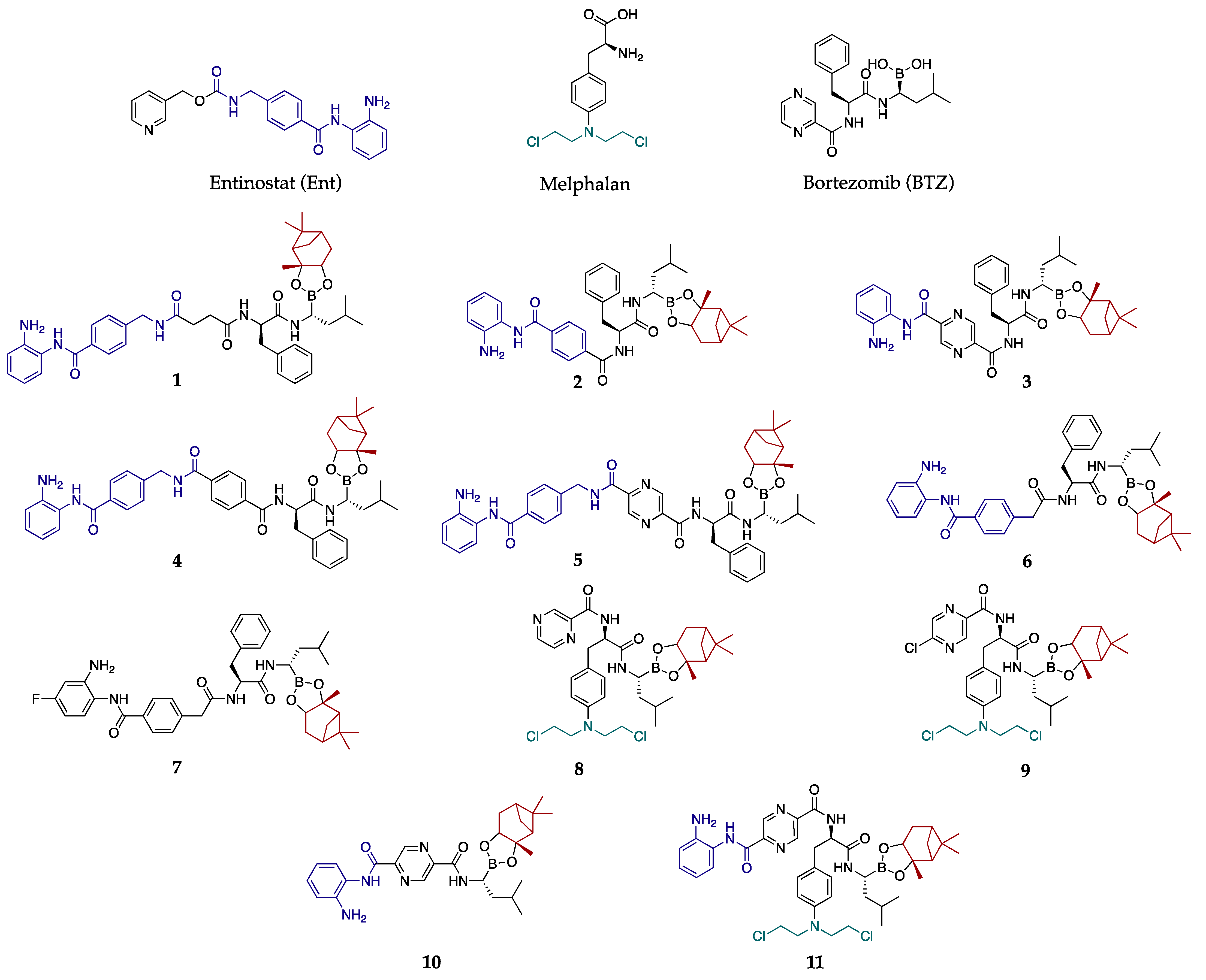
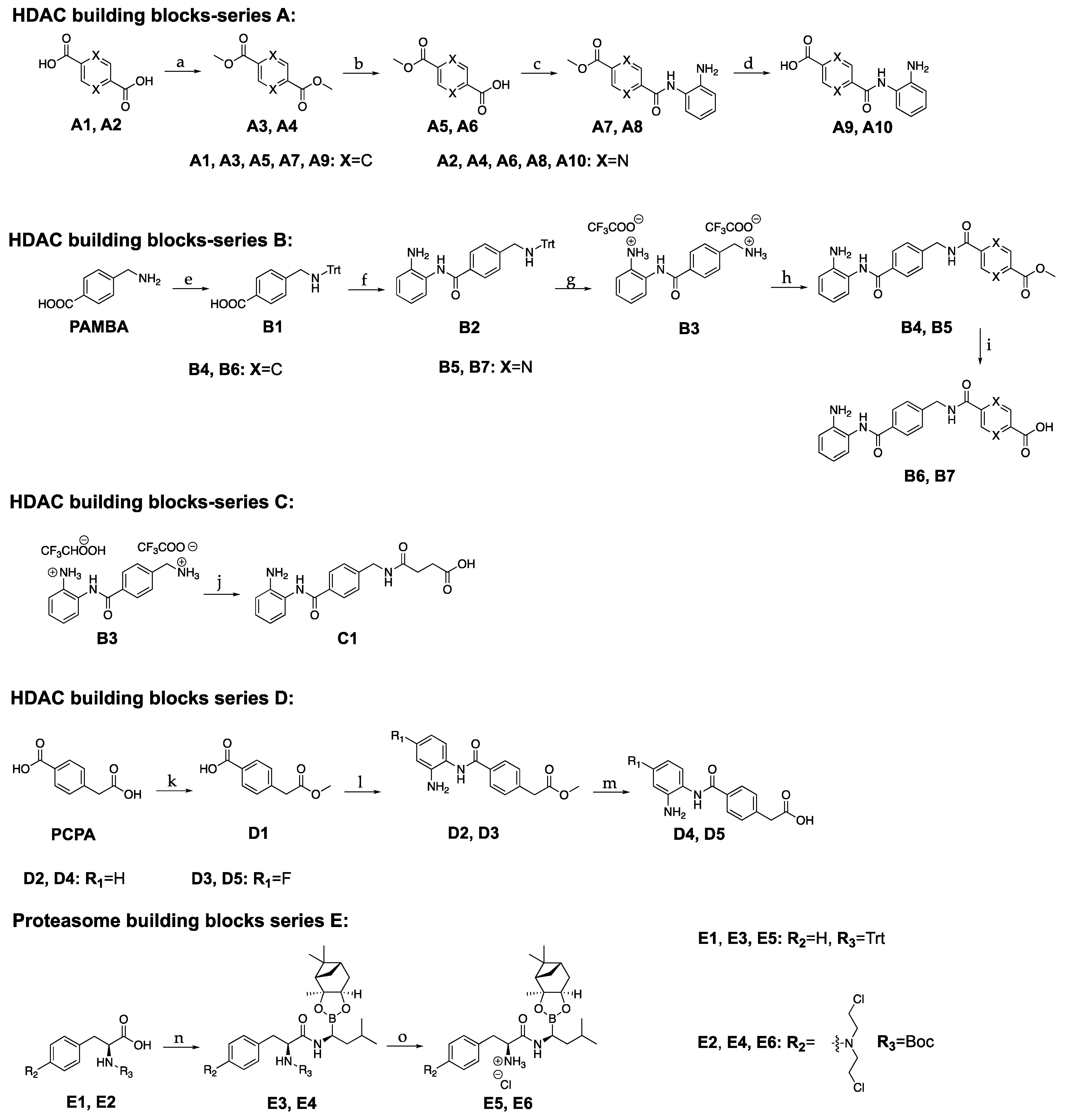
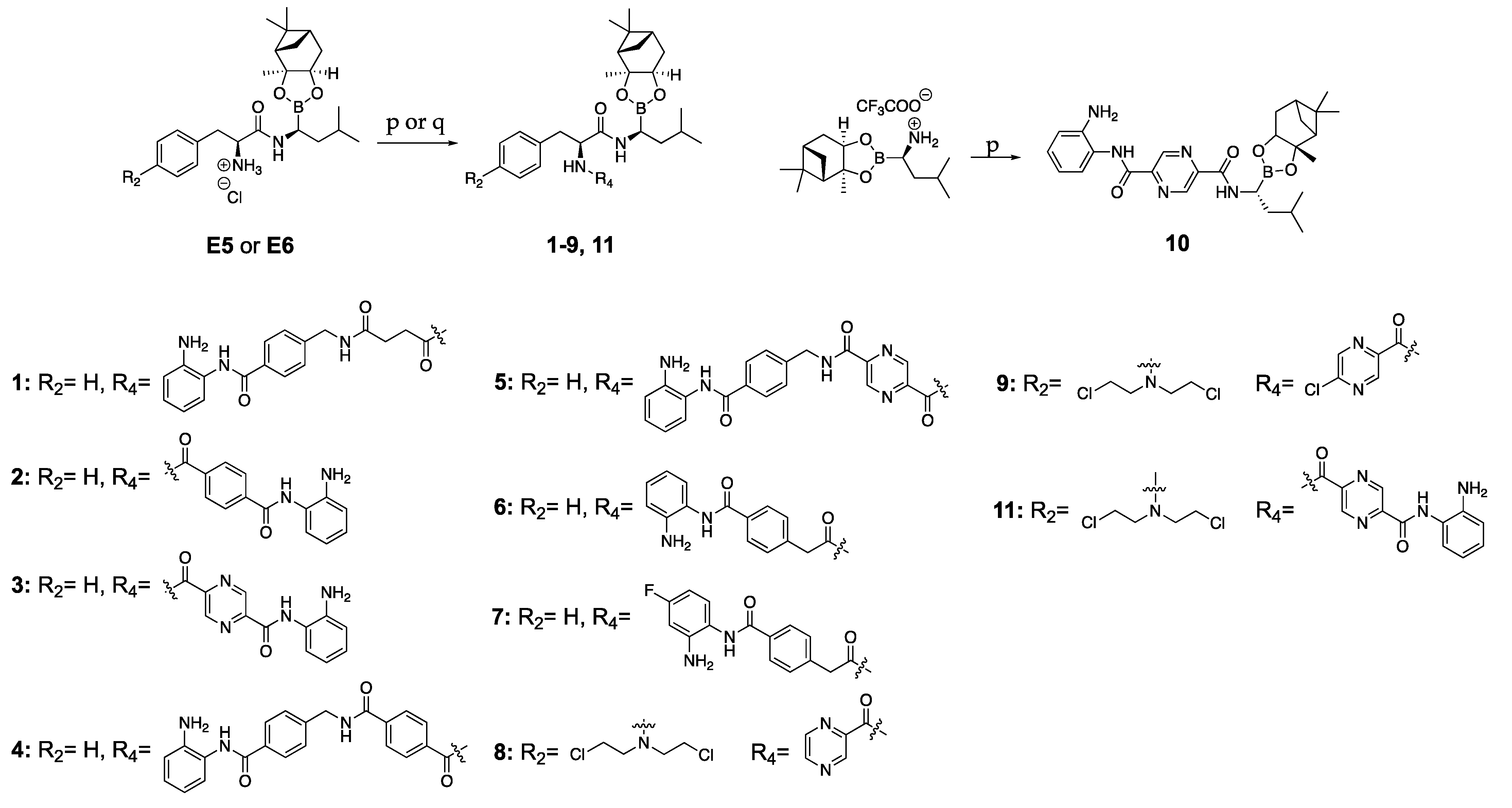
3.2. Synthesis
Dimethyl pyrazine-2,5-dicarboxylate (A4): To a suspension of pyrazine-2,5-dicarboxylic acid (3.15 g, 18.75 mmol) in anhydrous MeOH (40 mL), cooled at 0 °C. SOCl2 (1 mL, 13.8 mmol) was added dropwise, and the mixture was heated at 80 °C for 24 h. Upon completion, the suspension was filtered while hot and the filtrate was concentrated to dryness. The desired diester was afforded as pale-yellow fluffy crystals (1.08 g, 36% yield) after FCC purification using toluene/ethyl acetate 7:3 as eluent. Rf (PhMe/EtOAc 7:3) = 0.25; 1H NMR (CDCl3, 600.13 MHz) δ 9.39 (s, 2 H), 4.07 (s, 6 H); 13C NMR (CDCl3, 150.9 MHz) δ 163.5, 145.5, 145.2, 53.5.
5-(methoxycarbonyl)pyrazine-2-carboxylic acid (
A6): Into a suspension of dimethyl pyrazine-2,5-dicarboxylate) (0.3 g, 1.53 mmol) in MeOH (38.2 mL), 1 M NaOH in water (1.53 mmol) was dripped and the solution was stirred at room temperature for 24 h [
16]. The resulting mixture was concentrated to dryness under vacuum, and the residue was dissolved in water (10 mL). Then HCl
(conc.) (~5 mL) was dripped into the solution until a pale-yellow solid was precipitated. The suspension was filtrated under vacuum and air dried to give the acid as a pale-yellow solid (0.250 g, 98% yield), without further purification. R
f (DCE/MeOH/CH
3COOH) 8:2:0.5 = 0.2; MS (ESI, 30 eV):
m/
z 181.2 [M-H
+].
Methyl 5-((2-aminophenyl)carbamoyl)pyrazine-2-carboxylate (A8): To a stirring suspension of 5-(methoxycarbonyl)pyrazine-2-carboxylic acid (435 mg, 2.39 mmol) in anhydrous DCM (3 mL), Et3N (1.15 mL, 8.24 mmol) and HBTU (1.15 g, 3.03 mmol) were added and the mixture was cooled at 0 °C. Then, 1,2-phenylenediamine (890 mg, 8.24 mmol) was added and the mixture was stirred at room temperature for 24 h. Then the solvent was evaporated under reduced pressure, and the desired amide was afforded as a yellow solid (300 mg, 46% yield) after recrystallization of the dark brown residue, from toluene. Rf (PhMe/EtOAc) 1:1 = 0.12; MS (ESI, 30 eV): m/z 273.27 [M+H+], 295.51 [M+Na+]; 1H NMR (CDCl3, 600.13 MHz) δ 9.59 (d, J = 1.2 Hz, 2 H), 9.30 (d, J = 1.2 Hz, 1 H), 7.52 (dd, J = 1.8 Hz, J′ = 7.8 Hz, 1 H), 7.12 (td, J = 1.8 Hz, J′ = 7.8 Hz, 1 H), 6.88 (ddd, J =1.2 Hz, J′ = 7.8 Hz, J″ = 7.2 Hz, J‴ = 14.4 Hz, 2 H), 4.09 (s, 3 H); 13C NMR (CDCl3, 150.9 MHz) δ 163.8, 159.9, 146.3, 145.3, 144.1, 144.0, 140.0, 127.4, 124.4, 123.7, 120.0, 118.5, 53.5.
5-((2-aminophenyl)carbamoyl)pyrazine-2-carboxylic acid (A10): Into a stirring yellow suspension of methyl 5-((2-aminophenyl)carbamoyl)pyrazine-2-carboxylate (220 mg, 0.81 mmol) in DCM (4 mL), 3 M NaOH in MeOH (0.8 mL, 2.42 mmol) was dripped and the dark red solution was stirred at room temperature for 3 h. Upon consumption of the starting material the mixture was concentrated under vacuum and the residue was acidified with 5% citric acid(aq) up to pH 3.5-4. Then the mixture was concentrated to dryness and the desired acid was afforded as dark brown solid (100 mg, 48% yield) without further purification. Rf (DCE/MeOH/CH3COOH) 8:2:1 = 0.28; MS (ESI, 30 eV): m/z 257.34 [M-H+].
Dimethyl terephthalate (
A3): Suspension of terephthalic acid (4 g, 24.07 mmol) in MeOH (200 mL) was heated under reflux for 2 h [
17]. Then the suspension was cooled at 0 °C and SOCl
2 (50 mL, 485 mmol) was dripped carefully into the system. The reaction mixture was stirred at 80 °C for 24 h. The resulting solution was evaporated to dryness and the residue was subjected to FCC purification using toluene/ethyl acetate 8:2 as eluent, to give the desired diester as white fluffy crystals (4.1 g, 88% yield). R
f (PhMe/EtOAc) 8:2 = 0.56;
1H NMR (CDCl
3, 600.13 MHz) δ 8.10 (s, 4 H), 3.94 (s, 6 H);
13C NMR (CDCl
3, 150.9 MHz) δ 166.3, 133.9, 129.5, 52.4.
4-(methoxycarbonyl)benzoic acid (A5): Into a suspension of dimethyl terephthalate (2 g, 10.29 mmol) in MeOH (257.2 mL), 1 M NaOH in water (10.30 mmol) was dripped and the solution was stirred at room temperature for 24 h. The resulting mixture was concentrated to dryness under vacuum, and the residue was dissolved in water (~50 mL). Then HCl(conc.) (~70 mL) was dripped into the solution until a white solid was precipitated. The suspension was filtered under vacuum and air dried to give the acid as a white solid (1.8 g, 97% yield), without further purification. Rf (EtOAc) = 0.1.
Methyl 4-((2-aminophenyl)carbamoyl)benzoate (A7): To a stirring suspension of 4-(methoxycarbonyl)benzoic acid (400 mg, 2.22 mmol) in anhydrous DCM (3.2 mL), Et3N (560 μL, 4 mmol) and HATU (950 mg, 2.5 mmol) were added and the mixture was cooled at 0 °C. Then, 1,2-phenylenediamine (540 mg, 5 mmol) was added and the mixture was stirred at room temperature for 24 h. Upon completion, the mixture was concentrated to dryness and the residue was subjected to FCC purification using toluene/ethyl acetate 8:2 as eluent. The desired amide was afforded as pale-white solid (192 mg, 33% yield). Rf (PhMe/EtOAc) 8:2 = 0.16; MS (ESI, 30 eV): m/z 271.38 [M+H+], 293.30 [M+Na+]; 1H NMR (CDCl3, 600.13 MHz) δ 8.15 (d, J = 7.8 Hz, 2 H), 7.97 (d, J = 7.8 Hz, 2 H), 7.92 (s, 1 H), 7.37 (d, J = 7.8 Hz, 1 H), 7.11 (t, J = 7.8 Hz, 1 H), 6.87 (d, J = 7.8 Hz, 2 H), 3 96 (s, 3 H); 13C NMR (CDCl3, 150.9 MHz) δ 166.2, 140.4, 130.0, 127.3, 120.0, 118.7, 52.5.
4-((2-aminophenyl)carbamoyl)benzoic acid (
A9): Into a stirring suspension of methyl 4-((2-aminophenyl)carbamoyl)benzoate (240 mg, 0.88 mmol) in DCM (4.4 mL), 3 M NaOH in MeOH (0.88 mL, 2.64 mmol) was dripped and the dark yellow solution was stirred at room temperature for 3 h [
18]. Upon consumption of the starting material, the mixture was concentrated under vacuum and the residue was acidified with 5% citric acid
(aq) up to pH 4. Then the mixture was placed in a separatory funnel and extracted four times with DCM. The combined organic layers were washed with brine, dried over anhydrous Na
2SO
4 and concentrated to dryness under vacuum. The desired acid was afforded as pale-yellow solid (107 mg, 48% yield) without further purification. R
f (DCE/MeOH/CH
3COOH) 8:2:1 = 0.66; MS (ESI, 30 eV):
m/
z 255.20 [M-H
+];
1H NMR (CDCl
3, 600.13 MHz) δ 8.15–8.19 (m, 4 H), 7.48–7.52 (m, 4 H);
13C NMR (CDCl
3, 150.9 MHz) δ 167.3, 134.0, 129.5, 128.7, 127.9, 127.6, 126.4, 123.6.
4-((tritylamino)methyl)benzoic acid (B1): To a stirring suspension of 4-(aminomethyl) benzoic acid (4.53 g, 30 mmol) in a mixture of anhydrous DCM (52.5 mL) and ACN (7.5 mL), trimethylchlorosilane (4.19 mL, 33 mmol) was added, and the resulting mixture was heated under reflux for 30 min. After cooling at room temperature, the solution was cooled at 0 °C and triethylamine (17.5 mL, 120 mmol) was added dropwise into the system, followed by a portion-wise addition of TrtCl (8.78 g, 31.5 mmol) within 30 min. The resulting white emulsion was stirred vigorously for 1 h at 0 °C, and additionally for 3 h at room temperature. Then, the mixture was cooled at 0 °C, MeOH (3 mL) was added and after 30 min the mixture was concentrated to dryness. The resulting residue was diluted in 4 M NaOH(aq) (30 mL), placed in a separatory funnel, and twice extracted with diethyl ether. The aqueous layer was acidified with ice-cooled 5% citric acid(aq) up to pH 4–5 and twice extracted with ethyl acetate. The combined organic layers were, thereupon, washed with water and brine, dried over anhydrous Na2SO4, filtered and concentrated to dryness to afford the product as white foam (8 g, 68% yield) without further purification. Rf (PhMe/EtOAc) 8:2= 0.27; MS (ESI, 30 eV): m/z 392.32 [M-H+].
N-(2-aminophenyl)-4-((tritylamino)methyl)benzamide (B2): To a stirring suspension of 4-((tritylamino)methyl) benzoic acid (1 g, 2.54 mmol) in anhydrous DCM (3.6 mL), Et3N (1.3 mL, 10 mmol) and HATU (988 mg, 2.6 mmol) were added and the mixture was cooled at 0 °C. Then, 1,2-phenylenediamine (325 mg, 3 mmol) was added and the mixture was stirred at room temperature for 24 h. Upon completion, the mixture was placed in a separatory funnel and washed with ice-cooled 5% citric acid(aq), water and brine. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated to dryness. The residue was subjected to FCC purification using toluene/ethyl acetate 9:1 as eluent and the desired amide was afforded as white crystalline solid (812 mg, 66% yield). Rf (PhMe/EtOAc) 9:1 = 0.23; MS (ESI, 30 eV): m/z 243.91 [Trt+], 506.73 [M+Na+], 989.60 [2M+Na+]; 1H NMR (CDCl3, 600.13 MHz) δ 7.87 (d, J = 7.8 Hz, 2 H), 7.56 (d, J = 7.8 Hz, 5 H), 7.53 (d, J = 8.4 Hz, 2 H), 7.31 (t, J = 7.8 Hz, 7 H), 7.23 (t, J = 7.2 Hz, 3 H), 7.10 (t, J = 7.2 Hz, 1 H), 6.85–6.89 (m, 2 H), 3.44 (s, 2 H); 13C NMR (CDCl3, 150.9 MHz) δ 129.0, 128.6, 128.2, 128.0, 127.4, 126.6, 125.3, 47.7.
Bis (2-(4-(ammoniomethyl)benzamido)benzenaminium)) 2,2,2-trifluoroacetate salt (B3): Triethylsilane (380 μL, 2.37 mmol) and trifluoroacetic acid (386 μL, 5.04 mmol) were dripped into an ice-cooled solution of N-(2-aminophenyl)-4-((tritylamino)methyl) benzamide (812 mg, 1.68 mmol) in anhydrous DCM (3.5 mL) and the resulting mixture was initially stirred at 0 °C and then at room temperature for 1 h. Upon consumption of the starting material, concentration of the solution up to 0.2 mL took place, followed by the successive dropwise addition of diethyl ether and hexane, respectively. The white precipitate was twice washed with hexane and the desired salt was afforded as white amorphous solid (623 mg, 80% yield). MS (ESI, 30 eV): m/z 242.41 [M+H+], 264.39 [M+Na+].
Methyl 4-((4-((2-aminophenyl)carbamoyl)benzyl)carbamoyl)benzoate (B4): Bis (2-(4-(ammoniomethyl)benzamido)benzenaminium) 2,2,2-trifluoroacetate salt (105 mg, 0.22 mmol) and 4-(methoxycarbonyl)benzoic acid (41 mg, 0.23 mmol) were dissolved in anhydrous DMF (0.23 mL) and the solution was cooled at 0 °C. Then the addition of HBTU (102 mg, 0.27 mmol) took place, followed by dropwise addition of Et3N (94 μL, 0.68 mmol). The reaction mixture was initially stirred at 0 °C and then at room temperature for 10 h. Then, the solution was diluted in DCM and twice successively washed with water, 5% NaHCO3(aq) solution, and brine. The organic layer was dried over anhydrous Na2SO4, filtered, and concentrated to dryness to afford an oily residue. The desired amide was afforded as a pale-white solid (40 mg, 44% yield) after FCC purification of the residue using toluene/ethyl acetate 9:1 as eluent. Rf (PhMe/EtOAc) 9:1 = 0.2; MS (ESI, 30 eV): m/z 426.26 [M+Na+], 442.33 [M+K+]; 1H NMR (CDCl3, 600.13 MHz) δ 8.07–8.11 (m, 2 H), 7.85–7.89 (m, 2 H), 7.73–7.75 (m, 1 H), 7.44–7.46 (m, 1 H), 7.32–7.36 (m, 4 H), 7.01–7.04 (m, 2 H), 6.80–6.82 (m, 1 H), 5.07 (d, J = 1.8 Hz, 1 H), 4.66 (d, J = 5.4 Hz, 1 H), 3.94 (s, 3 H); 13C NMR (CDCl3, 150.9 MHz) δ 129.9, 128.5, 127.1, 126.7, 38.6.
4-((4-((2-aminophenyl)carbamoyl)benzyl)carbamoyl)benzoic acid (B6): 2 M LiOH in water (0.2 mL, 0.4 mmol) was dripped into a stirring suspension of methyl 4-((4-((2-aminophenyl)carbamoyl)benzyl)carbamoyl)benzoate (40 mg, 0.1 mmol) in THF (0.7 mL) and the dark yellow solution was stirred at room temperature for 3 h. Upon consumption of the starting material, the mixture was concentrated under vacuum and the residue was acidified with 5% citric acid(aq) up to pH 4. Then the mixture was placed in a separatory funnel and extracted four times with DCM. The combined organic layers were washed with brine, dried over anhydrous Na2SO4 and were concentrated to dryness under vacuum. The desired acid was afforded as pale-yellow solid (20 mg, 53% yield) without further purification.
Methyl 5-((4-((2-aminophenyl)carbamoyl)benzyl)carbamoyl)pyrazine-2-carboxylate (B5): Bis (2-(4-(ammoniomethyl)benzamido)benzenaminium) 2,2,2-trifluoroacetate salt (270 mg, 0.57 mmol) and 5-(methoxycarbonyl)pyrazine-2-carboxylic acid (100 mg, 0.55 mmol) were dissolved in anhydrous DCM (0.72 mL) and the solution was cooled at 0 °C. Then, the addition of HBTU (230 mg, 0.61 mmol) took place, followed by dropwise addition of Et3N (307 μL, 2.2 mmol). The reaction mixture was initially stirred at 0 °C and then at room temperature for 24 h. The desired amide was afforded as a yellow solid (200 mg, 89% yield) after recrystallization of the dark brown mixture, from toluene. Rf (PhMe/EtOAc) 9:1 = 0.38; MS (ESI, 30 eV): m/z 428.28 [M+Na+]; 1H NMR (CDCl3, 600.13 MHz) δ 9.53–9.54 (m, 1 H), 9.23–9.24 (m, 1 H), 8.25–8.26 (m, 1 H), 7.96 (d, J = 7.8 Hz, 1 H), 7.89 (d, J = 7.8 Hz, 1 H), 7.77 (d, J = 7.8 Hz, 1 H), 7.56 (d, J = 7.8 Hz, 1 H), 7.47 (d, J = 7.8 Hz, 1 H), 7.44 (d, J = 7.8 Hz, 1 H), 7.33–7.34 (m, 1 H), 7.08 (t, J = 7.2 Hz, 1 H), 6.87 (t, J = 7.8 Hz, 1 H), 4.75–4.76 (m, 1 H), 4.07 (s, 3 H); 13C NMR (CDCl3, 150.9 MHz) δ 163.8, 162.2, 146.1, 145.8, 145.6, 145.3, 144.4, 144.1, 144.0, 143.9, 141.8, 133.5, 128.0, 127.9, 127.3, 126.4, 124.7, 53.4, 43.2.
5-((4-((2-aminophenyl)carbamoyl)benzyl)carbamoyl)pyrazine-2-carboxylic acid (B7): 3 M NaOH in MeOH (0.74 mL, 2.22 mmol) was dripped into a stirring suspension of methyl 5-((4-((2-aminophenyl)carbamoyl)benzyl)carbamoyl)pyrazine-2-carboxylate (300 mg, 0.74 mmol) in DCM (3.7 mL) and the dark red solution was stirred at room temperature for 3 h. Upon consumption of the starting material, the mixture was concentrated under vacuum and the residue was acidified with 5% citric acid(aq) solution up to pH 3.5. Then the mixture was concentrated to dryness and the desired acid was afforded as pale-yellow solid (250 mg, 86% yield) without further purification. Rf (DCE/MeOH/CH3COOH) 8:2:0.5 = 0.3.
4-((4-((2-aminophenyl)carbamoyl)benzyl)amino)-4-oxobutanoic acid (C1): DIPEA (50 μL, 0.26 mmol) was dripped into a stirring solution of bis (2-(4-(ammoniomethyl)benzamido)benzenaminium) 2,2,2-trifluoroacetate salt (45 mg, 0.10 mmol) in a mixture of THF (400 μL) and DMF (100 μL) and the resulting solution was cooled at 0 °C. Then, succinic anhydride (120 mg, 1.2 mmol) was added, and the reaction mixture was stirred for 3 h at room temperature. Subsequently, the mixture was subjected to FCC purification using dichloromethane/methanol/acetic acid 85:10:5 as eluent, and the desired acid was afforded as pale-yellow oil (28 mg, 82% yield). Rf (DCE/MeOH/CH3COOH) 85:15:0.1 = 0.08; MS (ESI, 30 eV): m/z 364.65 [M+Na+]; 1H NMR (CDCl3, 600.13 MHz) δ 9.50 (s, 1 H), 8.07 (d, J = 8.4 Hz, 1 H), 7.87 (d, J = 7.8 Hz, 2 H), 7.55 (brs, 1 H), 7.34 (d, J = 7.8 Hz, 2 H), 7.16 (dd, J′ = 7.8, J″ = 1.8 Hz, 1 H), 7.11–7.12 (m, 1 H), 4.46 (d, J = 6 Hz, 2 H), 2.70 (dd, J′ = 7.8, J″ = 4.8 Hz, 2 H), 2.61–2.65 (m, 4 H), 2.52–2.54 (m, 2 H); 13C NMR (CDCl3, 150.9 MHz) δ 175.0, 133.0, 127.7, 127.3, 125.0, 52.0, 40.9, 31.8.
4-(2-methoxy-2-oxoethyl) benzoic acid (D1): 4-(carboxymethyl) benzoic acid (200 mg, 1.11 mmol) was added in MeOH (2.22 mL) and the suspension was cooled at 0 °C. Then, trimethylchlorosilane (10 μL, 0.05 mmol) was dripped into the mixture and the resulting solution was initially stirred at 0 °C and then at room temperature for 24 h. Upon completion of the reaction, the mixture was concentrated under vacuum to dryness, and diethyl ether was added to the residue. The suspension was thrice successively washed with 5% NaHCO3(aq) and water, and the combined aqueous layers were acidified with 1 M HCl(aq) up to the total precipitation of the white solid. The aqueous mixture was extracted five times with diethyl ether and the organic layers were washed with water and brine, dried over anhydrous Na2SO4 and concentrated to dryness under vacuum to afford the desired ester (130 mg, 60% yield) as colorless oil. Rf (DCM/MeOH) 9:1 = 0.45; MS (ESI, 30 eV): m/z 193.38 [M-H+]; 1H NMR (CDCl3, 600.13 MHz) δ 8.08 (d, J = 8.4 Hz, 2 H), 7.40 (d, J = 8.4 Hz, 2 H), 3.72 (s, 3 H), 3.71 (s, 2 H); 13C NMR (CDCl3, 150.9 MHz) δ 171.6, 171.2, 140.1, 130.5, 129.5, 128.2, 52.3, 41.2.
Methyl 2-(4-((2-aminophenyl)carbamoyl)phenyl)acetate (D2): To a stirring solution of 4-(2-methoxy-2-oxoethyl) benzoic acid (60 mg, 0.31 mmol) in anhydrous DMF (0.44 mL), Et3N (167 μL, 1.2 mmol) and HBTU (152 mg, 0.4 mmol) were added and the mixture was cooled at 0 °C. Then, 1,2-phenylenediamine (44 mg, 0.4 mmol) was added and the mixture was stirred at room temperature for 24 h. Upon completion, the mixture was diluted in DCM and twice successively washed with water, ice-cooled 5% NaHCO3(aq), water and brine. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated to dryness under vacuum to afford an oily residue. The desired amide was afforded as a pale-yellow solid (32 mg, 36% yield) after FCC purification using toluene/ethyl acetate 6:4 as eluent. Rf (PhMe/EtOAc) 6:4 = 0.26; MS (ESI, 30 eV): m/z 258.49 [M+H+], 307.22 [M+Na+]; 1H NMR (CDCl3, 600.13 MHz) δ 8.10 (s, 1 H), 7.83 (d, J = 7.8 Hz, 2 H), 7.35 (d, J = 7.8 Hz, 2 H), 7.28 (d, J = 8.4 Hz, 1 H), 7.06 (td, J = 7.8, J′ = 1.8 Hz, 1 H), 6.81–6.83 (m, 2 H), 3.71 (s, 3 H), 3.69 (s, 2 H); 13C NMR (CDCl3, 150.9 MHz) δ 171.4, 165.6, 140.6, 138.0, 133.0, 129.7, 127.6, 127.2, 125.3, 124.6, 124.6, 119.9, 119.8, 118.4, 52.2, 40.9.
2-(4-((2-aminophenyl)carbamoyl)phenyl)acetic acid (D4): 3 M NaOH in MeOH (0.35 mL, 1.05 mmol) was dripped into a stirring solution of methyl 2-(4-((2-aminophenyl)carbamoyl)phenyl)acetate (100 mg, 0.35 mmol) in DCM (1.75 mL) and the mixture was stirred at room temperature for 4 h. Upon consumption of the starting material, the mixture was concentrated under vacuum and the residue was acidified with 5% citric acid(aq) up to pH 4. The precipitated solid was diluted in methanol and the resulting mixture was then filtered and concentrated to dryness to afford the product as a pale-yellow oil, without further purification. (62 mg, 66% yield). Rf (DCE/MeOH/CH3COOH) 8:2:1 = 0.62.
Methyl 2-(4-((2-amino-4-fluorophenyl)carbamoyl)phenyl)acetate (D3): To a stirring solution of 4-(2-methoxy-2-oxoethyl) benzoic acid (42 mg, 0.22 mmol) in anhydrous DMF (0.31 mL), Et3N (92 μL, 0.66 mmol) and HBTU (114 mg, 0.3 mmol) were added and the mixture was cooled at 0 °C. Then, 1,2-diamino 4-fluorobenzene (38 mg, 0.3 mmol) was added and the mixture was stirred at room temperature for 24 h. Upon completion, the mixture was diluted in DCM and twice successively washed with water, ice-cooled 5% NaHCO3(aq), water and brine. The organic layer was dried over anhydrous Na2SO4, filtered, and concentrated to dryness under vacuum to afford an oily residue. The desired amide was afforded as a pale-yellow solid (19.2 mg, 30% yield) after FCC purification using toluene/ethyl acetate 6:4 as eluent. Rf (PhMe/EtOAc) 6:4 = 0.49; 1H NMR (CDCl3, 600.13 MHz) δ 7.88 (d, J = 7.8 Hz, 2 H), 7.40 (s, 2 H), 7.20 (s, 1 H), 6.57 (s, 2 H), 3.73 (s, 3 H), 3.71 (s, 2 H); 13C NMR (CDCl3, 150.9 MHz) δ 175.1, 148.0, 146.8, 137.4, 129.8, 129.7, 52.2, 40.9, 25.6.
2-(4-((2-amino-4-fluorophenyl)carbamoyl)phenyl) acetic acid (D5): 3 M NaOH in MeOH (0.29 mL, 0.85 mmol) was dripped into a stirring solution of methyl 2-(4-((2-amino-4-fluorophenyl)carbamoyl)phenyl)acetate (86 mg, 0.29 mmol) in DCM (1.49 mL) and the mixture was stirred at room temperature for 4 h. Upon consumption of the starting material, the mixture was concentrated under vacuum and the residue was acidified with 5% citric acid(aq) up to pH 3-4. The precipitated solid was diluted in methanol and the resulting mixture was then filtered and concentrated to dryness to afford the product as a pale-yellow solid, without further purification. (62 mg, 75% yield). Rf (DCE/MeOH/CH3COOH) 8:2:1 = 0.53.
(S)-3-(4-(bis(2-chloroethyl)amino)phenyl)-2-((tert-butoxycarbonyl)amino) propanoic acid (E2): To a suspension of melphalan (30 mg, 0.1 mmol) in anhydrous MeOH (0.33 mL), Et3N (14 μL, 0.1 mmol) and boc anhydride (23 mg, 0.1 mmol) were added and the mixture was stirred at room temperature for 4 h. The resulting solution was concentrated to dryness and the oily residue was diluted in DCM and washed successively with ice-cooled citric acid(aq) 5%, water and brine, two times. The organic layer was then dried over anhydrous Na2SO4, filtered and concentrated under vacuum. The desired product was afforded in pure form as a colorless oil (32 mg, 82% yield) after FCC purification of the residue using ethyl acetate as eluent. Rf (EtOAc/CH3COOH) 10:0.02 = 0.45; 1H NMR (CDCl3, 600.13 MHz) δ 7.07 (d, J = 7.8 Hz, 2 H), 6.62 (d, J = 9 Hz, 2 H), 4.94 (d, J = 7.8 Hz, 1 H), 4.54 (d, J = 9 Hz, 1 H), 3.71 (t, J = 7.2 Hz, 4 H), 3.61 (t, J = 7.2 Hz, 4 H), 3.09 (dd, J = 5.4 Hz, J′ = 14.4 Hz, 1 H), 3.00 (dd, J = 6 Hz, J′ = 15 Hz, 1 H), 1.43 (s, 9 H); 13C NMR (CDCl3, 150.9 MHz) δ 176.3, 155.5, 145.2, 130.7, 124.6, 112.1, 80.3, 60.4, 54.4, 53.5, 40.4, 36.7, 30.9, 28.3.
Tert-butyl((S)-3-(4-(bis(2-chloroethyl)amino)phenyl)-1-(((S)-3-methyl-1-((3aS,4S,6S,7aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxopropan-2-yl)carbamate (E4): To a stirring solution of N′-Boc-melphalan (180 mg, 0.44 mmol) in anhydrous DMF (0.88 mL), DIPEA (300 μL, 1.76 mmol) and HATU (186 mg, 0.49 mmol) were added and the mixture was cooled at 0 °C for 15 min. Then, (R)-boroleucine (1S,2S,3R,5S)-(+)-2,3-pinanediol ester trifluoroacetate salt (167 mg, 0.44 mmol) was added and the reaction mixture was left under stirring at 0 °C for 30 min and subsequently at room temperature for 20 h. Upon completion, the solution was diluted in DCM and washed with ice-cooled water and brine, twice. The organic layer was then dried over anhydrous Na2SO4, filtered and concentrated under vacuum. The pure product was afforded as a colorless oil (200 mg, 70% yield) after FCC purification of the oily residue, using toluene/ethyl acetate 99:1 as eluent. Rf (PhMe/EtOAc) 7:3 = 0.5; 1H NMR (CDCl3, 600.13 MHz) δ 7.10 (d, J = 7.8 Hz, 2 H), 6.60 (d, J = 8.4 Hz, 2 H), 4.30 (dd, J = 1.8 Hz, J′ = 8.4 Hz, 2 H), 3.70 (t, J = 7.2 Hz, 4 H), 3.61 (t, J = 7.2 Hz, 4 H), 3.07 (td, J = 4.2 Hz, J′ = 4.8 Hz, 1 H), 3.00 (dd, J = 6 Hz, J′ = 13.8 Hz, 1 H), 2.93 (dd, J = 7.2 Hz, J′ = 14.4 Hz, 1 H), 2.34–2.36 (m, 1 H), 2.32–2.33 (m, 1 H), 2.23–2.25 (m, 2 H), 2.00–2.05 (m, 3 H), 1.90–1.93 (m, 2 H), 1.88–1.90 (m, 1 H), 1.85–1.86 (m, 2 H), 1.41 (s, 9 H), 1.28 (s, 6 H), 0.85–0.87 (m, 6 H), 0.83 (s, 3 H); 13C NMR (CDCl3, 150.9 MHz) δ 167.7, 144.4, 140.4, 130.8, 129.1, 129.0, 118.3, 116.0, 112.2, 85.4, 84.7, 69.3, 53.5, 51.5, 40.4, 39.7, 39.4, 38.4, 38.2, 37.1, 35.7, 28.7, 28.6, 28.3, 27.2, 27.1, 26.3, 25.4, 24.1, 24.0, 23.1, 21.9, 13.7.
(S)-3-(4-(bis(2-chloroethyl)amino)phenyl)-1-(((S)-3-methyl-1-((3aS,4S,6S,7aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxopropan-2-aminium chloride salt (E6): Trifluoroethanol (9 μL, 0.12 mmol) and 4 M HCl in dioxane (35 μL, 0.14 mmol) were dripped into an ice-cooled solution of tert-butyl ((S)-3-(4-(bis(2-chloroethyl)amino)phenyl)-1-(((S)-3-methyl-1-((3aS,4S,6S,7aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxopropan-2-yl)carbamate (77 mg, 0.12 mmol) in anhydrous DCM (0.3 mL) and the resulting mixture was initially stirred at 0 °C and then at room temperature for 2 h. Addition of 4 M HCl in dioxane took place, if needed. Upon consumption of the starting material, concentration of the solution took place, followed by successive dropwise addition of diethyl ether and hexane, respectively. The white precipitate was washed thrice with hexane, and the desired salt was afforded as white amorphous solid (67 mg, 96% yield).
(2S)-N-((1R)-3-methyl-1-((3aS,4S,6S)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)-3-phenyl-2-(tritylamino)propenamide (E3): To a stirring solution of N′-trityl-L-phenylalanine (389 mg, 0.93 mmol) in anhydrous DMF (1.85 mL), DIPEA (500 μL, 2.77 mmol) and HATU (389 mg, 1.02 mmol) were added and the mixture was cooled at 0 °C for 15 min. Then, (R)-boroleucine (1S,2S,3R,5S)-(+)-2,3-pinanediol ester trifluoroacetate salt (350 mg, 0.92 mmol) was added and the reaction mixture was left under stirring at 0 °C for 30 min and subsequently at room temperature for 20 h. Upon completion, the solution was diluted in DCM and washed with ice-cooled water and brine, twice. The organic layer was then dried over anhydrous Na2SO4, filtered and concentrated under vacuum. The pure product was afforded as a white foam (410 mg, 68% yield) after FCC purification of the oily residue, using toluene/ethyl acetate 99:1 as eluent. Rf (PhMe/EtOAc) 95:5 = 0.24; 1H NMR (CDCl3, 600.13 MHz) δ 7.43 (d, J = 7.8 Hz, 7 H), 7.25–7.29 (m, 4 H), 7.20–7.22 (m, 7 H), 6.92 (d, J = 6.6 Hz, 2 H), 4.29 (d, J = 8.4 Hz, 1 H), 3.57 (t, J = 6 Hz, 1 H), 3.00–3.02 (m, 1 H), 2.96 (dd, J = 3.6 Hz, J′ = 13.2 Hz, 1 H), 2.33–2.35 (m, 1 H), 2.24–2.26 (m, 1 H), 2.16–2.18 (m, 1 H), 2.04 (dd, J = 6 Hz, J′ = 12 Hz, 2 H), 1.86–1.90 (m, 2 H), 1.61–1.63 (m, 3 H), 1.51–1.54 (hept, J = 6.6 Hz, 1 H), 1.42 (s, 3 H), 1.27 (s, 3 H), 0.95–0.96 (m, 1 H), 0.90 (d, J = 6.6 Hz, 3 H), 0.88 (d, J = 6.6 Hz, 3 H), 0.85 (s, 3 H); 13C NMR (CDCl3, 150.9 MHz) δ 175.7, 145.9, 136.0, 130.2, 128.9, 128.7, 128.6, 128.5, 128.2, 128.0, 126.8, 126.7, 84.9, 77.4, 72.0, 64.4, 57.0, 51.7, 40.5, 39.8, 38.1, 37.8, 36.0, 30.6, 28.9, 27.2, 26.5, 25.8, 24.1, 23.0, 22.3.
(2S)-1-(((1R)-3-methyl-1-((3aS,4S,6S)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-aminium chloride salt (E5): Trifluoroethanol (104 μL, 1.45 mmol) and 4 M HCl in dioxane (815 μL, 3.25 mmol) were dripped into an ice-cooled solution of (2S)-N-((1R)-3-methyl-1-((3aS,4S,6S)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)-3-phenyl-2-(tritylamino)propenamide (950 mg, 1.45 mmol) in anhydrous DCM (14.5 mL) and the resulting mixture was initially stirred at 0 °C and then at room temperature for 2 h. Upon consumption of the starting material, concentration of the solution up to 1 mL took place, followed by successive dropwise addition of diethyl ether and hexane, respectively. The white precipitate was washed with hexane thrice, and the desired salt was afforded as white amorphous solid (636 mg, 98% yield).
N1-(2-aminophenyl)-N4-((2R)-1-(((1R)-3-methyl-1-((3aS)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-yl)terephthalamide (2): To a stirring solution of 4-((2-aminophenyl) carbamoyl) benzoic acid (50 mg, 0.2 mmol) in a mixture of anhydrous DCM (133 μL) and DMF (260 μL), N′-methyl morpholine (90 μL, 0.8 mmol) and TBTU (83 mg, 0.26 mmol) were added and the mixture was cooled at 0 °C. Then, (2S)-1-(((1R)-3-methyl-1-((3aS,4S,6S)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-aminium chloride salt (89 mg, 0.2 mmol) was added and the reaction mixture was left under stirring at 0 °C for 30 min and subsequently at room temperature for 20 h. Upon completion, the mixture was subjected to FCC purification using toluene/ethyl acetate 8:2 as eluent, and the hybrid was afforded as pale-yellow solid (70 mg, 53% yield). Rf (PhMe/EtOAc) 8:2 = 0.15; IR (KBr): 2962, 1519, 1120, 1020 cm−1; HR-MALDI (m/z) [M+Νa+] calculated for [C38H47BN4NaO5]+ 673.3532. Found: 673.1960; 1H NMR (CDCl3, 600.13 MHz) δ 8.40 (brs, 1 H), 7.90 (s, 2 H), 7.72 (s, 2 H), 7.37 (s, 1 H), 7.11 (s, 2 H), 6.90 (s, 2 H), 6.17 (brs, 1 H), 4.80 (s, 1 H), 4.31 (d, J = 8.4 Hz, 1 H), 3.35–3.36 (m, 1 H), 3.13–3.15 (m, 3 H), 2.35–2.37 (m, 2 H), 2.19–2.20 (m, 1 H), 2.00–2.04 (hept, J = 5.4 Hz, 1 H), 1.87–1.89 (m, 3 H), 1.37–1.39 (m, 5 H), 1.28–1.30 (m, 8 H), 0.81–0.84 (m, 10 H); 13C NMR (CDCl3, 150.9 MHz) δ 13C NMR (151 MHz, CDCl3) δ 129.5, 128.6, 127.9, 127.7, 127.4, 127.0, 69.3, 54.5, 54.0, 51.4, 40.5, 40.0, 39.6, 39.0, 38.6, 38.2, 35.6, 29.7, 29.6, 28.7, 28.0, 27.8, 27.1, 26.4, 25.3, 24.1, 24.0, 23.0, 21.9.
N2-(2-aminophenyl)-
N5-((2
R)-1-(((1
R)-3-methyl-1-((3a
S)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[
d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-yl)pyrazine-2,5-dicarboxamide (
3): To a stirring solution of 5-((2-aminophenyl)carbamoyl)pyrazine-2-carboxylic acid (20 mg, 0.08 mmol) in a mixture of anhydrous DCM (95 μL) and DMF (103 μL),
N′-methyl morpholine (50 μL, 0.23 mmol) and TBTU (28 mg, 0.09 mmol) were added and the mixture was cooled at 0 °C. Then, (2
S)-1-(((1
R)-3-methyl-1-((3a
S,4
S,6
S)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[
d][
1,
3,
2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-aminium chloride salt (35 mg, 0.08 mmol) was added and the reaction mixture was left under stirring at 0 °C for 30 min and subsequently at room temperature for 20 h. Upon completion, the mixture was subjected to FCC purification using toluene/ethyl acetate 7:3 as eluent, and the hybrid was afforded as a bright yellow oil (25 mg, 50% yield). R
f (PhMe/EtOAc) 7:3 = 0.11; IR (KBr): 2962, 1652, 1519, 1020 cm
−1; HR-MALDI (
m/
z) [M+Νa
+] calculated for [C
36H
45BN
6NaO
5]
+ 675.3437. Found: 675.0880;
1H NMR (CDCl
3, 600.13 MHz) δ 9.59 (s, 1 H), 9.40 (d,
J = 1.2 Hz, 1 H), 9.31 (d,
J = 1.2 Hz, 1 H), 8.46 (d,
J = 8.4 Hz, 1 H), 7.52 (dd,
J = 1.2 Hz,
J′ = 7.8 Hz, 1 H), 7.28–7.30 (m, 3 H), 7.23 (tt,
J = 2.4 Hz,
J′ = 5.4 Hz, 1 H), 7.12 (td,
J = 1.8 Hz,
J′ = 7.8 Hz, 1 H), 6.87–6.89 (m, 2 H), 5.90 (d,
J = 5.4 Hz, 1 H), 4.80 (td,
J = 6.6 Hz,
J′ = 8.4 Hz, 1 H), 4.32 (dd,
J = 2.4 Hz,
J′ = 9 Hz, 1 H), 3.86–3.88 (m, 2 H), 3.17–3.19 (m, 3 H), 2.34 (ddt,
J = 3 Hz,
J′ = 8.4 Hz,
J″ = 14.4 Hz, 1 H), 2.23–2.25 (m, 3 H), 2.00–2.02 (m, 1 H), 1.91–1.93 (m, 1 H), 1.84 (ddd,
J = 1.2 Hz,
J′ = 2.4 Hz,
J″ = 14.4 Hz, 1 H), 1.38–1.40 (m, 5 H), 1.27–1.29 (m, 4 H), 0.85 (d,
J = 4.8 Hz, 6 H), 0.83 (d,
J = 3.6 Hz, 3 H);
13C NMR (150.9 MHz, CDCl
3) δ 170.2, 162.0, 160.2, 146.1, 142.6, 142.1, 140.1, 136.3, 129.4, 128.7, 127.4, 127.1, 124.5, 123.7, 119.9, 118.4, 86.0, 79.9, 54.4, 54.0, 51.4, 39.9, 39.6, 38.6, 38.2, 35.5, 29.7, 28.5, 28.0, 27.8, 27.1, 26.3, 25.3, 24.0, 23.0, 22.0.
N1-(4-((2-aminophenyl)carbamoyl)benzyl)-N4-((2R)-1-(((1R)-3-methyl-1-((3aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-yl)terephthalamide (4): To a stirring solution of 4-((4-((2-aminophenyl)carbamoyl)benzyl)carbamoyl)benzoic acid (42 mg, 0.11 mmol) in a mixture of anhydrous DCM (73 μL) and DMF (147 μL), N′-methyl morpholine (50 μL, 0.44 mmol) and TBTU (50 mg, 0.15 mmol) were added and the mixture was cooled at 0 °C. Then, (2S)-1-(((1R)-3-methyl-1-((3aS,4S,6S)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-aminium chloride salt (50 mg, 0.11 mmol) was added and the reaction mixture was left under stirring at 0 °C for 30 min and subsequently at room temperature for 20 h. Upon completion, the mixture was subjected to FCC purification using toluene/ethyl acetate 8:2 as eluent, and the hybrid was afforded as a colorless oil (39 mg, 45% yield). Rf (PhMe/EtOAc) 8:2 = 0.12; IR (KBr): 2962, 1544, 1120, 1020 cm−1; HR-MALDI (m/z) [M+Νa+] calculated for [C46H54BN5NaO6]+ 806.4059. Found: 806.1370; 1H NMR (CDCl3, 600.13 MHz) δ 9.58 (brs, 1 H), 9.10 (brs, 1 H), 8.01 (brs, 1 H), 7.95 (d, J = 7.8 Hz, 1 H), 7.81 (brs, 1 H), 7.56 (d, J = 7.2 Hz, 4 H), 7.51 (brs, 1 H), 7.41 (brs, 1 H), 7.28–7.32 (m, 5 H), 7.19–7.21 (m, 3 H), 7.09 (brs, 1 H), 7.02 (brs, 1 H), 5.87 (s, 1 H), 4.79 (d, J = 7.2 Hz, 1 H), 4.30–4.31 (m, 1 H), 3.42 (s, 1 H), 3.19–3.20 (m, 1 H), 3.11–3.12 (m, 1 H), 2.19–2.20 (m, 1 H), 2.02–2.03 (m, 1 H), 1.91–1.92 (m, 1 H), 1.85 (d, J = 14.4 Hz, 1 H), 1.60 (s, 4 H), 1.39 (m, 4 H), 1.28–1.29 (m, 6 H), 0.83–0.85 (m, 7 H); 13C NMR (150.9 MHz, CDCl3) δ 145.8, 136.5, 129.5, 129.0, 128.7, 128.6, 128.2, 128.1, 128.0, 127.4, 126.5, 86.0, 77.9, 54.0, 51.3, 40.0, 39.5, 38.7, 38.2, 35.5, 28.6, 27.1, 26.3, 25.3, 24.0, 23.0, 22.0.
N2-(4-((2-aminophenyl)carbamoyl)benzyl)-N5-((2R)-1-(((1R)-3-methyl-1-((3aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-yl)pyrazine-2,5-dicarboxamide (5): To a stirring solution of 5-((4-((2-aminophenyl)carbamoyl)benzyl)carbamoyl)pyrazine-2-carboxylic acid (30 mg, 0.08 mmol) in a mixture of anhydrous DCM (51 μL) and DMF (100 μL), N′-methyl morpholine (30 μL, 0.23 mmol) and TBTU (30 mg, 0.09 mmol) were added and the mixture was cooled at 0 °C. Then, (2S)-1-(((1R)-3-methyl-1-((3aS,4S,6S)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-aminium chloride salt (35 mg, 0.08 mmol) was added and the reaction mixture was left under stirring at 0 °C for 30 min and subsequently at room temperature for 20 h. Upon completion, the mixture was subjected to FCC purification using toluene/ethyl acetate 8:2 as eluent, and the hybrid was afforded as a yellow oil (24 mg, 40% yield). Rf (PhMe/EtOAc) 8:2 = 0.29; IR (KBr): 2962, 1729, 1616, 1120, 1020 cm−1; HR-MALDI (m/z) [M+Νa+] calculated for [C44H52BN7NaO6]+ 808.3964. Found: 808.2430; 1H NMR (CDCl3, 600.13 MHz) δ 10.05 (s, 1 H), 9.39 (s, 1 H), 9.26 (s, 1 H), 9.12 (s, 1 H), 8.43 (d, J = 8.4 Hz, 1 H), 7.93 (d, J = 7.8 Hz, 1 H), 7.76 (d, J = 7.8 Hz, 1 H), 7.60 (d, J = 7.8 Hz, 1 H), 7.39 (d, J = 7.8 Hz, 2 H), 7.28 (d, J = 4.8 Hz, 2 H), 5.90 (s, 1 H), 5.00 (s, 1 H), 4.78 (q, J = 7.2 Hz, 1 H), 4.32–4.34 (m, 2 H), 3.98 (dd, J = 4.8 Hz, J′ = 9 Hz, 1 H), 3.15–3.17 (m, 2 H), 2.55 (s, 1 H), 2.44–2.45 (m, 1 H), 2.33–2.34 (m, 1 H), 2.19–2.20 (m, 2 H), 2.01 (q, J = 5.4 Hz, 2 H), 1.92 (dt, J = 2.4 Hz, J′ = 6.6 Hz, 2 H), 1.82–1.83 (m, 1 H), 1.63 (ddd, J = 2.4 Hz, J′ = 5.4 Hz, J″ = 13.8 Hz, 1 H), 1.46 (s, 4 H), 1.37–1.39 (m, 3 H), 1.26–1.28 (m, 6 H), 0.82–0.85 (m, 9 H); 13C NMR (150.9 MHz, CDCl3) δ 165.7, 142.2, 129.4, 128.7, 127.8, 127.0, 126.5, 77.9, 54.0, 51.3, 40.5, 39.9, 39.5, 38.2, 35.5, 29.6, 28.5, 28.4, 28.0, 27.8, 27.1, 26.3, 25.3, 24.1, 24.0, 22.9, 22.0.
N1-(4-((2-aminophenyl)carbamoyl)benzyl)-N4-((2R)-1-(((1R)-3-methyl-1-((3aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-yl)succinimide (1): To a stirring solution of 4-((4-((2-aminophenyl) carbamoyl)benzyl)amino)-4-oxobutanoic acid (20 mg, 0.06 mmol) in a mixture of anhydrous DCM (40 μL) and DMF (80 μL), DIPEA (42 μL, 0.24 mmol) and pyBOP (52 mg, 0.1 mmol) were added and the mixture was cooled at 0 °C. Then, (2S)-1-(((1R)-3-methyl-1-((3aS,4S,6S)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-aminium chloride salt (27 mg, 0.06 mmol) was added and the reaction mixture was left under stirring at 0 °C for 30 min and subsequently at room temperature for 20 h. Upon completion, the mixture was subjected to FCC purification using ethyl acetate/methanol 9:1 as eluent, and the hybrid was afforded as a white solid (crystallization in ethyl acetate) (26 mg, 60% yield). Rf (EtOAc/MeOH) 9:1 = 0.09; IR (KBr): 1519, 1120, 1020 cm−1; HR-ESI (m/z) [M+H+] calculated for [C42H55BN5O6]+ 736.4240 Found: 736.4239; 1H NMR (CDCl3, 600.13 MHz) δ 8.41 (s, 1 H), 7.75 (d, J = 7.2 Hz, 2 H), 7.45–7.46 (m, 1 H), 7.30–7.33 (m, 3 H), 7.18–7.22 (m, 3 H), 6.80 (s, 1 H), 6.63 (s, 1 H), 6.51 (s, 1 H), 4.66 (q, J = 7.2 Hz, 1 H), 4.48 (dd, J = 6 Hz, J′ = 15.6 Hz, 1 H), 4.39 (dd, J = 5.4 Hz, J′ = 15 Hz, 1 H), 4.29 (dd, J = 1.8 Hz, J′ = 8.4 Hz, 1 H), 3.06 (qd, J = 6 Hz, J′ = 13.8 Hz, 2 H), 2.90–2.91 (m, 1 H), 2.85 (s, 3 H), 2.59–2.60 (m, 1 H), 2.44–2.48 (m, 3 H), 2.32–2.33 (m, 1 H), 2.17 (ddt, J = 1.8 Hz, J′ = 6 Hz, J″ = 7.8 Hz, 1 H), 2.02–2.04 (m, 2 H), 1.90–1.91 (m, 1 H), 1.82 (dt, J = 3 Hz, J′ = 14.4 Hz, 1 H), 1.75 (s, 3 H), 1.48 (dq, J = 6.6 Hz, J′ = 13.2 Hz, 1 H), 1.40 (s, 3 H), 1.34 (t, J = 7.2 Hz, 2 H), 1.28 (s, 3 H), 0.85 (s, 3 H), 0.80 (d, J = 6.6 Hz, 3 H), 0.76 (d, J = 6.6 Hz, 3 H); 13C NMR (150.9 MHz, CDCl3) δ 176.4, 172.3, 171.8, 129.6, 129.4, 128.6, 128.4, 127.7, 127.0, 85.3, 53.1, 51.6, 40.0, 39.7, 38.2, 35.8, 28.7, 27.2, 26.4, 25.2, 24.1, 23.0, 22.0.
N-(2-aminophenyl)-4-(2-(((2R)-1-(((1R)-3-methyl-1-((3aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-yl)amino)-2-oxoethyl)benzamide (6): To a stirring solution of 2-(4-((2-aminophenyl)carbamoyl)phenyl) acetic acid (31 mg, 0.12 mmol) in a mixture of anhydrous DCM (106 μL) and DMF (153 μL), N′-methyl morpholine (40 μL, 0.35 mmol) and TBTU (41 mg, 0.13 mmol) were added and the mixture was cooled at 0 °C. Then, (2S)-1-(((1R)-3-methyl-1-((3aS,4S,6S)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-aminium chloride salt (51 mg, 0.12 mmol) was added and the reaction mixture was left under stirring at 0 °C for 30 min and subsequently at room temperature for 20 h. Upon completion, the mixture was subjected to FCC purification using toluene/ethyl acetate 8:2 as eluent, and the hybrid was afforded as a colorless oil (47 mg, 62% yield). Rf (PhMe/EtOAc) 1:1 = 0.33; IR (KBr): 2925, 1662, 1533, 1027 cm−1; HR-ESI (m/z) [M+Na+] calculated for [C39H49BN4NaO5]+ 687.3688 Found: 687.3690; 1H NMR (CDCl3, 600.13 MHz) δ 8.25 (s, 1 H), 7.82 (d, J = 7.8 Hz, 2 H), 7.31 (d, J = 7.8 Hz, 1 H), 7.20–7.24 (m, 5 H), 7.07–7.13 (m, 3 H), 6.84 (dd, J = 8.4 Hz, J′ = 7.8 Hz, 2 H), 6.51 (d, J = 7.8 Hz, 1 H), 6.14 (d, J = 5.4 Hz, 1 H), 4.60 (q, J = 7.2 Hz, 1 H), 4.29 (dd, J = 2.4 Hz, J′ = 9 Hz, 1 H), 3.53 (s, 2 H), 3.10 (dt, J = 6 Hz, J′ = 9 Hz, 1 H), 2.96 (dd, J = 1.8 Hz, J′ = 6.6 Hz, 2 H), 2.32–2.34 (m, 1 H), 2.17-2.19 (m, 1 H), 2.02 (dt, J = 5.4 Hz, J′ = 10.8 Hz, 1 H), 1.89–1.91 (m, 3 H), 1.37–1.39 (m, 6 H), 1.27–1.29 (m, 6 H), 0.81–1.85 (m, 9 H); 13C NMR (150.9 MHz, CDCl3) δ 170.9, 170.0, 165.6, 140.8, 138.6, 136.3, 133.0, 129.6, 129.4, 128.5, 127.9, 127.3, 126.9, 124.6, 119.7, 118.3, 85.8, 77.8, 73.8, 69.2, 53.8, 51.4, 43.2, 40.5, 40.0, 39.5, 38.3, 38.2, 35.6, 29.6, 28.6, 28.0, 27.8, 27.1, 26.3, 25.3, 24.0, 23.0, 22.0.
N-(2-amino-4-fluorophenyl)-4-(2-(((2R)-1-(((1R)-3-methyl-1-((3aR)-3a,5,5-trimethyl-hexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-yl)amino)-2-oxoethyl)benzamide (7): To a stirring solution of 2-(4-((2-amino-4-fluorophenyl)carbamoyl)phenyl) acetic acid (37 mg, 0.13 mmol) in a mixture of anhydrous DCM (85 μL) and DMF (170 μL), N′-methyl morpholine (43 μL, 0.39 mmol) and TBTU (46 mg, 0.14 mmol) were added and the mixture was cooled at 0 °C. Then, (2S)-1-(((1R)-3-methyl-1-((3aS,4S,6S)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-aminium chloride salt (58 mg, 0.13 mmol) was added and the reaction mixture was left under stirring at 0 °C for 30 min and subsequently at room temperature for 20 h. Upon completion, the mixture was subjected to FCC purification using toluene/ethyl acetate 6:4 as eluent, and the hybrid was afforded as a colorless oil (56 mg, 63% yield). Rf (PhMe/EtOAc) 6:4 = 0.1; IR (KBr): 2925, 1662, 1533, 1027 cm−1; HR-ESI (m/z) [M+Na+] calculated for [C39H48BFN4NaO5]+ 705.3594 Found: 705.3599; 1H NMR (CDCl3, 600.13 MHz) δ 8.17 (s, 1 H), 7.81 (d, J = 7.8 Hz, 2 H), 7.20–7.24 (m, 5 H), 7.15 (dd, J = 6 Hz, J′ = 8.4 Hz, 1 H), 7.10–7.12 (m, 2 H), 6.56 (d, J = 7.8 Hz, 1 H), 6.50 (tt, J = 2.4 Hz, J′ = 9.6 Hz, 2 H), 6.11 (d, J = 5.4 Hz, 1 H), 4.59 (q, J = 7.2 Hz, 1 H), 4.28 (dd, J = 1.8 Hz, J′ = 8.4 Hz, 1 H), 3.52 (s, 2 H), 3.11 (dt, J = 1.2 Hz, J′ = 6 Hz, 1 H), 2.95–2.97 (m, 2 H), 2.33 (ddq, J = 2.4 Hz, J′ = 9 Hz, 1 H), 2.18–2.19 (m, 1 H), 2.02 (t, J = 5.4 Hz, 1 H), 1.90 (tt, J = 3 Hz, J′ = 6 Hz, 1 H), 1.82 (dt, J = 2.4 Hz, J′ = 14.4 Hz, 1 H), 1.43–1.45 (m, 1 H), 1.39 (s, 4 H), 1.34–1.35 (m, 2 H), 1.28 (s, 4 H), 1.19 (d, J = 10.8 Hz, 1 H), 0.83 (m, 9 H); 13C NMR (150.9 MHz, CDCl3) δ 170.9, 169.9, 165.9, 162.7, 161.1, 143.4, 138.8, 136.3, 132.7, 129.5, 129.4, 128.5, 127.9, 126.9, 119.8, 105.8, 105.7, 104.3, 104.1, 85.9, 77.8, 69.2, 53.9, 51.4, 43.1, 40.0, 39.5, 38.6, 38.4, 38.2, 35.5, 28.6, 27.1, 26.3, 25.3, 24.0, 22.9, 22.0.
N2-(2-aminophenyl)-N5-((1R)-3-methyl-1-((3aS)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)pyrazine-2,5-dicarboxamide (10): To a stirring solution of 5-((2-aminophenyl)carbamoyl)pyrazine-2-carboxylic acid (20 mg, 0.1 mmol) in a mixture of anhydrous DCM (52 μL) and DMF (104 μL), N′-methyl morpholine (30 μL, 24 mmol) and TBTU (28 mg, 0.1 mmol) were added and the mixture was cooled at 0 °C. Then, (R)-boroleucine (1S,2S,3R,5S)-(+)-2,3-pinanediol ester trifluoroacetate salt (30 mg, 0.1 mmol) was added and the reaction mixture was left under stirring at 0 °C for 30 min and subsequently at room temperature for 20 h. Upon completion, the mixture was subjected to FCC purification using toluene/ethyl acetate 9:1 as eluent, and the hybrid was afforded as a bright yellow oil (16 mg, 40% yield). Rf (PhMe/EtOAc) 9:1 = 0.27; IR (KBr): 2962, 1652, 1519, 1020, 802 cm−1; HR-ESI (m/z) [M+H+] calculated for [C27H40BN5O4]+ 506.2933 Found: 506.2938; 1H NMR (CDCl3, 600.13 MHz) δ 9.60 (s, 1 H), 9.41 (d, J = 1.8 Hz, 1 H), 9.36 (d, J = 1.8 Hz, 1 H), 7.94 (d, J = 6.6 Hz, 1 H), 7.52 (dd, J = 1.8 Hz, J = 7.8 Hz, 1 H), 7.11 (td, J = 1.2 Hz, J′ = 7.8 Hz, 1 H), 6.87–6.89 (m, 2 H), 4.37 (dd, J = 2.4 Hz, J′ = 9 Hz, 1 H), 3.63 (dt, J = 6 Hz, J′ = 9.6 Hz, 1 H), 2.35–2.37 (m, 1 H), 2.22–2.24 (m, 1 H), 2.07 (t, J = 5.4 Hz, 1 H), 1.91–1.93 (m, 3 H), 1.75 (ddt, J = 1.8 Hz, J′ = 6.6 Hz, 1 H), 1.66–1.68 (m, 1 H), 1.60–1.61 (m, 1 H), 1.45 (s, 3 H), 1.28–1.30 (m, 5 H), 0.96–1.00 (m, 6 H), 0.85 (s, 3 H); 13C NMR (150.9 MHz, CDCl3) δ 162.2, 146.6, 145.8, 142.4, 142.2, 127.3, 124.5, 123.8, 119.9, 118.3, 86.5, 78.2, 51.3, 40.2, 39.5, 38.2, 35.4, 28.5, 27.1, 26.4, 25.6, 24.0, 23.1, 22.0.
N2-(2-aminophenyl)-N5-((2R)-3-(4-(bis(2-chloroethyl)amino)phenyl)-1-(((1R)-3-methyl-1-((3aS)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxopropan-2-yl)pyrazine-2,5-dicarboxamide (11): To a stirring solution of 5-((2-aminophenyl)carbamoyl)pyrazine-2-carboxylic acid (10 mg, 0.04 mmol) in a mixture of anhydrous DCM (20 μL) and DMF (60 μL), N′-methyl morpholine (20 μL, 0.12 mmol) and TBTU (20 mg, 0.05 mmol) were added and the mixture was cooled at 0 °C. Then, (S)-3-(4-(bis(2-chloroethyl)amino)phenyl)-1-(((S)-3-methyl-1-((3aS,4S,6S,7aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxopropan-2-aminium chloride salt (25 mg, 0.04 mmol) was added and the reaction mixture was left under stirring at 0 °C for 30 min and subsequently at room temperature for 20 h. Upon completion, the mixture was subjected to FCC purification using toluene/ethyl acetate 6:4 as eluent, and the hybrid was afforded as a bright yellow oil (13 mg, 41% yield). Rf (PhMe/EtOAc) 1:1 = 0.18; IR (KBr): 2962, 1652, 1519, 1020, 802 cm−1; HR-ESI (m/z) [M+H+] calculated for [C40H53BCl2N7O5]+ 792.3573 Found: 792.3594; 1H NMR (CDCl3, 600.13 MHz) δ 9.60 (s, 1 H), 9.42 (d, J = 1.2 Hz, 1 H), 9.33 (d, J = 1.2 Hz, 1 H), 8.44 (d, J = 7.8 Hz, 1 H), 7.53 (dd, J = 1.2 Hz, J′ = 7.8 Hz, 1 H), 7.18 (d, J = 9 Hz, 2 H), 7.13 (td, J = 1.2 Hz, J′ = 7.2 Hz, 1 H), 6.89 (ddd, J = 1.2 Hz, J′ = 7.2 Hz, J″ = 13.2 Hz, 2 H), 6.61 (d, J = 8.4 Hz, 2 H), 5.97 (d, J = 5.4 Hz, 1 H), 4.75 (q, J = 7.2 Hz, 1 H), 4.34 (dd, J = 2.4 Hz, J′ = 9 Hz, 1 H), 3.97–3.99 (m, 1 H), 3.70 (dd, J = 1.8 Hz, J′ = 6 Hz, 4 H), 3.61 (t, J = 6.6 Hz, 4 H), 3.10–3.12 (m, 2 H), 2.38–2.40 (m, 1 H), 2.20–2.21 (m, 1 H), 2.03 (dd, J = 6 Hz, J′ = 7.8 Hz, 1 H), 1.92–1.93 (m, 1 H), 1.86 (dt, J = 2.4 Hz, J′ = 14.4 Hz, 1 H), 1.65–1.66 (m, 1 H), 1.48 (dq, J = 6.6 Hz, J′ = 13.2 Hz, 1 H), 1.39–1.42 (m, 4 H), 1.27–1.31 (m, 5 H), 0.95 (s, 1 H), 0.84–0.86 (m, 9 H); 13C NMR (150.9 MHz, CDCl3) δ 170.5, 162.0, 160.2, 146.2, 145.1, 142.6, 142.1, 140.1, 130.8, 127.4, 125.2, 124.5, 123.7, 119.9, 118.4, 112.2, 85.9, 77.9, 69.3, 54.4, 54.0, 53.5, 51.4, 40.5, 40.4, 40.0, 39.6, 38.2, 37.4, 35.6, 29.6, 28.6, 28.0, 27.8, 27.1, 26.3, 25.4, 24.0, 23.0, 22.0.
N-((2R)-3-(4-(bis(2-chloroethyl)amino)phenyl)-1-(((1R)-3-methyl-1-((3aS)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxopropan-2-yl)pyrazine-2-carboxamide (8): To a stirring solution of pyrazine-2-carboxylic acid (8 mg, 0.06 mmol) in DMF (100 μL), DIPEA (32 μL, 0.18 mmol) and HATU (26 mg, 0.07 mmol) were added and the mixture was cooled at 0 °C. Then, (S)-3-(4-(bis(2-chloroethyl)amino)phenyl)-1-(((S)-3-methyl-1-((3aS,4S,6S,7aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxopropan-2-aminium chloride salt (30 mg, 0.05 mmol) was added and the reaction mixture was left under stirring at 0 °C for 30 min and subsequently at room temperature for 20 h. Upon completion, the mixture was subjected to FCC purification using toluene/ethyl acetate 7:3 as eluent, and the hybrid was afforded as a bright yellow oil (13 mg, 38% yield). Rf (PhMe/EtOAc) 7:3 = 0.2; IR (KBr): 2925, 1662, 1533, 1027 cm−1; HR-ESI (m/z) [M+H+] calculated for [C33H47BCl2N5O4]+ 658.3093 Found: 658.3079; 1H NMR (CDCl3, 600.13 MHz) δ 9.35 (d, J = 1.8 Hz, 1 H), 8.74 (d, J = 2.4 Hz, 1 H), 8.53 (dd, J = 0.6 Hz, J′ = 1.8 Hz, 1 H), 8.33 (d, J = 8.4 Hz, 1 H), 7.17 (d, J = 8.4 Hz, 2 H), 6.60 (d, J = 8.4 Hz, 2 H), 5.95 (d, J = 4.8 Hz, 1 H), 4.74 (td, J = 1.2 Hz, J′ = 6.6 Hz, 1 H), 4.32 (dd, J = 1.8 Hz, J′ = 9 Hz, 1 H), 3.69 (t, J = 6.6 Hz, 4 H), 3.60 (t, J = 7.2 Hz, 4 H), 3.09–3.11 (m, 3 H), 2.33–2.34 (m, 1 H), 2.19 (ddd, J = 1.8 Hz, J′ = 6 Hz, J″ = 10.8 Hz, 1 H), 2.02 (t, J = 5.4 Hz, 1 H), 1.91 (tt, J = 3 Hz, J′ = 6 Hz, 1 H), 1.85 (dt, J = 2.4 Hz, J′ = 14.4 Hz, 1 H), 1.58–1.60 (m, 3 H), 1.48 (dq, J = 7.2 Hz, J′ = 13.8 Hz, 1 H), 1.41 (s, 3 H), 1.29 (s, 3 H), 0.82–0.86 (m, 9 H); 13C NMR (150.9 MHz, CDCl3) δ 170.7, 162.8, 147.4, 144.3, 144.1, 142.7, 130.8, 129.0, 128.2, 112.2, 85.8, 77.8, 54.2, 53.5, 51.4, 40.4, 40.0, 39.6, 38.2, 37.3, 35.6, 28.6, 27.1, 26.3, 25.4, 24.0, 23.0, 22.0.
N-((2R)-3-(4-(bis(2-chloroethyl)amino)phenyl)-1-(((1R)-3-methyl-1-((3aS)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxopropan-2-yl)-5-chloropyrazine-2-carboxamide (9): To a stirring solution of 5-chloropyrazine-2-carboxylic acid (10 mg, 0.06 mmol) in DMF (100 μL), DIPEA (32 μL, 0.18 mmol) and HATU (26 mg, 0.07 mmol) were added and the mixture was cooled at 0 °C. Then, (S)-3-(4-(bis(2-chloroethyl)amino)phenyl)-1-(((S)-3-methyl-1-((3aS,4S,6S,7aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxopropan-2-aminium chloride salt (30 mg, 0.05 mmol) was added and the reaction mixture was left under stirring at 0 °C for 30 min and subsequently at room temperature for 20 h. Upon completion, the mixture was subjected to FCC purification using toluene/ethyl acetate 9:1 as eluent, and the hybrid was afforded as a pale-yellow oil (15 mg, 43% yield). Rf (PhMe/EtOAc) 8:2 = 0.17; IR (KBr): 2925, 1662, 1533, 1027 cm−1; HR-ESI (m/z) [M+H+] calculated for [C33H46BCl3N5O4]+ 692.2703 Found: 692.2691; 1H NMR (CDCl3, 600.13 MHz) δ 8.71–8.73 (m, 2 H), 8.69 (d, J = 1.2 Hz, 1 H), 8.51 (ddd, J = 1.2 Hz, J′ = 4.8 Hz, J″ = 8.4 Hz, 1 H), 8.22 (d, J = 7.8 Hz, 1 H), 7.50 (ddd, J = 4.2 Hz, J′ = 5.4 Hz, 1 H), 7.15 (d, J = 9 Hz, 2 H), 6.60 (d, J = 8.4 Hz, 2 H), 5.98 (d, J = 5.4 Hz, 1 H), 4.70–4.71 (m, 1 H), 4.33 (dd, J = 2.4 Hz, J′ = 9 Hz, 1 H), 3.80–3.82 (m, 1 H), 3.70 (t, J = 6.6 Hz, 4 H), 3.61 (t, J = 7.2 Hz, 4 H), 3.09–3.11 (m, 2 H), 2.35 (ddt, J = 3 Hz, J′ = 5.4 Hz, J″ = 11.4 Hz, 1 H), 2.19–2.21 (m, 1 H), 2.02 (dt, J = 5.4 Hz, J′ = 6 Hz, 1 H), 1.92 (ddd, J = 3 Hz, 1 H), 1.85 (ddd, J = 1.2 Hz, J′ = 15 Hz, 1 H), 1.42 (s, 2 H), 1.38–1.39 (m, 1 H), 1.30–1.32 (m, 3 H), 1.25–1.27 (m, 1 H), 0.83–0.87 (m, 9 H); 13C NMR (150.9 MHz, CDCl3) δ 170.6, 161.6, 160.6, 152.0, 145.1, 142.1, 141.7, 140.6, 135.1, 130.7, 129.8, 125.2, 121.1, 112.1, 85.8, 65.5, 54.2, 53.5, 51.4, 40.4, 40.0, 39.6, 38.2, 37.4, 35.6, 28.6, 28.0, 27.1, 26.3, 25.4, 24.0, 23.0, 22.0.