Apricot Seed Shells and Walnut Shells as Unconventional Sugars and Lignin Sources
Abstract
1. Introduction
2. Results and Discussion
2.1. Raw Material Composition
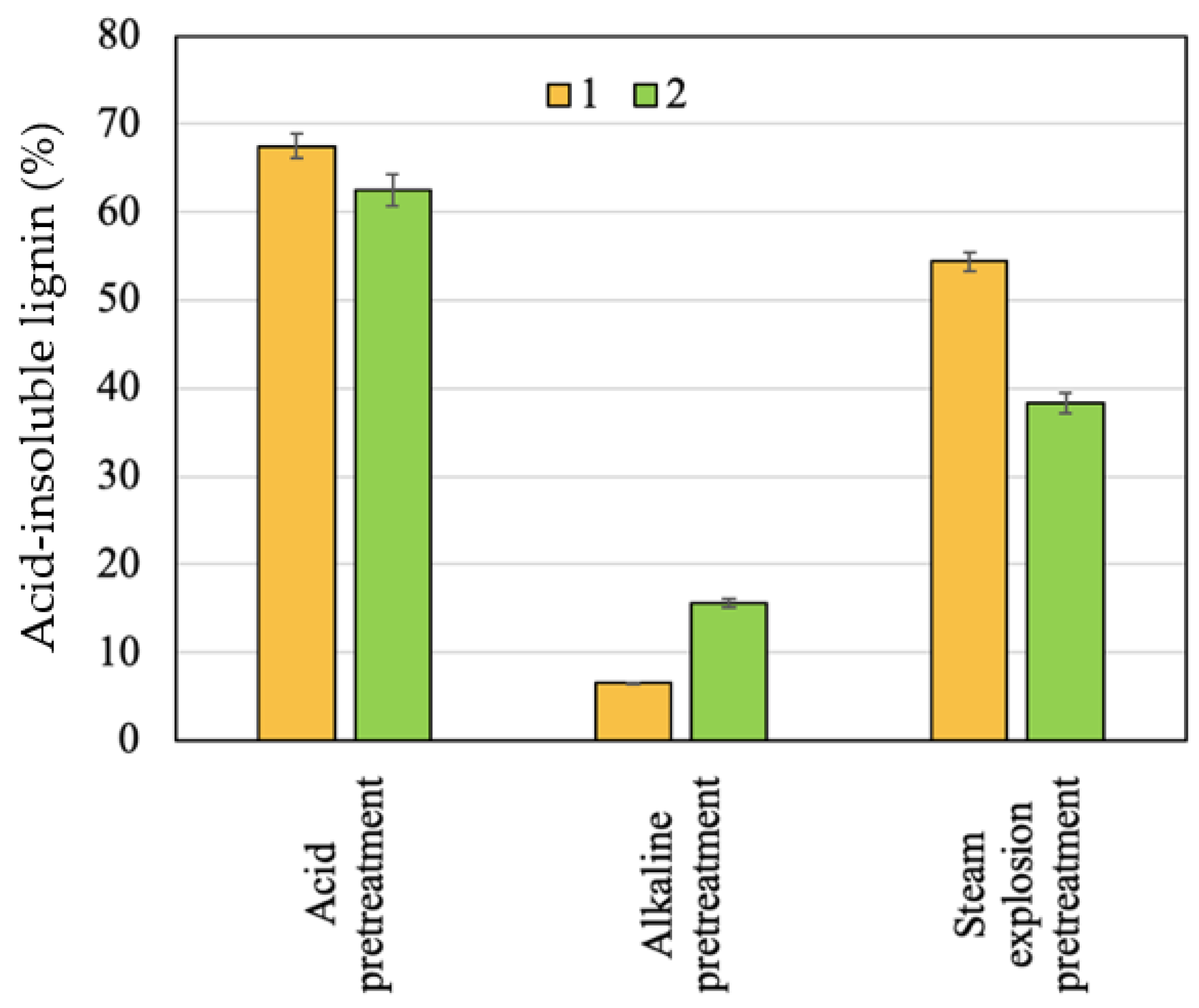
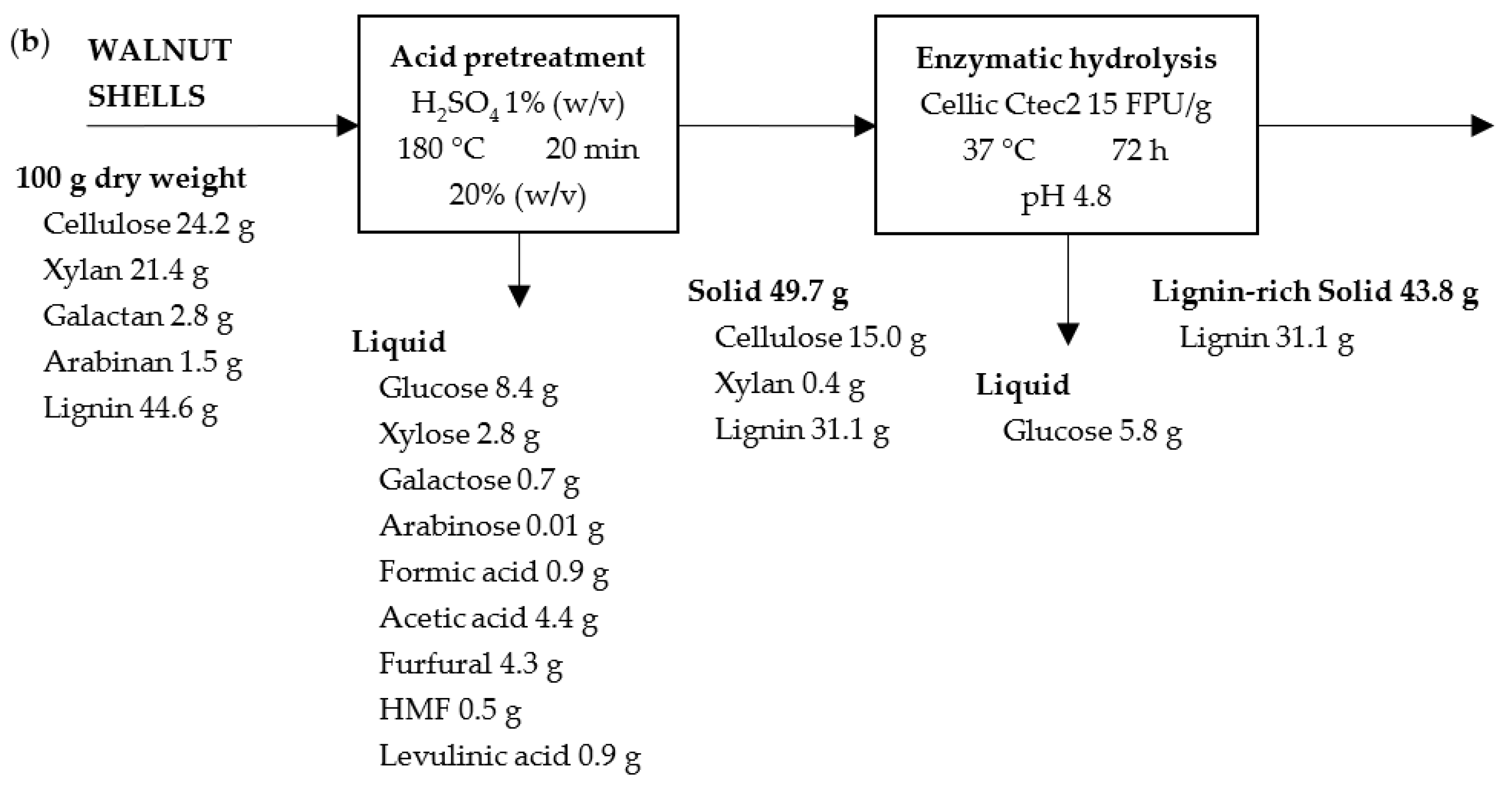
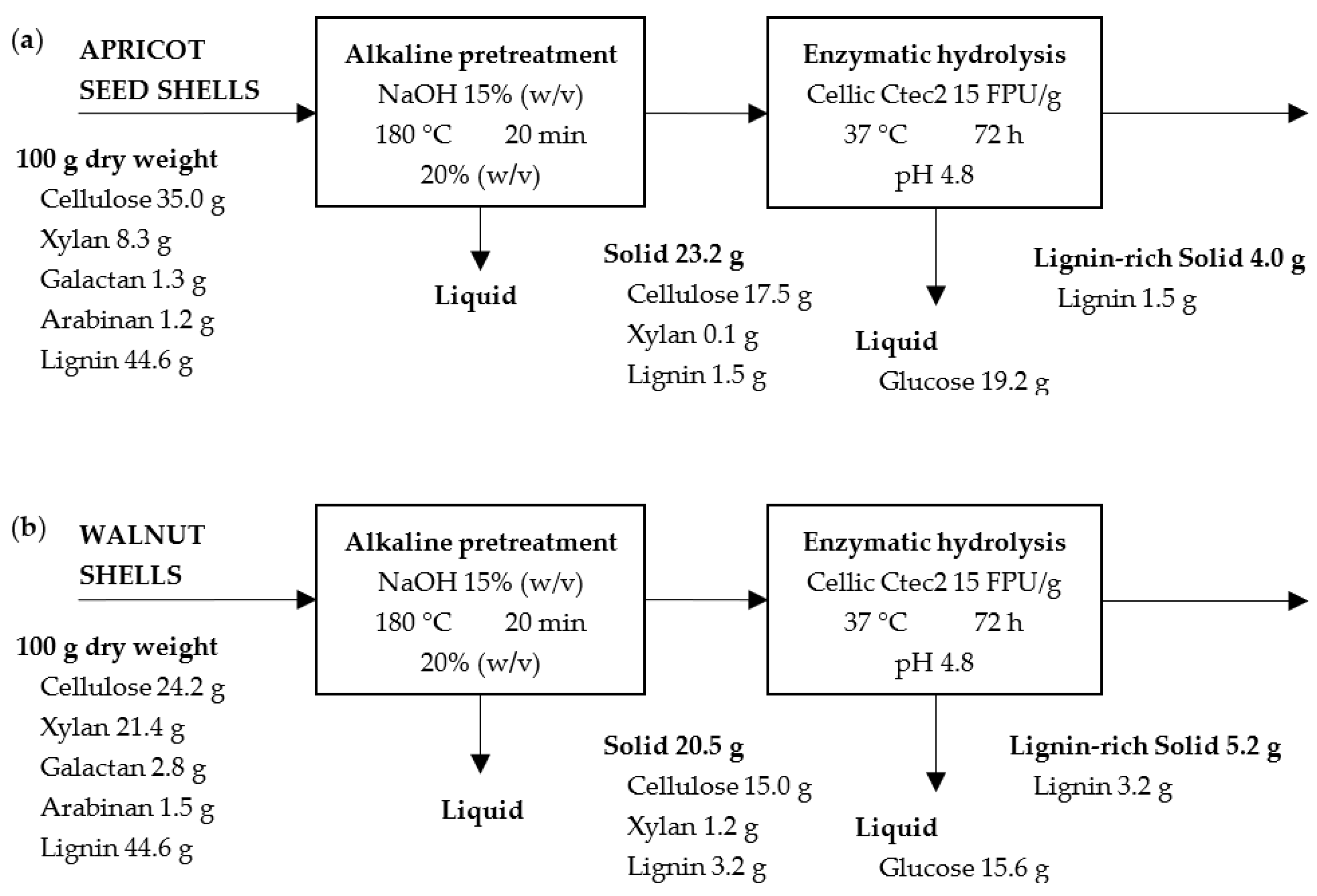
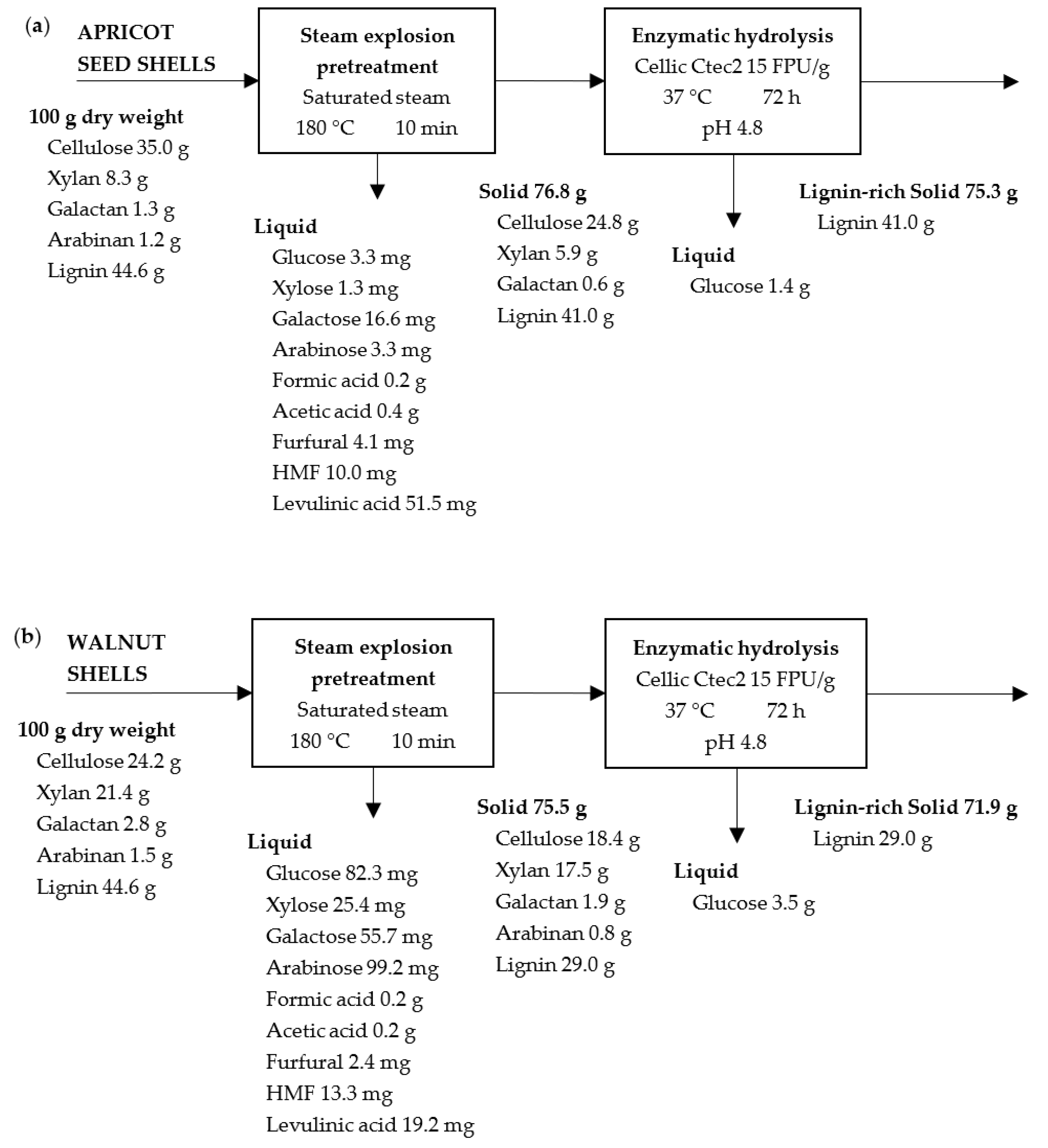
2.2. Effect of Pretreatment on Solid Recovery
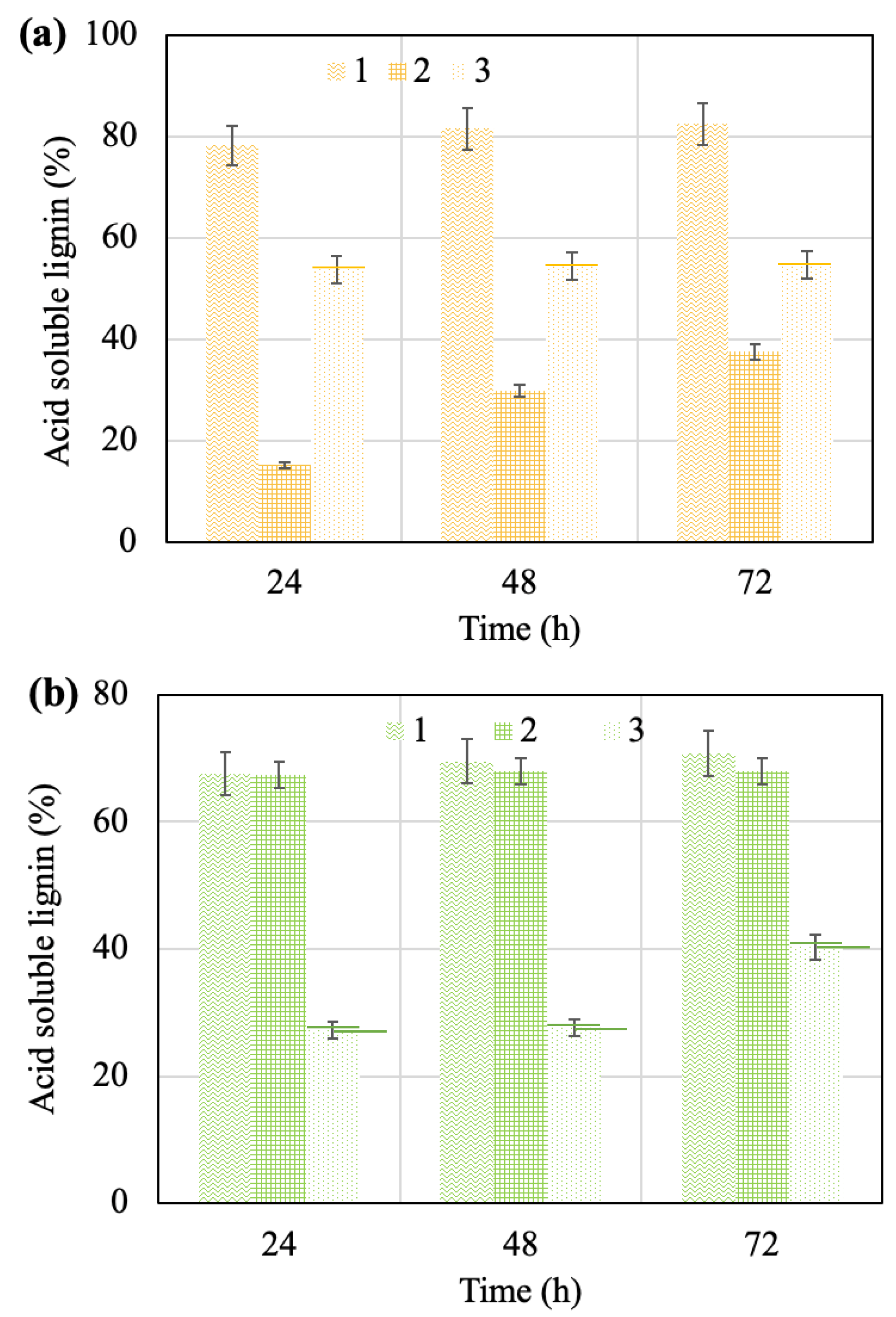
2.3. Effect of Pretreatment on Hydrolysate Characterization
2.4. Enzymatic Hydrolysis
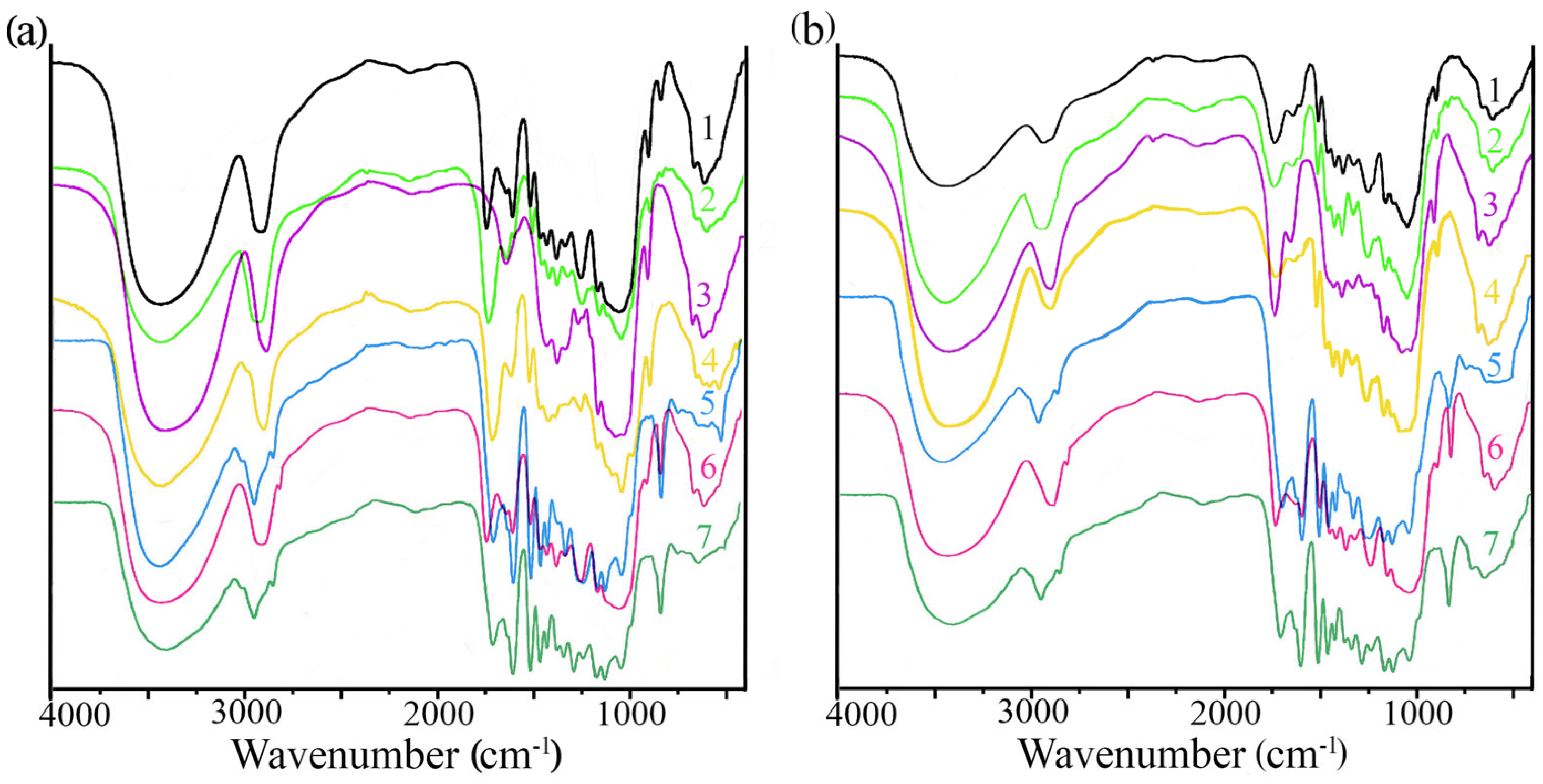
2.5. Solid Characterization after Enzymatic Hydrolysis
2.6. Comparison with Previous Studies
| Process Steps | Products | Ref. | ||
|---|---|---|---|---|
| Step-1 | Step-2 | Enzymatic Hydrolysis | ||
| Walnut shell | ||||
| p-TsOH pretreatment | H2O2 pretreatment | Cellic CTec 2 (40 FPU/g cellulose) | Fermentable sugars | [53] |
| HNO3 pretreatment | ZSL cellulose (40 FPU/g solid) | Fermentable sugars | [54] | |
| Deep eutectic solvent | Cellulase from Trichoderma viride (Novozymes) | Lignin nano-particles Fermentable sugars | [55] | |
| NaClO2-CH3COOH delignification | Alkaline extraction | Commercially available endo-1,4-β-xylanase | Xylooligosaccharides | [56] |
| Hydrothermal pretreatment | Organosolv delignification | Xylooligosaccharides Lignin Cellulose nanocrystals | [57] | |
| Sequential organosolv delignification (3n) | Hydrothermal treatment | Lignin Cellulose nanocrystals | [58] | |
| Apricot seed shells | ||||
| NH4OH pretreatment | Phosphorylated in an aqueous solution | Biosorbent | [59] | |
| “Sol-gel” technology. (CuO)x*(CoO)y*(NiO)z*(Fe2O3)k*(MoO3)m/HSZ based catalyst | Nanocarbon | [60] | ||
| H3PO4/KOH carbonization | Lignin-derived activated carbon | [61] | ||
| NH4OH pretreatment | Aminated using pyridine | Cellulose basic-ion exchangers | [62] | |
| KOH carbonization | Nanocarbon | [63] | ||
2.7. Mass Balance
3. Materials and Methods
3.1. Materials
3.2. Biomass Pretreatment
3.3. Enzymatic Hydrolysis
3.4. Raw Material, Residual Solid, and Liquid Fraction (Hydrolysate) Characterization
3.5. Temperature-Programmed Desorption Mass Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, R.G.; Sorrell, S.R. The future of oil supply. Philos. Trans. A Math. Phys. Eng. Sci. 2014, 372, 20130179. [Google Scholar] [CrossRef] [PubMed]
- Bay, M.S.; Karimi, K.; Nasr Esfahany, M.; Kumar, R. Structural modification of pine and poplar wood by alkali pretreatment to improve ethanol production. Ind. Crop. Prod. 2020, 152, 112506. [Google Scholar] [CrossRef]
- Grassi, M.C.B.; Pereira, G.A.G. Energy-cane and RenovaBio: Brazilian vectors to boost the development of Biofuels. Ind. Crop. Prod. 2019, 129, 201–205. [Google Scholar] [CrossRef]
- Patni, N.; Pillai, S.G.; Dwivedi, A.H. Wheat as a promising substitute of corn for bioethanol production. Procedia Eng. 2013, 51, 355–362. [Google Scholar] [CrossRef]
- Akao, S.; Yasutake, D.; Kondo, K.; Nagare, H.; Maeda, M.; Fujiwara, T. Effects of cultivation period on catch crop chemical composition and potential for bioenergy production. Ind. Crop. Prod. 2018, 111, 787–793. [Google Scholar] [CrossRef]
- Chavan, S.; Gaikwad, A. Optimization of enzymatic hydrolysis of bamboo biomass for enhanced saccharification of cellulose through Taguchi orthogonal design. J. Environ. Chem. Eng. 2020, 9, 104807. [Google Scholar] [CrossRef]
- Duruyurek, M.; Düşgün, C.; Gulhan, M.; Selamoglu, Z. Production of Bioethanol from Waste Potato. Turkish J. Agric.-Food Sci. Tech. 2015, 3, 331–334. [Google Scholar] [CrossRef]
- Nashiruddin, N.I.; Mansor, A.F.; Rahman, R.A.; Ilias, R.M.; Yussof, H.W. Process parameter optimization of pretreated pineapple leaves fiber for enhancement of sugar recovery. Ind. Crop. Prod. 2020, 152, 112514. [Google Scholar] [CrossRef]
- Kartel, M.; Galysh, V. New composite sorbents for Caesium and Strontium ions sorption. Chem. J. Mold. 2017, 12, 37–44. [Google Scholar] [CrossRef]
- Deykun, I.; Halysh, V.; Barbash, V. Rapeseed straw as an alternative for pulping and papermaking. Cellulose Chem. Technol. 2018, 52, 833–839. [Google Scholar]
- Halysh, V.; Trembus, I.; Deykun, I.; Ostapenko, A.; Nikolaichuk, A.; Ilnitska, G. Development of effective technique for the disposal of the Prunus Armeniaca seed shells. East-Eur. J. Enterp. Technol. 2018, 1, 4–9. [Google Scholar] [CrossRef]
- Mahari, W.A.V.; Waiho, K.; Fazhan, H.; Necibi, M.C.; Hafsa, J.; Mrid, R.B.; Fal, S.; Arrousi, H.E.; Peng, W.; Tabatabaei, M.; et al. Progress in valorisation of agriculture, aquaculture and shellfish biomass into biochemicals and biomaterials towards sustainable bioeconomy. Chemosphere 2021, 291, 133036. [Google Scholar] [CrossRef]
- Peinemann, J.C.; Pleissner, D. Continuous pretreatment, hydrolysis, and fermentation of organic residues for the production of biochemicals. Bioresour. Technol. 2020, 295, 122256. [Google Scholar] [CrossRef]
- Gilna, P.; Lynd, L.R.; Mohnen, D.; Davis, M.F.; Davison, B.H. Progress in understanding and overcoming biomass recalcitrance: A BioEnergy Science Center (BESC) perspective. Biotechnol. Biofuels 2017, 10, 285. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, M.; Su, J.; Hu, H.; Yang, M.; Huang, Z.; Chen, D.; Wu, J.; Feng, Z. Overcoming biomass recalcitrance by synergistic pretreatment of mechanical activation and metal salt for enhancing enzymatic conversion of lignocellulose. Biotechnol. Biofuels 2019, 12, 12. [Google Scholar] [CrossRef]
- Meng, X.; Pu, Y.; Yoo, C.G.; Li, M.; Bali, G.; Park, D.Y.; Gjersing, E.; Davis, M.F.; Muchero, W.; Tuskan, G.A.; et al. An in-depth understanding of biomass recalcitrance using natural poplar variants as the feedstock. Chem. Sus. Chem. 2017, 10, 139–150. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Momayez, F.; Karimi, K.; Karimi, S.; Horváth, I.S. Efficient hydrolysis and ethanol production from rice straw by pretreatment with organic acids and effluent of biogas plant. RSC Adv. 2017, 7, 50537–50545. [Google Scholar] [CrossRef]
- Xing, Y.; Yu, H.; Zhu, L.; Jiang, J. Efficient enzymatic hydrolysis of bamboo by pretreatment with steam explosion and alkaline peroxide. BioResources 2013, 8, 5392–5408. [Google Scholar] [CrossRef]
- Cutrim, F.M.; Ramos, E.C.S.S.; Abreu, M.C.C.; Godinho, A.S.; Maciel, A.P.; Mendonça, C.J.S.; Cavalcante, K.S.B. A study of chemical composition and enzymatic hydrolysis of solid organic waste from Agrosilvopastoral systems. J. Braz. Chem. Soc. 2019, 30, 1955–1963. [Google Scholar] [CrossRef]
- Kontogianni, N.; Barampouti, E.M.; Mai, S.; Malamis, D.; Loizidou, M. Effect of alkaline pretreatments on the enzymatic hydrolysis of wheat straw. Environ. Sci. Pollut. Res. 2019, 26, 35648–35656. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, D.M.; Sevastyanova, O.; Penna, L.S.; de Silva, B.P.; Lindström, M.E.; Colodette, J.L. Assessment of chemical transformations in eucalyptus, sugarcane bagasse and straw during hydrothermal, dilute acid, and alkaline pretreatments. Ind. Crop. Prod. 2015, 73, 118–126. [Google Scholar] [CrossRef]
- Romero-García, J.M.; Lama-Muñoz, A.; Rodríguez-Gutiérrez, G.; Moya, M.; Ruiz, E.; Fernández-Bolaños, J.; Castro, E. Obtaining sugars and natural antioxidants from olive leaves by steam-explosion. Food Chem. 2016, 210, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; He, W.; Yuan, L. Aqueous ammonia pretreatment of sugar beet pulp for enhanced enzymatic hydrolysis. Bioprocess Biosyst. Eng. 2017, 40, 1603–1609. [Google Scholar] [CrossRef]
- FAO—Food and Agriculture Organization. Food and Agriculture Data. Available online: https://www.fao.org/faostat/en/#home (accessed on 18 January 2023).
- Halysh, V.; Trus, I.; Nikolaichuk, A.; Skiba, M.; Radovenchyk, I.; Deykun, I.; Vorobyova, V.; Vasylenko, I.; Sirenko, L. Spent biosorbents as additives in cement production. J. Ecol. Eng. 2020, 21, 131–138. [Google Scholar] [CrossRef]
- Halysh, V.; Sevastyanova, O.; de Carvalho, D.M.; Riazanova, A.V.; Lindström, M.E.; Gomelya, M. Effect of oxidative treatment on composition and properties of sorbents prepared from sugarcane residue. Ind. Crop. Prod. 2019, 139, 111566. [Google Scholar] [CrossRef]
- Trembus, I.V.; Trophimchuk, J.S.; Galysh, V.V. Preparation of pulp from sunflower stalks using peroxy acids. Voprosy Khimii i Khimicheskoi Tekhnologii 2018, 2, 122–127. [Google Scholar]
- Trembus, I.; Hondovska, A.; Halysh, V.; Deykun, I.; Cheropkina, R. Feasible Technology for Agricultural Residues Utilization for the Obtaining of Value-Added Products. Ecol. Eng. Environ. Technol. 2022, 2, 107–112. [Google Scholar] [CrossRef]
- Yu, Z.; Jameel, H.; Chang, H.M.; Park, S. The effect of delignification of forest biomass on enzymatic hydrolysis. Bioresour. Technol. 2011, 102, 9083–9089. [Google Scholar] [CrossRef]
- Park, J.; Shin, H.; Yoo, S.; Zoppe, J.O.; Park, S. Delignification of lignocellulosic biomass and its effect on subsequent enzymatic hydrolysis. BioResources 2015, 10, 2732–2743. [Google Scholar] [CrossRef]
- Corbett, D.B.; Kohan, N.; Machado, G.; Jing, C.; Nagardeolekar, A.; Bujanovic, B.M. Chemical composition of apricot pit shells and effect of hot-water extraction. Energies 2015, 8, 9640–9654. [Google Scholar] [CrossRef]
- Queirós, C.; Cardoso, S.; Lourenço, A.; Ferreira, J.; Miranda, I.; Lourenço, M.; Pereira, H. Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Conv. Bioref. 2020, 10, 175–188. [Google Scholar] [CrossRef]
- Pirayesh, H.; Khazaeian, A.; Tabarsa, T. The potential for using walnut (Juglans regia L.) shell as a raw material for wood-based particleboard manufacturing. Compos. Part B—Eng. 2012, 43, 3276–3280. [Google Scholar] [CrossRef]
- Wu, Z.; Hao, H.; Tu, Y.; Hu, Z.; Wei, F.; Liu, Y.; Zhou, Y.; Wang, Y.; Xie, G.; Gao, C.; et al. Diverse cell wall composition and varied biomass digestibility in wheat straw for bioenergy feedstock. Biomass Bioenergy 2014, 70, 347–355. [Google Scholar] [CrossRef]
- Oka, D.; Kobayashi, K.; Isobe, N.; Ogawa, Y.; Yokoyama, T.; Kimura, S.; Kim, U.; Tokuyasu, K.; Wada, M. Enzymatic hydrolysis of wood with alkaline treatment. J. Wood Sci. 2013, 59, 484–488. [Google Scholar] [CrossRef]
- Siddhu, M.A.H.; Li, W.; He, Y.; Liu, G.; Chen, C. Steam explosion pretreatment of rice straw to improve structural carbohydrates anaerobic digestibility for biomethanation. Environ. Sci. Pollut. Res. 2019, 26, 22189–22196. [Google Scholar] [CrossRef]
- Benjamin, Y.; Cheng, H.; Görgens, J.F. Optimization of dilute sulfuric acid pretreatment to maximize combined sugar yield from sugarcane bagasse for ethanol production. Appl. Biochem. Biotechnol. 2014, 172, 610–630. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Mafa, M.S.; Malgas, S.; Bhattacharya, A.; Rashamuse, K.; Pletschke, B.I. The effects of alkaline pretreatment on agricultural biomasses (corn cob and sweet sorghum bagasse) and their hydrolysis by a termite-derived enzyme cocktail. Agronomy 2020, 10, 1211. [Google Scholar] [CrossRef]
- Herbaut, M.; Zoghlami, A.; Habrant, A.; Falourd, X.; Foucat, L.; Chabbert, B.; Paës, G. Multimodal analysis of pretreated biomass species highlights generic markers of lignocellulose recalcitrance. Biotechnol. Biofuels 2018, 11, 52. [Google Scholar] [CrossRef]
- Steinbach, D.; Kruse, A.; Sauer, J.; Storz, J. Is Steam Explosion a Promising Pretreatment for Acid Hydrolysis of Lignocellulosic Biomass. Processes 2020, 8, 1626. [Google Scholar] [CrossRef]
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the structural and chemical changes of plant biomass following steam explosion pretreatment. Biotechnol. Biofuels 2017, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ziegler-Devin, I.; Chrusciel, L.; Ngwa Obame, S.; Hong, L.; Lu, X.; Brosse, N. Lignin-first integrated steam explosion process for green wood adhesive application. ACS Sustain. Chem. Eng. 2020, 8, 5380–5392. [Google Scholar] [CrossRef]
- Kemppainen, K.; Inkinen, J.; Uusitalo, J.; Nakari-Setälä, T.; Siika-aho, M. Hot water extraction and steam explosion as pretreatments for ethanol production from spruce bark. Biores Technol. 2012, 117, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Liu, L.S. Steam Explosion Pretreatment Technique and Application in Biomass Conversion. Adv. Mater. Res. 2010, 113–116, 525–528. [Google Scholar] [CrossRef]
- Singh, J.; Suhag, M.; Dhaka, A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review. Carbohydr. Polym. 2015, 117, 624–631. [Google Scholar] [CrossRef]
- Díaz-Villanueva, M.; Cara-Corpas, C.; Ruiz-Ramos, E.; Romero-Pulido, I.; Castro-Galiano, E. Olive tree pruning as an agricultural residue for ethanol production. Fermentation of hydrolysates from dilute acid pretreatment. Span. J. Agric. Res. 2012, 10, 643–648. [Google Scholar] [CrossRef]
- Baadhe, R.R.; Potumarthi, R.; Mekala, N.K. Influence of dilute acid and alkali pretreatment on reducing sugar production from corncobs by crude enzymatic method: A comparative study. Bioresour. Technol. 2014, 162, 213–217. [Google Scholar] [CrossRef]
- Shimizu, F.L.; Monteiro, P.Q.; Ghiraldi, P.H.C.; Melati, R.B.; Pagnocca, F.C.; Souza, W.; Sant’Anna, C.; Brienzo, M. Acid, alkali and peroxide pretreatments increase the cellulose accessibility and glucose yield of banana pseudostem. Ind. Crop. Prod. 2018, 115, 62–68. [Google Scholar] [CrossRef]
- Lebedev, A. Mass Spectrometry in Organic Chemistry; Binom: Moscow, Russia, 2003; 493p. [Google Scholar]
- Palianytsia, B.; Kulik, T.; Dudik, O.; Cherniavska, T.; Tonkha, O. Study of the thermal decomposition of some components of biomass by desorption mass spectrometry. In Proceedings of the International Congress on Energy Efficiency and Energy Related Materials (ENEFM2013), Antalya, Turkey, 9–12 October 2013; pp. 19–25. [Google Scholar]
- Zhu, J.; Jiao, N.; Li, H.; Xu, G.; Zhang, H.; Xu, Y. P-Toluenesulfonic Acid Combined with Hydrogen Peroxide-Assisted Pretreatment Improves the Production of Fermentable Sugars from Walnut (Juglans regia L.) Shells. Bioresour. Technol. 2022, 355, 127300. [Google Scholar] [CrossRef]
- Tan, M.; Ma, L.; Rehman, M.S.U.; Ahmed, M.A.; Sajid, M.; Xu, X.; Sun, Y.; Cui, P.; Xu, J. Screening of Acidic and Alkaline Pretreatments for Walnut Shell and Corn Stover Biorefining using Two Way Heterogeneity Evaluation. Renew. Energy 2019, 132, 950–958. [Google Scholar] [CrossRef]
- Li, H.; Liang, J.; Chen, L.; Ren, M.; Zhou, C. Utilization of Walnut Shell by Deep Eutectic Solvents: Enzymatic Digestion of Cellulose and Preparation of Lignin Nanoparticles. Ind. Crop. Prod. 2023, 192, 116034. [Google Scholar] [CrossRef]
- Cebin, A.V.; Ralet, M.; Vigouroux, J.; Karača, S.; Martinić, A.; Komes, D.; Bonnin, E. Valorisation of Walnut Shell and Pea Pod as Novel Sources for the Production of Xylooligosaccharides. Carbohydr. Polym. 2021, 263, 117932. [Google Scholar] [CrossRef]
- Morales, A.; Labidi, J.; Gullón, P. Hydrothermal Treatments of Walnut Shells: A Potential Pretreatment for Subsequent Product Obtaining. Sci. Total Environ. 2021, 764, 142800. [Google Scholar] [CrossRef]
- Morales, A.; Labidi, J.; Gullón, P. Integral Valorisation of Walnut Shells Based on a Three-Step Sequential Delignification. J. Environ. Manag. 2022, 310, 114730. [Google Scholar] [CrossRef]
- Yelatontsev, D. Production of Versatile Biosorbent Via Eco-Friendly Utilization of Non-Wood Biomass. Chem. Eng. J. 2023, 451, 138811. [Google Scholar] [CrossRef]
- Yelatontsev, D.A.; Mukhachev, A.P. Synthesis and Properties of Ion Exchangers Derived from Non-Wood Cellulose. ChemChemTech 2020, 63, 88–95. [Google Scholar] [CrossRef]
- Saha, D.; Taylor, B.; Alexander, N.; Joyce, D.F.; Faux, G.I.; Lin, Y.; Shteyn, V.; Orkoulas, G. One-Step Conversion of Agro-Wastes to Nanoporous Carbons: Role in Separation of Greenhouse Gases. Bioresour. Technol. 2018, 256, 232–240. [Google Scholar] [CrossRef]
- Wang, L.; Feng, X.; Li, X.; Ma, H.; Wu, J.; Chen, Y.; Zhou, J. Valorization of Lignin: Application of Lignin-Derived Activated Carbon in Capacitors and Investigation of its Textural Properties and Electrochemical Performance. Diam. Relat. Mat. 2022, 122, 108791. [Google Scholar] [CrossRef]
- Xolmirzayeva, H.N.; Fayzullayev, N.I. Obtaining Nanocarbon from Local Raw Materials and Studying its Textural and Sorption Properties. Int. J. Eng. Trends Technol. 2022, 70, 163–171. [Google Scholar] [CrossRef]
- NREL—National Renewable Energy Laboratory. Determination of Structural Carbohydrates and Lignin in Biomass. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 1 January 2022).
- NREL—National Renewable Energy Laboratory. Ash in Biomass. Available online: https://www.nrel.gov/docs/gen/fy08/42622.pdf (accessed on 1 January 2022).
- NREL—National Renewable Energy Laboratory. Extractives in Biomass. Available online: https://www.nrel.gov/docs/gen/fy08/42619.pdf (accessed on 1 January 2022).
- NREL—National Renewable Energy Laboratory. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples. Available online: https://www.nrel.gov/docs/gen/fy08/42623.pdf (accessed on 1 January 2022).
- Kulyk, K.; Palianytsia, B.; Alexander, J.D.; Azizova, L.; Borysenko, M.; Kartel, M.; Larsson, M.; Kulik, T. Kinetics of Valeric Acid Ketonization and Ketenization in Catalytic Pyrolysis on Nanosized SiO2, γ-Al2O3, CeO2/SiO2, Al2O3/SiO2 and TiO2/SiO2. Chem. Phys. Chem. 2017, 18, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Kulyk, K.; Borysenko, M.; Kulik, T.; Mikhalovska, L.; Alexander, J.D.; Palianytsia, B. Chemisorption and thermally induced transformations of polydimethylsiloxane on the surface of nanoscale silica and ceria/silica. Polym. Degrad. Stab. 2015, 120, 203–211. [Google Scholar] [CrossRef]
- Kulik, T.; Palianytsia, B.; Larsson, M. Catalytic pyrolysis of aliphatic carboxylic acids into symmetric ketones over ceria-based catalysts: Kinetics, isotope effect and mechanism. Catalysts 2020, 10, 179. [Google Scholar] [CrossRef]


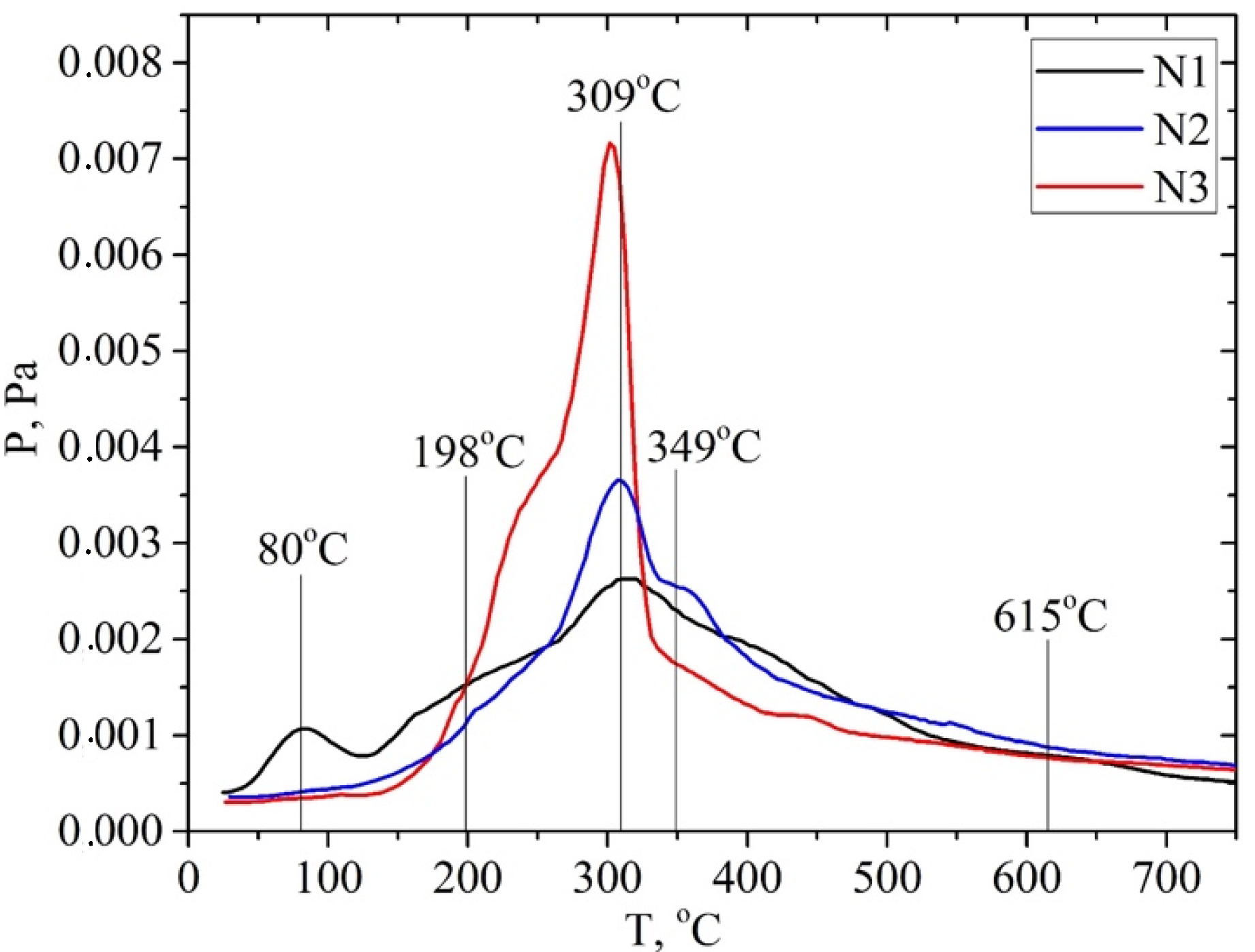
 ; walnut shell
; walnut shell  ; hemicellulose: apricot seed shell
; hemicellulose: apricot seed shell  ; walnut shell
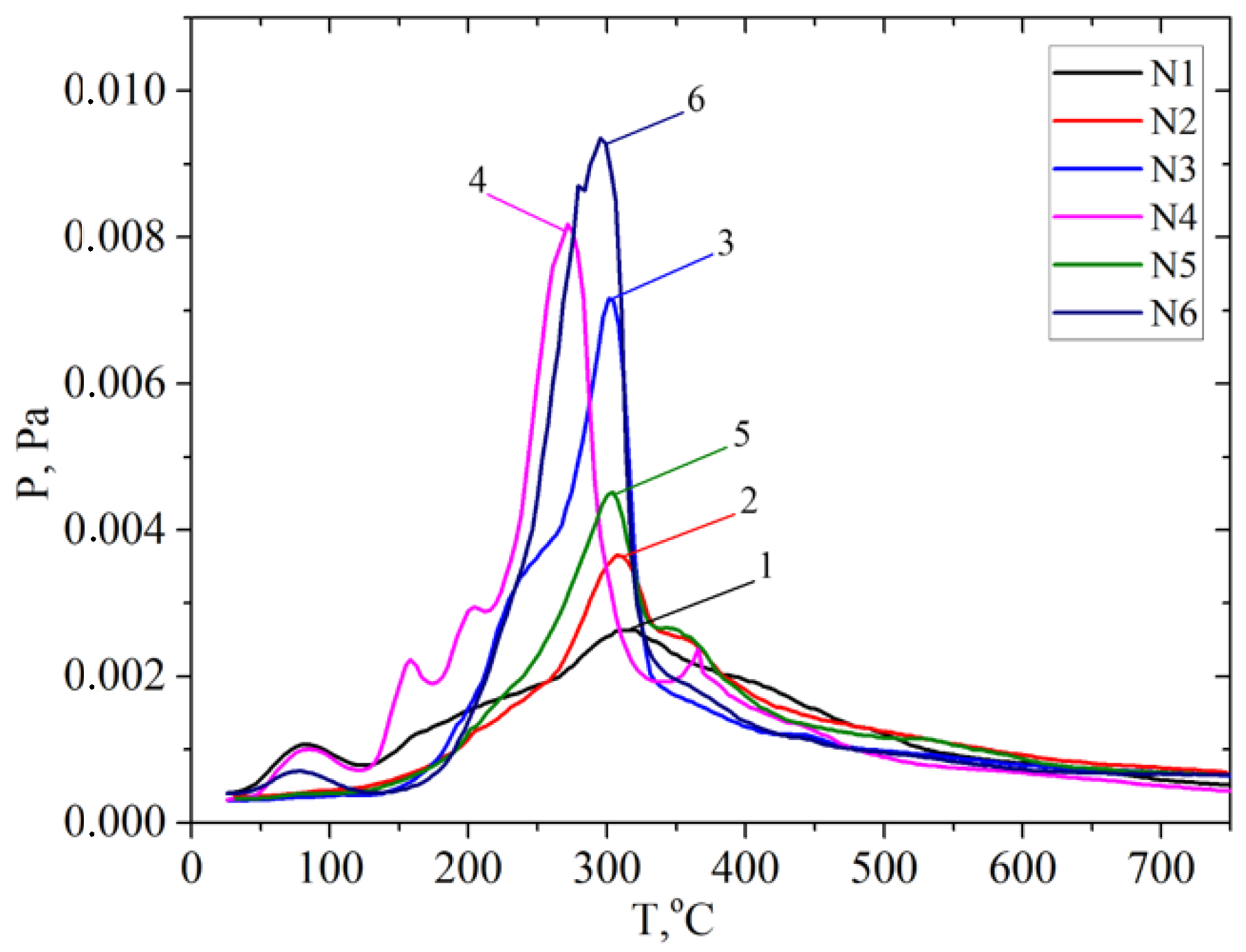
; walnut shell . In addition, the ion with m/z 60 ([H2C=C(OH)2]+) is the most intense one in the mass spectrum of acetic acid. Hemicellulose pyrolysis is accompanied by the intensive desorption of acetic acid due to the elimination of acetyl groups. Therefore, according to the intensity of peaks on the TPD curve for the ion with m/z 60 at ~200 °C (150–250 °C), one can identify the relative amount of hemicelluloses in the biomass samples, and according to the intensity of the peak at ~300 °C (250–350 °C), one can identify the relative amount of cellulose, as shown in Figures S1–S6 (see SM file). The TPD MS data are in good agreement with the data in Table 3. The highest peak intensity on the TPD curve for m/z 60 at around ~200 °C is observed for the samples of apricot seed shells and walnut shells after the pretreatment with steam explosion (Figures S3 and S6). The lowest intensities are observed for the samples of apricot seed shells and walnut shells after the acid pretreatment (Figures S2 and S5), since acid hydrolysis leads to the almost complete dissolution and removal of hemicellulose from the biomass. In addition, the acid treatment leads to the hydrolysis of acetyl groups with the formation of acetic acid. This process can be used in “green technologies” to produce renewable bio-based acetic acid.
. In addition, the ion with m/z 60 ([H2C=C(OH)2]+) is the most intense one in the mass spectrum of acetic acid. Hemicellulose pyrolysis is accompanied by the intensive desorption of acetic acid due to the elimination of acetyl groups. Therefore, according to the intensity of peaks on the TPD curve for the ion with m/z 60 at ~200 °C (150–250 °C), one can identify the relative amount of hemicelluloses in the biomass samples, and according to the intensity of the peak at ~300 °C (250–350 °C), one can identify the relative amount of cellulose, as shown in Figures S1–S6 (see SM file). The TPD MS data are in good agreement with the data in Table 3. The highest peak intensity on the TPD curve for m/z 60 at around ~200 °C is observed for the samples of apricot seed shells and walnut shells after the pretreatment with steam explosion (Figures S3 and S6). The lowest intensities are observed for the samples of apricot seed shells and walnut shells after the acid pretreatment (Figures S2 and S5), since acid hydrolysis leads to the almost complete dissolution and removal of hemicellulose from the biomass. In addition, the acid treatment leads to the hydrolysis of acetyl groups with the formation of acetic acid. This process can be used in “green technologies” to produce renewable bio-based acetic acid.
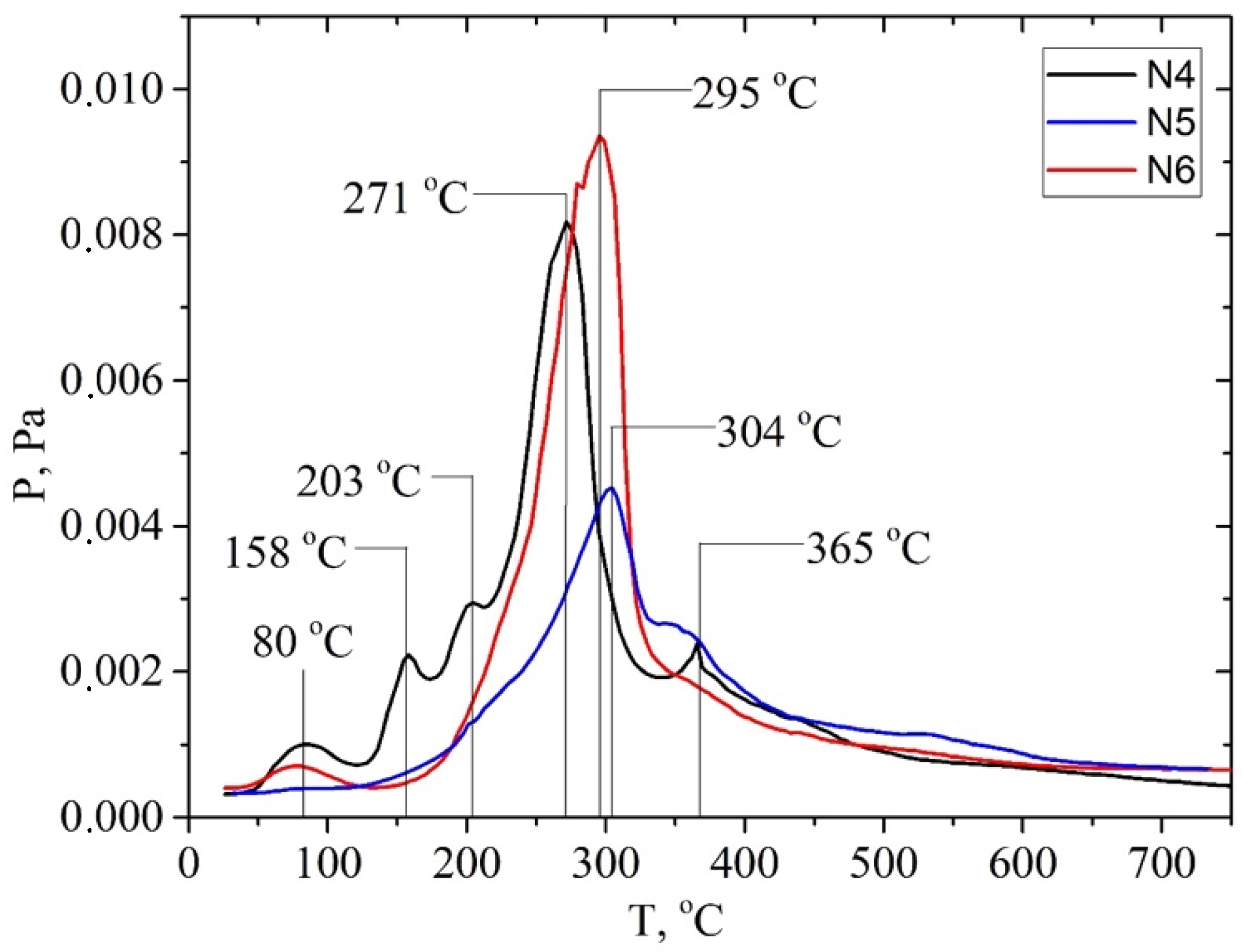
 ; walnut shell
; walnut shell  ; hemicellulose: apricot seed shell
; hemicellulose: apricot seed shell  ; walnut shell
; walnut shell . In addition, the ion with m/z 60 ([H2C=C(OH)2]+) is the most intense one in the mass spectrum of acetic acid. Hemicellulose pyrolysis is accompanied by the intensive desorption of acetic acid due to the elimination of acetyl groups. Therefore, according to the intensity of peaks on the TPD curve for the ion with m/z 60 at ~200 °C (150–250 °C), one can identify the relative amount of hemicelluloses in the biomass samples, and according to the intensity of the peak at ~300 °C (250–350 °C), one can identify the relative amount of cellulose, as shown in Figures S1–S6 (see SM file). The TPD MS data are in good agreement with the data in Table 3. The highest peak intensity on the TPD curve for m/z 60 at around ~200 °C is observed for the samples of apricot seed shells and walnut shells after the pretreatment with steam explosion (Figures S3 and S6). The lowest intensities are observed for the samples of apricot seed shells and walnut shells after the acid pretreatment (Figures S2 and S5), since acid hydrolysis leads to the almost complete dissolution and removal of hemicellulose from the biomass. In addition, the acid treatment leads to the hydrolysis of acetyl groups with the formation of acetic acid. This process can be used in “green technologies” to produce renewable bio-based acetic acid.
. In addition, the ion with m/z 60 ([H2C=C(OH)2]+) is the most intense one in the mass spectrum of acetic acid. Hemicellulose pyrolysis is accompanied by the intensive desorption of acetic acid due to the elimination of acetyl groups. Therefore, according to the intensity of peaks on the TPD curve for the ion with m/z 60 at ~200 °C (150–250 °C), one can identify the relative amount of hemicelluloses in the biomass samples, and according to the intensity of the peak at ~300 °C (250–350 °C), one can identify the relative amount of cellulose, as shown in Figures S1–S6 (see SM file). The TPD MS data are in good agreement with the data in Table 3. The highest peak intensity on the TPD curve for m/z 60 at around ~200 °C is observed for the samples of apricot seed shells and walnut shells after the pretreatment with steam explosion (Figures S3 and S6). The lowest intensities are observed for the samples of apricot seed shells and walnut shells after the acid pretreatment (Figures S2 and S5), since acid hydrolysis leads to the almost complete dissolution and removal of hemicellulose from the biomass. In addition, the acid treatment leads to the hydrolysis of acetyl groups with the formation of acetic acid. This process can be used in “green technologies” to produce renewable bio-based acetic acid.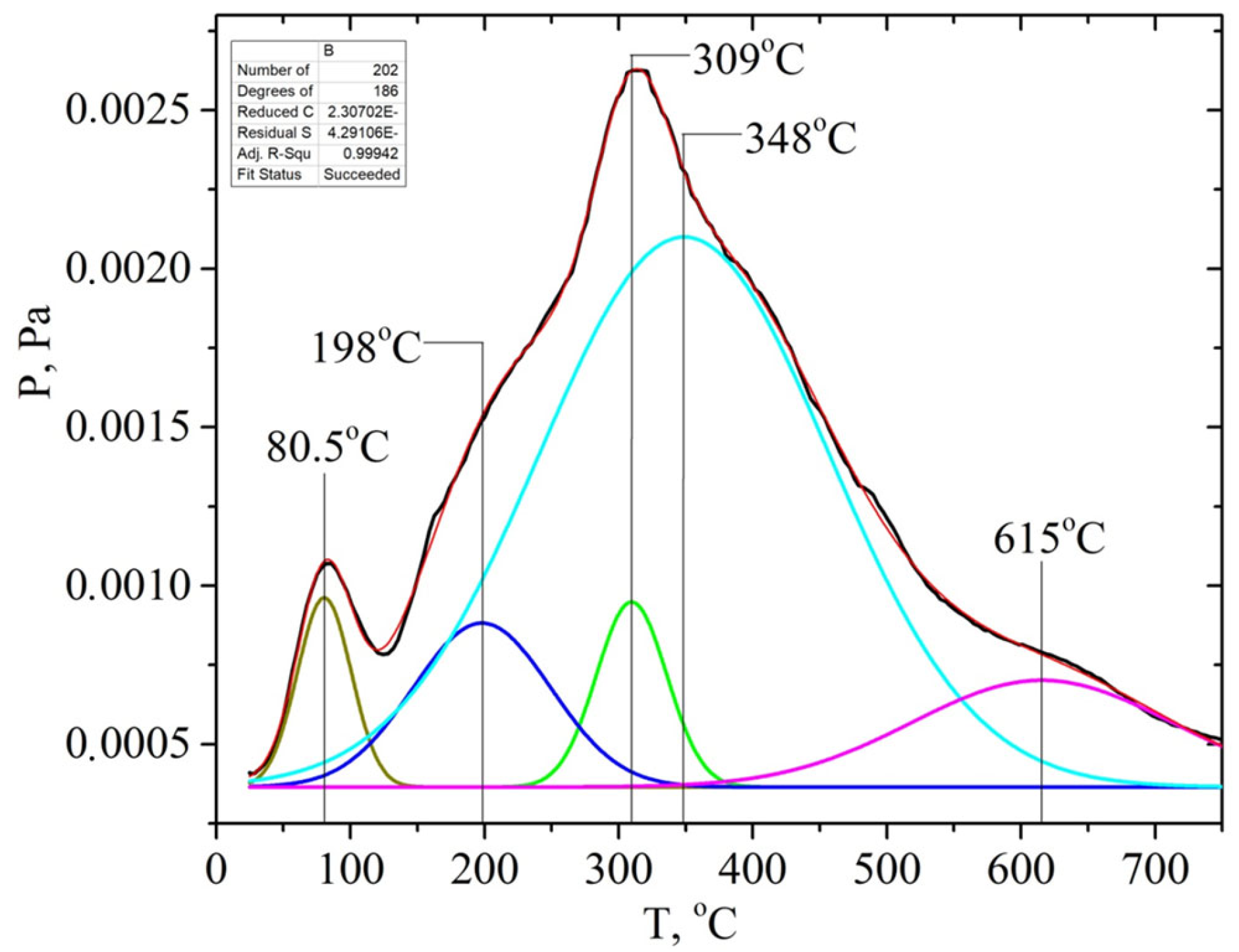
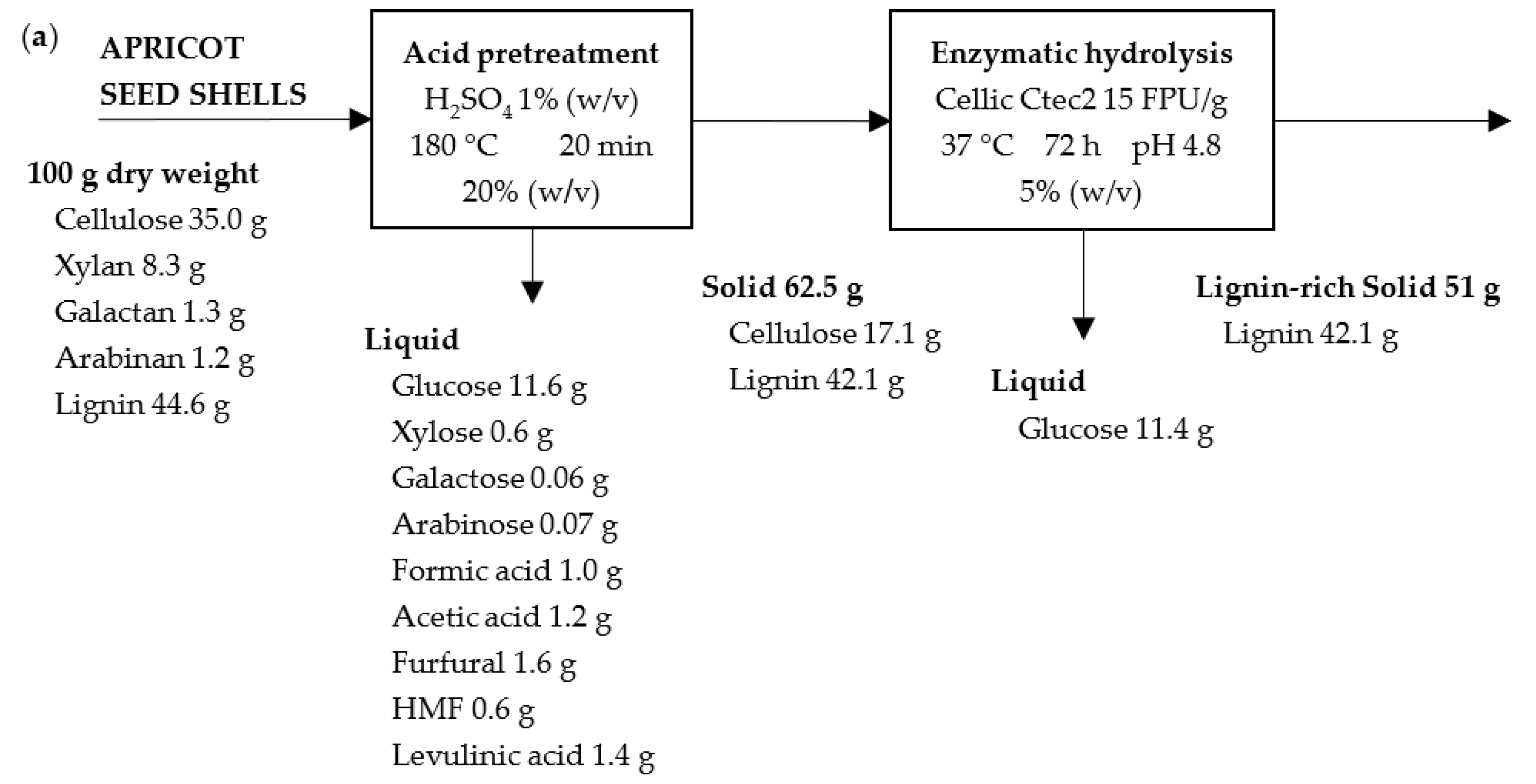
| Components | Apricot Seed Shells | Walnut Shells |
|---|---|---|
| Extractives | 9.97 ± 0.51 | 11.41 ± 2.23 |
| Cellulose | 35.01 ± 0.42 | 24.19 ± 0.68 |
| Hemicellulose Xylan Galactan Arabinan Mannan | 10.77 ± 0.14 8.33 ± 0.09 1.28 ± 0.05 1.16 ± 0.02 - | 25.68 ± 1.70 21.35 ± 1.83 2.78 ± 0.07 1.55 ± 0.01 - |
| Lignin Acid-Soluble Lignin Acid-Insoluble Lignin | 44.55 ± 1.05 1.12 ± 0.01 43.43 ± 1.04 | 44.63 ± 1.01 1.14 ± 0.01 43.49 ± 1.00 |
| Ash Acid-insoluble ash | 0.23 ± 0.01 - | 0.37 ± 0.01 0.12 ± 0.00 |
| Acetyl groups Acetic acid | 0.85 ± 0.02 1.21 ± 0.03 | 3.87 ± 0.09 5.53 ± 0.12 |
| Material | Sugars Content (%) | Acid-Insoluble Ash (%) | ||||
|---|---|---|---|---|---|---|
| Glucan | Xylan | Galactan | Arabinan | Mannan | ||
| Acid pretreatment | ||||||
| Apricot seed shells | 27.43 ± 0.29 | - | - | - | - | - |
| Walnut shells | 30.30 ± 0.64 | 0.90 ± 0.06 | - | - | - | 0.05 ± 0.00 |
| Alkaline pretreatment | ||||||
| Apricot seed shells | 75.58 ± 1.14 | 3.59 ± 0.28 | - | - | - | - |
| Walnut shells | 73.38 ± 1.58 | 5.57 ± 0.09 | - | - | - | - |
| Steam explosion pretreatment | ||||||
| Apricot seed shells | 32.32 ± 0.64 | 7.75 ± 0.29 | 0.57 ± 0.09 | - | - | - |
| Walnut shells | 24.35 ± 0.12 | 23.25 ± 0.39 | 2.58 ± 0.06 | 1.24 ± 0.05 | - | - |
| Composition | Acid Pretreatment | Steam Explosion Pretreatment | ||
|---|---|---|---|---|
| Apricot Seed Shells | Walnut Shells | Apricot Seed Shells | Walnut Shells | |
| Hemicellulosic sugars recovery (%) | 4.21 | 10.91 | 2.02 | 5.24 |
| Glucose recovery (%) | 37.16 | 35.67 | 0.02 | 3.52 |
| Sugars content (g/L) | ||||
| Glucose | 27.86 ± 0.08 | 18.98 ± 0.07 | 0.02 ± 0.00 | 0.68 ± 0.01 |
| Xylose | 1.35 ± 0.02 | 6.33 ± 0.02 | 0.08 ± 0.00 | 0.21 ± 0.01 |
| Galactose | 0.17 ± 0.00 | 1.55 ± 0.01 | 0.10 ± 0.00 | 0.46 ± 0.01 |
| Arabinose | - | 0.19 ± 0.00 | 0.02 ± 0.00 | 0.82 ± 0.01 |
| Mannose | 0.17 ± 0.00 | - | - | - |
| Inhibitors (g/L) | ||||
| Formic acid | 2.45 ± 0.01 | 1.96 ± 0.02 | 1.42 ± 0.01 | 1.87 ± 0.02 |
| Acetic acid | 2.91 ± 0.01 | 9.92 ± 0.03 | 2.16 ± 0.02 | 1.59 ± 0.01 |
| Furfural | 3.74 ± 0.02 | 9.74 ± 0.03 | 0.00 ± 0.00 | 0.02 ± 0.00 |
| Hydroxymethylfurfural | 1.47 ± 0.01 | 1.06 ± 0.01 | 0.06 ± 0.00 | 0.11 ± 0.00 |
| Levulinic acid | 3.31 ± 0.02 | 2.05 ± 0.02 | 0.31 ± 0.01 | 0.16 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halysh, V.; Romero-García, J.M.; Vidal, A.M.; Kulik, T.; Palianytsia, B.; García, M.; Castro, E. Apricot Seed Shells and Walnut Shells as Unconventional Sugars and Lignin Sources. Molecules 2023, 28, 1455. https://doi.org/10.3390/molecules28031455
Halysh V, Romero-García JM, Vidal AM, Kulik T, Palianytsia B, García M, Castro E. Apricot Seed Shells and Walnut Shells as Unconventional Sugars and Lignin Sources. Molecules. 2023; 28(3):1455. https://doi.org/10.3390/molecules28031455
Chicago/Turabian StyleHalysh, Vita, Juan Miguel Romero-García, Alfonso M. Vidal, Tetiana Kulik, Borys Palianytsia, Minerva García, and Eulogio Castro. 2023. "Apricot Seed Shells and Walnut Shells as Unconventional Sugars and Lignin Sources" Molecules 28, no. 3: 1455. https://doi.org/10.3390/molecules28031455
APA StyleHalysh, V., Romero-García, J. M., Vidal, A. M., Kulik, T., Palianytsia, B., García, M., & Castro, E. (2023). Apricot Seed Shells and Walnut Shells as Unconventional Sugars and Lignin Sources. Molecules, 28(3), 1455. https://doi.org/10.3390/molecules28031455









