Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a highly malignant tumor with an extremely poor prognosis and low survival rate. Due to its inconspicuous symptoms, PDAC is difficult to diagnose early. Most patients are diagnosed in the middle and late stages, losing the opportunity for surgery. Chemotherapy is the main treatment in clinical practice and improves the survival of patients to some extent. However, the improved prognosis is associated with higher side effects, and the overall prognosis is far from satisfactory. In addition to resistance to chemotherapy, PDAC is significantly resistant to targeted therapy and immunotherapy. The failure of multiple treatment modalities indicates great dilemmas in treating PDAC, including high molecular heterogeneity, high drug resistance, an immunosuppressive microenvironment, and a dense matrix. Nanomedicine shows great potential to overcome the therapeutic barriers of PDAC. Through the careful design and rational modification of nanomaterials, multifunctional intelligent nanosystems can be obtained. These nanosystems can adapt to the environment’s needs and compensate for conventional treatments’ shortcomings. This review is focused on recent advances in the use of well-designed nanosystems in different therapeutic modalities to overcome the PDAC treatment dilemma, including a variety of novel therapeutic modalities. Finally, these nanosystems’ bottlenecks in treating PDAC and the prospect of future clinical translation are briefly discussed.
1. Introduction
1.1. Current Status of and Dilemmas in PDAC Treatment
Pancreatic cancer is one of the most aggressive and lethal solid tumors worldwide and is the third leading cause of death in men and women combined, with a very low five-year overall survival rate of 11% [1]. Although the five-year survival rate (9%) [2,3] for pancreatic cancer has increased slightly in the past few years, its incidence has continued to increase by about 1% annually [1], and it is predicted to be the second leading cause of cancer death in the United States by 2030 [4]. Due to the aggressive nature of pancreatic cancer and the difficulty of early diagnosis, the number of deaths and cases is almost equal. According to a report, 496,000 cases of pancreatic cancer were diagnosed worldwide in 2020, including 466,000 deaths [5]. Pancreatic cancers are generally divided into endocrine and exocrine cancers, among which pancreatic ductal adenocarcinoma (PDAC) is an exocrine tumor, accounting for the majority (90%) of all pancreatic cancers [6,7]. PDAC rarely shows clinical symptoms until it develops into the advanced stage, resulting in a delay in diagnosis and treatment [8].
The current clinical treatment for PDAC mainly relies on surgery and chemotherapy. For resectable PDAC, surgery is the first choice, followed by adjuvant chemotherapy. Although surgical treatment improves the five-year survival rate, only 15–20% of cases qualify for surgical resection and the recurrence rate after surgery is as high as 85% [9]. The standard first-line chemotherapy regimen for patients with advanced PDAC is gemcitabine (GEM) plus albumin-bound (nab) paclitaxel or FOLFIRONOX/modified FOLFIRONOX (a combination of 5-fluorouracil (5-FU), leucovorin, irinotecan, and oxaliplatin) [10,11]. Nab-paclitaxel plus GEM had a significant survival benefit compared with GEM alone (8.5 months vs. 6.7 months). The FOLFIRINOX regimen further improved the median survival to 11.1 months [12]. Although these chemotherapy regimens represent a major breakthrough in the treatment of PDAC, they have been associated with more severe side effects. Moreover, although chemotherapy does improve the survival of patients to some extent, it is far from satisfactory, and the overall prognosis of PDAC is still poor. In addition to chemotherapy, researchers are eager to improve outcomes using other therapies, such as radiotherapy, targeted therapy, and immunotherapy. Unfortunately, the results have been disappointing. These therapies, which have been successful in many other types of tumor, have yet to make breakthroughs in PDAC [13].
The failure of multiple treatments indicates that there are complex dilemmas and challenges in the treatment of PDAC. In fact, PDAC is a very unique malignancy. The characteristics of PDAC, such as high molecular heterogeneity [14], drug resistance [15], an immunosuppressive microenvironment [16], a highly fibrotic dense matrix [16], and high hypoxia [17], pose great difficulties in its treatment. High molecular heterogeneity is one of the dilemmas in the treatment of PDAC, and gives PDAC obvious invasive and metastatic ability [14]. Drug resistance is another major dilemma in the treatment of PDAC which makes it difficult for chemotherapy drugs and targeted therapies to play a role [15]. The immunosuppressive microenvironment makes it difficult for traditional immunotherapy to achieve satisfactory results in PDAC [16]. The highly fibrotic dense matrix is another huge dilemma in the treatment of PDAC; it is not only related to tumor progression and metastasis, but also forms a tight barrier for drug penetration. In addition, the highly hypoxic microenvironment, as another major dilemma, has a significant impact on tumor invasiveness, metabolism, and treatment resistance [17]. Our understanding of the complex molecular composition and specific microenvironment of PDAC has made great progress, and the key is to overcome these dilemmas in a targeted way. Therefore, novel experimental designs and well-designed therapeutic approaches that specifically address this challenge are needed to achieve effective treatment.
1.2. Well-Designed Therapeutic Nanosystems for PDAC
In recent years, the application of nanotechnology in cancer research has received increasing attention. Nanomaterials show great potential in drug delivery, imaging, and cancer treatment due to their unique properties [18,19,20]. Compared with traditional drugs, nanomedicines have many advantages, such as passive targeting of tumor tissue through the enhanced permeation and retention (EPR) effect, improved circulation, high drug loading capacity, and even intrinsic diagnostic and therapeutic properties [21]. In 2013, nab-paclitaxel plus GEM was reported to improve overall survival and progression-free survival in patients with metastatic PDAC, and was approved for first-line treatment in patients with advanced PDAC [22]. After years of clinical application, nab-paclitaxel has been proven to improve the prognosis of patients with PDAC effectively. In recent years, an increasing number of nanomedicines for PDAC treatment have been used in clinical trials (Table 1), which indicates that nanomedicine has great transformation potential in PDAC treatment. In addition to traditional nanomaterials (such as micelles and liposomes), new functional nanomaterials are also being developed, such as lanthanide nanoparticles (NPs), superparamagnetic iron oxide (SPIO) NPs, gold (Au) NPs, quantum dots (QDs), carbon nanotubes (CNTs), mesoporous silica NPs (MSNPs), metal–organic framework (MOF)-based materials, biomimetic nanomaterials, etc., which can incorporate imaging agents and/or therapeutic drugs [23]. The superior properties of these nanomaterials allow them to be refined and engineered, resulting in intelligent and well-designed nanosystems that integrate multiple functions. This review does not just refer to NPs for the treatment of PDAC. What we call a nanosystem is a multifunctional system based on a variety of nanomaterials such as NPs. NPs are the most basic and important components of nanosystems. There are other components, including a modification component, a drug component, a targeting component, a stimulus-response component, etc., which ultimately constitute a well-designed nanosystem. This well-designed nanosystem can specifically target defects in tumors to achieve a precision treatment effect. Through the rational design of nanosystems, researchers have constructed nanosystems that can overcome PDAC drug resistance, penetrate PDAC matrix barriers, reverse PDAC immune suppression, etc. In addition to conventional chemotherapy, those well-designed nanosystems show great potential for use in various novel cancer treatment modalities. This review aims to summarize the current state of the art in well-designed nanosystems for PDAC therapy. We emphasize the specificity of these nanosystems in overcoming the therapeutic dilemma of PDAC. This review will discuss the logical design and functionality of these nanosystems in terms of different therapeutic modalities, including chemotherapy, gene therapy, immunotherapy, anti-stroma therapy, photothermal therapy (PTT), photodynamic therapy (PDT), sonodynamic therapy (SDT), and chemodynamic therapy (CDT) (Figure 1). Finally, the bottleneck of these nanotechnologies in PDAC treatment and the prospect of clinical translation in the future will be briefly discussed. Through an in-depth summary and analysis, this review aims to provide cutting-edge ideas for further research on strategies to overcome the dilemma of PDAC treatment based on well-designed nanosystems.

Table 1.
Clinical trials of nanosystem therapeutic strategies for PDAC (obtained from https://clinicaltrials.gov/, accessed on 1 October 2022).
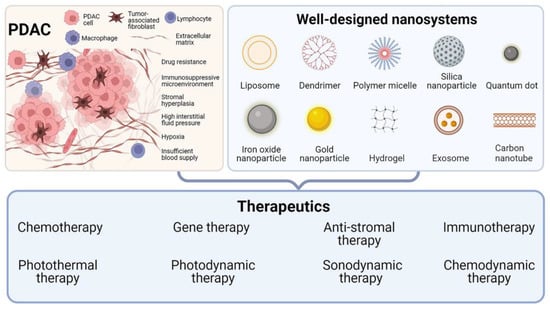
Figure 1.
Well-designed nanosystems for different PDAC therapeutics. Created using BioRender.com.
2. Well-Designed Nanosystems for Different PDAC Therapeutics
2.1. Nanosystems Designed for PDAC Chemotherapy
A variety of chemotherapy drugs, including GEM, paclitaxel, irinotecan, fluorouracil, oxaliplatin, capecitabine, doxorubicin, and curcumin, are used in the treatment of PDAC. However, the efficacy of the drugs is limited by non-specific distribution, drug metabolism, and intrinsic toxicity [24]. To address these issues, a promising option is to implement nanomaterial-based therapeutics in PDAC. Table 2 summarized recent studies on the delivery of PDAC chemotherapy drugs based on nanodelivery systems. GEM is considered the gold standard and is the first FDA-approved agent as monotherapy for advanced PDAC. Despite initial clinical success as a first-line treatment for PDAC, the therapeutic efficacy of GEM is limited by rapid metabolism, high-dose toxicity, and drug resistance [25]. GEM is easily metabolized to inactive 2′, 2′-difluorodeoxyuridine by deoxycytidine deaminase present in the plasma and liver, thus resulting in a very short plasma half-life of 15–20 min. To overcome these limitations and improve safety, a variety of GEM delivery systems have been developed. In addition to traditional liposomes, micelles, and polymer NPs, many novel nanomaterials such as MOF, QDs, hydrogels, and exosomes have also been developed (Table 2). Loading GEM in nanocarriers improves its pharmacokinetic parameters and facilitates entry into target cells via passive or active targeting, thus improving drug availability and reducing high-dose toxicity. Li et al. reported an autologous exosome (ExoGEM) as a drug carrier for targeted delivery of GEM for the treatment of PDAC [26]. ExoGEM showed superior therapeutic efficacy against PDAC, extending survival in a dose-responsive manner with minimal damage to normal tissue. Various stimulus-responsive (such as pH, enzymes, temperature, redox reactions, magnetic fields, light, ultrasound, etc.) nanodelivery systems have been developed [27]. Based on the properties of the tumor microenvironment (TME) or external stimuli, nanosystems can release active therapeutic components at specific sites in a controlled and targeted manner. Strategies for size or morphology transformation in response to stimuli have emerged in recent years, which can make the best use of nanosystems in different situations. Sheng et al. designed a GEM-loaded matrix metalloproteinase 2 (MMP-2)-responsive transformable beaded nanofibril, which integrates the merits of nanofibril and small-sized NPs [28]. In general, the longer the length of the fibril, the longer the circulation time, but the lower the penetration and internalization. Instead, small-size NPs with positive charges are the best choice for penetration and internalization. This nanosystem can self-assemble into negatively charged beaded nanofibrils with prolonged circulation time under physiological conditions. However, upon reaching the tumor tissue, in response to MMP-2, the nanofibrils transform into positively charged small particles, which can be effectively internalized by tumor cells. This fiber-to-sphere transition strategy takes full advantage of nanomaterials with different sizes and morphologies, allowing GEM to overcome multiple obstacles to be internalized into cells.
Due to its hydrophilic nature, it is difficult for GEM to enter cells via passive diffusion and it relies on human nucleoside transporters (hNTs) instead. Human NT is a family of membrane proteins containing a variety of protein subtypes, among which human equilibrative nucleoside transporter 1 (hENT1) mediates most GEM transport. Therefore, hENT1 deficiency is one of the most important reasons for cell resistance to GEM. To overcome GEM resistance induced by low hENT1 expression, Guo et al. reported a GEM-loaded human serum albumin NP (GEM-HSA-NP) which could be taken into tumor cells through endocytosis, thus bypassing the transport of hENT1 [29]. In vivo and in vitro studies showed that GEM-HSA-NP was more effective than GEM in inhibiting tumor growth regardless of whether hENT1 expression level was high or low. Another study developed hyaluronic acid-functionalized pH-sensitive liposomes (HA-pSL) to investigate whether intracellular delivery could overcome GEM resistance [30]. They hypothesized that HA-pSL would be internalized through CD44-mediated cellular endocytosis. The outcomes demonstrated that the nanosystem showed increased cell uptake and in vitro cytotoxicity, but did not eliminate resistant GR2000 cells. In vivo efficacy studies showed that the nanosystem only slightly enhanced the efficacy of GEM therapy, and the tumor was not eliminated. Their results suggest that not all cases are simply “transport-based resistance”. Before designing better nanosystems, the properties of nanomaterials and the complex biological interactions with tumor cells and TME need to be fully understood.
Although FOLFIRINOX has a better response rate than GEM, the side effects associated with the treatment are also more severe. Irinotecan, a component of the FOLFIRINOX regimen, significantly affects this toxic response. Although MM-398 (nanoliposomal irinotecan) has been approved by the FDA for the treatment of patients with PDAC who do not respond to GEM therapy, its gastrointestinal and bone marrow toxicity effects remain relatively high. To address the possible failure of liposomal carriers to improve the safety of highly toxic drugs such as irinotecan, Liu et al. developed lipid bilayer-coated mesoporous silica NPs (LB-MSNPs) for high-dose irinotecan loading [31]. The stability and loading capacity of the nanocarrier improved the biodistribution, circulating half-life, and drug tumor content of irinotecan compared with the MM-398 liposome, resulting in a more effective anticancer effect and lower toxicity in a robust treatment-resistant KRAS-induced pancreatic cancer (KPC) model.
In addition to single-drug delivery, a variety of co-delivery vehicles are also being developed. For instance, Shabana et al. designed a thermosensitive and biodegradable hydrogel encapsulating targeted NPs for the local and sustained co-delivery of GEM and paclitaxel in PDAC [32]. Chen et al. constructed a pH-activatable core-shell nanobomb for the co-delivery of autophagy inhibitor and GEM [33]. Lei et al. developed polymeric micelles bearing active formats of irinotecan and oxaliplatin [34]. These findings suggested that combined delivery, enabled by nanotechnology, may further improve the feasibility of multidrug treatment for advanced PDAC.
Collectively, the main difficulties in PDAC chemotherapy are poor bioavailability, drug resistance, and toxic side effects. Therefore, the main design concepts of nanosystems in PDAC chemotherapy are to improve drug bioavailability, overcome drug tolerance, and reduce drug side effects. To this end, researchers have designed a series of drug delivery nanosystems, including a ligand-targeted delivery system, a stimulus-response delivery system, a biomimetic delivery system, a collaborative treatment system, and a cascade-targeted delivery system. Recently, preclinical research has been devoted to developing novel nanocarriers. However, the nanocarriers that have entered clinical application mainly focus on liposomes. Although the development of new nanomaterials and vectors is a promising area, perhaps researchers should pay more attention to further studying the performance of existing vectors to ensure higher clinical translational potential.

Table 2.
Recent advances in chemotherapy drug delivery nanosystems for PDAC.
Table 2.
Recent advances in chemotherapy drug delivery nanosystems for PDAC.
| Chemotherapy Drugs | Nanosystems | Animal model | Ref |
|---|---|---|---|
| GEM | GEM-conjugated polymeric micelle | MIA PaCa-2 orthotopic model | [35] |
| GEM | MOF NPs | PANC-1 cells | [36] |
| GEM | GEM-loaded albumin NPs | PDAC patient-derived xenograft (PDX) subcutaneous model | [29] |
| GEM | Self-healing pH- and enzyme stimulus-responsive hydrogels | AsPC-1 subcutaneous model | [37] |
| GEM | Autologous exosomes | PANC-1 subcutaneous model | [26] |
| GEM | Plectin-1-targeted AuNPs | PANC-1 orthotopic model | [38] |
| GEM | Transferrin (Tf)-conjugated polymer-coated MSNPs | MIA PaCa-2 cells | [39] |
| GEM | Dual enzymatic reaction-assisted CdSe/ZnS QDs | BxPC-3 subcutaneous model | [40] |
| GEM and paclitaxel | Lipid-coated MSNPs | PANC-1 subcutaneous model | [41] |
| GEM and paclitaxel | Thermosensitive and biodegradable hydrogel encapsulating targeted NPs | PANC-1 cells | [32] |
| GEM and doxorubicin | Protein–gold cluster-capped MSNPs | MIA PaCa-2 subcutaneous model | [42] |
| GEM and doxorubicin | Photo- and thermoresponsive multicompartment hydrogels | - | [43] |
| Irinotecan | Lipid bilayer-coated MSNPs | KPC-derived orthotopic model | [31] |
| Curcumin | Tf-targeted PEGylated curcumin-loaded MSNPs | MIA PaCa-2 subcutaneous model | [44] |
| Curcumin | SPIO NPs of curcumin | HPAF-II orthotopic model | [45] |
2.2. Gene Therapy through Tailored Nanosystems
Most instances of PDAC arise from a precursor lesion called pancreatic intraepithelial neoplasia (PanIN), which progresses by acquiring genetic alterations and eventually develops into overt PDAC [8]. Multiple genetic alterations are present in PDAC, the most common of which is the activation of the KRAS proto-oncogene, which is present in more than 95% of human PDAC [46,47,48]. Oncogenic KRAS may be the main driving force behind PDAC, which was identified in PanIN [47]. Several other common gene mutations included TP53, SMAD4, and CDKN2A, each of which was mutated in >50% of different but mostly overlapping patients [49,50]. Mutations in genes with an incidence of 5–10% have also been identified, including KDM6A, BCORL1, RBM10, MLL3 (also known as KMT2C), ARID1A, and TGFBR2 [51,52,53]. The pathogenesis of PDAC is the continuous accumulation of somatic mutations in oncogenes and tumor suppressor genes. Therefore, gene therapy is a promising approach to PDAC treatment. Gene therapy, defined as the deletion, insertion, modification, or addition of genes in target cells, has shown significant therapeutic effects in recent preclinical and clinical studies [54]. Therapeutic genes include plasmid DNA (pDNA), mRNA, microRNA (miRNA), short interfering RNA (siRNA), and antisense oligonucleotides [54]. Their effects are limited by poor stability in vivo, immune system activation, insufficient tumor delivery, and barriers to transport across the plasma membrane to the cytoplasm [55]. Vectors used to deliver genes include viral vectors and non-viral vectors. Viral vectors have shown high transduction efficiency. However, their potential carcinogenicity, immunogenicity, and high production cost limit their application [54]. Therefore, non-viral vectors have received a great deal of attention. In addition to traditional cationic liposomes and polymers, a variety of emerging nanomaterials such as gold nanoclusters, layered double hydroxides (LDH), CNTs, and graphene oxide (GO) have been developed for gene delivery (Table 3).

Table 3.
Recent advances in nanosystem-mediated gene therapy for PDAC.
KRASG12D has a mutation rate of more than 90% in PDAC, which promotes tumorigenesis, development, and metastasis [56]. Therefore, KRAS mutation inhibition holds great promise for effective gene therapy strategies for PDAC. Unfortunately, traditional gene therapy faces many huge biological and technical challenges. Yu et al. used a synthetic nucleic acid analog called peptide nucleic acid (PNA) [56]. Compared with conventional negatively charged DNA or RNA, neutral PNA is more stable in recognizing and forming PNA-DNA hybrids with target mutant sequences. LDH nanosheets have attracted great interest due to their good stability and dispersion and high gene delivery efficiency. Therefore, they designed a PNA-based gene therapy strategy. LDH is used as a PNA carrier to effectively deliver PNA to PDAC cells and achieve mutation silencing of KRAS. Through the proton sponge effect of LDH, PNA is able to be released from lysosomes into the nucleus after being phagocytosed by the cell, thus having a significant therapeutic effect on PDAC [56]. In addition, to overcome the instability and low absorption efficiency of siRNA in vivo, Yin et al. used functionalized GO nanosheets as gene carriers to co-deliver HDAC1 and KRAS siRNA. Double silencing of HDAC1 and KRAS significantly inhibited cell proliferation and tumor growth [70].
Traditional cationic polymeric gene vectors are closely connected to negatively charged DNA through electrostatic interaction, which is difficult to dissociate after reaching cells, resulting in low transfection efficiency. To this end, Zhang et al. constructed a reactive oxygen species (ROS)-responsive gene delivery vector by encapsulating the polymer B-PDEAEA and DNA complex in IR780-loaded liposomes [67]. IR780 in the nanosystem can produce a large number of ROS under ultrasound irradiation, triggering the charge reversal of the redox-responsive polymer, thus releasing TRAIL pDNA [67]. TRAIL is a therapeutic gene that specifically eliminates tumor cells without damaging normal cells. Additionally, the modification of polyethylene glycol (PEG) can further maintain the stability of therapeutic genes and prolong blood circulation time, thus improving the efficiency of gene transfection. Most importantly, a combination of this gene delivery system and ultrasound can achieve superior biocompatibility and therapeutic effect in vitro and in vivo.
In addition to targeting PDAC tumor cells, Jiang et al. designed a gene delivery strategy targeting pancreatic stellate cells (PSCs) [62]. Due to the large number of PSCs present in the PDAC microenvironment and located near blood vessels, targeting PSC helps overcome the difficulty of delivering genes to PDAC tumor cells [62]. In their study, branched polyethyleneimine was used to deliver the TRAIL gene to PSC and induce PSC to produce TRAIL protein, which was non-toxic to PSC but could induce the death of neighboring PDAC cells. In conclusion, they demonstrate that PSC, as a major stromal component in the PDAC microenvironment, is an ideal host for gene therapy strategies and in developing novel modalities for gene therapy for PDAC.
In addition to the KRAS mutations observed in 90% of patients with PDAC, mutations in the tumor suppressor gene P53 are also observed in 50–80% of patients. Moreover, about 55% of patients have both KRAS and P53 mutations, which lead to an increase in glucose metabolites, including deoxycytidine triphosphate (dCTP) [58]. dCTP can be inserted into DNA strands for replication and will compete with the active metabolite of GEM in the nucleus, thus making PDAC resistant to GEM. In this case, overcoming drug resistance by simultaneously inhibiting the KRAS and P53 genes can lead to effective PDAC therapy. The clustered regularly interspaced short palindromic repeats-associated protein 9 (CRISPR-Cas9) system is an emerging gene editing therapy [71]. The adenine base editor (ABE) is a more advanced gene editing strategy [72]. Won et al. constructed a Cas9 and ABE-based ribonucleoprotein gene editor that utilizes a biocompatible liposome as a delivery nanosystem for the simultaneous KRAS and P53 gene editing of PDAC [58]. Multiple biological analyses of in vitro and in vivo models verified the effective killing of PDAC cancer cells after co-treatment with double gene editing and GEM [58]. Overall, they provide a combined therapeutic strategy utilizing gene editing systems and may be a promising approach for the treatment of drug-resistant PDAC.
In conclusion, nanosystem-based gene therapy has great potential in PDAC treatment because it can prevent DNA/RNA degradation and enhance in vivo stability and gene transfection efficiency. A large number of preclinical studies have shown that gene therapy may bring new hope for PDAC treatment. However, developing safe and efficient vectors is extremely important to achieve effective treatment. The development of a variety of novel nano-gene delivery systems may uncover a new solution to PDAC treatment in the future. Furthermore, considering the heterogeneity of PDAC tumors and the complexity of the TME, single-target gene therapy may have limited efficacy. Therefore, multi-mutation gene therapy or a combination of multiple therapeutic modalities may achieve a better synergistic therapeutic effect.
2.3. Nanosystems Designed to Overcome PDAC Stroma
An important reason for the poor prognosis of PDAC is its specific TME, which includes a highly fibrotic tumor stroma. The stromal component of PDAC is up to 90% of the entire tumor [12]. This specific TME is a highly dynamic system composed of multiple cellular components and an extracellular matrix (ECM). The cellular components include PSCs, cancer-associated fibroblasts (CAFs), immune cells, and endothelial cells. ECM is composed of hyaluronic acid, collagen fiber, fibronectin, and soluble growth factors such as transforming growth factor-β (TGF-β), vascular endothelial growth factor, and fibroblast growth factor [12]. The dense tumor stroma of PDAC results in elevated interstitial fluid pressure, poor blood supply, and hypoxia in the TME. The interaction between stroma and tumor cells has a significant impact on the progression, invasion, and metastasis of PDAC [6]. Additionally, the dense fibrotic stroma severely impedes drug penetration into the PDAC tumor tissue, which leads to poor efficacy of the PDAC drug treatment. Therefore, modulation of the PDAC stroma to facilitate treatment is a highly effective strategy. A series of nanosystem-based matrix modulation strategies have been developed, including targeting PSCs, CAFs, ECM components, matrix-related signal transduction pathways, etc. (Table 4).

Table 4.
Recent advances in nanosystem-mediated modulation of PDAC stroma.
In the PDAC stroma, PSCs are the main source of CAFs. Activated PSCs are converted into CAFs, which form a dense ECM network by producing large amounts of fibronectin, collagen, and α smooth muscle actin (α-SMA) [74]. There is increasing evidence that PSCs present in the stroma of PDAC promote the invasion and metastasis of cancer cells by remodeling TME [84]. Therefore, targeting PSCs and remodeling PDAC stroma has proven to be a promising strategy for the treatment of PDAC [85]. Calcipotriol is a ligand for vitamin D receptors, which can inhibit the activation of PSCs and restore them to the resting state [86]. However, its poor water solubility, short plasma half-life, and adverse reactions limit its clinical application. Wang et al. constructed a self-assembled polymer nanosystem for the co-delivery of calcipotriol and the chemotherapy drug SN38 [73]. Their results showed that this nanosystem improved the water solubility of calcipotriol and prolonged the blood circulation time of calcipotriol significantly. After systemic administration, this nanosystem effectively inhibited ECM production, reducing collagen and fibronectin content. This nanosystem effectively promoted the penetration of chemotherapeutic drugs, showing inhibitory effects on primary and metastatic tumors in a PDAC mouse model. In another study, researchers inhibited the activation of PSCs to produce ECM by delivering nitric oxide (NO) [74]. NO is an important biological signaling molecule that can regulate a variety of physiological processes. They used liposomes loaded with a glutathione (GSH)-sensitive NO donor, S-nitroso-N-acetylpenicillamine (SNAP). NO can be rapidly released from SNAP when stimulated by high concentrations of GSH at the tumor site. Next, NO reduced the production of ECM components in PSCs by inhibiting the TGF-β1 pathway and cutting off its downstream pro-fibrotic signal transduction [74]. Due to the reduction in stroma induced by NO, enhanced liposome-GEM intratumor penetration was achieved in the PDAC mouse model. In addition, Feng et al. used polymer NPs coated with CREKA peptide and loaded α-mangostin (CRE-NP(α-M)) to target CAFs and interfere with the TGF-β/Smad signaling pathway to inhibit CAFs from producing ECM [76]. The sequential targeted drug delivery regimen after CRE-NP(α-M) pretreatment showed strong tumor growth inhibition in the orthotopic tumor model.
The TGF-β1 signal transduction pathway not only plays an important role in promoting stroma formation in PDAC [87], but also mediates drug resistance through autocrine or paracrine mechanisms. PDAC tumor cells, or PSCs, contribute to the production of TGF-β1, which can activate PSCs and maintain their myofibroblast phenotype through an autocrine positive feedback mechanism [88]. As a result, the activated PSCs continue to secrete large amounts of ECM components, forming a dense ECM network [89]. Based on the key role of TGF-β1 in the course of forming PDAC stroma, targeting TGF-β1 signal transduction represents a promising approach. Vactosertib (VAC) is a selective small-molecule inhibitor of TGF-β1 receptor kinase. Zhao et al. designed a size-switchable nanoplatform by incorporating paclitaxel into the hydrophobic layer of small nanospheres (NS-TAX), and then, encapsulating them in liposomes loaded with VAC (NS-TAX@Lipo-VAC) [77]. NS-TAX@Lipo-VAC can target the ECM of PDAC and accumulate in the matrix due to its large size. The released NS-TAX is able to penetrate deep into tumors because of its small size. At a later stage, VAC inhibited TGF-β1 signaling in the tumor stroma, leading to a significant decrease in ECM protein, which further promoted the penetration of NS-TAX in tumor tissues. Their nanoplatform effectively reduced ECM production and inhibited tumor progression in fibrosis-rich PDAC animal models. This cascade of penetration strategies combining TGF-β1 signal regulation and size-switchable nanoplatforms has great potential to overcome stroma barriers in PDAC and improve therapeutic outcomes. In another study, Li et al. developed an MMP-2-responsive alternating copolymer carrying LY2109761 and CPI-613 [78]. LY2109761 is a novel TGF-β receptor kinase inhibitor that blocks the interaction between tumor cells and PSCs. CPI-613 is an anti-mitochondrial metabolic agent that can disrupt mitochondrial metabolism in cancer cells. The MMP-2-responsive connector is cleaved in the TME, resulting in dissociation of the nanosystem and the subsequent release of LY2109761 and CPI-613. This strategy significantly inhibited the growth of PDAC tumors by simultaneously blocking stroma production and disrupting mitochondrial metabolism in tumor cells.
In addition to overcoming the barrier of drug penetration by adjusting the matrix composition, effective deep intratumoral penetration can also be achieved through rational design of the nanosystems. Endothelial cells can actively transport cargo through caveolae-mediated transcytosis to transport it across the capillary wall to the tumor tissue [90]. The cationization of nanocarriers can effectively induce adsorption-mediated transendocytosis and promote the penetration of the carrier through multiple cell layers [91]. Based on this, Zhou et al. designed a γ-glutamyl transpeptidase (GGT)-responsive charge reversal nanosystem [79]. The nanosystem remains neutral or microanionic during blood circulation to ensure effective long circulation. After reaching the tumor site, GGT overexpressed in tumor vascular endothelial cells and on the tumor cell surface triggers the charge reversal of the nanosystem to cationization. This results in rapid caveola-mediated transendocytosis, enabling transendothelial and transcellular transport into the tumor (Figure 2).
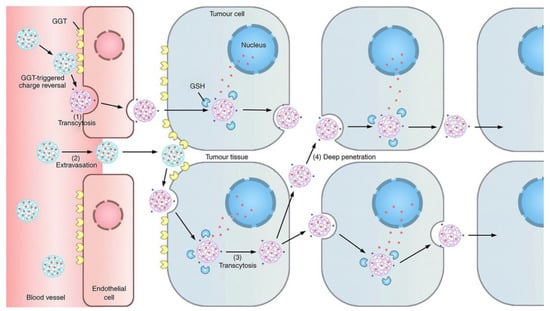
Figure 2.
Scheme of the GGT-activatable nanosystem for active tumor penetration through cationization-triggered transcytosis. Reprinted with permission from [79]. Copyright 2019, Springer Nature.
In addition to targeting cellular components such as PSCs and CAFs in the stroma, various strategies have been developed to target the ECM. Hyaluronic acid and collagen are the main components of ECM, and several studies have shown that manipulation of these ECM components can effectively alleviate matrix obstruction. For example, ongoing phase III trials of PEGylated recombinant human hyaluronidase in combination with GEM have shown prolonged survival in PDAC treatment [92]. Collagenase has also been used in several studies to degrade collagen in the ECM, reducing interstitial stress and facilitating therapeutic drug penetration [82,83].
To summarize, the stroma composition of PDAC is complex and variable. It is not only related to the progression and metastasis of PDAC, but also affects the immune microenvironment and cell metabolism [93,94]. Anti-stromal therapy holds great promise for the treatment of PDAC, and it can help overcome the drug penetration dilemma of PDAC therapy to promote efficacy. A series of nanosystems have been designed to eliminate the dense stroma of PDAC, including targeting PSCs, CAFs, ECM components, etc. In addition, the design of transformable nanosystems is also a promising strategy for overcoming stroma obstruction. It is important that the complexity of the PDAC stroma system be fully considered in the design of nanosystems, so as to design a more reasonable and refined stroma regulation strategy.
2.4. Immunosuppressive Microenvironment-Regulating Nanosystems
Although immunotherapy has greatly changed the outlook for some tumors, such as melanoma and lung cancer, it has had little success in the treatment of PDAC. PDAC is almost completely unable to use FDA-approved immunotherapies, except in <1% of patients with high microsatellite instability [95]. At present, many clinical trials have attempted to evaluate the effects of immunotherapies on PDAC, including immune checkpoint inhibitors (ICIs), cancer vaccines, adoptive cell transfer, etc., but none have shown satisfactory results [16]. The main reason for the hindered efficacy of immunotherapy in PDAC is its highly inhibitory immune microenvironment. This highly inhibitory immune microenvironment lacks cytotoxic T cell (CTL) infiltration but is rich in immunosuppressive cells including dendritic cells (DCs) and tumor-associated macrophages (TAMs), as well as myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) [96,97,98,99]. Therefore, PDAC is immunologically referred to as an immune “cold” tumor. Researchers in the field of nanomedicine are working hard on new approaches that could effectively change the dilemma of PDAC immunotherapy and are trying to improve the situation of immunotherapy for PDAC by utilizing the advantages of nanomaterials.
The combination of immunogenic cell death (ICD) inducers and ICIs has shown satisfactory results in preclinical studies in a variety of tumors. Some chemotherapeutic agents, such as doxorubicin and oxaliplatin, can increase the response of CTL to “cold” tumors and reverse them to “hot” tumors by inducing ICD. This immunogenic effect occurs due to the ability of these chemotherapeutic agents to trigger an endoplasmic reticulum (ER) stress response that translocates calreticulin (CRT) to the surface of tumor cells [100]. CRT serves as a signal for antigen-presenting cells (APCs) to phagocytose cancer cells, enabling APCs to present endogenous tumor-associated antigens to naive T cells [101]. Moreover, disintegration of the tumor nucleus leads to the release of high-mobility group box 1 protein, which acts as an adjuvant by binding to Toll-like receptor 4 (TLR4) to APCs. In addition, autophagy and ATP release can also increase APC recruitment and immune effects [102]. ICD promotes antigen uptake and presentation by APC through the release of tumor antigens, damage-associated molecular patterns, and proinflammatory cytokines, and ultimately elicits antigen-specific antitumor immune responses [103]. However, the existence of an immune checkpoint can inhibit the killing effect of CTLs. Therefore, ICIs are an effective means of ensuring the tumor killing function of CTLs. Although some progress has been made in the development of ICD inducers and ICI combinations, several challenges remain in clinical translation. Many studies have focused on improving the efficacy of ICD and ICI combination therapy through nanosystems. Liu et al. used lipid-coated MSNPs loaded with irinotecan to explore the immunogenic effect of irinotecan [104]. The results showed that encapsulated irinotecan caused lysosomal alkalinization and ER stress, inducing immunogenicity and PD-L1 expression. In general, the lack of PD-L1 expression in PDAC tumor cells is an important reason for the poor response to the PD-1/PD-L1 blockade [104]. The silica nanosystem induces a more potent chemoimmunotherapeutic response than either free drugs or liposomal irinotecan. Moreover, by inducing the expression of PD-L1, the combined treatment with anti-PD-1 can significantly inhibit tumor growth and improve the survival rate, and is far better than free drugs plus anti-PD-1. In another study, the authors also used lipid-coated MSNPs to load oxaliplatin [105]. In an orthotopic KPC model, the nanosystem showed superior pharmacokinetic profiles and intratumoral drug delivery to free drugs, while inducing significant ICD effects. In addition, combined treatment with anti-PD-1 antibody further enhanced the effect of chemoimmunotherapy. Overall, improving the efficacy of ICD inducers through nanocarriers and combined treatment with ICI may be an effective strategy to improve the treatment dilemma of PDAC. This combinatorial effect raises the possibility of using the multifunctional properties of the nanosystem to co-produce immunomodulators selected to intervene in a range of impediments in PDAC immunotherapy. For example, Luo et al. used this liposomal silica to load the ICD inducer irinotecan into the pores of MSNPs, further loading the outer lipid layer with TLR agonists capable of activating innate immunity [106]. Although ICD effects can improve the delivery of tumor antigens to APCs, the activation, maturation, and enhanced function of APCs require additional co-stimulation [106]. The experimental results showed that the dual-drug delivery vector produced an effective anti-PDAC immune response, including increased DC activation, an increased number of CTLs, and significant tumor shrinkage. These results not only demonstrate the efficacy of co-delivered drug vehicles, but also expand the scope of chemoimmunotherapy for PDAC. Although most studies have focused on the adaptive immune system, the immune microenvironment of PDAC is rich in a large number of dysfunctional APCs. Based on this, Lorkowski et al. designed highly effective innate immune-stimulating NPs to induce strong activation and expansion of APC in TME [107]. The nanosystem is loaded with two innate immune activators, including the pathway agonist of stimulator of interferon genes and the TLR4 pathway activator. The nanosystem stimulated more significant APC amplification than either agonist alone, resulting in a powerful synergistic antitumor immune effect.
In addition to ICD induced by chemotherapeutic agents, other physical mechanisms can also induce ICD while killing tumor cells, such as photothermal and photodynamic effects. Jang et al. constructed a tumor cell-derived exosome loaded with chlorin e6 (Ce6) to target tumor cells and generate ROS in tumor cells after laser irradiation [108]. The ROS produced can not only kill tumor cells, but also further induce anti-tumor immune responses, and eventually, inhibit tumor growth, recurrence, and metastasis. In another study, Yu et al. developed a stimulus-responsive size-transformed liposome in combination with mild hyperthermia and ICI treatment [109]. Mild hyperthermia alleviated tumor hypoxia and increased immune cell infiltration, and combined with ICI therapy, effectively inhibited PDAC tumor growth and metastasis. In addition, the nanosystem can achieve size conversion under temperature stimulation to penetrate deep into tumor tissue and overcome the dense stromal barrier of PDAC. The dense matrix of PDAC is also a major obstacle to the efficacy of immunotherapy; it blocks not only drug penetration but also effector immune cell infiltration. Tong et al. designed a pH-sensitive tumor-penetrating nanosystem that self-assembled into NPs (120 nm) at neutral pH and transformed into small particles (10 nm) at acidic pH (Figure 3) [110]. The released small particles promote the deep penetration of GEM into tumor tissues, induce anti-tumor immune effects, enhance the infiltration of immune cells, and up-regulate the expression level of PD-L1. Ultimately, the combination of anti-PD-1 antibodies with the nanosystem induced robust antitumor immunity.
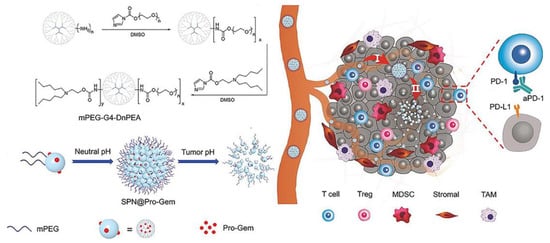
Figure 3.
Scheme of the design and mechanism of the pH-sensitive size-transformable tumor-penetrating nanosystem SPN@Pro-Gem. Reprinted with permission from [110]. Copyright 2021, John Wiley and Sons.
In addition to immunotherapeutic strategies to activate APCs and enhance the effects of CTLs, TAM is also an important cell in the immunosuppressive microenvironment of PDAC. TAM exhibits two phenotypes, M1-TAM and M2-TAM, in which M2-TAM promotes tumor progression and metastasis and inhibits DCs and CD8+T cell function. In contrast, M1-TAM has antitumor effects. Therefore, the polarization of TAM toward the M1 type is an effective antitumor immunotherapy strategy. Zhou et al. developed an exosome-based dual delivery nanosystem for the delivery of oxaliplatin and galectin-9 siRNA [111]. Oxaliplatin not only kills PDAC cells by inhibiting DNA synthesis and repair, but also further induces the ICD effect. Moreover, galectin-9 siRNA polarized M2-TAM by blocking galectin-9/dectin-1. The final results showed that this dual delivery system polarized inhibitory TAM, recruited CTLs, and down-regulated Treg, thus eliciting a potent antitumor immune response. In another study, Parayath et al. constructed M2 peptide-modified polymer NPs for TAM-targeted miR-125b delivery [112]. The results showed that intraperitoneal administration of M2-targeted NPs showed preferential accumulation at the tumor site, and the ratio of M1-to-M2-TAM increased more than fourfold. Due to the poor immunogenicity of PDAC, a single target may not produce a satisfactory therapeutic effect. Li et al. designed an M2-TAM-targeted micelle for simultaneous delivery of the PI3K-γ inhibitor and CSF-1R siRNA [113]. The micelles specifically targeted TAM, effectively reduced the number of M2-TAM, increased the number of M1-TAM, and inhibited the infiltration of MDSCs through dual blockade of the two pathways. This dual inhibitory strategy provided a new approach for the TME remodeling of PDAC and the coordinated activation of antitumor immune effects.
In conclusion, although the low immunogenicity and highly suppressive immune microenvironment of PDAC have largely limited the effectiveness of immunotherapy in PDAC, the development of nanosystems has brought new hope for PDAC immunotherapy. While it is difficult for monotherapy to be effective in PDAC, the nanosystem helps to combine different parts of the immune response pathway to induce effective antitumor immune responses through multiple pathways simultaneously. It is believed that these well-designed nanosystems can achieve new breakthroughs in the field of immunotherapy for PDAC in the future.
2.5. Nanosystems Designed for PDAC Photothermal Therapy (PTT)
PTT has become a new and powerful treatment technique because of its non-invasiveness, low damage to normal tissue, and strong therapeutic effect [114,115]. A variety of photothermal agents, such as inorganic (metal NPs, nanosheets, and transition metal oxides) and organic materials (such as polyaniline, cyanine dye, and indocyanine), have been widely developed [116]. Table 5 summarize recent advances in PTT for the treatment of PDAC. Local hyperthermia caused by PTT can increase the blood flow and microvascular permeability of tumor tissue, thus promoting the deep penetration of drugs. Zhao et al. constructed a gold nanoshell-coated mesopore silica nanosystem for combined PTT and chemotherapy treatment of PDAC [117]. The nanosystem provides a cascade-targeting strategy that promotes drug penetration and accumulation through both photothermal and molecular targeting. After inducing PTT at the tumor site, blood perfusion and vascular permeability were improved, which greatly promoted the infiltration and accumulation of nanosystems in tumor tissues. The second step of cascade targeting was to target the TfR on the surface of tumor cells via Tf modification of the nanosystem. Their research demonstrated that the more nanosystems accumulated in the tumor, the better the photothermal warming, which, in turn, promoted the accumulation of more nanosystems, creating a positive feedback loop. At the same time, the photothermal effect greatly enhanced the sensitivity of PDAC cells to chemotherapy, and finally, effectively inhibited tumor growth through the combination of PTT and chemotherapy. In another study of PTT in combination with chemotherapy, Zhan et al. designed a polymer micelle that was both ROS- and temperature-sensitive [118]. The excess ROS in TME can trigger a surface charge change in micelles, thereby releasing the outer indocyanine green (ICG). After ICG release, the micelles become smaller and penetrate more deeply into the tumor. At the same time, ICG can produce a photothermal effect under 808 nm laser irradiation, triggering the polymer to change from hydrophobic to hydrophilic, and releasing the loaded camptothecin into deep tumors to realize chemotherapy. In vitro and in vivo experiments showed that the combined treatment could effectively combat PDAC and improve the therapeutic effect. Semiconductor polymer NPs (SPN) composed of completely organic compounds have become photothermal agents for cancer treatment [119,120]. Compared with other inorganic nanomaterials, SPNs have unique characteristics such as better biocompatibility, and higher photothermal conversion efficiency and controllability. The utilization of SPNs for PTT has been well explored, but there are few reports on combination therapy. In their study, Shi et al. reported a 177Lu-labeled glucose-dependent insulinotropic polypeptide-targeting SPN for combined radiotherapy and PTT of PDAC [120]. As a result, these combined treatment techniques complemented each other and provided superior results to PTT or radiotherapy alone. This is a simple and effective way to fabricate SPN-based multifunctional diagnostic and therapeutic nanosystems. In addition to these widely studied materials, many novel nanomaterials have also been developed for PDAC PTT, such as single-walled CNTs [121] and graphene QDs [122], which exhibit excellent photothermal conversion efficiency and stability.
Although PTT by itself is only suitable for local cancer treatment, combinations of PTT with other therapies have shown the benefits of inducing distant effects, in which local treatment suppressed not only the treated tumor but also the distal metastatic tumor [123]. Polydopamine (DP) is a kind of bionic coating material with excellent biocompatibility and photothermal conversion ability. In order to induce powerful anti-primary and metastatic tumor effects, Sun et al. designed a DP-coated ultra-small nanocarrier for the delivery of GEM and IDO inhibitors [123]. The nanosystem can not only inhibit tumor growth through the combination of chemotherapy and immunotherapy, but also further enhance the inhibitory effect on primary and metastatic tumors under the effect of PTT. Various nanosystems have been developed for phototherapy combined with immunotherapy. Nanomaterials can enhance the stability of photothermal agents or immune adjuvants, reduce side effects, and enhance therapeutic effect. In addition, these nanomaterials not only possess excellent photothermal properties, but can also induce remodeling of the tumor immune microenvironment. For example, Wang et al. constructed a nanosystem based on iron oxide loaded with imiquimod as an immune adjuvant and ICG as a photothermal agent (IMQ@IONs/ICG) [124]. Tumor antigen release was induced by PTT to stimulate DC cell activation and maturation. Moreover, IMQ@IONs/ICG could induce the polarization of M2-TAM to M1-TAM (Figure 4). This strategy ultimately eliminated the primary tumor and induced a robust immune response against metastasis and recurrence. Additionally, the multifunctional nanosystem also features MR imaging guidance and real-time temperature monitoring. It can accurately monitor the temperature change in tumor tissue during treatment. In another experiment, Li et al. assembled immunoregulatory thymopentin and ICG into nanofibrils for local combined PTT for PDAC [125]. The high aspect ratio of fibrillary nanostructures showed more pronounced retention in tumor tissue than that of other nanostructures. Their results showed that the combination of rapid PTT of tumor tissue and moderate systemic immune modulation, after a single injection and a single irradiation treatment, effectively suppressed tumor growth and tumor metastasis and minimized systemic side effects. PTT not only induced the anti-tumor immune response through ICD, but some studies have also shown that local hyperthermia can reduce the amount of CAF in PDAC, alleviate stromal disorders, and enhance the effect of chemotherapy [126]. Teng et al. reported an Abraxane-loaded MoSe2 nanosheet for chemotherapy combined with PTT [126]. Importantly, a reduction in the amount of CAF in response to the photothermal effects was confirmed via immunofluorescence analysis of α-SMA and vimentin. This therapeutic effect may provide a new perspective on PDAC treatment.
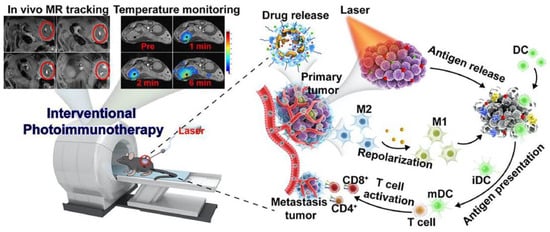
Figure 4.
Schematic representation of IMQ@IONs/ ICG-mediated photothermal immunotherapy and real-time temperature monitoring for PDAC. Reprinted with permission from [124]. Copyright 2022, Elsevier.
Although photothermal agents have led to progress in hyperthermia ablation, triggering drug release, and immune activation, their potential applications are still limited. Most photothermal agents are absorbed in the near-infrared (NIR) I region (600–1000 nm), which has limited light penetration depth. In contrast, NIR-II (1000–1700 nm) light is able to penetrate to deeper tissues and reduce light scattering, which is more suitable for PTT in deep tissues [127,128]. PTT in the NIR-II region can not only achieve high therapeutic effects and deep treatment, but can also minimize side effects. Geng et al. reported a platelet membrane-coated NIR-II nanosystem for tumor-targeted photothermal therapy [129]. The coating of the platelet membrane improved the hydrophilicity and targeting ability of NIR-II dye IR1048, which has excellent tumor suppressor effects in a variety of tumor models, including PDAC. The results showed that the nanosystem exhibited excellent photoacoustic imaging capability and significantly improved the photothermal conversion efficiency in the NIR-II window.
Overall, PTT has a strong and stable therapeutic effect in preclinical cancer studies. Current research focuses on designing targeted or stimulus-responsive nanosystems to improve the tumor specificity of photothermal agents, providing better results and fewer side effects. Especially when PTT is combined with chemotherapy or immunotherapy, it can produce a significant synergistic therapeutic effect. Based on the safety and efficacy of PTT, it has great translational potential in the treatment of PDAC.

Table 5.
Summary of recent advances in well-designed nanosystem-mediated PTT, PDT, SDT, and CDT for the treatment of PDAC.
Table 5.
Summary of recent advances in well-designed nanosystem-mediated PTT, PDT, SDT, and CDT for the treatment of PDAC.
| Agents | Nanosystem | Treatment Strategies | Animal Model | Ref | |
|---|---|---|---|---|---|
| PTT | Au nanoshell | Tf-GNRS-GEM | PTT + GEM | MIA PaCa-2 subcutaneous model | [117] |
| Graphene | Graphene@Gold Nanostar/Lipid | PTT + gene therapy | Capan-1 subcutaneous model | [130] | |
| MoSe2 | Abraxane@MoSe2 | PTT reduced CAFs + Abraxane | PDX model | [126] | |
| ICG | CPT@PAAB@ICG | PTT + chemotherapy | BXPC3 subcutaneous model | [118] | |
| SPN | 177Lu-SPN-GIP | PTT + RT + SPECT/CT | CFPAC-1 subcutaneous model | [120] | |
| SWNT | IGF-1R-targeted SWNT | PTT | BXPC-3 orthotopic model | [121] | |
| ICG | IMQ@IONs/ICG | IPTT + immunotherapy + MRI | Panc02-H7 orthotopic model | [124] | |
| Conjugated small molecule | DCTBT-loaded liposomes | PTT + PDT + FLI | PANC-1 orthotopic model | [131] | |
| Dopamine | NLG/PGEM/dp NPs | PTT + GEM + immunotherapy | Panc02 subcutaneous model | [123] | |
| PDT | SiPc | PFC/SiPc@PS@PNIPAM-Au980-DOX | PDT + PTT + chemotherapy | MIA PaCa-2 orthotopic model | [132] |
| PPIX | Perfluoropentane-doped oxygen MBs | PDT + O2 | Panc02 orthotopic model | [133] | |
| DiD | PF11DG | PDT + O2 + GEM + immunotherapy | Panc02 subcutaneous model | [134] | |
| RB, Ce6 | UCNP/RB, Ce6 | Dual PDT | PANC-1 subcutaneous model | [135] | |
| PPa | Supramolecular prodrug nanosystem | PDT + immunotherapy | Panc02 subcutaneous model | [136] | |
| Ce6 | Ce6-GVS NPs | PDT + chemotherapy + pro-apoptotic signal | PANC-1 subcutaneous model | [137] | |
| TBD-3C | Membrane-anchoring photosensitizer | PDT + pyroptosis + immunotherapy | KPC orthotopic model | [138] | |
| SDT | TiO2 | Tablet-like TiO2/C nanocomposites | SDT | Panc02 subcutaneous model | [139] |
| IR780 | IR780@O2-FHMON | SDT + O2 | PANC-1 subcutaneous model | [140] | |
| TCPP | Ti-TCPP MOF | SDT | BxPC-3 orthotopic model | [141] | |
| PPIX | PMPS NDs | SDT + immunotherapy | Panc02 orthotopic model | [142] | |
| IR780 | Pt@ZIF-90@Gem@IR780 | SDT + chemotherapy | BxPC-3 subcutaneous model | [143] | |
| CDT | Cu | HAS-MnO2-CuS | CDT + PTT | Panc02 subcutaneous model | [144] |
| Fe2+ | HFePQS | CDT + immunotherapy | KPC orthotopic model | [81] | |
| HPPH | HMON-Au-Col@Cu-TA-PVP | CDT + PDT | BxPC-3 subcutaneous model | [145] | |
| AIPH | AIPH@Cu-MOF | CDT + SDT | Panc02 orthotopic model | [146] |
2.6. Enhanced Photodynamic Therapy (PDT) Based on Tailored Nanosystems
PDT is a minimally invasive treatment that involves shining a laser light on a photosensitizer (PS) that transfers absorbed photon energy to the surrounding oxygen, resulting in the generation of ROS primarily consisting of singlet oxygen (1O2) [147]. The ROS produced can trigger oxidative damage to mitochondria, leading to apoptosis [148]. PDT has shown advantages in existing clinical trials and has emerged as a potential alternative therapy for patients with advanced PDAC [149,150]. However, there are still some shortcomings in the clinical application of PDT, including poor water solubility of PS and easy aggregation, tumor hypoxia, and limited efficacy in invasive tumors [147]. In preclinical studies, nanosystem-mediated PDT has been shown to overcome these deficiencies through the design of various corresponding strategies (Table 5). Hypoxia at the tumor site and the non-specific accumulation of PS are the key factors limiting the efficacy of PDT. Based on this, Xu et al. developed a PS- and oxygen-targeted delivery system to alleviate tumor hypoxia, reduce the non-specific accumulation of PS, and enhance the efficacy of PDT [133]. They prepared a perfluoropentane-doped oxygen microbubble (OPMB) for efficient oxygen delivery and an ultra-small PEG-modified protoporphyrin IX micelle (PPM) for PDT treatment. Protoporphyrin IX is a heme prerequisite with excellent photosensitive activity, but its hydrophobicity and non-target tissue accumulation limit its application [133]. Conjugated PEG and protoporphyrin IX self-assembled into ultra-small PPMs, which resolved the hydrophobicity of protoporphyrin IX and reduced nonspecific accumulation. Furthermore, the disruption of OPMB with ultrasound resulted in an approximately 2.2-fold increase in tumor-specific PPM accumulation. In addition, tumor oxygenation was increased from 22% to 50% by the MB disruption of oxygen release, which not only increased 1O2 production but also significantly decreased the expression of HIF-1α, thus preventing angiogenesis and epithelial–mesenchymal transition. This strategy has been shown to significantly prolong the survival of mice with orthotopic PDAC. In another study, Zhang et al. designed a cascade therapy strategy tailored for hypoxic orthotopic PDAC [132]. They prepared a nanosystem called PSPP-Au980-D, which was composed of a bilayer functionalized polymer encapsulating perfluorocarbon (PFC). In their design, the first step is to irradiate the nanosystem with a 980 nm laser to induce photothermal effects, triggering the explosive release of oxygen and doxorubicin. The second step is to use a 680 nm laser to trigger PDT under high oxygen content. The controlled release of the “oxygen bomb” and the programmed cascade therapy strategy provided a new way to suppress hypoxic tumors (Figure 5). In addition to improving the efficacy of PDT by increasing the oxygen content, highly cytotoxic free radicals are produced via the type I pathway and oxygen dependence is significantly reduced. Therefore, type I PDT is capable of producing highly potent toxic free radicals under hypoxia. However, the development of type I PS with long-wavelength absorption regions for deep tissue penetration remains a great challenge. Based on this, Li et al. prepared a PS with aggregation-induced emission (AIE) properties, namely DCTBT [131]. Through clever molecular engineering, DCTBT has powerful AIE properties, NIR-II absorption, and type I PDT and PTT function. DCTBT was encapsulated by liposomes modified with targeted peptides, and then, delivered to the tumor site. The results showed that DCTBT-loaded liposomes exhibited significant tumor growth inhibition in a PDAC mouse model following NIR-II fluorescence imaging-guided synergistic treatment of type I PDT and PTT. The NIR-IIb region (1500 to 1700 nm) has recently been identified as a promising region for deeper tissue penetration [135]. Based on this, Pham et al. developed a dual PDT nanosystem with a 1550 nm light response to effectively promote 1O2 production [135]. Rose red (RB) and Ce6 were loaded into SiO2-coated core-shell upconverted NPs to form a nanosystem. This nanosystem can further activate RB and Ce6 by emitting green (~550 nm) and red (~670 nm) light under laser irradiation at 1550 nm. The simultaneous excitation of double PS produced a large amount of 1O2, thus producing a powerful PDT effect. In vitro and in vivo experiments demonstrated that this dual PDT nanosystem exhibited enhanced antitumor effects at a single dose of treatment compared to single PS. The combination of enhanced dual PDT and 1550 nm photoexcitation is expected to provide new avenues for PDAC treatment.
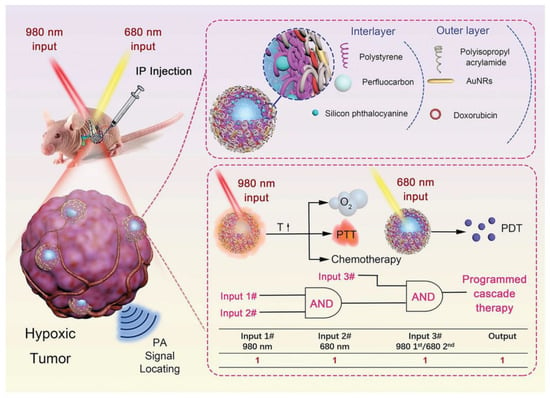
Figure 5.
Schematic diagram of PSPP-Au980-D-mediated controllable release of “oxygen bomb” and programmed cascade therapy process. Reprinted with permission from [132]. Copyright 2022, John Wiley and Sons.
In addition to strategies that enhance the effects of PDT, such as alleviating hypoxia and double PS, synergistic combinations of multiple therapeutic strategies are expected to improve the dismal efficacy of PDAC (for example, the combination of PDT and chemotherapy or immunotherapy). Zhu et al. developed a PDAC treatment strategy that combines PDT, chemotherapy, and pro-apoptotic signals [137]. The nanosystem consists of Ce6 and a pro-apoptotic peptide-GEM conjugate. The pro-apoptotic peptide-GEM conjugate was linked by a ROS-sensitive linker, and then, self-assembled with Ce6 to form Ce6-GVS NPs. Upon irradiation using a 660 nm laser, the nanosystem generated ROS and triggered the release of GEM and pro-apoptotic peptides. The strong tumor-suppressive effect of the combined strategy was confirmed in vitro and in vivo. Sun et al. designed a supramolecular prodrug nanoplatform to promote PDAC photodynamic immunotherapy by regulating glucose metabolism [136]. The prodrug NPs were prepared via the host–guest interaction between β-cyclodextrin-grafted hydronic acid and a supramolecular prodrug nanosystem to deliver pyropheophorbide a (PPa) and a bromodomain and extraterminal protein 4 inhibitor (JQ1) for the photoimmunotherapy of PDAC. PPa can produce PDT effects under 671 nm laser irradiation, thereby activating the ICD effect and causing the activation and infiltration of CD8+T cells. JQ1 can inhibit PDT-enhanced glycolysis and down-regulate the expression of PD-L1 in tumor cells to inhibit PDT-induced immune tolerance. Therefore, this is the first study to demonstrate the role of supramolecular NPs in the regulation of tumor glycolytic and immune microenvironments and may be a novel strategy to promote photodynamic immunotherapy for PDAC. Hypoxia not only severely limits the efficacy of PDT, but also can regulate a variety of immune cells to promote the tumor phenotype and enhance the immunosuppressive function of MDSC and Treg, resulting in a significant immunosuppressive effect. Therefore, improving tumor hypoxia can effectively enhance the antitumor response mediated by PDT and immunomodulators. Wang et al. reported an oxygen delivery PFC carrier for increasing oxygen levels in tumors and enhancing photodynamic immunotherapy effects [134]. The nanosystem specifically accumulated and enhanced oxygen levels within the tumor site. Under laser irradiation, DiD produced a large number of ROS, induced the generation of the ICD effect, and triggered the responsive release of GEM; this effectively stimulated CD8+T cells and NK cells and inhibited MDSC, and finally produced a strong anti-tumor effect. Previous studies have shown that PDT can induce apoptosis, necrosis, autophagy, and ICD [151]. Recently, a novel membrane-targeted PS (TBD-3C) has been shown to activate cancer cell pyroptosis [152]. Wang et al. further evaluated the immune activation effect of TBD-3C-induced pyroptosis [138]. They found that PDT induced by TBD-3C could induce pyroptosis, activate antitumor immunity, and significantly inhibit the growth of PDAC. Their findings lay the foundation for photo-controlled antitumor immunotherapy induced by pyroptosis.
In summary, PDT is a powerful and effective treatment for cancer. The main obstacles to PDT treatment in PDAC are severe internal hypoxia and limited light penetration depth. A variety of nanosystem design strategies to alleviate hypoxia and enhance light penetration depth have been widely developed. Recent research aims to overcome the shortcomings of PDT treatment through multifunctional nanosystems and enhance the efficacy of PDT by combining multiple treatment modalities. Although the integration of multiple functions in one nanosystem is beneficial in overcoming the drawbacks of each treatment modality, the corresponding increase in the complexity of the manufacturing process makes clinical translation difficult. Future research may require more efforts to fabricate nanosystems that are beneficial in overcoming multiple dilemmas through simple and convenient synthetic routes.
2.7. Elaborate Nanosystems for PDAC Sonodynamic Therapy (SDT)
SDT, as a promising non-invasive treatment, has attracted more and more attention in recent years. SDT relies on low-intensity ultrasound to trigger the production of cytotoxic ROS by sonosensitizers, thereby inducing cell damage [153]. Compared with PDT, SDT has greater advantages in tissue penetration depth. Using the penetration of ultrasound into deep tissues, SDT has thus emerged as a promising alternative for the treatment of deep tumors while still maintaining its advantages over conventional cancer treatment modalities. Although preclinical studies of SDT have shown promising results in various tumor cells, its therapeutic efficacy of tumor elimination in vivo is still far from satisfactory [153]. Driven by advances in nanotechnology, many new nanosystems have been developed in this emerging field to enhance the therapeutic efficacy of SDT (Table 5). Conventional SDT still relies on the intratumoral supply of oxygen, which is required for ROS production. However, the utility of SDT in PDAC is limited due to inherent intratumoral hypoxia. Recent studies have shown that titanium dioxide (TiO2) has the characteristic of responding to ultrasound [139]. Ultrasound can induce TiO2 to mediate the production of cytotoxic free radicals and superoxide, and can achieve efficient oxygen-independent type I SDT. However, the single TiO2 material has some limitations related to its stability and energy transfer efficiency. To this end, Cao et al. prepared a TiO2/C nanocomposite for type I SDT-mediated PDAC treatment [139]. This novel TiO2/C nanocomposite has excellent stability and can effectively produce ROS even under hypoxic conditions. Another key determinant of the efficacy of SDT is the subcellular localization of the sonosensitizer, as most ROS generated have a very short lifetime and limited diffusion length [154]. Recent evidence suggests that sonosensitizers located near DNA are able to induce more intense oxidative damage, thereby achieving a powerful therapeutic effect [155]. Zhang et al. reported a nuclear-targeted Ti-tetrakis(4-carboxyphenyl)porphyrin (TCPP) MOF that can exert type I SDT efficacy as a sonosensitizer [141]. This novel MOF can effectively target the nucleus and directly break DNA through the SDT effect. In addition, the MOF can efficiently produce ROS in hypoxic environments, and thus, has great prospects for the treatment of hypoxic tumors. In another study, Chen et al. designed an ER-targeted nanodroplet that selectively accumulated in the ER, generated large amounts of ROS, induced intense ER stress, and finally, triggered strong ICD effects [142]. In addition to enhancing the ability to produce ROS under hypoxic conditions through type I SDT, another strategy is to enhance the efficacy of type II SDT by providing oxygen. Chen et al. constructed an oxygen-generated nanoplatform mediated by modified fluorocarbon (FC) chains for efficient hypoxic SDT treatment [140]. The nanoplatform consisted of MSNPs functionalized with FC chains loaded with the sonosensitizer IR780. This oxygen delivery protocol effectively alleviated the intratumor hypoxia state of PDAC and produced sufficient ROS to kill tumor cells under the induction of ultrasound. In another study, Chen et al. designed a pH/ATP dual responsive nanoplatform based on zeolitic imidazolate framework-90 to alleviate PDAC hypoxia and enhance SDT efficacy [143]. In this nanosystem (Pt@ZIF-90@Gem@IR780), platinum (Pt) NPs were added to exert catalase activity and promote the conversion of hydrogen peroxide into oxygen in the tumor to increase the oxygen content. IR780 and GEM were co-loaded onto the nanoplatform as SDT therapeutic agents and chemotherapy agents. After catalysis by Pt NPs, the hypoxia situation in the tumor was relieved, ultimately leading to an enhanced SDT effect and chemotherapy sensitivity (Figure 6). Ultrasound-targeted MB destruction is an emerging technology in the field of drug delivery that involves the use of low-intensity ultrasound to destroy MBs at the target site to release loaded cargo or encapsulated gas [156]. When oxygen and sonosensitizer are loaded into MBs, under the stimulation of ultrasound, on the one hand, the cavitation effect of MBs is used to rupture the MBs and release oxygen; on the other hand, the sonosensitizer can produce a large amount of ROS efficiently under the promotion of oxygen. Nesbitt et al. combined oxygen MB-GEM with RB-functionalized oxygen MB for chemo-sonodynamic therapy, which effectively inhibited tumor growth and resulted in the decreased expression of markers related to PDAC progression, demonstrating great potential for the treatment of PDAC [157]. In another study, Sheng et al. added magnetic NPs into MB shells to deliver MBs in a targeted manner under the action of an external magnetic field [158]. They constructed oxygenated magnetic MBs with 5-FU and RB attached to the surface for PDAC-targeted therapy. The combination of an external magnetic field and ultrasound resulted in a 48.3% reduction in tumor volume in orthotopic PDAC compared with controls. Their results demonstrated the effectiveness of using a magnetic targeting MB platform for the highly targeted therapy of PDAC.
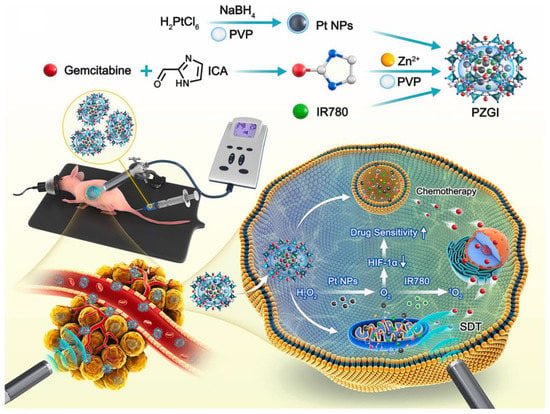
Figure 6.
Schematic diagram the pH/ATP dual responsive Pt@ZIF-90@Gem@IR780 for synergistic chemo-sonodynamic therapy of PDAC. Reprinted with permission from [143]. Copyright 2021, Elsevier.
To summarize, SDT is a new treatment that has been developed for PDAC in recent years, and there are few related studies. However, SDT has great potential for the treatment of PDAC due to its multiple advantages, especially its deep penetration ability. Future studies should focus more on the enrichment of sonosensitizer at the tumor site, subcellular localization, tumor hypoxia, and other issues to achieve a more powerful therapeutic effect of SDT.
2.8. Custom-Made Nanosystems for Enhanced Chemodynamic Therapy (CDT)
Another type of treatment that damages cells by producing hydroxyl radicals (·OH) is called CDT [159] (Table 5). Different from PDT or SDT, CDT does not depend on light or ultrasound, but on the Fenton reaction chemical process of endogenous hydrogen peroxide (H2O2) to OH [159]. Therefore, CDT is an effective therapeutic strategy to overcome tissue depth and the hypoxic microenvironment. However, the insufficient H2O2 content in the microenvironment will weaken the therapeutic effect of CDT [144]. Therefore, increasing the in situ production of H2O2, and thereby enhancing Fenton or Fenton-like reaction, is a promising strategy to enhance CDT efficacy. Ultra-small AuNPs are a kind of nanoenzyme with glucose oxidase activity, and can catalyze the formation of H2O2 from glucose [160]. Using this property, Li et al. designed a nanosystem based on hollow mesoporous organosilica nanoparticles (HMONs) for PDT- and CDT-mediated PDAC treatment [145]. The photosensitizer HPPH was hybridized to the HMON skeleton through in situ growth. Ultra-small AuNPs were immobilized in the HMON cavity via thiol groups. Finally, Cu2+-chelated tannic acid was deposited on the surface of the HMONs. The nanosystem first catalyzed glucose to produce H2O2, which promoted the occurrence of Fenton-like reactions and induced strong CDT therapeutic effects. In addition, under NIR irradiation, the resulting PDT effect further synergistically killed tumor cells. This well-designed therapeutic cascade effectively enhanced ROS-mediated anti-PDAC therapy. Recent studies have found that hyperthermia produced by PTT can promote the catalytic efficiency of the Fenton reaction and contribute to the realization of efficient synergistic cancer therapy [161]. Based on this, Sun et al. developed a photothermal Fenton nanocatalyst for PDAC synergistic PTT and CDT [144]. The nanosystem was synthesized by combining copper sulfide (CuS) with manganese dioxide coated with human serum albumin. CuS acted as a nanocatalyst to produce toxic ·OH in a Fenton-like reaction with H2O2. Furthermore, NIR-II irradiation not only ablated tumor cells via hyperthermia, but also accelerated the Fenton-like response. The results showed that the synergistic treatment of PTT and CDT completely inhibited tumor growth in Panc02 tumor-bearing mice. In addition to the combination of PTT, Sun et al. designed a Cu-MOF for SDT and CDT, which was able to efficiently induce cellular free radical production under hypoxic conditions [146]. On the one hand, this Cu-MOF will degrade under hypoxic conditions, releasing Cu2+ and sonosensitizer. When exposed to ultrasound, the sonosensitizer produced nitrogen bubbles and alkyl radicals in an oxygen-independent manner. On the other hand, the released Cu2+ consumed a high level of GSH in the tumor and transited into Cu+, which then underwent a Fenton-like reaction with H2O2 to produce ·OH. This hypoxic TME responsive sono-chemodynamic therapy strategy provided an effective way to achieve oxygen-independent free radical generation.
It was found that Fe(III)/Fe(II) could induce polarization of M2-TAM to the M1-TAM phenotype [162]. Because of the stimulative role of M2-TAM in PDAC stromal formation, Fe(III)/Fe(II) is expected to comprehensively improve PDAC therapy by simultaneously killing tumor cells via CDT and reprogramming the PDAC stromal microenvironment. To test this hypothesis, Chen et al. constructed a nanosystem to simultaneously kill tumor cells and reshape the matrix [81]. Fe2+ and a strong acid environment are essential for the Fenton reaction to occur. Therefore, they introduced SO2 into the nanosystem as a reducing agent to convert Fe3+ to Fe2+ and produce sulfuric acid. In addition, GSH-responsive groups were introduced into the nanosystem to achieve controllability. The excess GSH in tumor cells triggered the rapid release of Fe3+ and SO2, which further promoted the conversion of Fe3+ to Fe2+ and the production of sulfuric acid. Finally, the Fenton reaction produced a large amount of ·OH to kill tumor cells. However, in TAM cells, the Fenton response was not triggered due to insufficient GSH. Instead, Fe3+ innocuously induced TAM to polarize to the M1 phenotype, ultimately downregulating pro-fiber signaling and alleviating stromal disorders. Thus, this carefully tailored nanosystem exploited the unique redox homeostasis within the TME for different therapeutic purposes.
Overall, CDT is a promising treatment modality for PDAC, and its potent free radical production capacity can exert potent cytotoxic effects. In addition, it is independent of external energy and is not limited by penetration depth, which also helps to overcome the difficulties of PDAC treatment. However, CDT also has its associated limitations, such as insufficient H2O2 levels, highly acidic environments, and the catalytic efficiency of Fenton reactions. The design of more sophisticated nanosystems is needed to fully consider the limitations of CDT and make up for its shortcomings.
3. Conclusions and Challenges
PDAC is a malignant tumor with an extremely poor prognosis, and its survival prognosis has not improved significantly over the years. The main reason is that the existence of various dilemmas and obstacles makes the disease resistant to a variety of treatment methods. The resistance to and side effects of chemotherapy drugs have greatly limited their efficacy in the treatment of PDAC. Researchers have attempted to improve outcomes by targeting specific pathways, but have experienced many failures. In addition, initial clinical attempts to target tumor stroma have failed. Even immunotherapies that have been successful in a variety of human tumors have been unable to break down the barriers to PDAC treatment. The development of nanotechnology brings new hope for the treatment of PDAC. Well-designed smart nanosystems have been extensively developed to overcome various barriers in PDAC therapy, such as charge-reversal nanosystems, size-transform nanosystems, cascade-targeted nanosystems, and cluster bomb nanosystems [80]. These nanosystems are endowed with a variety of powerful capabilities that can target therapeutic needs, and have shown encouraging results in preclinical trials.
However, only a small number of nanomaterials have been approved for clinical use and they are focused on relatively simple products such as liposomes. Therefore, the clinical translation of nanomedicine is one of the major challenges for researchers designing nanosystems. Many factors hinder the clinical translation of nanomaterials, including the stability of nanomaterials, effective biodistribution, in vivo toxicity, and biodegradation mechanisms [21]. For example, Vadim et al. reported that tiny rare-earth fluoride NPs carry the risk of promoting tumor cell growth through electrical polar interactions [163]. Other nanomaterials such as gold NPs, MSNPs, and ceria NPs have been reported to have tumor-promoting properties [164,165,166]. It is estimated that about 20% of nanomaterials in clinical trials fail due to safety issues. Therefore, the toxicity of nanomaterials is a non-negligible problem, so the safety of nanomaterials must be guaranteed before entering into clinical transformation. The development of toxicological analyses of nanomaterials has helped to identify key physicochemical characteristics that contribute to their toxicity and to design safer strategies [167]. By optimizing the physicochemical properties of nanomaterials, the toxicity of nanomaterials can be minimized while their biological efficacy is maximized. In addition, issues related to raw material costs, manufacturing costs, and standardized mass production are also factors to be considered. Designing ever more complex nanosystems will not enable us to escape this transformation mess. To escape this predicament, we must critically rethink the current and future directions of nanomedicine. In addition to the development of intelligence and versatility, more attention should be paid to the practical transformation value of nanosystems. For example, in the process of designing and manufacturing nanomaterials, the physical and chemical characterization of nanomaterials should be fully completed to ensure the stability and biological safety of the materials [168].
The transport of nanosystems in PDAC tumor tissues is another challenge limiting their effectiveness. Most nanomedicines that are currently in clinical trials rely on EPR effects. However, the stromal barrier of PDAC severely limits the EPR effect. Recent studies have found that transcytosis is an effective targeting strategy that can complement the EPR effects of PDAC drug delivery [169]. Related studies include the discovery of vesico-vacuolar organelle-like structures in PDAC [170,171], the iRGD peptide-triggered mass transcytosis pathway [172], caveolae-mediated transcytosis mediated by albumin-based nanosystems [173,174], urokinase plasminogen activator receptor-mediated transcytosis [175], and macropinocytosis-mediated transcytosis activated by cationic NPs [79]. These studies suggest that the active transport mode of transcytosis can bypass the barrier of passive diffusion and enable the active penetration of nanomaterials into tumors. Future research designs should take into account the lack of EPR effects, as well as the advantages of active transport.
On the other hand, anti-stroma therapy strategies developed in preclinical studies have shown enhanced efficacy, but many of these strategies have resulted in clinical failure [6]. These results have sparked debate about the pro-tumor and anti-tumor effects of the stroma in PDAC. Our understanding of the biological mechanisms of the PDAC stroma is relatively preliminary compared to other PDAC-related mechanisms [6]. In fact, the PDAC stroma is a heterogeneous equilibrium system, and disruption of this equilibrium may have harmful consequences, such as promoting tumor invasion and metastasis [176,177]. The two-sided nature of the PDAC stroma should be fully considered in the design of nanosystems, and the stroma regulation strategy should be carefully evaluated and formulated.
Current immunotherapy strategies tend to use combined regulatory approaches [176]. However, the immune microenvironment of PDAC is extremely complex and variable. There are still significant limits to our understanding and regulation of the immune microenvironment. It is important to recognize that patients with PDAC may have more than one immune deficiency, and what is even more challenging is that immune deficiency may change during the treatment [178]. Therefore, choosing the right combination of treatments at the right point in time is critical.
Additionally, nanomaterials face the challenge of recognition and clearance by the immune system. The successful use of Abraxane® (nab-paclitaxel) illustrates the advantages of endogenous proteins as drug carriers [179]. Cell-derived exosomes are also being used in clinical trials for PDAC diagnosis (NCT03032913). The evidence suggests that biomimetic nanomaterials may have greater potential.
Finally, given the complexity of the TME of PDAC, the selection of tumor models in preclinical studies is of great importance. Subcutaneous xenograft models may struggle to mimic the stromal microenvironment of PDAC, even when tumor cells are co-injected with PSCs. The genetically engineered mouse model can better summarize the molecular biological and histopathological features of human PDAC, including similar precursor lesions, the dense fibrotic matrix, and the immunosuppressive microenvironment [180]. In addition, the PDX model has high fidelity in PDAC modeling and is a powerful platform for both basic and translational research [181]. However, due to its high cost and long duration, the PDX model is not an ideal platform for large-scale research. Patient tumor-derived organoid models that retain the genetic and phenotypic characteristics of PDAC are rapidly developing as an effective method for mimicking patient disease in vitro [181].
Collectively, a series of well-designed smart nanosystems hold infinite promise for the treatment of PDAC. However, there is still a long way to go to overcome the dilemma of PDAC treatment and realize clinical transformation. With the joint efforts of researchers from multiple disciplines, nanotechnology is expected to achieve new breakthroughs for PDAC treatment.
Author Contributions
Conceptualization, X.-Y.Y.; Formal Analysis, Y.-F.L.; Investigation, J.-X.X.; Writing—Original Draft Preparation, X.-Y.Y.; Writing—Review and Editing, Y.-Z.D. and R.-S.Y.; Supervision, R.-S.Y.; Funding Acquisition, R.-S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported, in part, by grants from the Natural Science Foundation of Zhejiang province (LZ22H180002) and the National Natural Science Foundation of China (General Program: 82171998).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
AIE: aggregation-induced emission; APCs: antigen-presenting cells; CAFs: cancer-associated fibroblasts; CDT: chemodynamic therapy; Ce6: chlorin e6; CNTs: carbon nanotubes; CRT: calreticulin; CTL: cytotoxic T cell; CuS: copper sulfide; DCs: dendritic cells; dCTP: deoxycytidine triphosphate; ECM: extracellular matrix; ER: endoplasmic reticulum; 5-FU: 5-fluorouracil; GEM: gemcitabine; GEM-HSA-NP: GEM-loaded human serum albumin nanoparticle; GGT: γ-glutamyl transpeptidase; GO: graphene oxide; GSH: glutathione; hENT1: human equilibrative nucleoside transporter 1; hNTs: human nucleoside transporters; H2O2: hydrogen peroxide; ICD: immunogenic cell death; ICIs: immune checkpoint inhibitors; KPC: KRAS-induced pancreatic cancer; LDH: layered double hydroxides; MDSCs: myeloid-derived suppressor cells; miRNA: microRNA; MMP-2: matrix metalloproteinase 2; MOF: metal–organic framework; MSNPs: mesoporous silica NPs; NO: nitric oxide; NPs: nanoparticles; ·OH: hydroxyl radicals; PanIN: pancreatic intraepithelial neoplasia; PDAC: pancreatic ductal adenocarcinoma; pDNA: plasmid DNA; PDT: photodynamic therapy; PDX: patient-derived xenograft; PFC: perfluorocarbon; PS: photosensitizer; PSC: pancreatic stellate cell; PTT: photothermal therapy; QDs: quantum dots; RB: rose red; SDT: sonodynamic therapy; siRNA: short interfering RNA; α-SMA: α smooth muscle actin; SNAP: S-nitroso-N-acetylpenicillamine; SPIO: superparamagnetic iron oxide; TAMs: tumor-associated macrophages; Tf: transferrin; TGF-β: transforming growth factor-β; TLR: Toll-like receptor; Tregs: regulatory T cells; VAC: vactosertib.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lu, T.; Prakash, J. Nanomedicine Strategies to Enhance Tumor Drug Penetration in Pancreatic Cancer. Int. J. Nanomed. 2021, 16, 6313–6328. [Google Scholar] [CrossRef]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Lee, J.S.; Rhee, T.M.; Pietrasz, D.; Bachet, J.B.; Laurent-Puig, P.; Kong, S.Y.; Takai, E.; Yachida, S.; Shibata, T.; Lee, J.W.; et al. Circulating tumor DNA as a prognostic indicator in resectable pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 16971. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Chiorean, E.G.; Czito, B.; Scaife, C.; Narang, A.K.; Fountzilas, C.; Wolpin, B.M.; Al-Hawary, M.; Asbun, H.; et al. Pancreatic Adenocarcinoma, Version 1.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 202–210. [Google Scholar] [CrossRef]
- Perri, G.; Prakash, L.; Qiao, W.; Varadhachary, G.R.; Wolff, R.; Fogelman, D.; Overman, M.; Pant, S.; Javle, M.; Koay, E.J.; et al. Response and Survival Associated With First-line FOLFIRINOX vs Gemcitabine and nab-Paclitaxel Chemotherapy for Localized Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2020, 155, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Neesse, A.; Algul, H.; Tuveson, D.A.; Gress, T.M. Stromal biology and therapy in pancreatic cancer: A changing paradigm. Gut 2015, 64, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Connor, A.A.; Gallinger, S. Pancreatic cancer evolution and heterogeneity: Integrating omics and clinical data. Nat. Rev. Cancer 2022, 22, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, C.; Xie, K.-P. Therapeutic resistance of pancreatic cancer: Roadmap to its reversal. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1875, 188461. [Google Scholar] [CrossRef]
- Schizas, D.; Charalampakis, N.; Kole, C.; Economopoulou, P.; Koustas, E.; Gkotsis, E.; Ziogas, D.; Psyrri, A.; Karamouzis, M.V. Immunotherapy for pancreatic cancer: A 2020 update. Cancer Treat. Rev. 2020, 86, 102016. [Google Scholar] [CrossRef]
- Tao, J.; Yang, G.; Zhou, W.; Qiu, J.; Chen, G.; Luo, W.; Zhao, F.; You, L.; Zheng, L.; Zhang, T.; et al. Targeting hypoxic tumor microenvironment in pancreatic cancer. J. Hematol. Oncol. 2021, 14, 14. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy. Curr. Drug Metab. 2019, 20, 416–429. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in cancer diagnosis: Progress, challenges and opportunities. J. Hematol. Oncol. 2019, 12, 137. [Google Scholar] [CrossRef]
- Asadujjaman, M.; Cho, K.H.; Jang, D.J.; Kim, J.E.; Jee, J.P. Nanotechnology in the arena of cancer immunotherapy. Arch. Pharm. Res. 2020, 43, 58–79. [Google Scholar] [CrossRef]
- Van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Y.; Cheng, R.; Yang, Z.; Tian, Z.M. Nanotechnology for Cancer Therapy Based on Chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Xi, X.; Batra, S.K.; Bronich, T.K. Combination Therapies and Drug Delivery Platforms in Combating Pancreatic Cancer. J. Pharmacol. Exp. Ther. 2019, 370, 682–694. [Google Scholar] [CrossRef]
- Paroha, S.; Verma, J.; Dubey, R.D.; Dewangan, R.P.; Molugulu, N.; Bapat, R.A.; Sahoo, P.K.; Kesharwani, P. Recent advances and prospects in gemcitabine drug delivery systems. Int. J. Pharm. 2021, 592, 120043. [Google Scholar] [CrossRef]
- Li, Y.J.; Wu, J.Y.; Wang, J.M.; Hu, X.B.; Cai, J.X.; Xiang, D.X. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2020, 101, 519–530. [Google Scholar] [CrossRef]
- Liu, G.; Lovell, J.F.; Zhang, L.; Zhang, Y. Stimulus-Responsive Nanomedicines for Disease Diagnosis and Treatment. Int. J. Mol. Sci. 2020, 21, 6380. [Google Scholar] [CrossRef]
- Sheng, Q.; Li, T.; Tang, X.; Zhao, W.; Guo, R.; Cun, X.; Zang, S.; Zhang, Z.; Li, M.; He, Q. Comprehensively enhanced delivery cascade by transformable beaded nanofibrils for pancreatic cancer therapy. Nanoscale 2021, 13, 13328–13343. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, F.; Di, Y.; Yao, L.; Yu, X.; Fu, D.; Li, J.; Jin, C. Antitumor effect of gemcitabine-loaded albumin nanoparticle on gemcitabine-resistant pancreatic cancer induced by low hENT1 expression. Int. J. Nanomed. 2018, 13, 4869–4880. [Google Scholar] [CrossRef]
- Tang, M.; Svirskis, D.; Leung, E.; Kanamala, M.; Wang, H.; Wu, Z. Can intracellular drug delivery using hyaluronic acid functionalised pH-sensitive liposomes overcome gemcitabine resistance in pancreatic cancer? J. Control. Release 2019, 305, 89–100. [Google Scholar] [CrossRef]
- Liu, X.; Situ, A.; Kang, Y.; Villabroza, K.R.; Liao, Y.; Chang, C.H.; Donahue, T.; Nel, A.E.; Meng, H. Irinotecan Delivery by Lipid-Coated Mesoporous Silica Nanoparticles Shows Improved Efficacy and Safety over Liposomes for Pancreatic Cancer. ACS Nano 2016, 10, 2702–2715. [Google Scholar] [CrossRef] [PubMed]
- Shabana, A.M.; Kambhampati, S.P.; Hsia, R.C.; Kannan, R.M.; Kokkoli, E. Thermosensitive and biodegradable hydrogel encapsulating targeted nanoparticles for the sustained co-delivery of gemcitabine and paclitaxel to pancreatic cancer cells. Int. J. Pharm. 2021, 593, 120139. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tao, Y.; He, M.; Deng, M.; Guo, R.; Sheng, Q.; Wang, X.; Ren, K.; Li, T.; He, X.; et al. Co-delivery of autophagy inhibitor and gemcitabine using a pH-activatable core-shell nanobomb inhibits pancreatic cancer progression and metastasis. Theranostics 2021, 11, 8692–8705. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Xi, X.; Rachagani, S.; Seshacharyulu, P.; Talmon, G.A.; Ponnusamy, M.P.; Batra, S.K.; Bronich, T.K. Nanoscale platform for delivery of active IRINOX to combat pancreatic cancer. J. Control. Release 2021, 330, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Kattel, K.; Mondal, G.; Lin, F.; Kumar, V.; Mahato, R.I. Biodistribution of Self-Assembling Polymer-Gemcitabine Conjugate after Systemic Administration into Orthotopic Pancreatic Tumor Bearing Mice. Mol. Pharm. 2017, 14, 1365–1372. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, V.; Maksimenko, A.; Anand, R.; Monti, S.; Agostoni, V.; Couvreur, P.; Lampropoulou, M.; Yannakopoulou, K.; Gref, R. Efficient “green” encapsulation of a highly hydrophilic anticancer drug in metal-organic framework nanoparticles. J. Drug Target. 2015, 23, 759–767. [Google Scholar] [CrossRef]
- Bilalis, P.; Skoulas, D.; Karatzas, A.; Marakis, J.; Stamogiannos, A.; Tsimblouli, C.; Sereti, E.; Stratikos, E.; Dimas, K.; Vlassopoulos, D.; et al. Self-Healing pH- and Enzyme Stimuli-Responsive Hydrogels for Targeted Delivery of Gemcitabine To Treat Pancreatic Cancer. Biomacromolecules 2018, 19, 3840–3852. [Google Scholar] [CrossRef]
- Pal, K.; Al-Suraih, F.; Gonzalez-Rodriguez, R.; Dutta, S.K.; Wang, E.; Kwak, H.S.; Caulfield, T.R.; Coffer, J.L.; Bhattacharya, S. Multifaceted peptide assisted one-pot synthesis of gold nanoparticles for plectin-1 targeted gemcitabine delivery in pancreatic cancer. Nanoscale 2017, 9, 15622–15634. [Google Scholar] [CrossRef]
- Saini, K.; Bandyopadhyaya, R. Transferrin-Conjugated Polymer-Coated Mesoporous Silica Nanoparticles Loaded with Gemcitabine for Killing Pancreatic Cancer Cells. ACS Appl. Nano Mater. 2019, 3, 229–240. [Google Scholar] [CrossRef]
- Han, H.; Valdeperez, D.; Jin, Q.; Yang, B.; Li, Z.; Wu, Y.; Pelaz, B.; Parak, W.J.; Ji, J. Dual Enzymatic Reaction-Assisted Gemcitabine Delivery Systems for Programmed Pancreatic Cancer Therapy. ACS Nano 2017, 11, 1281–1291. [Google Scholar] [CrossRef]
- Meng, H.; Wang, M.; Liu, H.; Liu, X.; Situ, A.; Wu, B.; Ji, Z.; Chang, C.H.; Nel, A.E. Use of a Lipid-Coated Mesoporous Silica Nanoparticle Platform for Synergistic Gemcitabine and Paclitaxel Delivery to Human Pancreatic Cancer in Mice. ACS Nano 2015, 9, 3540–3557. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Zhang, D.; Alsaiari, S.; Lu, J.; Deng, L.; Tamanoi, F.; AlMalik, A.M.; Zink, J.I.; Khashab, N.M. Protein-gold clusters-capped mesoporous silica nanoparticles for high drug loading, autonomous gemcitabine/doxorubicin co-delivery, and in-vivo tumor imaging. J. Control. Release 2016, 229, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, G.; Liu, G.; Hu, J.; Liu, S. Photo- and thermo-responsive multicompartment hydrogels for synergistic delivery of gemcitabine and doxorubicin. J. Control. Release 2017, 259, 149–159. [Google Scholar] [CrossRef] [PubMed]
- R S, P.; Mal, A.; Valvi, S.K.; Srivastava, R.; De, A.; Bandyopadhyaya, R. Noninvasive Preclinical Evaluation of Targeted Nanoparticles for the Delivery of Curcumin in Treating Pancreatic Cancer. ACS Appl. Bio. Mater. 2020, 3, 4643–4654. [Google Scholar] [CrossRef]
- Khan, S.; Setua, S.; Kumari, S.; Dan, N.; Massey, A.; Hafeez, B.B.; Yallapu, M.M.; Stiles, Z.E.; Alabkaa, A.; Yue, J.; et al. Superparamagnetic iron oxide nanoparticles of curcumin enhance gemcitabine therapeutic response in pancreatic cancer. Biomaterials 2019, 208, 83–97. [Google Scholar] [CrossRef]
- Choi, M.; Bien, H.; Mofunanya, A.; Powers, S. Challenges in Ras therapeutics in pancreatic cancer. Semin. Cancer Biol. 2019, 54, 101–108. [Google Scholar] [CrossRef]
- Stoica, A.F.; Chang, C.H.; Pauklin, S. Molecular Therapeutics of Pancreatic Ductal Adenocarcinoma: Targeted Pathways and the Role of Cancer Stem Cells. Trends Pharmacol. Sci. 2020, 41, 977–993. [Google Scholar] [CrossRef]
- Gillson, J.; Ramaswamy, Y.; Singh, G.; Gorfe, A.A.; Pavlakis, N.; Samra, J.; Mittal, A.; Sahni, S. Small Molecule KRAS Inhibitors: The Future for Targeted Pancreatic Cancer Therapy? Cancers 2020, 12, 1341. [Google Scholar] [CrossRef]
- Qian, Z.R.; Rubinson, D.A.; Nowak, J.A.; Morales-Oyarvide, V.; Dunne, R.F.; Kozak, M.M.; Welch, M.W.; Brais, L.K.; Da Silva, A.; Li, T.; et al. Association of Alterations in Main Driver Genes With Outcomes of Patients With Resected Pancreatic Ductal Adenocarcinoma. JAMA Oncol. 2018, 4, e173420. [Google Scholar] [CrossRef]
- Collisson, E.A.; Bailey, P.; Chang, D.K.; Biankin, A.V. Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 207–220. [Google Scholar] [CrossRef]
- Chang, D.K.; Grimmond, S.M.; Biankin, A.V. Pancreatic cancer genomics. Curr. Opin. Genet. Dev. 2014, 24, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Waddell, N.; Pajic, M.; Patch, A.M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.M.; Gingras, M.C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, W.; Wang, Y.; Han, S.; Yuan, Y.; Huang, J.; Shuai, X.; Peng, Z. Recent development of gene therapy for pancreatic cancer using non-viral nanovectors. Biomater. Sci. 2021, 9, 6673–6690. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, D.; Sivak, L.; Jogdeo, C.M.; Kumar, R.; Khan, R.; Hang, Y.; Oupicky, D. Gene silencing delivery systems for the treatment of pancreatic cancer: Where and what to target next? J. Control. Release 2021, 331, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Hu, P.; Xu, Y.; Bao, Q.; Ni, D.; Wei, C.; Shi, J. Efficient Gene Therapy of Pancreatic Cancer via a Peptide Nucleic Acid (PNA)-Loaded Layered Double Hydroxides (LDH) Nanoplatform. Small 2020, 16, e1907233. [Google Scholar] [CrossRef]
- Zhao, X.; Li, F.; Li, Y.; Wang, H.; Ren, H.; Chen, J.; Nie, G.; Hao, J. Co-delivery of HIF1alpha siRNA and gemcitabine via biocompatible lipid-polymer hybrid nanoparticles for effective treatment of pancreatic cancer. Biomaterials 2015, 46, 13–25. [Google Scholar] [CrossRef]
- Won, E.J.; Park, H.; Chang, S.H.; Kim, J.H.; Kwon, H.; Cho, Y.S.; Yoon, T.J. One-shot dual gene editing for drug-resistant pancreatic cancer therapy. Biomaterials 2021, 279, 121252. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Li, J.; Zhang, Z.; Huang, C.; Lian, G.; Yang, K.; Chen, S.; Lin, Y.; Wang, L.; et al. Co-delivery of microRNA-21 antisense oligonucleotides and gemcitabine using nanomedicine for pancreatic cancer therapy. Cancer Sci. 2017, 108, 1493–1503. [Google Scholar] [CrossRef]
- Lin, L.; Fan, Y.; Gao, F.; Jin, L.; Li, D.; Sun, W.; Li, F.; Qin, P.; Shi, Q.; Shi, X.; et al. UTMD-Promoted Co-Delivery of Gemcitabine and miR-21 Inhibitor by Dendrimer-Entrapped Gold Nanoparticles for Pancreatic Cancer Therapy. Theranostics 2018, 8, 1923–1939. [Google Scholar] [CrossRef]
- Luo, D.; Xu, X.; Iqbal, M.Z.; Zhao, Q.; Zhao, R.; Farheen, J.; Zhang, Q.; Zhang, P.; Kong, X. siRNA-Loaded Hydroxyapatite Nanoparticles for KRAS Gene Silencing in Anti-Pancreatic Cancer Therapy. Pharmaceutics 2021, 13, 1428. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, S.; Zhou, X.; Wang, L.; Ye, L.; Zhou, Z.; Tang, J.; Liu, X.; Teng, L.; Shen, Y. New path to treating pancreatic cancer: TRAIL gene delivery targeting the fibroblast-enriched tumor microenvironment. J. Control. Release 2018, 286, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Y.; Hu, M.; Wang, M.; Liu, X.; Huang, L. Relaxin gene delivery modulates macrophages to resolve cancer fibrosis and synergizes with immune checkpoint blockade therapy. Sci. Adv. 2021, 7, eabb6596. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.J.; Xie, Z.B.; Gao, Y.; Zhang, Y.F.; Yao, L.; Fu, D.L. LyP-1-fMWNTs enhanced targeted delivery of MBD1siRNA to pancreatic cancer cells. J. Cell. Mol. Med. 2020, 24, 2891–2900. [Google Scholar] [CrossRef]
- Gokita, K.; Inoue, J.; Ishihara, H.; Kojima, K.; Inazawa, J. Therapeutic Potential of LNP-Mediated Delivery of miR-634 for Cancer Therapy. Mol. Ther. Nucleic Acids 2020, 19, 330–338. [Google Scholar] [CrossRef]
- Das, M.; Shen, L.; Liu, Q.; Goodwin, T.J.; Huang, L. Nanoparticle Delivery of RIG-I Agonist Enables Effective and Safe Adjuvant Therapy in Pancreatic Cancer. Mol. Ther. 2019, 27, 507–517. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Song, Y.; Luo, J.; Jin, P.; Wang, X.; Xin, L.; Qiu, F.; Yao, J.; Wang, G.; et al. Ultrasound-Enhanced Reactive Oxygen Species Responsive Charge-Reversal Polymeric Nanocarriers for Efficient Pancreatic Cancer Gene Delivery. ACS Appl. Mater. Interfaces 2022, 14, 2587–2596. [Google Scholar] [CrossRef]
- Xin, X.; Kumar, V.; Lin, F.; Kumar, V.; Bhattarai, R.; Bhatt, V.R.; Tan, C.; Mahato, R.I. Redox-responsive nanoplatform for codelivery of miR-519c and gemcitabine for pancreatic cancer therapy. Sci. Adv. 2020, 6, eabd6764. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, L.; Xie, Y.; Xianyu, Y.; Zhang, L.; Wang, P.; Hamada, Y.; Jiang, K.; Zheng, W.; Jiang, X. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat. Commun. 2017, 8, 15130. [Google Scholar] [CrossRef]
- Yin, F.; Hu, K.; Chen, Y.; Yu, M.; Wang, D.; Wang, Q.; Yong, K.T.; Lu, F.; Liang, Y.; Li, Z. SiRNA Delivery with PEGylated Graphene Oxide Nanosheets for Combined Photothermal and Genetherapy for Pancreatic Cancer. Theranostics 2017, 7, 1133–1148. [Google Scholar] [CrossRef]
- Jang, H.K.; Song, B.; Hwang, G.H.; Bae, S. Current trends in gene recovery mediated by the CRISPR-Cas system. Exp. Mol. Med. 2020, 52, 1016–1027. [Google Scholar] [CrossRef]
- Ryu, S.M.; Koo, T.; Kim, K.; Lim, K.; Baek, G.; Kim, S.T.; Kim, H.S.; Kim, D.E.; Lee, H.; Chung, E.; et al. Adenine base editing in mouse embryos and an adult mouse model of Duchenne muscular dystrophy. Nat. Biotechnol. 2018, 36, 536–539. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Zhou, Q.; Gu, S.; Liu, X.; Huang, J.; Jiang, H.; Wang, H.; Cao, L.; Sun, J.; et al. Prodrug nanoparticles rationally integrating stroma modification and chemotherapy to treat metastatic pancreatic cancer. Biomaterials 2021, 278, 121176. [Google Scholar] [CrossRef]
- Chen, X.; Jia, F.; Li, Y.; Deng, Y.; Huang, Y.; Liu, W.; Jin, Q.; Ji, J. Nitric oxide-induced stromal depletion for improved nanoparticle penetration in pancreatic cancer treatment. Biomaterials 2020, 246, 119999. [Google Scholar] [CrossRef]
- Yu, Q.; Qiu, Y.; Li, J.; Tang, X.; Wang, X.; Cun, X.; Xu, S.; Liu, Y.; Li, M.; Zhang, Z.; et al. Targeting cancer-associated fibroblasts by dual-responsive lipid-albumin nanoparticles to enhance drug perfusion for pancreatic tumor therapy. J. Control. Release 2020, 321, 564–575. [Google Scholar] [CrossRef]
- Feng, J.; Xu, M.; Wang, J.; Zhou, S.; Liu, Y.; Liu, S.; Huang, Y.; Chen, Y.; Chen, L.; Song, Q.; et al. Sequential delivery of nanoformulated alpha-mangostin and triptolide overcomes permeation obstacles and improves therapeutic effects in pancreatic cancer. Biomaterials 2020, 241, 119907. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, X.; Wang, X.; Zhao, X.; Zhang, Y.; Liu, S.; Anderson, G.J.; Kim, S.J.; Li, Y.; Nie, G. Penetration Cascade of Size Switchable Nanosystem in Desmoplastic Stroma for Improved Pancreatic Cancer Therapy. ACS Nano 2021, 15, 14149–14161. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Liu, H.; Fetse, J.P.; Jain, A.; Lin, C.Y.; Cheng, K. Development of a Tumor-Responsive Nanopolyplex Targeting Pancreatic Cancer Cells and Stroma. ACS Appl. Mater. Interfaces 2019, 11, 45390–45403. [Google Scholar] [CrossRef]
- Zhou, Q.; Shao, S.; Wang, J.; Xu, C.; Xiang, J.; Piao, Y.; Zhou, Z.; Yu, Q.; Tang, J.; Liu, X.; et al. Enzyme-activatable polymer-drug conjugate augments tumour penetration and treatment efficacy. Nat. Nanotechnol. 2019, 14, 799–809. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, Z.; Zhao, Z.; Li, Q.; Wu, Y.; Yan, S.; Shen, Y.; Huang, P. Enzyme-Triggered Transcytosis of Dendrimer-Drug Conjugate for Deep Penetration into Pancreatic Tumors. ACS Nano 2020, 14, 4890–4904. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Zhou, S.; Sun, M.; Chen, L.; Wang, J.; Xu, M.; Liu, S.; Liang, K.; Zhang, Q.; et al. Tailored Chemodynamic Nanomedicine Improves Pancreatic Cancer Treatment via Controllable Damaging Neoplastic Cells and Reprogramming Tumor Microenvironment. Nano Lett. 2020, 20, 6780–6790. [Google Scholar] [CrossRef] [PubMed]
- Zinger, A.; Koren, L.; Adir, O.; Poley, M.; Alyan, M.; Yaari, Z.; Noor, N.; Krinsky, N.; Simon, A.; Gibori, H.; et al. Collagenase Nanoparticles Enhance the Penetration of Drugs into Pancreatic Tumors. ACS Nano 2019, 13, 11008–11021. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Cao, J.; Ma, G.; Wang, X.; Sun, Y.; Zhang, C.; Shi, Z.; Zeng, Y.; Zhang, T.; Huang, P. Collagenase-Loaded H-TiO2 Nanoparticles Enhance Ultrasound Imaging-Guided Sonodynamic Therapy in a Pancreatic Carcinoma Xenograft Model via Digesting Stromal Barriers. ACS Appl. Mater. Interfaces 2022, 14, 40535–40545. [Google Scholar] [CrossRef] [PubMed]
- Koikawa, K.; Ohuchida, K.; Ando, Y.; Kibe, S.; Nakayama, H.; Takesue, S.; Endo, S.; Abe, T.; Okumura, T.; Iwamoto, C.; et al. Basement membrane destruction by pancreatic stellate cells leads to local invasion in pancreatic ductal adenocarcinoma. Cancer Lett. 2018, 425, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014, 159, 80–93. [Google Scholar] [CrossRef]
- Pickup, M.; Novitskiy, S.; Moses, H.L. The roles of TGFbeta in the tumour microenvironment. Nat. Rev. Cancer 2013, 13, 788–799. [Google Scholar] [CrossRef]
- Lee, H.; Lim, C.; Lee, J.; Kim, N.; Bang, S.; Lee, H.; Min, B.; Park, G.; Noda, M.; Stetler-Stevenson, W.G.; et al. TGF-beta signaling preserves RECK expression in activated pancreatic stellate cells. J. Cell. Biochem. 2008, 104, 1065–1074. [Google Scholar] [CrossRef]
- Apte, M.V.; Park, S.; Phillips, P.A.; Santucci, N.; Goldstein, D.; Kumar, R.K.; Ramm, G.A.; Buchler, M.; Friess, H.; McCarroll, J.A.; et al. Desmoplastic Reaction in Pancreatic Cancer: Role of Pancreatic Stellate Cells. Pancreas 2004, 29, 179–187. [Google Scholar] [CrossRef]
- Fung, K.Y.Y.; Fairn, G.D.; Lee, W.L. Transcellular vesicular transport in epithelial and endothelial cells: Challenges and opportunities. Traffic 2018, 19, 5–18. [Google Scholar] [CrossRef]
- Syvanen, S.; Eden, D.; Sehlin, D. Cationization increases brain distribution of an amyloid-beta protofibril selective F(ab’)2 fragment. Biochem. Biophys. Res. Commun. 2017, 493, 120–125. [Google Scholar] [CrossRef]
- Wang-Gillam, A. Targeting Stroma: A Tale of Caution. J. Clin. Oncol. 2019, 37, 1041–1043. [Google Scholar] [CrossRef]
- Davidson, S.M.; Jonas, O.; Keibler, M.A.; Hou, H.W.; Luengo, A.; Mayers, J.R.; Wyckoff, J.; Del Rosario, A.M.; Whitman, M.; Chin, C.R.; et al. Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nat. Med. 2017, 23, 235–241. [Google Scholar] [CrossRef]
- Kraman, M.; Bambrough, P.J.; Arnold, J.N.; Roberts, E.W.; Magiera, L.; Jones, J.O.; Gopinathan, A.; Tuveson, D.A.; Fearon, D.T. Suppression of Antitumor Immunity by Stromal Cells Expressing Fibroblast Activation Protein–α. Science 2010, 330, 827–830. [Google Scholar] [CrossRef]
- Bear, A.S.; Vonderheide, R.H.; O’Hara, M.H. Challenges and Opportunities for Pancreatic Cancer Immunotherapy. Cancer Cell 2020, 38, 788–802. [Google Scholar] [CrossRef]
- Anderson, K.G.; Stromnes, I.M.; Greenberg, P.D. Obstacles Posed by the Tumor Microenvironment to T cell Activity: A Case for Synergistic Therapies. Cancer Cell 2017, 31, 311–325. [Google Scholar] [CrossRef]
- Jang, J.E.; Hajdu, C.H.; Liot, C.; Miller, G.; Dustin, M.L.; Bar-Sagi, D. Crosstalk between Regulatory T Cells and Tumor-Associated Dendritic Cells Negates Anti-tumor Immunity in Pancreatic Cancer. Cell Rep. 2017, 20, 558–571. [Google Scholar] [CrossRef]
- Markowitz, J.; Brooks, T.R.; Duggan, M.C.; Paul, B.K.; Pan, X.; Wei, L.; Abrams, Z.; Luedke, E.; Lesinski, G.B.; Mundy-Bosse, B.; et al. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid-derived suppressor cells upon progression of disease. Cancer Immunol. Immunother. 2015, 64, 149–159. [Google Scholar] [CrossRef]
- Pergamo, M.; Miller, G. Myeloid-derived suppressor cells and their role in pancreatic cancer. Cancer Gene Ther. 2017, 24, 100–105. [Google Scholar] [CrossRef]
- Kepp, O.; Menger, L.; Vacchelli, E.; Locher, C.; Adjemian, S.; Yamazaki, T.; Martins, I.; Sukkurwala, A.Q.; Michaud, M.; Senovilla, L.; et al. Crosstalk between ER stress and immunogenic cell death. Cytokine Growth Factor Rev. 2013, 24, 311–318. [Google Scholar] [CrossRef]
- Bezu, L.; Gomes-de-Silva, L.C.; Dewitte, H.; Breckpot, K.; Fucikova, J.; Spisek, R.; Galluzzi, L.; Kepp, O.; Kroemer, G. Combinatorial strategies for the induction of immunogenic cell death. Front. Immunol. 2015, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Martins, I.; Sukkurwala, A.Q.; Adjemian, S.; Ma, Y.; Pellegatti, P.; Shen, S.; Kepp, O.; Scoazec, M.; Mignot, G.; et al. Autophagy-Dependent Anticancer Immune Responses Induced by Chemotherapeutic Agents in Mice. Science 2011, 334, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Jin, F.; Xu, X.; Du, Y. Combination Cancer Immunotherapy of Nanoparticle-Based Immunogenic Cell Death Inducers and Immune Checkpoint Inhibitors. Int. J. Nanomed. 2021, 16, 1435–1456. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, J.; Liao, Y.P.; Tang, I.; Zheng, E.; Qiu, W.; Lin, M.; Wang, X.; Ji, Y.; Mei, K.C.; et al. Combination Chemo-Immunotherapy for Pancreatic Cancer Using the Immunogenic Effects of an Irinotecan Silicasome Nanocarrier Plus Anti-PD-1. Adv. Sci. 2021, 8, 2002147. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, J.; Chang, C.H.; Liao, Y.P.; Lodico, J.J.; Tang, I.; Zheng, E.; Qiu, W.; Lin, M.; Wang, X.; et al. Development of Facile and Versatile Platinum Drug Delivering Silicasome Nanocarriers for Efficient Pancreatic Cancer Chemo-Immunotherapy. Small 2021, 17, e2005993. [Google Scholar] [CrossRef]
- Luo, L.; Wang, X.; Liao, Y.P.; Chang, C.H.; Nel, A.E. Nanocarrier Co-formulation for Delivery of a TLR7 Agonist plus an Immunogenic Cell Death Stimulus Triggers Effective Pancreatic Cancer Chemo-immunotherapy. ACS Nano 2022, 16, 13168–13182. [Google Scholar] [CrossRef]
- Lorkowski, M.E.; Atukorale, P.U.; Bielecki, P.A.; Tong, K.H.; Covarrubias, G.; Zhang, Y.; Loutrianakis, G.; Moon, T.J.; Santulli, A.R.; Becicka, W.M.; et al. Immunostimulatory nanoparticle incorporating two immune agonists for the treatment of pancreatic tumors. J. Control. Release 2021, 330, 1095–1105. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, H.; Yoon, S.; Lee, H.; Hwang, J.; Jung, J.; Chang, J.H.; Choi, J.; Kim, H. Exosome-based photoacoustic imaging guided photodynamic and immunotherapy for the treatment of pancreatic cancer. J. Control. Release 2021, 330, 293–304. [Google Scholar] [CrossRef]
- Yu, Q.; Tang, X.; Zhao, W.; Qiu, Y.; He, J.; Wan, D.; Li, J.; Wang, X.; He, X.; Liu, Y.; et al. Mild hyperthermia promotes immune checkpoint blockade-based immunotherapy against metastatic pancreatic cancer using size-adjustable nanoparticles. Acta Biomater. 2021, 133, 244–256. [Google Scholar] [CrossRef]
- Tong, Q.S.; Miao, W.M.; Huang, H.; Luo, J.Q.; Liu, R.; Huang, Y.C.; Zhao, D.K.; Shen, S.; Du, J.Z.; Wang, J. A Tumor-Penetrating Nanomedicine Improves the Chemoimmunotherapy of Pancreatic Cancer. Small 2021, 17, e2101208. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 2021, 268, 120546. [Google Scholar] [CrossRef] [PubMed]
- Parayath, N.N.; Hong, B.V.; Mackenzie, G.G.; Amiji, M.M. Hyaluronic acid nanoparticle-encapsulated microRNA-125b repolarizes tumor-associated macrophages in pancreatic cancer. Nanomedicine 2021, 16, 2291–2303. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, M.; Yang, Y.; Liu, Y.; Xie, H.; Yu, Q.; Tian, L.; Tang, X.; Ren, K.; Li, J.; et al. Remodeling tumor immune microenvironment via targeted blockade of PI3K-gamma and CSF-1/CSF-1R pathways in tumor associated macrophages for pancreatic cancer therapy. J. Control. Release 2020, 321, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, W.; Ji, W.; Wang, J.; Wang, N.; Wu, W.; Wu, Q.; Hou, X.; Hu, W.; Li, L. Near infrared photothermal conversion materials: Mechanism, preparation, and photothermal cancer therapy applications. J. Mater. Chem. B 2021, 9, 7909–7926. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; He, S.; Wang, Y.; Zhu, X. Noble Metal Nanomaterials for NIR-Triggered Photothermal Therapy in Cancer. Adv. Healthc. Mater. 2021, 10, e2001806. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, H.M.; Li, Z. Near-infrared inorganic nanomaterial-based nanosystems for photothermal therapy. Nanoscale 2021, 13, 8751–8772. [Google Scholar] [CrossRef]
- Zhao, R.; Han, X.; Li, Y.; Wang, H.; Ji, T.; Zhao, Y.; Nie, G. Photothermal Effect Enhanced Cascade-Targeting Strategy for Improved Pancreatic Cancer Therapy by Gold Nanoshell@Mesoporous Silica Nanorod. ACS Nano 2017, 11, 8103–8113. [Google Scholar] [CrossRef]
- Zhan, X.; Nie, X.; Gao, F.; Zhang, C.; You, Y.Z.; Yu, Y. An NIR-activated polymeric nanoplatform with ROS- and temperature-sensitivity for combined photothermal therapy and chemotherapy of pancreatic cancer. Biomater. Sci. 2020, 8, 5931–5940. [Google Scholar] [CrossRef]
- Li, J.; Pu, K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 2019, 48, 38–71. [Google Scholar] [CrossRef]
- Shi, X.; Li, Q.; Zhang, C.; Pei, H.; Wang, G.; Zhou, H.; Fan, L.; Yang, K.; Jiang, B.; Wang, F.; et al. Semiconducting polymer nano-radiopharmaceutical for combined radio-photothermal therapy of pancreatic tumor. J. Nanobiotechnol. 2021, 19, 337. [Google Scholar] [CrossRef]
- Lu, G.H.; Shang, W.T.; Deng, H.; Han, Z.Y.; Hu, M.; Liang, X.Y.; Fang, C.H.; Zhu, X.H.; Fan, Y.F.; Tian, J. Targeting carbon nanotubes based on IGF-1R for photothermal therapy of orthotopic pancreatic cancer guided by optical imaging. Biomaterials 2019, 195, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chan, K.K.; Xu, G.; Yin, M.; Lin, G.; Wang, X.; Lin, W.-J.; Birowosuto, M.D.; Zeng, S.; Ogi, T.; et al. Biodegradable Polymer-Coated Multifunctional Graphene Quantum Dots for Light-Triggered Synergetic Therapy of Pancreatic Cancer. ACS Appl. Mater. Interfaces 2019, 11, 2768–2781. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wan, Z.; Xu, J.; Luo, Z.; Ren, P.; Zhang, B.; Diao, D.; Huang, Y.; Li, S. Tumor size-dependent abscopal effect of polydopamine-coated all-in-one nanoparticles for immunochemo-photothermal therapy of early- and late-stage metastatic cancer. Biomaterials 2021, 269, 120629. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; Wang, M.; Liu, K.; Hoover, A.R.; Li, M.; Towner, R.A.; Mukherjee, P.; Zhou, F.; Qu, J.; et al. Synergistic interventional photothermal therapy and immunotherapy using an iron oxide nanoplatform for the treatment of pancreatic cancer. Acta Biomater. 2021, 138, 453–462. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; Xing, R.; Yuan, C.; Xue, H.; Yan, X. Supramolecular Nanofibrils Formed by Coassembly of Clinically Approved Drugs for Tumor Photothermal Immunotherapy. Adv Mater. 2021, 33, e2100595. [Google Scholar] [CrossRef]
- Teng, T.; Lin, R.; Lin, Z.; Ke, K.; Lin, X.; Pan, M.; Zhang, D.; Huang, H. Photothermal augment stromal disrupting effects for enhanced Abraxane synergy chemotherapy in pancreatic cancer PDX mode. Biomater. Sci. 2020, 8, 3278–3285. [Google Scholar] [CrossRef]
- Alifu, N.; Zebibula, A.; Qi, J.; Zhang, H.; Sun, C.; Yu, X.; Xue, D.; Lam, J.W.Y.; Li, G.; Qian, J.; et al. Single-Molecular Near-Infrared-II Theranostic Systems: Ultrastable Aggregation-Induced Emission Nanoparticles for Long-Term Tracing and Efficient Photothermal Therapy. ACS Nano 2018, 12, 11282–11293. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Zhen, X.; Xie, C.; Pu, K. Dual-Peak Absorbing Semiconducting Copolymer Nanoparticles for First and Second Near-Infrared Window Photothermal Therapy: A Comparative Study. Adv. Mater. 2018, 30, 1705980. [Google Scholar] [CrossRef]
- Geng, X.; Gao, D.; Hu, D.; Liu, Q.; Liu, C.; Yuan, Z.; Zhang, X.; Liu, X.; Sheng, Z.; Wang, X.; et al. Active-Targeting NIR-II Phototheranostics in Multiple Tumor Models Using Platelet-Camouflaged Nanoprobes. ACS Appl. Mater. Interfaces 2020, 12, 55624–55637. [Google Scholar] [CrossRef]
- Jia, X.; Xu, W.; Ye, Z.; Wang, Y.; Dong, Q.; Wang, E.; Li, D.; Wang, J. Functionalized Graphene@Gold Nanostar/Lipid for Pancreatic Cancer Gene and Photothermal Synergistic Therapy under Photoacoustic/Photothermal Imaging Dual-Modal Guidance. Small 2020, 16, e2003707. [Google Scholar] [CrossRef]
- Li, D.; Chen, X.; Wang, D.; Wu, H.; Wen, H.; Wang, L.; Jin, Q.; Wang, D.; Ji, J.; Tang, B.Z. Synchronously boosting type-I photodynamic and photothermal efficacies via molecular manipulation for pancreatic cancer theranostics in the NIR-II window. Biomaterials 2022, 283, 121476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Z.; Wang, Q.; Liu, Q.; Yuan, W.; Feng, W.; Li, F. An NIR-II Photothermally Triggered “Oxygen Bomb” for Hypoxic Tumor Programmed Cascade Therapy. Adv. Mater. 2022, 34, e2201978. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, R.; Yang, H.; Qu, S.; Qian, L.; Dai, Z. Enhancing Photodynamic Therapy Efficacy Against Cancer Metastasis by Ultrasound-Mediated Oxygen Microbubble Destruction to Boost Tumor-Targeted Delivery of Oxygen and Renal-Clearable Photosensitizer Micelles. ACS Appl. Mater. Interfaces 2022, 14, 25197–25208. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, X.; Li, J.; Wang, H.; Xu, X.; Li, Y.; Sha, X.; Zhang, Z. Oxygen-Delivering Polyfluorocarbon Nanovehicles Improve Tumor Oxygenation and Potentiate Photodynamic-Mediated Antitumor Immunity. ACS Nano 2021, 15, 5405–5419. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.Y.; Wang, L.C.; Hsieh, C.C.; Hsu, Y.P.; Chang, L.C.; Su, W.P.; Chien, Y.H.; Yeh, C.S. 1550 nm excitation-responsive upconversion nanoparticles to establish dual-photodynamic therapy against pancreatic tumors. J. Mater. Chem. B 2021, 9, 694–709. [Google Scholar] [CrossRef]
- Sun, F.; Zhu, Q.; Li, T.; Saeed, M.; Xu, Z.; Zhong, F.; Song, R.; Huai, M.; Zheng, M.; Xie, C.; et al. Regulating Glucose Metabolism with Prodrug Nanoparticles for Promoting Photoimmunotherapy of Pancreatic Cancer. Adv. Sci. 2021, 8, 2002746. [Google Scholar] [CrossRef]
- Zhu, L.; Lin, S.; Cui, W.; Xu, Y.; Wang, L.; Wang, Z.; Yuan, S.; Zhang, Y.; Fan, Y.; Geng, J. A nanomedicine enables synergistic chemo/photodynamic therapy for pancreatic cancer treatment. Biomater. Sci. 2022, 10, 3624–3636. [Google Scholar] [CrossRef]
- Wang, M.; Wu, M.; Liu, X.; Shao, S.; Huang, J.; Liu, B.; Liang, T. Pyroptosis Remodeling Tumor Microenvironment to Enhance Pancreatic Cancer Immunotherapy Driven by Membrane Anchoring Photosensitizer. Adv. Sci. 2022, 9, e2202914. [Google Scholar] [CrossRef]
- Cao, J.; Sun, Y.; Zhang, C.; Wang, X.; Zeng, Y.; Zhang, T.; Huang, P. Tablet-like TiO2/C nanocomposites for repeated type I sonodynamic therapy of pancreatic cancer. Acta Biomater. 2021, 129, 269–279. [Google Scholar] [CrossRef]
- Chen, J.; Luo, H.; Liu, Y.; Zhang, W.; Li, H.; Luo, T.; Zhang, K.; Zhao, Y.; Liu, J. Oxygen-Self-Produced Nanoplatform for Relieving Hypoxia and Breaking Resistance to Sonodynamic Treatment of Pancreatic Cancer. ACS Nano 2017, 11, 12849–12862. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, Y.; Cao, J.; Luo, J.; Wang, J.; Jiang, Z.; Huang, P. Intrinsic nucleus-targeted ultra-small metal-organic framework for the type I sonodynamic treatment of orthotopic pancreatic carcinoma. J. Nanobiotechnol. 2021, 19, 315. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Feng, L.; Jin, P.; Shen, J.; Lu, J.; Song, Y.; Wang, G.; Chen, Q.; Huang, D.; Zhang, Y.; et al. Cavitation assisted endoplasmic reticulum targeted sonodynamic droplets to enhanced anti-PD-L1 immunotherapy in pancreatic cancer. J. Nanobiotechnol. 2022, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bao, Y.; Song, Y.; Zhang, C.; Qiu, F.; Sun, Y.; Xin, L.; Cao, J.; Jiang, Y.; Luo, J.; et al. Hypoxia-alleviated nanoplatform to enhance chemosensitivity and sonodynamic effect in pancreatic cancer. Cancer Lett. 2021, 520, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, Y.; Chen, S.; Wang, R.; Chen, Q.; Li, J.; Luo, Y.; Wang, X.; Chen, H. Photothermal Fenton Nanocatalysts for Synergetic Cancer Therapy in the Second Near-Infrared Window. ACS Appl. Mater. Interfaces 2020, 12, 30145–30154. [Google Scholar] [CrossRef]
- Li, L.; Yang, Z.; Fan, W.; He, L.; Cui, C.; Zou, J.; Tang, W.; Jacobson, O.; Wang, Z.; Niu, G.; et al. In Situ Polymerized Hollow Mesoporous Organosilica Biocatalysis Nanoreactor for Enhancing ROS-Mediated Anticancer Therapy. Adv. Funct. Mater. 2020, 30, 1907716. [Google Scholar] [CrossRef]
- Sun, Y.; Cao, J.; Wang, X.; Zhang, C.; Luo, J.; Zeng, Y.; Zhang, C.; Li, Q.; Zhang, Y.; Xu, W.; et al. Hypoxia-Adapted Sono-chemodynamic Treatment of Orthotopic Pancreatic Carcinoma Using Copper Metal-Organic Frameworks Loaded with an Ultrasound-Induced Free Radical Initiator. ACS Appl. Mater. Interfaces 2021, 13, 38114–38126. [Google Scholar] [CrossRef]
- Sivasubramanian, M.; Chuang, Y.C.; Lo, L.-W. Evolution of Nanoparticle-Mediated Photodynamic Therapy: From Superficial to Deep-Seated Cancers. Molecules 2019, 24, 520. [Google Scholar] [CrossRef]
- Tait, S.W.G.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef]
- Huggett, M.T.; Jermyn, M.; Gillams, A.; Illing, R.; Mosse, S.; Novelli, M.; Kent, E.; Bown, S.G.; Hasan, T.; Pogue, B.W.; et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br. J. Cancer 2014, 110, 1698–1704. [Google Scholar] [CrossRef]
- DeWitt, J.M.; Sandrasegaran, K.; O’Neil, B.; House, M.G.; Zyromski, N.J.; Sehdev, A.; Perkins, S.M.; Flynn, J.; McCranor, L.; Shahda, S. Phase 1 study of EUS-guided photodynamic therapy for locally advanced pancreatic cancer. Gastrointest. Endosc. 2019, 89, 390–398. [Google Scholar] [CrossRef]
- Hirschberg, H.; Berg, K.; Peng, Q. Photodynamic therapy mediated immune therapy of brain tumors. Neuroimmunol. Neuroinflamm. 2018, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, X.; Chen, H.; Duan, Y.; Liu, J.; Pan, Y.; Liu, B. Activation of Pyroptosis by Membrane-Anchoring AIE Photosensitizer Design: New Prospect for Photodynamic Cancer Cell Ablation. Angew. Chem. Int. Ed. Engl. 2021, 60, 9093–9098. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Deng, X.; Ma, P.a.; Cheng, Z.; Lin, J. Recent Advances in Nanomaterial-Assisted Combinational Sonodynamic Cancer Therapy. Adv. Mater. 2020, 32, 2003214. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Fan, J.-H.; Zhao, L.-P.; Fan, G.-L.; Zheng, R.-R.; Qiu, X.-Z.; Yu, X.-Y.; Li, S.-Y.; Zhang, X.-Z. Chimeric peptide engineered exosomes for dual-stage light guided plasma membrane and nucleus targeted photodynamic therapy. Biomaterials 2019, 211, 14–24. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, K.; Liao, X.; Rees, T.W.; Chen, Y.; Zhao, Z.; Ji, L.; Chao, H. Nucleus-targeting ultrasmall ruthenium(iv) oxide nanoparticles for photoacoustic imaging and low-temperature photothermal therapy in the NIR-II window. Chem. Commun. 2020, 56, 3019–3022. [Google Scholar] [CrossRef]
- Xing, L.; Shi, Q.; Zheng, K.; Shen, M.; Ma, J.; Li, F.; Liu, Y.; Lin, L.; Tu, W.; Duan, Y.; et al. Ultrasound-Mediated Microbubble Destruction (UMMD) Facilitates the Delivery of CA19-9 Targeted and Paclitaxel Loaded mPEG-PLGA-PLL Nanoparticles in Pancreatic Cancer. Theranostics 2016, 6, 1573–1587. [Google Scholar] [CrossRef]
- Nesbitt, H.; Sheng, Y.; Kamila, S.; Logan, K.; Thomas, K.; Callan, B.; Taylor, M.A.; Love, M.; O’Rourke, D.; Kelly, P.; et al. Gemcitabine loaded microbubbles for targeted chemo-sonodynamic therapy of pancreatic cancer. J. Control. Release 2018, 279, 8–16. [Google Scholar] [CrossRef]
- Sheng, Y.; Beguin, E.; Nesbitt, H.; Kamila, S.; Owen, J.; Barnsley, L.C.; Callan, B.; O’Kane, C.; Nomikou, N.; Hamoudi, R.; et al. Magnetically responsive microbubbles as delivery vehicles for targeted sonodynamic and antimetabolite therapy of pancreatic cancer. J. Control. Release 2017, 262, 192–200. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angew. Chem. Int. Ed. Engl. 2019, 58, 946–956. [Google Scholar] [CrossRef]
- Luo, W.; Zhu, C.; Su, S.; Li, D.; He, Y.; Huang, Q.; Fan, C. Self-Catalyzed, Self-Limiting Growth of Glucose Oxidase-Mimicking Gold Nanoparticles. ACS Nano 2010, 4, 7451–7458. [Google Scholar] [CrossRef]
- Liang, R.; Li, Y.; Huo, M.; Lin, H.; Chen, Y. Triggering Sequential Catalytic Fenton Reaction on 2D MXenes for Hyperthermia-Augmented Synergistic Nanocatalytic Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 42917–42931. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Semashko, V.V.; Pudovkin, M.S.; Cefalas, A.C.; Zelenikhin, P.V.; Gavriil, V.E.; Nizamutdinov, A.S.; Kollia, Z.; Ferraro, A.; Sarantopoulou, E. Tiny Rare-Earth Fluoride Nanoparticles Activate Tumour Cell Growth via Electrical Polar Interactions. Nanoscale Res. Lett. 2018, 13, 370. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Li, J.; Zhang, Y.; Rong, H.; Lu, W.; Jiang, L. Effects of aggregation and the surface properties of gold nanoparticles on cytotoxicity and cell growth. Nanomedicine 2012, 8, 46–53. [Google Scholar] [CrossRef]
- Huang, X.; Zhuang, J.; Teng, X.; Li, L.; Chen, D.; Yan, X.; Tang, F. The promotion of human malignant melanoma growth by mesoporous silica nanoparticles through decreased reactive oxygen species. Biomaterials 2010, 31, 6142–6153. [Google Scholar] [CrossRef]
- Cheng, H.; Liao, Z.-L.; Ning, L.-H.; Chen, H.-Y.; Wei, S.-S.; Yang, X.-C.; Guo, H. Alendronate-anchored PEGylation of ceria nanoparticles promotes human hepatoma cell proliferation via AKT/ERK signaling pathways. Cancer Med. 2017, 6, 374–381. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2021, 22, 385. [Google Scholar] [CrossRef]
- Aillon, K.L.; Xie, Y.; El-Gendy, N.; Berkland, C.J.; Forrest, M.L. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv. Drug Deliv. Rev. 2009, 61, 457–466. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Meng, H. Transcytosis-An effective targeting strategy that is complementary to “EPR effect” for pancreatic cancer nano drug delivery. Theranostics 2019, 9, 8018–8025. [Google Scholar] [CrossRef]
- Dvorak, A.M.; Kohn, S.; Morgan, E.S.; Fox, P.; Nagy, J.A.; Dvorak, H.F. The vesiculo-vacuolar organelle (VVO): A distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. J. Leukoc. Biol. 1996, 59, 100–115. [Google Scholar] [CrossRef]
- Liu, X.; Lin, P.; Perrett, I.; Lin, J.; Liao, Y.P.; Chang, C.H.; Jiang, J.; Wu, N.; Donahue, T.; Wainberg, Z.; et al. Tumor-penetrating peptide enhances transcytosis of silicasome-based chemotherapy for pancreatic cancer. J. Clin. Investig. 2017, 127, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Greenwald, D.R.; Ruoslahti, E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 2010, 328, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Di, Y.; Xie, C.; Song, Y.; He, H.; Li, H.; Pu, X.; Lu, W.; Fu, D.; Jin, C. An in vitro and in vivo study of gemcitabine-loaded albumin nanoparticles in a pancreatic cancer cell line. Int. J. Nanomed. 2015, 10, 6825–6834. [Google Scholar] [CrossRef]
- Chen, N.; Brachmann, C.; Liu, X.; Pierce, D.W.; Dey, J.; Kerwin, W.S.; Li, Y.; Zhou, S.; Hou, S.; Carleton, M.; et al. Albumin-bound nanoparticle (nab) paclitaxel exhibits enhanced paclitaxel tissue distribution and tumor penetration. Cancer Chemother. Pharmacol. 2015, 76, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Mao, H.; Cao, Z.; Wang, Y.A.; Peng, X.; Wang, X.; Sajja, H.K.; Wang, L.; Duan, H.; Ni, C.; et al. Molecular imaging of pancreatic cancer in an animal model using targeted multifunctional nanoparticles. Gastroenterology 2009, 136, 1514–1525.e1512. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, D.; Zhang, C.; Li, C. Nanoparticle-based delivery systems modulate the tumor microenvironment in pancreatic cancer for enhanced therapy. J. Nanobiotechnol. 2021, 19, 384. [Google Scholar] [CrossRef]
- Vennin, C.; Murphy, K.J.; Morton, J.P.; Cox, T.R.; Pajic, M.; Timpson, P. Reshaping the Tumor Stroma for Treatment of Pancreatic Cancer. Gastroenterology 2018, 154, 820–838. [Google Scholar] [CrossRef]
- Upadhrasta, S.; Zheng, L. Strategies in Developing Immunotherapy for Pancreatic Cancer: Recognizing and Correcting Multiple Immune “Defects” in the Tumor Microenvironment. J. Clin. Med. 2019, 8, 1472. [Google Scholar] [CrossRef]
- Sofias, A.M.; Dunne, M.; Storm, G.; Allen, C. The battle of “nano” paclitaxel. Adv. Drug Deliv. Rev. 2017, 122, 20–30. [Google Scholar] [CrossRef]
- Mallya, K.; Gautam, S.K.; Aithal, A.; Batra, S.K.; Jain, M. Modeling pancreatic cancer in mice for experimental therapeutics. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1876, 188554. [Google Scholar] [CrossRef]
- Huang, L.; Bockorny, B.; Paul, I.; Akshinthala, D.; Frappart, P.-O.; Gandarilla, O.; Bose, A.; Sanchez-Gonzalez, V.; Rouse, E.E.; Lehoux, S.D.; et al. PDX-derived organoids model in vivo drug response and secrete biomarkers. JCI Insight 2020, 5, e135544. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).