Intraspecific Variability of Stinging Nettle (Urtica dioica L.)
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Chemical Analysis
3.2.1. Analysis of Phenolics by HPLC
3.2.2. The Content of Chlorophylls and Carotenoids
3.3. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Grosse-Veldmann, B.; Weigend, M. The geometry of gender: Hyper–diversification of sexual systems in Urtica L. (Urticaceae). Cladistics 2017, 34, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Kręgiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef] [PubMed]
- Dhouibi, R.; Affes, H.; Ben Salem, M.; Hammami, S.; Sahnoun, Z.; Zeghal, K.M.; Ksouda, K. Screening of pharmacological uses of Urticadioica and others benefits. Prog. Biophys. Mol. Biol. 2020, 150, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Grauso, L.; De Falco, B.; Lanzotti, V.; Motti, R. Stinging nettle, Urtica dioica L.: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2020, 19, 1341–1377. [Google Scholar] [CrossRef]
- Grosse-Veldmann, B.; Weigend, M. Weeding the nettles III: Named nonsense versus named morphotypes in European Urtica dioica L. (Urticaceae) Phytotaxa 2015, 208, 239–260. [Google Scholar] [CrossRef]
- Cronk, Q.; Hidalgo, O.; Pellicer, J.; Percy, D.; Leitch, I.J. Salix transect of Europe: Variation in ploidy and genome size in willow–associated common nettle, Urtica dioica L. sens.lat., from Greece to arctic Norway. Biodivers. Data J. 2016, 4, e10003. [Google Scholar] [CrossRef]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2017. [Google Scholar]
- European Pharmacopoeia. European Directorate for the Quality of Medicines and Health Care (EDQM), 10th ed.; Council of Europe: Strasbourg, France, 2019. [Google Scholar]
- Upton, R. Stinging nettles leaf (Urtica dioica L.): Extraordinary vegetable medicine. J. Herb. Med. 2013, 3, 9–38. [Google Scholar] [CrossRef]
- Di Virgilio, N.; Papazoglou, E.G.; Jankauskiene, Z.; Di Lonardo, S.; Praczyk, M.; Wielgusz, K. The potential of stinging nettle (Urtica dioicaL.) as a crop with multiple uses. Ind. Crops Prod. 2015, 68, 42–49. [Google Scholar] [CrossRef]
- EMA (European Medicines Agency). Assessment report on Urtica dioica L., Urtica urens L., folium. Committee on Herbal Me-dicinal Products. EMA/HMPC/508013/2007. Available online: https://www.ema.europa.eu/en/documents/herbal–report/final–assessment–report–urtica–dioica–l–urtica–urens–l–folium_en.pdf (accessed on 29 October 2022).
- Kalia, A.N.; Joshi, B.C.; Mukhija, M. Pharmacognostical review of Urtica dioica L. Int. J. Green Pharm. 2014, 8, 201. [Google Scholar] [CrossRef]
- Đurović, S.; Pavlić, B.; Šorgić, S.; Popov, S.; Savić, S.; Petronijević, M.; Radojković, M.; Cvetanović, A.; Zeković, Z. Chemical composition of stinging nettle leaves obtained by different analytical approaches. J. Funct. Foods 2017, 32, 18–26. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Rebolloso-Fuentes, M.M.; Isasa, M.E.T. Fatty acids and carotenoids from stinging nettle (Urtica dioica L.). J. Food Compos. Anal. 2003, 16, 111–119. [Google Scholar] [CrossRef]
- Gül, S.; Demirci, B.; Başer, K.H.C.; Akpulat, H.A.; Aksu, P. Chemical Composition and In Vitro Cytotoxic, Genotoxic Effects of Essential Oil from Urtica dioica L. Bull. Environ. Contam. Toxicol. 2012, 88, 666–671. [Google Scholar] [CrossRef]
- Rafajlovska, V.; Kavrakovski, Z.; Simonovska, J.; Srbinoska, M. Determination of protein and mineral contents in stinging nettle. Qual. Life Banja Luka APEIRON 2013, 7, 1–2. [Google Scholar] [CrossRef]
- Devkota, H.P.; Paudel, K.R.; Khanal, S.; Baral, A.; Panth, N.; Adhikari-Devkota, A.; Jha, N.K.; Das, N.; Singh, S.K.; Chellappan, D.K.; et al. Stinging Nettle (Urtica dioica L.): Nutritional Composition, Bioactive Compounds, and Food Functional Properties. Molecules 2022, 27, 5219. [Google Scholar] [CrossRef]
- Lamer-Zarawska, E.; Kowal-Gierczak, B.; Niedworek, J. Fitoterapia i Leki Roślinne; Wydawnictwo Lekarskie PZWL: Warsaw, Poland, 2014. [Google Scholar]
- Jinous, A.; Razieh, M. Phytochemistry and pharmacologic properties of Urtica dioica L. J. Med. Plant Res. 2012, 6, 5714–5719. [Google Scholar]
- Rutto, L.K.; Xu, Y.; Ramirez, E.; Brandt, M. Mineral properties and dietary value of raw and processed stinging nettle (Urtica dioica L.). Int. J. Food Sci. 2013, 2013. [Google Scholar] [CrossRef]
- Külcü, B.D.; Gökışık, D.C.; Aydın, S. An investigation of antibacterial and antioxidant activity of nettle (Urtica dioica L.), mint (Mentha piperita), thyme (Thyme serpyllum) and Chenopodium album L. plants from Yaylacık Plateau, Giresun, Turkey. Turk. J. Agric. Food Sci. Technol. 2019, 7, 73–80. [Google Scholar]
- Bhusal, K.K.; Magar, S.K.; Thapa, R.; Lamsal, A.; Bhandari, S.; Maharjan, R.; Shrestha, S.; Shrestha, J. Nutritional and pharmacological importance of stinging nettle (Urticadioica L.): A review. Heliyon 2022, 8, e09717. [Google Scholar] [CrossRef]
- Kosolapov, V.M.; Cherniavskih, V.I.; Zarudny, V.A.; Mazur, K.; Konieczna, A.; Tseiko, L.; Dumacheva, E.V.; Dumachev, D.V. Observations on the Productivity of Breeding Specimens of Urtica dioica L. from European Russian Ecotopes in Comparison with the Breeding Variety under Field Crop Conditions. Agronomy 2021, 12, 76. [Google Scholar] [CrossRef]
- Napoli, E.; Siracusa, L.; Ruberto, G. New tricks for old guys: Recent developments in the chemistry, biochemistry, applications and exploitation of selected species from the Lamiaceae family. Chem. Biodivers. 2020, 17, e1900677. [Google Scholar] [CrossRef]
- Opačić, N.; Radman, S.; FabekUher, S.; Benko, B.; Voća, S.; ŠicŽlabur, J. Nettle Cultivation Practices—From Open Field to Modern Hydroponics: A Case Study of Specialized Metabolites. Plants 2022, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Paulauskienė, A.; Tarasevičienė, Ž.; Laukagalis, V. Influence of Harvesting Time on the Chemical Composition of Wild Stinging Nettle (Urtica dioica L.). Plants 2021, 10, 686. [Google Scholar] [CrossRef]
- Nkhabu, K.; Liphoto, M.; Ntahane, T.; Senoko, K. Genetic Diversity of Stinging Nettle (Urtica dioica) by Agro Morphological Markers. Eur. J. Bot. Plant Sci. Phytol. 2021, 6, 51–68. [Google Scholar]
- Jankauskiene, Z.; Gruzdevienė, E. Changes in the productivity of wild and cultivated stinging nettle (Urtica dioica L.) as influenced by the planting density and crop age. Zemdirb. Agric. 2015, 102, 31–40. [Google Scholar] [CrossRef]
- Grevsen, K.; Fretté, X.; Christensen, L.P. Concentration and composition of flavonol glycosides and phenolic acids in aerial parts of stinging nettle (Urtica dioica L.) are affected by high nitrogen fertilization and by harvest time. Eur. J. Hortic. Sci. 2008, 73, 20–27. [Google Scholar]
- Biesiada, A.; Wołoszczak, E. The effect of method of plantation establishing on yield and chlorophyll concentration of stinging nettle (Urtica dioica L.) in the first year of cultivation. Herba Pol. 2007, 53, 85–89. [Google Scholar]
- Radman, S.; Fabek, S.; Žutić, I.; Benko, B.; Toth, N. Stinging nettle cultivation in floating hydropon. Contemp. Agric. 2014, 63, 215–223. [Google Scholar]
- Radman, S.; Ivanka, I.; Žutić, I.; Fabek, S.; Jana, J.; Ic, I.; Žlabur, J.; Bo, B.; Benko, B.; Toth, N.; et al. Influence of Nitrogen Fertilization on Chemical Composition of Cultivated Nettle. Emir. J. Food Agric. 2015, 27, 889–896. [Google Scholar] [CrossRef]
- Biesiada, A.; Wołoszczak, E.; Sokόł-Łętowska, A.; Kucharska, A.Z.; Nawirska-Olszańska, A. The effect of nitrogen form and dose on yield, chemical composition and antioxidant activity of stinging nettle (Urtica dioica L.). Herba Pol. 2009, 55, 84–93. [Google Scholar]
- Marotti, I.; Frassineti, E.; Trebbi, G.; Alpi, M.; D’Amen, E.; Dinelli, G. Health-promoting phytochemicals of stinging nettle (Urtica dioica L.) grown under organic farming in Italian environments. Ind. Crops Prod. 2022, 182, 114903. [Google Scholar] [CrossRef]
- Weglarz, Z.; Karaczum, W. Influence of plantation age and date of harvesting of herb on yield and chemical constitute of the nettle (Urtica dioica L.). Herba Pol. 1996, 42, 88–95. [Google Scholar]
- Węglarz, Z.; Rosłon, W. Developmental and chemical differentiation of male and female underground organs of nettle (Urtica dioica L.). Herba Pol. 2000, 46, 324–331. [Google Scholar]
- Dumacheva, E.V.; Cherniavskih, V.I.; Prisniy, A.V.; Vorobyova, O.V.; Gorbacheva, A.A.; Glubsheva, T.N.; Grigorenko, S.E. Studies of biological resources of Urtica dioica L. as initial material for breeding. J. Int. Pharm. Res. 2018, 45, 473. [Google Scholar]
- Kohlmünzer, S. Farmakognozja: Podręcznik Dla Studentów Farmacji; Wydawnictwo Lekarskie PZWL: Warsaw, Poland, 2017. [Google Scholar]
- Pinelli, P.; Ieri, F.; Vignolini, P.; Bacci, L.; Baronti, S.; Romani, A. Extraction and HPLC Analysis of Phenolic Compounds in Leaves, Stalks, and Textile Fibers of Urtica dioicaL. J. Agric. Food Chem. 2008, 56, 9127–9132. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A.; Frankowski, R.; Grześkowiak, T.; Jeszka, A.M. Variation in the Content of Bioactive Compounds in Infusions Prepared from Different Parts of Wild Polish Stinging Nettle (Urtica dioica L.). Molecules 2022, 27, 4242. [Google Scholar] [CrossRef]
- Zeković, Z.; Cvetanović, A.; Švarc-Gajić, J.; Gorjanović, S.; Sužnjević, D.; Mašković, P.; Đurović, S. Chemical and biological screening of stinging nettle leaves extracts obtained by modern extraction techniques. Ind. Crops Prod. 2017, 108, 423–430. [Google Scholar] [CrossRef]
- Repajić, M.; Cegledi, E.; Kruk, V.; Pedisić, S.; Çınar, F.; Kovačević, D.B.; Žutić, I.; Dragović-Uzelac, V. Accelerated solvent extraction as a green tool for the recovery of polyphenols and pigments from wild nettle leaves. Processes 2020, 8, 803. [Google Scholar] [CrossRef]
- Otles, S.; Yalcin, B. Phenolic Compounds Analysis of Root, Stalk, and Leaves of Nettle. Sci. World J. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Lattanzio, V. Phenolic Compounds: Introduction. In Natural Product; Ramawat, K., Merillon, J.M., Eds.; Springer: Berlin, Germany, 2013. [Google Scholar] [CrossRef]
- Chowdhary, V.; Alooparampil, S.V.; Pandya, R.G.; Tank, J. Physiological function of phenolic compounds in plant defense system. In Phenolic Compounds–Chemistry, Synthesis, Diversity, Non–Conventional Industrial, Pharmaceutical and Therapeutic Applications; Badria, F.A., Blumenberg, M., Eds.; Bod Third Party Titles: London, UK, 2021. [Google Scholar]
- Biesiada, A.; Kucharska, A.; Sokół-Łętowska, A.; Kuś, A. Effect of the Age of Plantation and Harvest Term on Chemical Composition and Antioxidant Avctivity of Stinging Nettle (Urtica dioica L.). Ecol. Chem. Eng. 2010, 17, 1061–1068. [Google Scholar]
- Kőszegi, K.; Békássy-Molnár, E.; Koczka, N.; Kerner, T.; Stefanovits-Bányai, É. Changes in total polyphenol content and an-tioxidant capacity of stinging nettle (Urtica dioica L.) from spring to autumn. Period. Polytech. Chem. Eng. 2020, 64, 548–554. [Google Scholar] [CrossRef]
- Droštinová, L.; Braniša, J.; Bončíková, D.; Jomová, K. Effect of Drying Methods on Content of Some Natural Pigments in Urtica Dioica L. and Melissa Officinalis L. J. Microbiol. Biotechnol. Food Sci. 2015, 5, 182–185. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Tumolo, T.; Lanfer-Marquez, U.M. Copper chlorophyllin: A food colorant with bioactive properties? Int. Food Res. J. 2012, 46, 451–459. [Google Scholar] [CrossRef]
- Recovery & Dilution Procedures. Available online: https://standards.chromadex.com/Documents/Tech%20Tips/techtip0003-recoverydilutionprocedures_nl_pw.pdf (accessed on 2 December 2022).
- Lichtenthaler, H.; Wellburn, A. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 603, 591–592. [Google Scholar] [CrossRef]
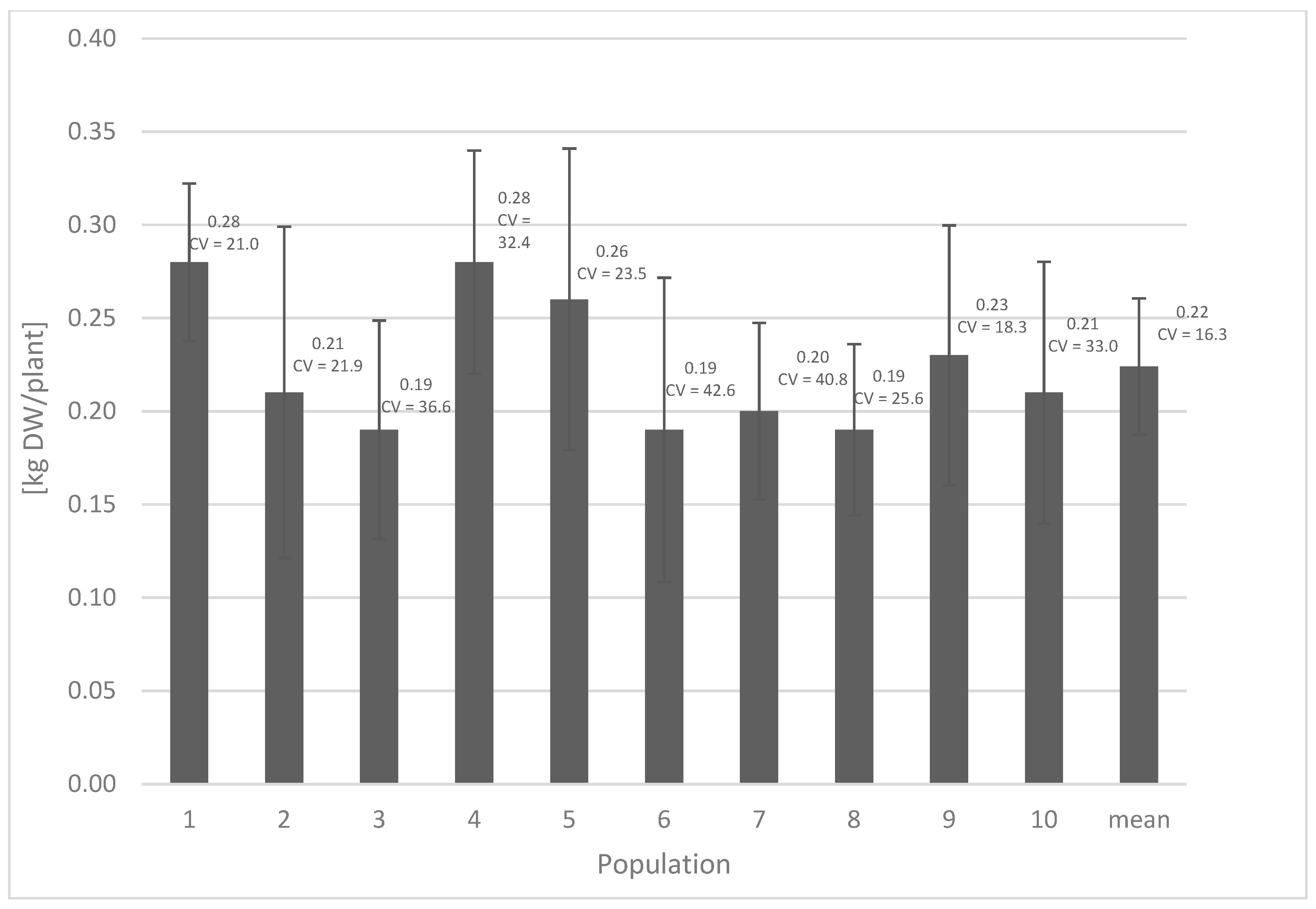
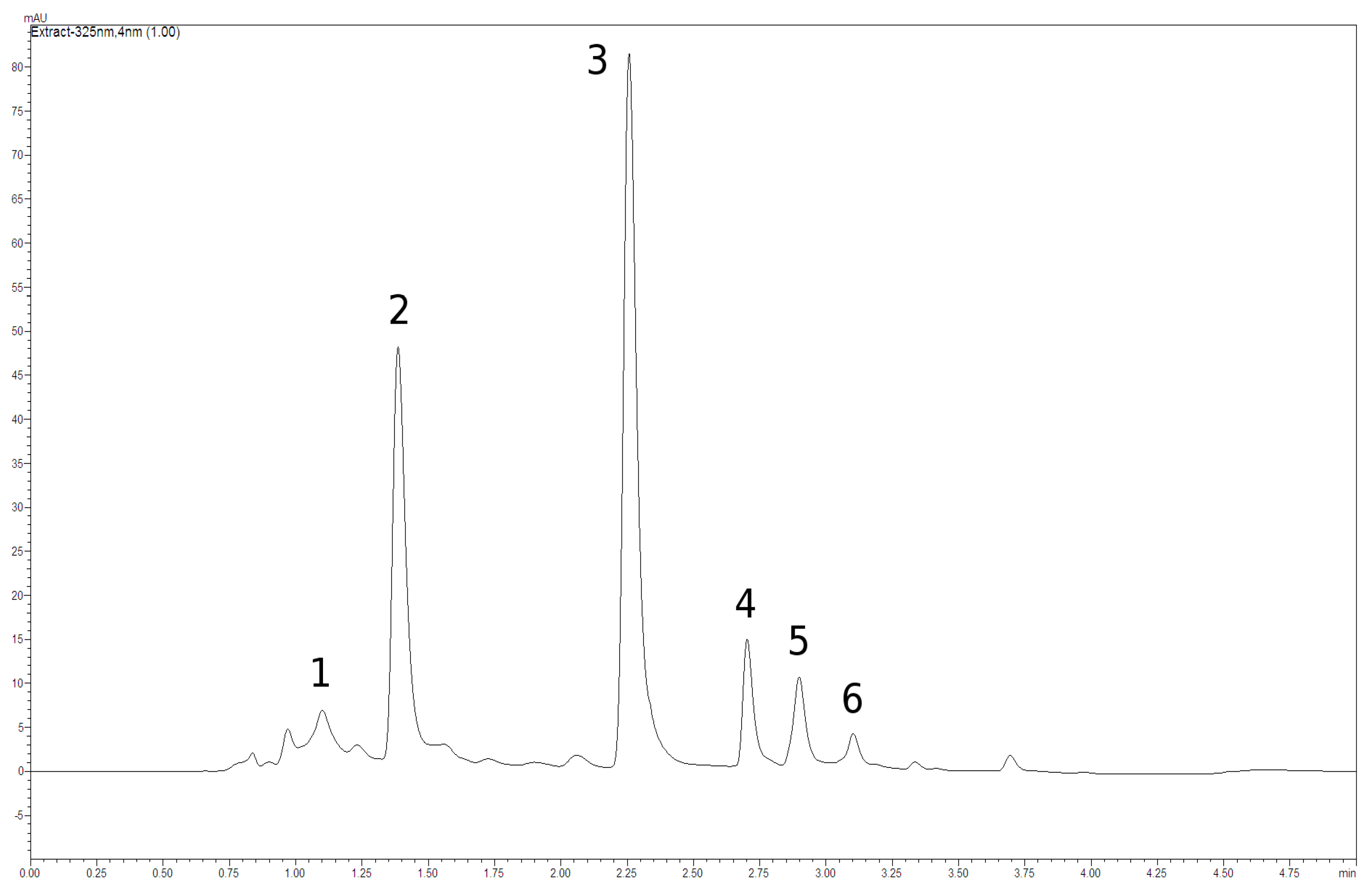
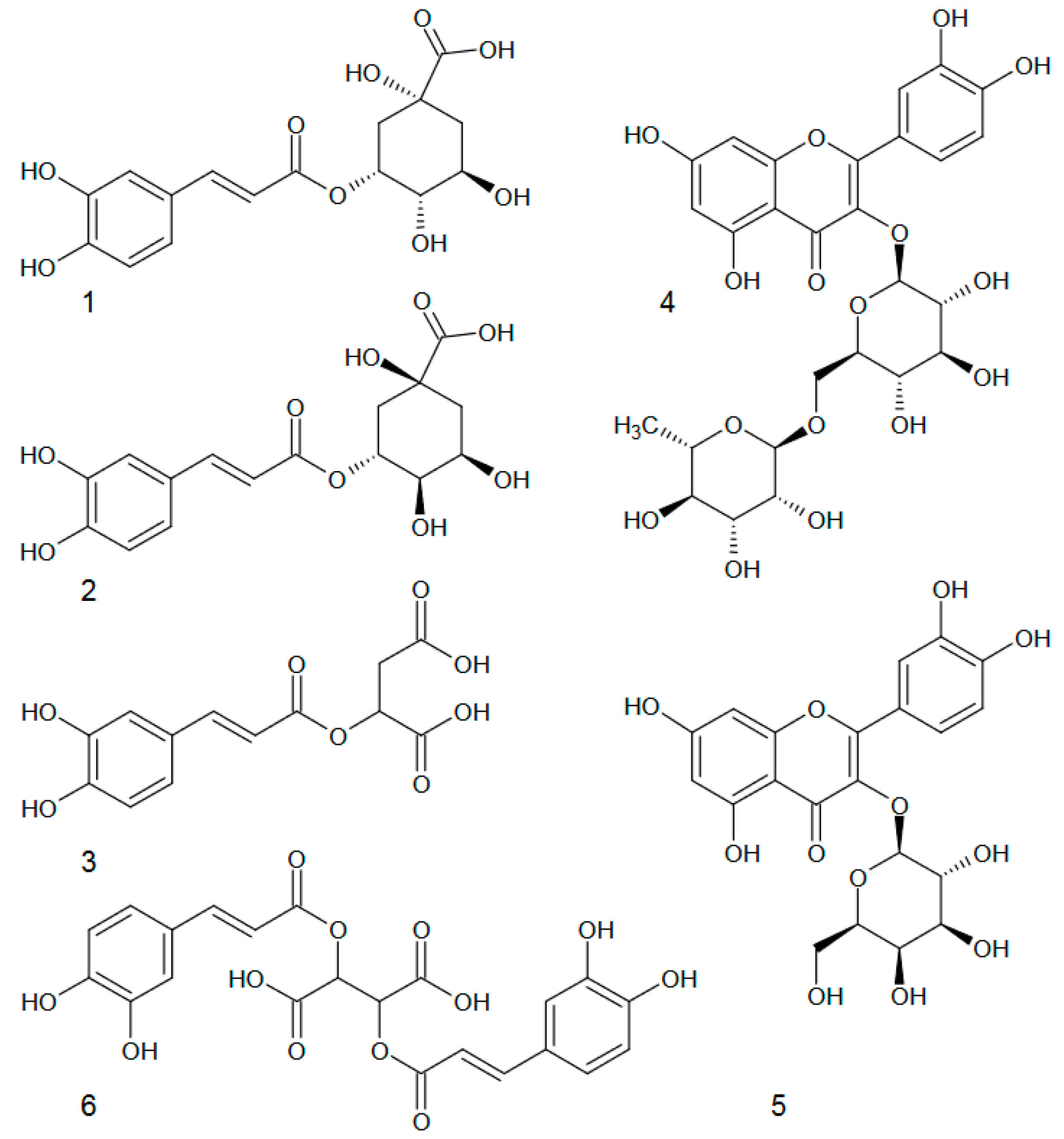
| Population | Neochlorogenic Acid | Chlorogenic Acid | Caffeoylmalic Acid | Cichoric Acid | Sum of Chlorogenic and Caffeoylmalic Acids | Sum of All Detected Phenolic Acids | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CV (%) | CV (%) | CV (%) | CV (%) | |||||||
| 1 | 204.5 ± 102.6 | 50.2 | 548.4 ± 234.0 | 43.8 | 1126.9 ± 288.3 | 25.6 | 189.5 ± 72.5 | 38.3 | 1675.3 ± 470.8 | 2069.3 ± 547.6 |
| 2 | 214.1 ± 65.6 | 30.6 | 1070.8 ± 503.6 | 47.0 | 1367.4 ± 431.8 | 31.6 | 184.3 ± 64.9 | 35.2 | 2438.2 ± 853.7 | 2836.6 ± 46.2 |
| 3 | 200.4 ± 78.2 | 39.0 | 513.6 ± 213.7 | 41.6 | 1114.7 ± 480.4 | 43.1 | 107.4 ± 47.9 | 44.6 | 1628.5 ± 803.2 | 1936.3 ± 79.9 |
| 4 | 150.5 ± 92.8 | 61.7 | 535.7 ± 367.5 | 68.6 | 801.7 ± 313.1 | 39.1 | 61.9 ± 21.7 | 35.1 | 1337.4 ± 750.1 | 1549.8 ± 16.2 |
| 5 | 242.7 ± 125.2 | 51.6 | 625.2 ± 315.4 | 50.4 | 851.7 ± 222.9 | 26.2 | 72.5 ± 31.2 | 42.9 | 1476.9 ± 533.4 | 1792.1 ± 82.6 |
| 6 | 284.8 ± 114.8 | 40.3 | 704.9 ± 300.8 | 42.7 | 1008.7 ± 288.2 | 28.6 | 85.0 ± 25.3 | 29.8 | 1713.7 ± 518.9 | 2083.4 ± 58.5 |
| 7 | 226.8 ± 84.0 | 37.0 | 578.5 ± 247.1 | 42.7 | 916.7 ± 225.4 | 24.6 | 86.8 ± 32.3 | 37.2 | 1495.2 ± 349.8 | 1808.8 ± 55.2 |
| 8 | 114.6 ± 62.6 | 54.6 | 601.6 ± 293.1 | 48.7 | 962.9 ± 303.2 | 31.5 | 72.2 ± 18.1 | 25.1 | 1564.5 ± 541.2 | 1751.2 ± 01.3 |
| 9 | 194.0 ± 61.4 | 31.7 | 419.1 ± 228.9 | 54.6 | 735.3 ± 242.6 | 33.0 | 72.4 ± 21.1 | 29.8 | 1154.3 ± 489.2 | 1420.7 ± 72.5 |
| 10 | 169.1 ± 54.7 | 32.3 | 352.8 ± 175.1 | 49.6 | 571.0 ± 235.5 | 41.2 | 58.3 ± 10.4 | 17.9 | 923.8 ± 413.7 | 1151.1 ± 413.8 |
| Mean | 200.1 | 595.1 | 945.7 | 99.0 | 1540.8 | 1839.9 | ||||
| CV | 24.0 | 32.7 | 23.9 | 48.8 | 26.0 | 24.7 |
| Population | Rutoside | Hyperoside | Sum | ||
|---|---|---|---|---|---|
| CV * (%) | CV (%) | ||||
| 1 | 1329.7 ± 845.10 | 63.6 | 42.0 ± 33.4 | 79.5 | 1246.4 ± 925.6 |
| 2 | 1375.9 ± 686.1 | 49.9 | 289.5 ± 170.8 | 59.0 | 1665.3 ± 816.7 |
| 3 | 917.1 ± 462.5 | 50.4 | 62.0 ± 50.7 | 81.9 | 873.3 ± 518.3 |
| 4 | 1937.4 ± 594.1 | 30.7 | 270.0 ± 167.4 | 62.0 | 2191.0 ± 698.1 |
| 5 | 1391.2 ± 851.1 | 61.2 | 114.8 ± 110.7 | 96.4 | 1270.0 ± 992.8 |
| 6 | 1479.9 ± 801.5 | 54.2 | 249.0 ± 128.9 | 51.7 | 1704.3 ± 895.8 |
| 7 | 1083.3 ± 512.1 | 47.3 | 99.8 ± 78.1 | 78.3 | 922.0 ± 711.6 |
| 8 | 1749.8 ± 661.2 | 37.8 | 160.2 ± 81.0 | 50.6 | 1910.0 ± 674.1 |
| 9 | 1223.3 ± 662.2 | 54.1 | 160.1 ± 99.0 | 61.8 | 1504.0 ± 682.9 |
| 10 | 1381.7 ± 601.0 | 43.5 | 115.3 ± 75.7 | 65.7 | 1368.9 ± 722.7 |
| Mean | 1386.9 | 156.3 | 1465.5 | ||
| CV | 21.3 | 55.6 | 28.5 | ||
| Population | Chlorophyll a | Chlorophyll b | Total Carotenoids | |||
|---|---|---|---|---|---|---|
| CV * (%) | CV (%) | CV (%) | ||||
| 1 | 4.2 ± 0.6 | 14.7 | 1.9 ± 0.5 | 24.7 | 2.6 ± 0.4 | 14.6 |
| 2 | 3.8 ± 0.5 | 13.5 | 1.6 ± 0.4 | 23.3 | 2.3 ± 0.3 | 11.1 |
| 3 | 4.3 ± 0.3 | 8.0 | 1.9 ± 0.3 | 15.5 | 2.6 ± 0.2 | 7.6 |
| 4 | 4.1 ± 0.3 | 7.8 | 1.9 ± 0.4 | 19.9 | 2.5 ± 0.2 | 6.8 |
| 5 | 4.1 ± 1.1 | 26.0 | 2.0 ± 0.5 | 23.4 | 2.6 ± 0.3 | 11.4 |
| 6 | 4.2 ± 0.6 | 15.0 | 1.8 ± 0.5 | 25.5 | 2.5 ± 0.3 | 12.2 |
| 7 | 4.1 ± 0.6 | 14.8 | 1.9 ± 0.5 | 25.7 | 2.4 ± 0.3 | 13.0 |
| 8 | 4.1 ± 0.5 | 11.4 | 1.8 ± 0.3 | 18.6 | 2.4 ± 0.2 | 9.5 |
| 9 | 4.5 ± 0.6 | 12.6 | 2.2 ± 0.5 | 22.8 | 2.6 ± 0.2 | 8.8 |
| 10 | 4.1 ± 0.6 | 13.7 | 1.7 ± 0.4 | 22.2 | 2.4 ± 0.3 | 11.9 |
| Mean | 4.2 | 1.9 | 2.5 | |||
| CV | 4.2 | 8.9 | 3.8 |
| pH | NO3− | NH4+ | P | K | Ca | Mg | Cl | Na | Cu | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6.78 | 63 | 12 | 82 | 158 | 727 | 127 | 42 | 42 | 2.9 | 49.7 | 5.7 | 5.3 |
| Population No. | Location | Voivodeship | Geographical Coordinates | Altitude | |
|---|---|---|---|---|---|
| Latitude | Longitude | ||||
| 1 | Bożejewo | Podlaskie | 53°11′09.8″ N | 22°17′45.3″ E | 104 |
| 2 | Grądy-Woniecko | Podlaskie | 53°09′35.9″ N | 22°23′29.8″ E | 113 |
| 3 | Świniary | Masovian | 52°30′26.5″ N | 22°15′65.2″ E | 178 |
| 4 | Sosnówek | Masovian | 53°16′57.3″ N | 20°58′47.6″ E | 123 |
| 5 | Łazy | Lubelskie | 51°91′71.1″ N | 22°42′11.5″ E | 159 |
| 6 | WolaSękowa | Subcarpathian | 49°30′26.5″ N | 22°00′30.9″ E | 408 |
| 7 | Karlików | Subcarpathian | 49°26′06.3″ N | 22°04′33.8″ E | 478 |
| 8 | Szczawne | Subcarpathian | 49°24′14.4″ N | 22°09′02.3″ E | 394 |
| 9 | Siedliska | Subcarpathian | 49°57′22.3″ N | 21°56′46.5″ E | 229 |
| 10 | Mszana | Subcarpathian | 49°49′46.2″ N | 21°64′84.7″ E | 453 |
| Months | Temperature (°C) | Rainfall (mm) | Air Humidity (%) | Sun Days |
|---|---|---|---|---|
| April | 8 | 21.8 | 69 | 23 |
| May | 16 | 8.2 | 62 | 31 |
| June | 21 | 22.5 | 66 | 26 |
| July | 23 | 20.8 | 60 | 29 |
| August | 25 | 9.0 | 55 | 30 |
| September | 16 | 7.8 | 60 | 28 |
| October | 14 | 3.5 | 69 | 31 |
| No. | Compound | Precision Intra–Day (CV%) | Precision Int er–Day (CV%) | Calibration Equation | R2 * | Linear Ran ge (mg/mL) | LOD a (µg/L) | LOQ b (µg/L) |
|---|---|---|---|---|---|---|---|---|
| 1 | 5-O-caffeoylquinic acid (Neochlorogenic acid) | 0.27 | 0.78 | y = 1809.0 x − 1539.8 | 0.9999 | 0.39–392.0 | 18.39 | 61.31 |
| 2 | 3-O-Caffeoylquinic acid (Chlorogenic acid) | 1.32 | 1.63 | y = 6517.4 x − 12,016.6 | 0.9997 | 0.40–39.47 | 20.97 | 69.90 |
| 3 | Caffeoylmalic acid | 1.32 | 1.63 | y = 6517.4 x − 12,016.6 | 0.9997 | 0.40–39.47 | 20.97 | 69.90 |
| 4 | Quercetin 3-O-rutinoside (Rutoside) | 0.37 | 0.86 | y = 1434.0 x − 5093.0 | 0.9999 | 0.90–90.67 | 7.46 | 24.88 |
| 5 | Quercetin 3-O-galactoside (Hyperoside) | 1.25 | 2.14 | y = 3435.5 x − 6882.2 | 0.9999 | 0.38–38.40 | 4.12 | 12.24 |
| 6 | Cichoric acid | 0.18 | 0.49 | y = 3230.70 x + 6882.20 | 0.9998 | 0.46–456.96 | 11.47 | 38.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koczkodaj, S.; Przybył, J.L.; Kosakowska, O.; Węglarz, Z.; Bączek, K.B. Intraspecific Variability of Stinging Nettle (Urtica dioica L.). Molecules 2023, 28, 1505. https://doi.org/10.3390/molecules28031505
Koczkodaj S, Przybył JL, Kosakowska O, Węglarz Z, Bączek KB. Intraspecific Variability of Stinging Nettle (Urtica dioica L.). Molecules. 2023; 28(3):1505. https://doi.org/10.3390/molecules28031505
Chicago/Turabian StyleKoczkodaj, Sylwia, Jarosław L. Przybył, Olga Kosakowska, Zenon Węglarz, and Katarzyna B. Bączek. 2023. "Intraspecific Variability of Stinging Nettle (Urtica dioica L.)" Molecules 28, no. 3: 1505. https://doi.org/10.3390/molecules28031505
APA StyleKoczkodaj, S., Przybył, J. L., Kosakowska, O., Węglarz, Z., & Bączek, K. B. (2023). Intraspecific Variability of Stinging Nettle (Urtica dioica L.). Molecules, 28(3), 1505. https://doi.org/10.3390/molecules28031505








