± 1.48 a | 18.90 ± 1.23 a | 82.14 ± 2.88 b |
| AEO-loaded Film | 92.09 ± 1.33 c | 102.48 ± 3.71 b | 11.08 ± 0.17 b | 45.18 ± 1.22 b | 11.46 ± 1.15 c | 103.51 ± 3.60 a |
| NLP/AEO-loaded Film | 105.42 ± 2.51 a | 124.02 ± 3.37 a | 10.37 ± 0.08 c | 40.91 ± 1.62 c | 16.37 ± 1.02 b | 63.09 ± 2.74 c |
| Microbial Strain | Inhibition Diameter (mm) | ||||
|---|---|---|---|---|---|
| Day | NLP (Negative Control) | AEO | NLP/AEO | Chloramphenicol (Positive Control) | |
| Staphylococcus aureus | 3rd | 6.7 ± 0.3 | 16.3 ± 0.5 | 14.5 ± 0.9 | 20.3 ± 0.5 |
| 30th | 6.3 ± 0.3 | 9.7 ± 0.7 | 12.4 ± 0.8 | 14.6 ± 0.8 | |
| Escherichia coli | 3rd | 6.5 ± 0.4 | 12.9 ± 0.9 | 10.3 ± 0.6 | 15.1 ± 1.1 |
| 30th | 6.2 ± 0.2 | 7.3 ± 0.6 | 9.8 ± 0.5 | 11.2 ± 0.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekrami, M.; Ekrami, A.; Hosseini, M.A.; Emam-Djomeh, Z. Characterization and Optimization of Salep Mucilage Bionanocomposite Films Containing Allium jesdianum Boiss. Nanoliposomes for Antibacterial Food Packaging Utilization. Molecules 2022, 27, 7032. https://doi.org/10.3390/molecules27207032
Ekrami M, Ekrami A, Hosseini MA, Emam-Djomeh Z. Characterization and Optimization of Salep Mucilage Bionanocomposite Films Containing Allium jesdianum Boiss. Nanoliposomes for Antibacterial Food Packaging Utilization. Molecules. 2022; 27(20):7032. https://doi.org/10.3390/molecules27207032
Chicago/Turabian StyleEkrami, Mohammad, Ali Ekrami, Mohammad Ali Hosseini, and Zahra Emam-Djomeh. 2022. "Characterization and Optimization of Salep Mucilage Bionanocomposite Films Containing Allium jesdianum Boiss. Nanoliposomes for Antibacterial Food Packaging Utilization" Molecules 27, no. 20: 7032. https://doi.org/10.3390/molecules27207032
APA StyleEkrami, M., Ekrami, A., Hosseini, M. A., & Emam-Djomeh, Z. (2022). Characterization and Optimization of Salep Mucilage Bionanocomposite Films Containing Allium jesdianum Boiss. Nanoliposomes for Antibacterial Food Packaging Utilization. Molecules, 27(20), 7032. https://doi.org/10.3390/molecules27207032






