Anti-Trypanosomatidae Activity of Essential Oils and Their Main Components from Selected Medicinal Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Essential Oil Extraction
2.2. EO Analysis
2.3. Pure Compounds
2.4. Anti-Parasitic Activity In Vitro
2.5. Cytotoxicity of Pure Compounds
2.6. Statistical Analysis
3. Results
3.1. Antiprotozoal Activity of EOs from Lamiaceae and Asteraceae Plants
3.2. Chemical Composition of Essential Oils
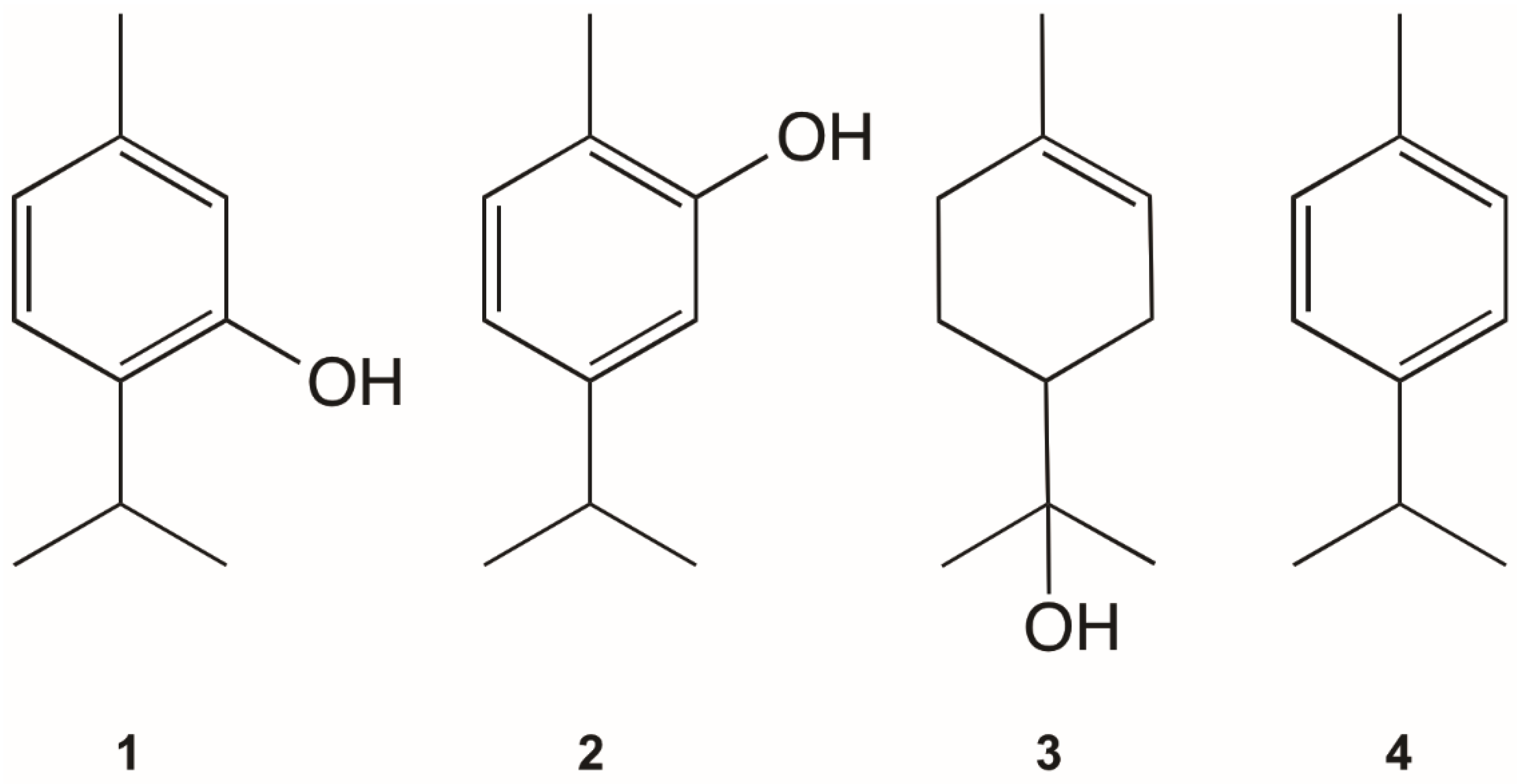
3.3. Anti-Phytomonas and Cytotoxic Activity of Pure Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Stuart, K.; Brun, R.; Croft, S.; Fairlamb, A.; Gürtler, R.E.; McKerrow, J.; Reed, S.; Tarleton, R. Kinetoplastids: Related protozoan pathogens, different diseases. J. Clin. Investig. 2008, 118, 1301. [Google Scholar] [CrossRef]
- Sunter, J.; Gull, K. Shape, form, function and Leishmania pathogenicity: From textbook descriptions to biological understanding. Open Biol. 2017, 7, 170165. [Google Scholar] [CrossRef]
- Yurchenko, V.; Butenko, A.; Kostygov, A.Y. Genomics of Trypanosomatidae: Where we stand and what needs to be done? Pathogens 2021, 10, 1124. [Google Scholar] [CrossRef]
- Jaskowska, E.; Butler, C.; Preston, G.; Kelly, S. Phytomonas: Trypanosomatids adapted to plant environments. PLoS Pathog. 2015, 11, e1004927. [Google Scholar] [CrossRef]
- Georgiadou, S.P.; Makaritsis, K.P.; Dalekos, G.N. Leishmaniasis revisited: Current aspects on epidemiology, diagnosis and treatment. J. Transl. Int. Med. 2015, 3, 43–50. [Google Scholar] [CrossRef]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- Sasidharan, S.; Saudagar, P. Leishmaniasis: Where are we and where are we heading? Parasitol. Res. 2021, 120, 1541–1554. [Google Scholar] [CrossRef]
- Santos, A.L.S.; d’Avila-Levy, C.M.; Elias, C.G.R.; Vermelho, A.B.; Branquinha, M.H. Phytomonas serpens: Immunological similarities with the human Trypanosomatid pathogens. Microbes Infect. 2007, 9, 915–921. [Google Scholar] [CrossRef]
- Lopes, A.H.; Souto-Padrón, T.; Dias, F.A.; Gomes, M.T.; Rodrigues, G.C.; Zimmermann, L.T.; Alves e Silva, T.L.; Vermelho, A.B. Trypanosomatids: Odd organisms, devastating diseases. Open Parasitol. J. 2010, 4, 30–59. [Google Scholar] [CrossRef]
- Ginsburg, H.; Deharo, E. A Call for using natural compounds in the development of new antimalarial treatments—An introduction. Malar. J. 2011, 10, S1. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Al-Sadi, A.M. Biosynthesis of essential oils in aromatic plants: A review. Food Rev. Int. 2016, 32, 117–160. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Fampa, P.; Florencio, M.; Santana, R.C.; Rosa, D.; Soares, D.C.; Leonel De Matos Guedes, H.; Cordeiro Da Silva, A.; Siqueira, D.; Chaves, A.; Pinto-Da-Silva, L.H. Anti-Leishmania effects of volatile oils and their isolates. Rev. Bras. Farmacogn. 2021, 31, 561–578. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Cianfaglione, K.; Bajalan, I.; Morshedloo, M.R.; Lupidi, G.; Romano, D.; et al. Microemulsions for delivery of apiaceae essential oils—Towards highly effective and eco-friendly mosquito larvicides? Ind. Crops Prod. 2019, 129, 631–640. [Google Scholar] [CrossRef]
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlock, G.E.; Schulz, H.; et al. essential oils as multicomponent mixtures and their potential for human health and well-being. Front. Pharmacol. 2022, 13, 2645. [Google Scholar] [CrossRef]
- Andrade, M.A.; Azevedo, C.D.S.; Motta, F.N.; Santos, M.L.D.; Silva, C.L.; Santana, J.M.D.; Bastos, I.M.D. Essential Oils: In vitro activity against Leishmania amazonensis, cytotoxicity and chemical composition. BMC Complement. Altern. Med. 2016, 16, 444. [Google Scholar] [CrossRef]
- Kalaivani, K.; Senthil-Nathan, S.; Murugesan, A.G. biological activity of selected Lamiaceae and Zingiberaceae plant essential oils against the dengue vector Aedes aegypti L. (Diptera: Culicidae). Parasitol. Res. 2012, 110, 1261–1268. [Google Scholar] [CrossRef]
- Vieira-Araújo, F.M.; Macedo Rondon, F.C.; Pinto Vieira, Í.G.; Pereira Mendes, F.N.; Carneiro de Freitas, J.C.; Maia de Morais, S. Sinergism between alkaloids piperine and capsaicin with meglumine antimoniate against Leishmania infantum. Exp. Parasitol. 2018, 188, 79–82. [Google Scholar] [CrossRef]
- Hammi, K.M.; Essid, R.; Tabbene, O.; Elkahoui, S.; Majdoub, H.; Ksouri, R. Antileishmanial activity of Moringa oleifera leaf extracts and potential synergy with Amphotericin B. S. Afr. J. Bot. 2020, 129, 67–73. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Guzmán, M.; González-Coloma, A.; Fe Andrés, M.; Navarro-Rocha, J.; Martínez-Díaz, R.A. Biological evaluation of essential oils from selected medicinal plants and their main components against Phytomonas davidi (Kinetoplastea: Trypanosomatidae). Chem. Biodivers. 2020, 17, e2000521. [Google Scholar] [CrossRef]
- Monzote, L.; Geroldinger, G.; Tonner, M.; Scull, R.; de Sarkar, S.; Bergmann, S.; Bacher, M.; Staniek, K.; Chatterjee, M.; Rosenau, T.; et al. Interaction of ascaridole, carvacrol, and caryophyllene oxide from essential oil of Chenopodium ambrosioides L. with mitochondria in Leishmania and other eukaryotes. Phytother. Res. 2018, 32, 1729–1740. [Google Scholar] [CrossRef]
- Bailén, M.; Díaz-Castellanos, I.; Azami-Conesa, I.; Alonso Fernández, S.; Martínez-Díaz, R.A.; Navarro-Rocha, J.; Gómez-Muñoz, M.T.; González-Coloma, A. Anti-Trichomonas gallinae activity of essential oils and main compounds from Lamiaceae and Asteraceae plants. Front. Vet. Sci. 2022, 9, 981763. [Google Scholar] [CrossRef]
- Julio, L.F.; Barrero, A.F.; Herrador Del Pino, M.M.; Arteaga, J.F.; Burillo, J.; Andres, M.F.; Díaz, C.E.; González-Coloma, A. Phytotoxic and nematicidal components of Lavandula luisieri. J. Nat. Prod. 2016, 79, 261–266. [Google Scholar] [CrossRef]
- Postell, F.J.; Mcghee, R.B. An Ultrastructural study of Phytomonas davidi Lafont (Trypanosomatidae)1. J. Protozool. 1981, 28, 78–83. [Google Scholar] [CrossRef]
- Sainz, P.; Andrés, M.F.; Martínez-Díaz, R.A.; Bailén, M.; Navarro-Rocha, J.; Díaz, C.E.; González-Coloma, A. Chemical composition and biological activities of Artemisia pedemontana subsp. assoana essential oils and hydrolate. Biomolecules 2019, 9, 558. [Google Scholar] [CrossRef]
- Escobar, P.; Leal, S.M.; Herrera, L.V.; Martinez, J.R.; Stashenko, E. Chemical composition and antiprotozoal activities of Colombian lippia spp. essential oils and their major components. Mem. Inst. Oswaldo Cruz 2010, 105, 184–190. [Google Scholar] [CrossRef]
- Youssefi, M.R.; Moghaddas, E.; Tabari, M.A.; Moghadamnia, A.A.; Hosseini, S.M.; Hosseini Farash, B.R.; Ebrahimi, M.A.; Mousavi, N.N.; Fata, A.; Maggi, F.; et al. In vitro and in vivo effectiveness of carvacrol, thymol and linalool against Leishmania infantum. Molecules 2019, 24, 2072. [Google Scholar] [CrossRef]
- Leal, S.M.; Pino, N.; Stashenko, E.E.; Martínez, J.R.; Escobar, P. Antiprotozoal activity of essential oils derived from Piper spp. grown in Colombia. J. Essent. Oil Res. 2013, 25, 512–519. [Google Scholar] [CrossRef]
- Zaman, W.; Ye, J.; Saqib, S.; Liu, Y.; Shan, Z.; Hao, D.; Chen, Z.; Xiao, P. Predicting potential medicinal plants with phylogenetic topology: Inspiration from the research of traditional Chinese medicine. J. Ethnopharmacol. 2021, 281, 114515. [Google Scholar] [CrossRef]
- Moraes Neto, R.N.; Setúbal, R.F.B.; Higino, T.M.M.I.; Brelaz-De-Castro, M.C.A.; da Silva, L.C.N.; dos Santos Aliança, A.S. Asteraceae plants as sources of compounds against leishmaniasis and Chagas disease. Front. Pharmacol. 2019, 10, 477. [Google Scholar] [CrossRef]
- Silva, K.P.; de Carvalho Santos, T.A.; Moutinho, B.L.; da Silva, R.S.; dos Santos Pinto, V.; Blank, A.F.; Corrêa, C.B.; Scher, R.; Fernandes, R.P.M. Using Varronia curassavica (Cordiaceae) essential oil for the biocontrol of Phytomonas serpens. Ind. Crops Prod. 2019, 139, 111523. [Google Scholar] [CrossRef]
- Pereira, K.L.G.; Nizio, D.A.D.C.; de Lima, P.C.N.; Fernandes, R.P.M.; Arrigoni-Blank, M.D.F.; de Sá Filho, J.C.F.; Nascimento, L.F.D.A.; de Souza, V.T.; Silva, K.P.; Blank, A.F. Seasonal variance in the chemical composition of essential oils from Lantana camara accessions and their trypanocidal activity on Phytomonas serpens. Bol. Latinoam. Caribe Plantas Med. Aromat. 2022, 21, 737–756. [Google Scholar] [CrossRef]
- Ben Hamida, N.; Martínez-Díaz, R.A.; Hela, M.; Msaada, K.; Ouerghi, Z.; Andres, M.F.; González-Coloma, A. Effect of salinity on the antiparasitic activity of hyssop essential oil. J. Essent. Oil Res. 2020, 32, 74–83. [Google Scholar] [CrossRef]
- Božovic, M.; Pirolli, A.; Ragno, R. Mentha suaveolens Ehrh. (Lamiaceae) essential oil and its main constituent piperitenone oxide: Biological activities and chemistry. Molecules 2015, 20, 8605–8633. [Google Scholar] [CrossRef]
- Menezes, L.R.A.; Santos, N.N.; Meira, C.S.; Ferreira Dos Santos, J.A.; Guimarães, E.T.; Soares, M.B.P.; Nepel, A.; Barison, A.; Costa, E.V. A new source of (R)-limonene and rotundifolone from leaves of Lippia pedunculosa (Verbenaceae) and their trypanocidal properties. Nat. Prod. Commun. 2014, 9, 737–739. [Google Scholar] [CrossRef]
- Matos-Rocha, T.J.; dos Santos Cavalcanti, M.G.; Barbosa-Filho, J.M.; Lúcio, A.S.S.C.; Veras, D.L.; Feitosa, A.P.S.; de Siqueira Júnior, J.P.; de Almeida, R.N.; Marques, M.O.M.; Alves, L.C.; et al. In vitro evaluation of schistosomicidal activity of essential oil of Mentha x villosa and some of its chemical constituents in adult worms of Schistosoma mansoni. Planta Med. 2013, 79, 1307–1312. [Google Scholar] [CrossRef]
- Lima, T.C.; da Silva, T.K.M.; Silva, F.L.; Barbosa-Filho, J.M.; Marques, M.O.M.; Santos, R.L.C.; Cavalcanti, S.C.D.H.; de Sousa, D.P. Larvicidal activity of Mentha x villosa Hudson essential oil, rotundifolone and derivatives. Chemosphere 2014, 104, 37–43. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Abdallah, H.M.; Mohamed, G.A.; Farag, M.A.; Alshali, K.Z.; Alsherif, E.A.; Ross, S.A. Volatile oil profile of some Lamiaceous plants growing in Saudi Arabia and their biological activities. J. Biosci. 2017, 72, 35–41. [Google Scholar] [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Bakri, Y.; Talbaui, A.; Fellah, H.; Abrini, J.; Dakka, N. Chemical composition of Mentha pulegium and Rosmarinus officinalis essential oils and their antileishmanial, antibacterial and antioxidant activities. Microb. Pathog. 2017, 111, 41–49. [Google Scholar] [CrossRef]
- Machado, M.; Santoro, G.; Sousa, M.C.; Salgueiro, L.; Cavaleiro, C. Activity of essential oils on the growth of Leishmania infantum promastigotes. Flavour Fragr. J. 2010, 25, 156–160. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Cantrell, C.L.; Astatkie, T.; Hristov, A. Yield, content, and composition of peppermint and spearmints as a function of harvesting time and drying. J. Agric. Food Chem. 2010, 58, 11400–11407. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Agholi, M.; Ghanbariasad, A.; Ranjbar, A.; Osanloo, M. A nanoemulsion-based nanogel of citrus limon essential oil with leishmanicidal activity against Leishmania tropica and Leishmania major. J. Parasit. Dis. 2021, 45, 441–448. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Jalali Sendi, J.; Ziaee, M.; Krutmuang, P. Acaricidal, insecticidal, and nematicidal efficiency of essential oils isolated from the Satureja genus. Int. J. Environ. Res. Public Health 2021, 18, 6050. [Google Scholar] [CrossRef]
- Jafari, F.; Farmani, F.; Zomorodian, K.; Moein, M.; Faridi, P.; Zarshenas, M.M. A Study on essential oil chemical compositions, antioxidant, and antimicrobial activities of native and endemic Satureja species growing in Iran. Pharm. Chem. J. 2018, 52, 63–68. [Google Scholar] [CrossRef]
- Tepe, B.; Cilkiz, M.A. Pharmacological and phytochemical overview on Satureja. Pharm. Biol. 2015, 54, 375–412. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Rodrigues, A.M.; Sena, I.; Moiteiro, C.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity of Ruta graveolens and Satureja montana essential oils on Solanum tuberosum hairy roots and Solanum tuberosum hairy roots with Meloidogyne chitwoodi co-cultures. J. Agric. Food Chem. 2016, 64, 7452–7458. [Google Scholar] [CrossRef]
- Navarro-Rocha, J.; Andrés, M.F.; Díaz, C.E.; Burillo, J.; González-Coloma, A. Composition and biocidal properties of essential oil from pre-domesticated Spanish Satureja montana. Ind. Crops Prod. 2020, 145, 111958. [Google Scholar] [CrossRef]
- Kheirandish, F.; Chegeni, R.; Delfan, B.; Jabari, M.; Ebrahimzadeh, F.; Rashidipour, M. The cytotoxic and antileishmanial effects of Satureja khuzestanica essential oil. Herb. Med. J. 2016, 1, 11–17. [Google Scholar] [CrossRef]
- Mohammadpour, G.; Marzony, E.T.; Farahmand, M. Evaluation of the anti-Leishmania major activity of Satureja bakhtiarica essential oil in vitro. Nat. Prod. Commun. 2012, 7, 133–136. [Google Scholar] [CrossRef]
- Tariku, Y.; Hymete, A.; Hailu, A.; Rohloff, J. Essential-oil composition, antileishmanial, and toxicity study of Artemisia abyssinica and Satureja punctata ssp. punctata from Ethiopia. Chem. Biodivers. 2010, 7, 1009–1018. [Google Scholar] [CrossRef]
- Machado, M.; Martins, N.; Salgueiro, L.; Cavaleiro, C.; Sousa, M.C. Lavandula luisieri and Lavandula viridis essential oils as upcoming anti-protozoal agents: A key focus on leishmaniasis. Appl. Sci. 2019, 9, 3056. [Google Scholar] [CrossRef]
- Shokri, A.; Saeedi, M.; Fakhar, M.; Morteza-Semnani, K.; Keighobadi, M.; Teshnizi, S.H.; Kelidari, H.R.; Sadjadi, S. Antileishmanial activity of Lavandula angustifolia and Rosmarinus officinalis essential oils and nano-emulsions on Leishmania major (MRHO/IR/75/ER). Iran. J. Parasitol. 2017, 12, 622–631. [Google Scholar]
- Bouyahya, A.; Et-Touys, A.; Abrini, J.; Talbaoui, A.; Fellah, H.; Bakri, Y.; Dakka, N. Lavandula stoechas essential oil from Morocco as novel source of antileishmanial, antibacterial and antioxidant activities. Biocatal. Agric. Biotechnol. 2017, 12, 179–184. [Google Scholar] [CrossRef]
- Machado, M.; Dinis, A.M.; Santos-Rosa, M.; Alves, V.; Salgueiro, L.; Cavaleiro, C.; Sousa, M.C. Activity of Thymus capitellatus volatile extract, 1,8-cineole and borneol against Leishmania Species. Vet. Parasitol. 2014, 200, 39–49. [Google Scholar] [CrossRef]
- Güler, E.; Özbilgin, A.; Becer, E.; Hanoğlu, A.; Şanlidağ, T. An endemic plant of Cyprus, Origanum majorana: Is it a new alternative natural product for malaria treatment? Mikrobiyol. Bul. 2020, 54, 463–478. [Google Scholar] [CrossRef]
- Méabed, E.M.H.; El- Sayed, N.M.; Abou-Sreea, A.I.B.; Roby, M.H.H. Chemical analysis of aqueous extracts of Origanum majorana and Foeniculum vulgare and their efficacy on Blastocystis spp. cysts. Phytomedicine 2018, 43, 158–163. [Google Scholar] [CrossRef]
- Babili, F.E.; Bouajila, J.; Souchard, J.P.; Bertrand, C.; Bellvert, F.; Fouraste, I.; Moulis, C.; Valentin, A. Oregano: Chemical analysis and evaluation of its antimalarial, antioxidant, and cytotoxic activities. J. Food Sci. 2011, 76, C512–C518. [Google Scholar] [CrossRef]
- Teles, A.M.; Rosa, T.D.D.S.; Mouchrek, A.N.; Abreu-Silva, A.L.; da Silva Calabrese, K.; Almeida-Souza, F. Cinnamomum zeylanicum, Origanum vulgare, and Curcuma longa essential oils: Chemical composition, antimicrobial and antileishmanial activity. Evid. Based Complement. Altern. Med. 2019, 2019, 2421695. [Google Scholar] [CrossRef]
- Sanchez-Suarez, J.F.; Riveros, I.; Delgado, G. Evaluation of the leishmanicidal and cytotoxic potential of essential oils derived from ten Colombian plants. Iran. J. Parasitol. 2013, 8, 129. [Google Scholar]
- Tasdemir, D.; Kaiser, M.; Demirci, B.; Demirci, F.; Hüsnü Can Baser, K. Antiprotozoal activity of Turkish Origanum onites essential oil and its components. Molecules 2019, 24, 4421. [Google Scholar] [CrossRef]
- Silva, A.R.S.T.; Scher, R.; Santos, F.V.; Ferreira, S.R.; Cavalcanti, S.C.H.; Correa, C.B.; Bueno, L.L.; Alves, R.J.; Souza, D.P.; Fujiwara, R.T.; et al. Leishmanicidal activity and structure-activity relationships of essential oil constituents. Molecules 2017, 22, 815. [Google Scholar] [CrossRef]
- De Morais, S.M.; Vila-Nova, N.S.; Bevilaqua, C.M.L.; Rondon, F.C.; Lobo, C.H.; de Alencar Araripe Noronha Moura, A.; Sales, A.D.; Rodrigues, A.P.R.; de Figuereido, J.R.; Campello, C.C.; et al. Thymol and eugenol derivatives as potential antileishmanial agents. Bioorg. Med. Chem. 2014, 22, 6250–6255. [Google Scholar] [CrossRef]
- Polanco-Hernández, G.M.; Giménez-Turba, A.; Salamanca, E.; Getti, G.; Rai, R.; Acosta-Viana, K.Y.; Ermilo Arana-Argáez, V.; Torres-Romero, J.C.; Fernández-Martín, K.G.; Segura-Campos, M.R.; et al. Leishmanicidal activity and immunomodulatory effect of a mixture of lupenone and β-caryophyllene oxide. Rev. Bras. Farmacogn. 2021, 31, 199–206. [Google Scholar] [CrossRef]
- Pastor, J.; García, M.; Steinbauer, S.; Setzer, W.N.; Scull, R.; Gille, L.; Monzote, L. Combinations of ascaridole, carvacrol, and caryophyllene oxide against Leishmania. Acta Trop. 2015, 145, 31–38. [Google Scholar] [CrossRef]
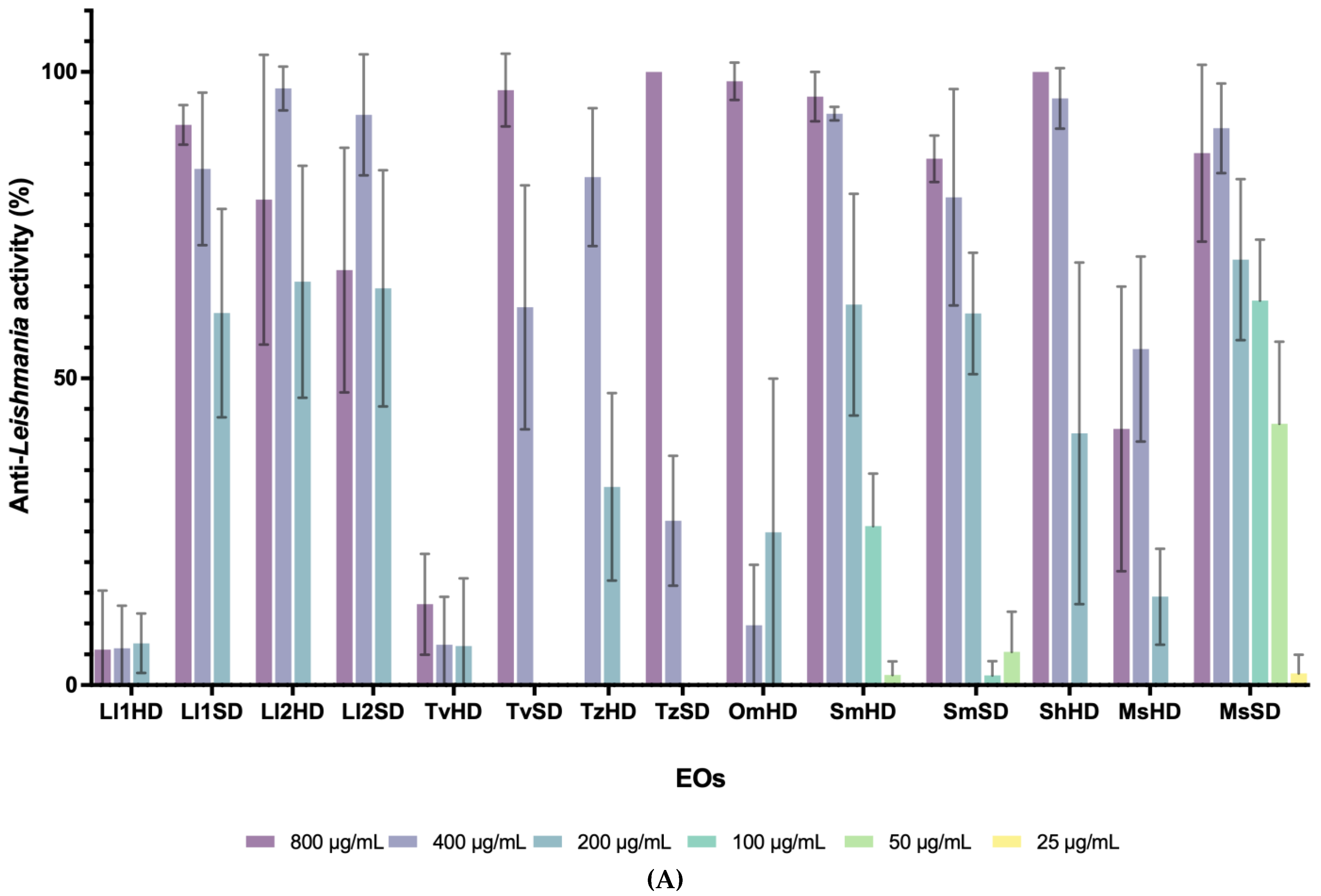
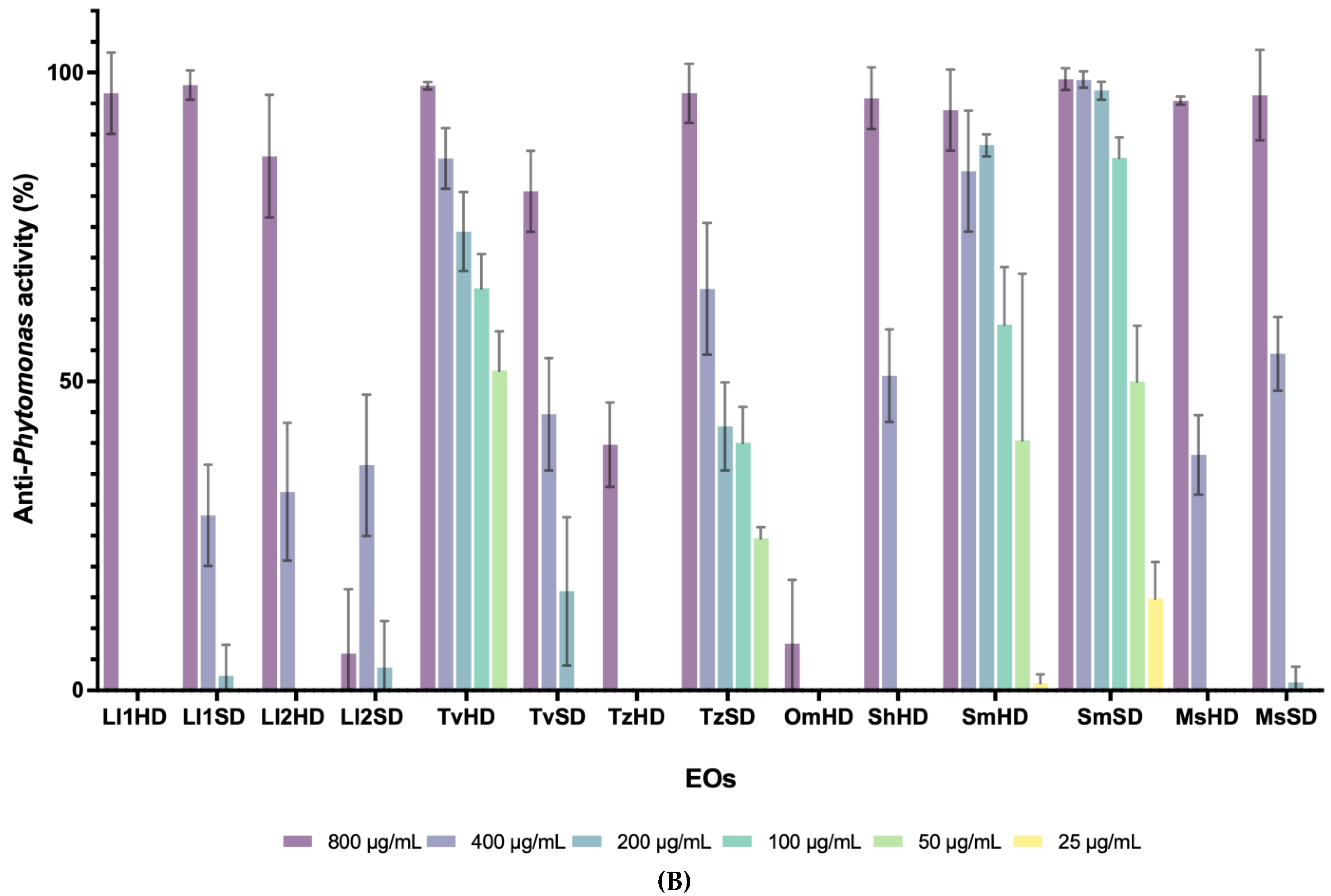
| Family | Genus | Species | Extraction Method | L. infantum (IC50) | P. davidi (IC50) |
|---|---|---|---|---|---|
| Asteraceae | Tanacetum | Tanacetum vulgare | HD | 325.5 (300.0, 353.1) | >800 |
| Santolina | Santolina chamaecyparissus | HD | 451.2 (391.4–520.2) | 599.2 (580.7–618.3) | |
| Ditrichia | Ditrichia graveolens | HD | 350.8 (303.4–405.4) | 341.5 (288.8–403.8) | |
| SD | 306.8 (246.7–381.5) | >800 | |||
| Lamiaceae | Lavandula | Lavandula lanata | HD | >800 | >800 |
| Lavandula luisieri 1 | HD | >800 | 572.5 (557.5–587.9) | ||
| SD | <200 | 439.5 (394.8–489.3) | |||
| Lavandula luisieri 2 | HD | 247.4 (227.7–268.8) | 573.9 (485.6–678.2) | ||
| SD | 74.3 (61.6–89.5) | >800 | |||
| Lavandula angustifolia | HD | 461.2 (402.2–528.8) | >800 | ||
| SD | 512.2 (477.1–550.0) | >800 | |||
| Lavandula x intermedia “Abrial” | HD | 634.0 (528.5–760.7) | >800 | ||
| SD | 319.1 (294.8–345.3) | >800 | |||
| Lavandula x intermedia “Super” | HD | 466.5 (404.2–538.3) | 343.8 (259.3–455.8) | ||
| SD | 640.7 (603.6–680.1) | >800 | |||
| Lavandula x intermedia “Grosso” | HD | >800 | 496.6 (417.9–590.1) | ||
| SD | 633.8 (595.0–675.0) | >800 | |||
| Lavandula mallete | HD | 397.9 (362.4–436.9 | 436.5 (367.4–518.6) | ||
| SD | 293.2 (233.8–367.6) | >800 | |||
| Origanum | Origanum majorana | HD | 547.9 (521.4–575.7) | >800 | |
| Origanum virens | HD | 781.2 (742.7–821.7) | 507.8 (486.8–529.7) | ||
| SD | 742.6 (664.7–829.6) | 521.9 (499.0–545.8) | |||
| Rosmarinus | Rosmarinus officinalis | HD | 718.9 (667.1–774.7) | 599.6 (495.4–725.7) | |
| SD | 367.1 (307.8–437.7) | 446.5 (321.7–619.7) | |||
| Satureja | Satureja montana | HD | 170.9 (154.0–189.6) | 83.1 (67.2–102.7) | |
| SD | 194.1 (176.7–213.3)) | 49.7 (46.0–53.7) | |||
| Mentha | Mentha suaveolens | HD | 117.9 (101.3–137.4) | 431.6 (404.5–460.6) | |
| SD | 88.2 (73.2–106.4) | 396.0 (374.0–419.3) | |||
| Salvia | Salvia officinalis | HD | 680.8 (621.8–745.4) | >800 | |
| SD | <200 | 329.3 (280.6–386.4) | |||
| Salvia hybrid | HD | 184.7 (154.8–220.3) | 406,3 (387.2–426,3) | ||
| Salvia blancoana | HD | >800 | 514.2 (474.1–557.7) | ||
| Salvia sclarea | HD | >800 | >800 | ||
| Thymus | Thymus mastichina | HD | 743.7 (682.4–810.5) | >800 | |
| Thymus vulgaris | HD | >800 | 43.3 (33.5–56.0) | ||
| SD | 375.6 (331.8–425.1) | 426.4 (369.9–491.4) | |||
| Thymus zygis | HD | 141.8 (99.1–202.8) | >800 | ||
| SD | 433.0 (394.9–474.7) | 182.3 (155.7–213.4) |
| Plant Species | EM | Compounds (% Relative Abundance) |
|---|---|---|
| M. suaveolens | HD | piperitenone (53%), piperitenone oxide (23%), limonene (6%) |
| SD | piperitenone oxide (37%), piperitenone (21%), limonene (7%), germacrene D (7%), β -caryophyllene (6%) | |
| L. luisieri 1 * | HD | fenchone (20%), camphor (13%), trans-α-necrodyl acetate (12%), lavandulyl acetate (6%), α-pinene (6%), 1,8-cineole (5%) |
| SD | camphor (35%), trans-α-necrodyl acetate (19%), lavandulyl acetate (6%), α-pinene (5%) | |
| L. luisieri 2 * | HD | camphor (49%), trans-α-necrodyl acetate (13%), lavandulol (6%) |
| SD | trans-α-necrodyl acetate (18%), lavandulol (8%), germacrene D (8%), camphor (5%) | |
| S. hybrid * | HD | 1,8-cineole (21%), camphor (14%), trans-bornyl acetate (13%), β-pinene (11%), camphene (7%) |
| S. montana * | HD | carvacrol (33%), p-cymene (18%), thymol (17%), γ-terpinene (12%) |
| SD | carvacrol (41%), p-cymene (12%), γ-terpinene (12%), thymol (7%), β-caryophyllene (6%) | |
| T. vulgaris * | HD | thymol (43%), p-cymene (22%), γ-terpinene (6%) |
| SD | carvacrol (41%), p-cymene (31%), thymol (28%), β-caryophyllene (6%) | |
| T. zygis * | HD | thymol (39%), p-cymene (18%), γ-terpinene (9%), linalool (5%), borneol (6%), carvacrol (5%) |
| SD | thymol (21%), linalyl acetate (18%), linalool (12%), p-cymene (8%), carvacrol (5%), α-bisabolol (5%) |
| Compound | AL Activity | AP Activity | ||
|---|---|---|---|---|
| PCC | p | PCC | p | |
| p-cymene | −0.048 | 0.773 | −0.441 | 0.005 |
| α-terpineol | 0.408 | 0.010 | 0.247 | 0.129 |
| piperitenone oxide | −0.338 | 0.035 | −0.146 | 0.377 |
| thymol | −0029 | 0.859 | −0.396 | 0.013 |
| carvacrol | −0242 | 0.138 | −0.517 | 0.001 |
| Compound | Vero Cells [25] | P. davidi | L. infantum | ||
|---|---|---|---|---|---|
| CC50 (µg/mL) a | IC50 (µg/mL) b | SI c | IC50 (µg/mL) b | SI c | |
| thymol (1) | ≈100 | 45.0 (38.4–52.7) | 2.22 | 7.22 (6.22–8.62) [29] | 13.85 |
| carvacrol (2) | >100 | 79.1 (71.9–86.9) | 1.26 | 9.8 (8.51–11.7) [29] | 10.20 |
| α-terpineol (3) | >100 | >100 | 1 | nd | - |
| p-cymene (4) | >100 | >100 | 1 | >100 [28] | 0.67 |
| amphotericin B | - | 0.2 (0.1–0.2) | - | 0.01 [30] | 10.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailén, M.; Illescas, C.; Quijada, M.; Martínez-Díaz, R.A.; Ochoa, E.; Gómez-Muñoz, M.T.; Navarro-Rocha, J.; González-Coloma, A. Anti-Trypanosomatidae Activity of Essential Oils and Their Main Components from Selected Medicinal Plants. Molecules 2023, 28, 1467. https://doi.org/10.3390/molecules28031467
Bailén M, Illescas C, Quijada M, Martínez-Díaz RA, Ochoa E, Gómez-Muñoz MT, Navarro-Rocha J, González-Coloma A. Anti-Trypanosomatidae Activity of Essential Oils and Their Main Components from Selected Medicinal Plants. Molecules. 2023; 28(3):1467. https://doi.org/10.3390/molecules28031467
Chicago/Turabian StyleBailén, María, Cristina Illescas, Mónica Quijada, Rafael Alberto Martínez-Díaz, Eneko Ochoa, María Teresa Gómez-Muñoz, Juliana Navarro-Rocha, and Azucena González-Coloma. 2023. "Anti-Trypanosomatidae Activity of Essential Oils and Their Main Components from Selected Medicinal Plants" Molecules 28, no. 3: 1467. https://doi.org/10.3390/molecules28031467
APA StyleBailén, M., Illescas, C., Quijada, M., Martínez-Díaz, R. A., Ochoa, E., Gómez-Muñoz, M. T., Navarro-Rocha, J., & González-Coloma, A. (2023). Anti-Trypanosomatidae Activity of Essential Oils and Their Main Components from Selected Medicinal Plants. Molecules, 28(3), 1467. https://doi.org/10.3390/molecules28031467