Nano-Liquid Chromatography with a New Monolithic Column for the Analysis of Coenzyme Q10 in Pistachio Samples
Abstract
:1. Introduction
2. Results and Discussion
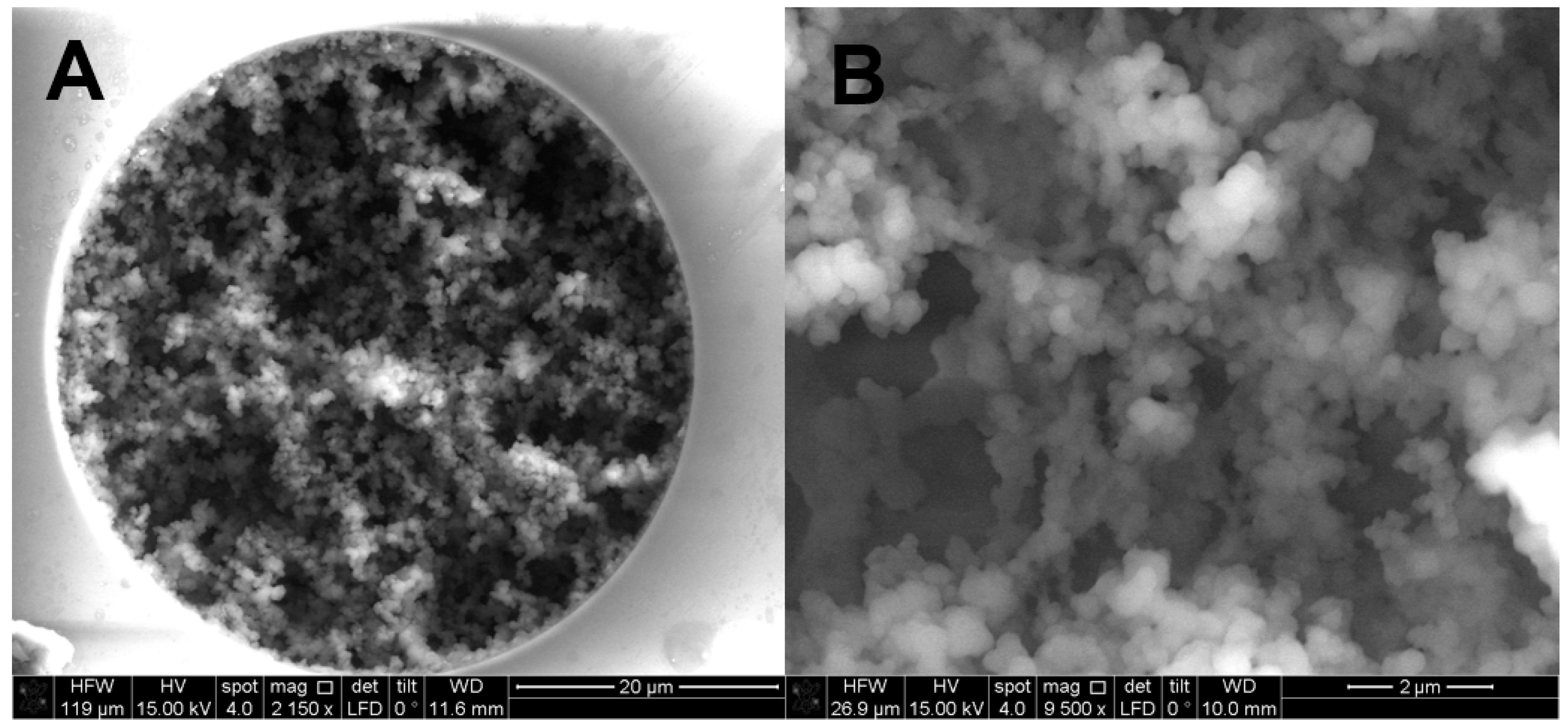
2.1. Preparation and Characterization of the Monolithic Column with 50 µm i.d.
2.2. Study Design
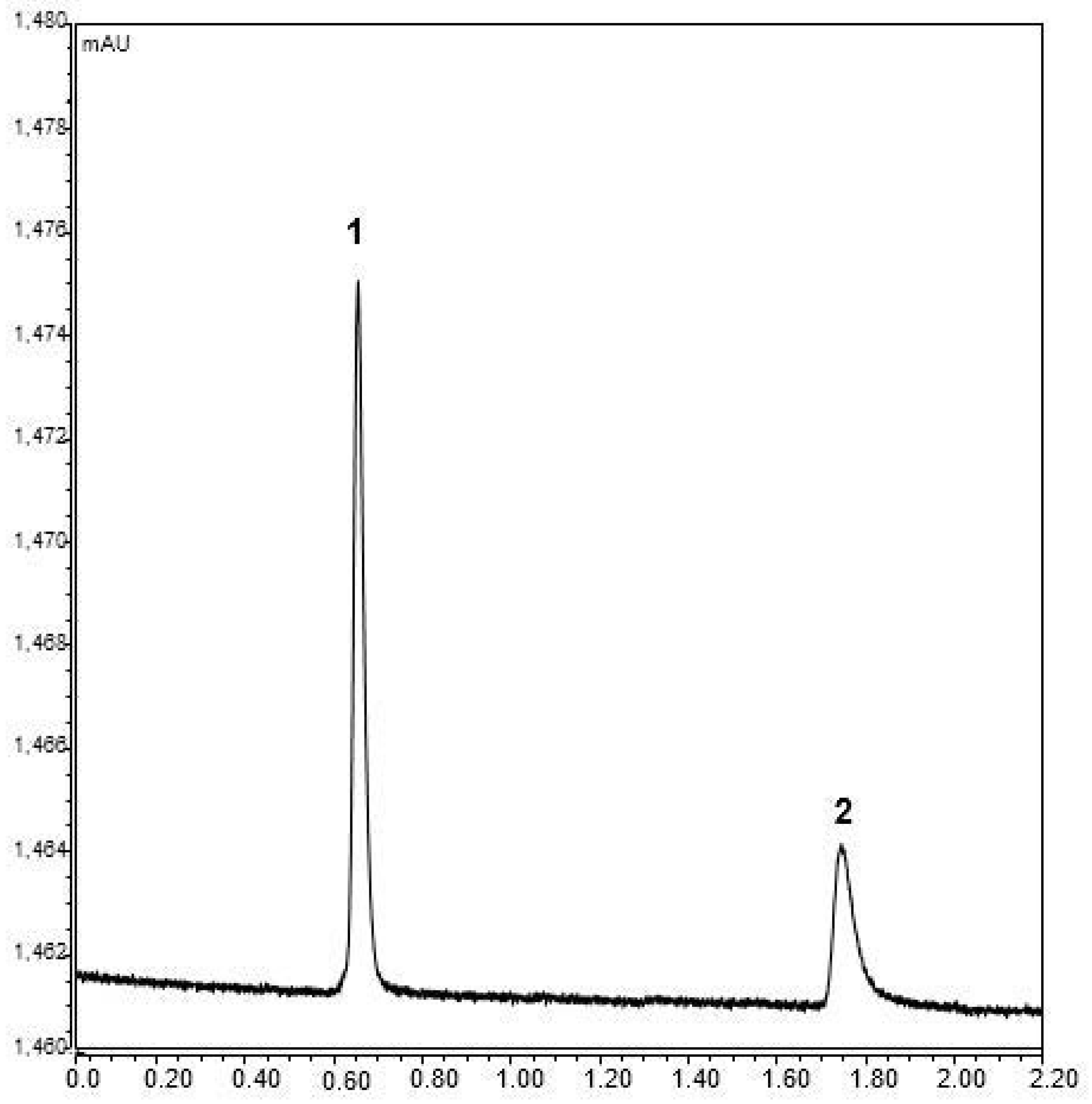
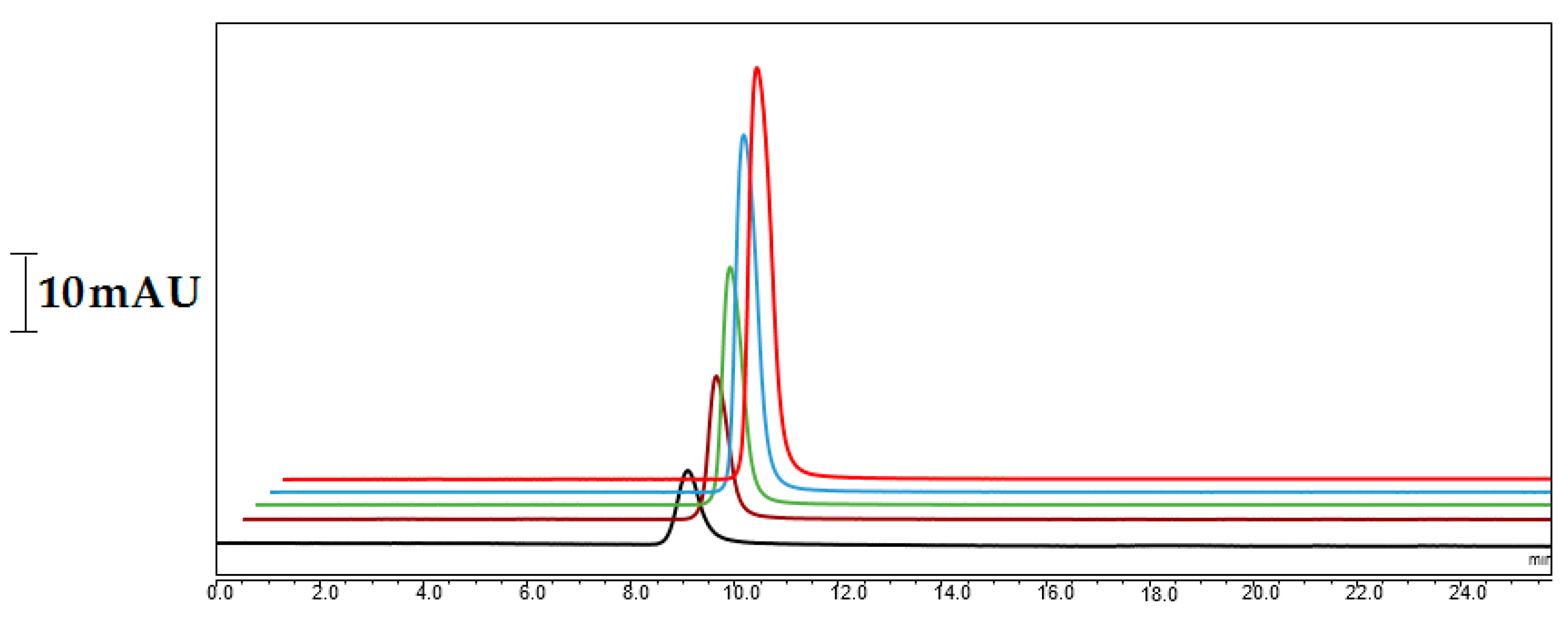
2.3. Loading Capacity
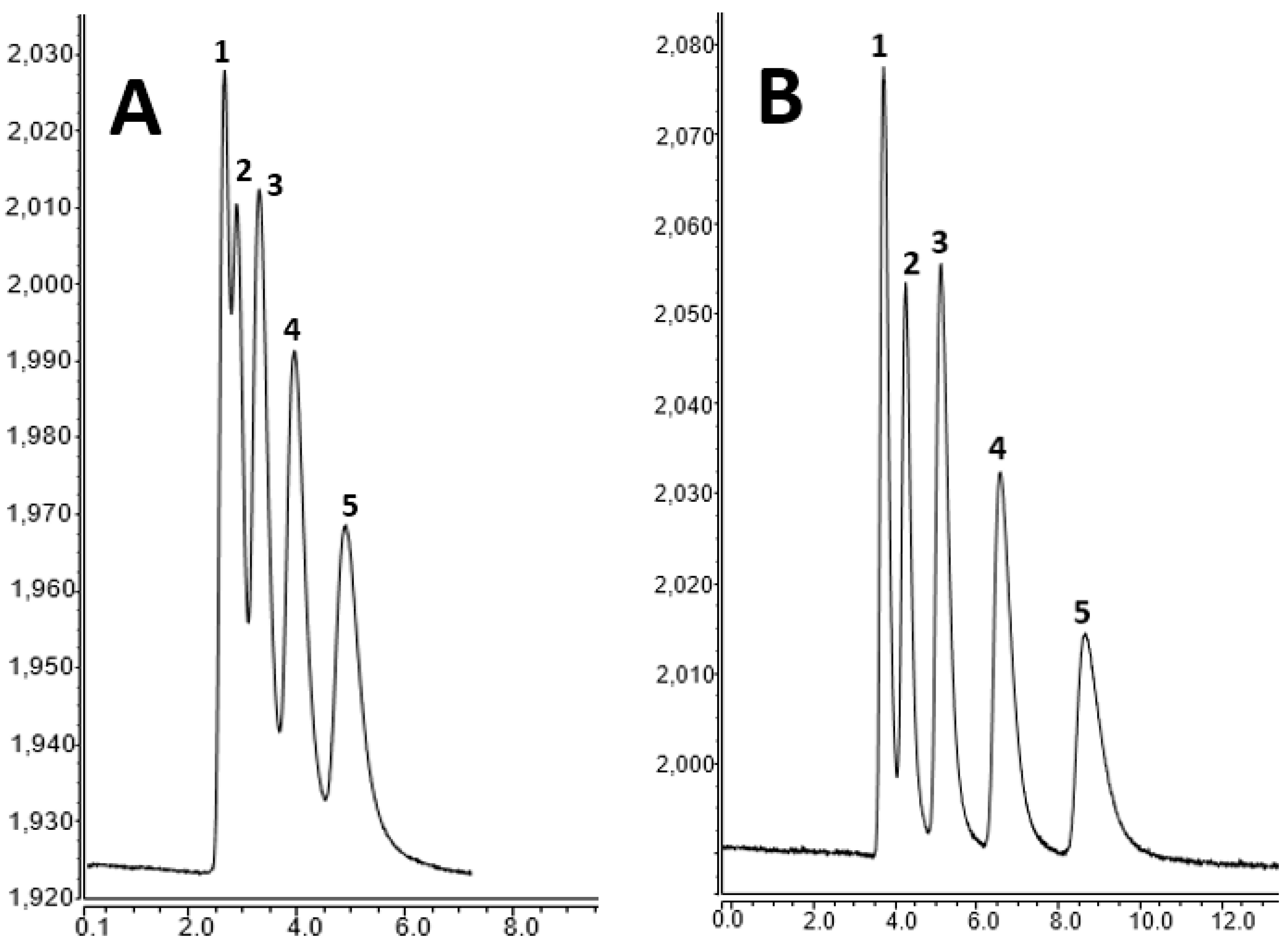
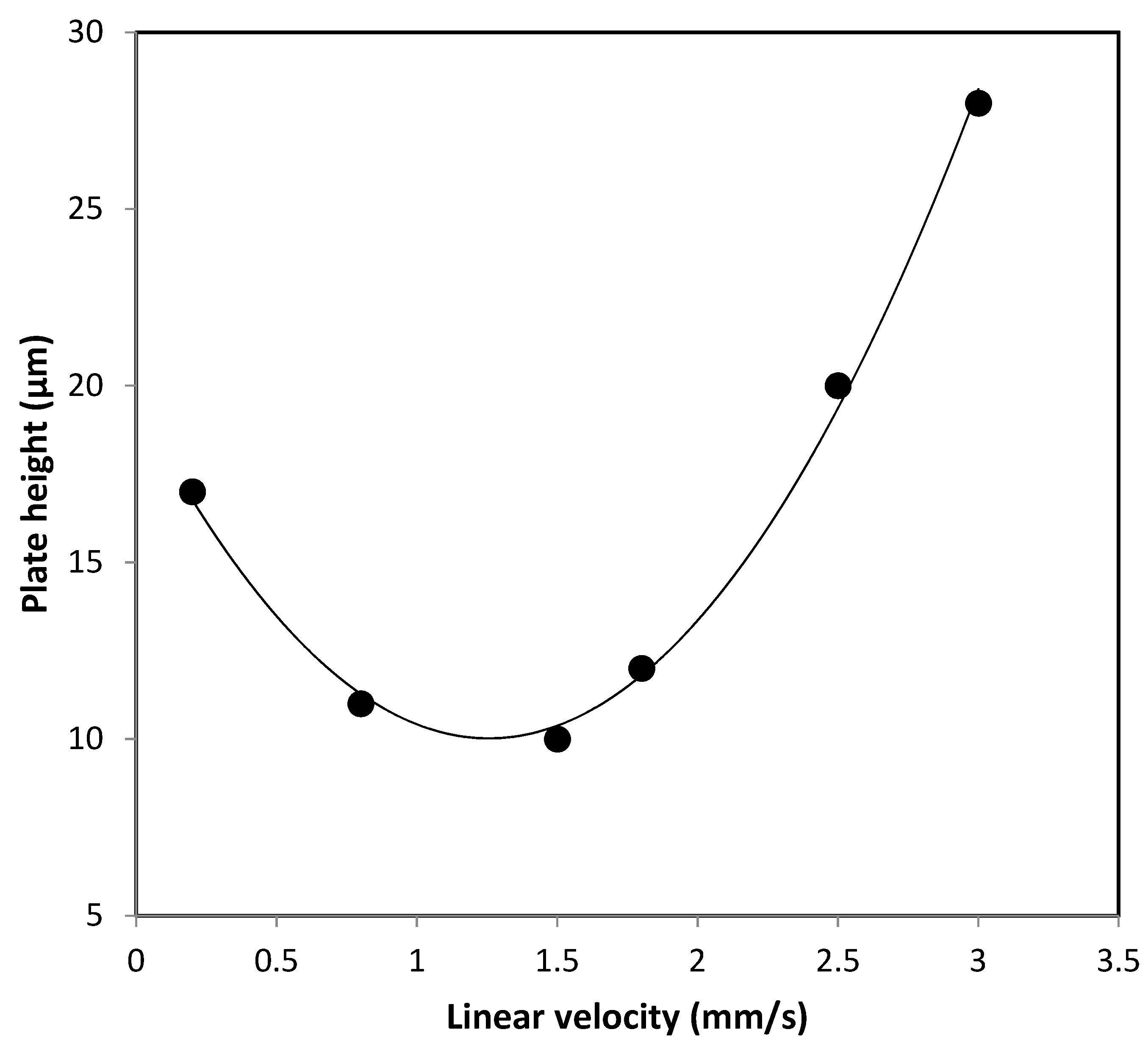
2.4. The Optimization of Sample Preparation and Chromatography Conditions for Nano-LC
2.5. Method Validation
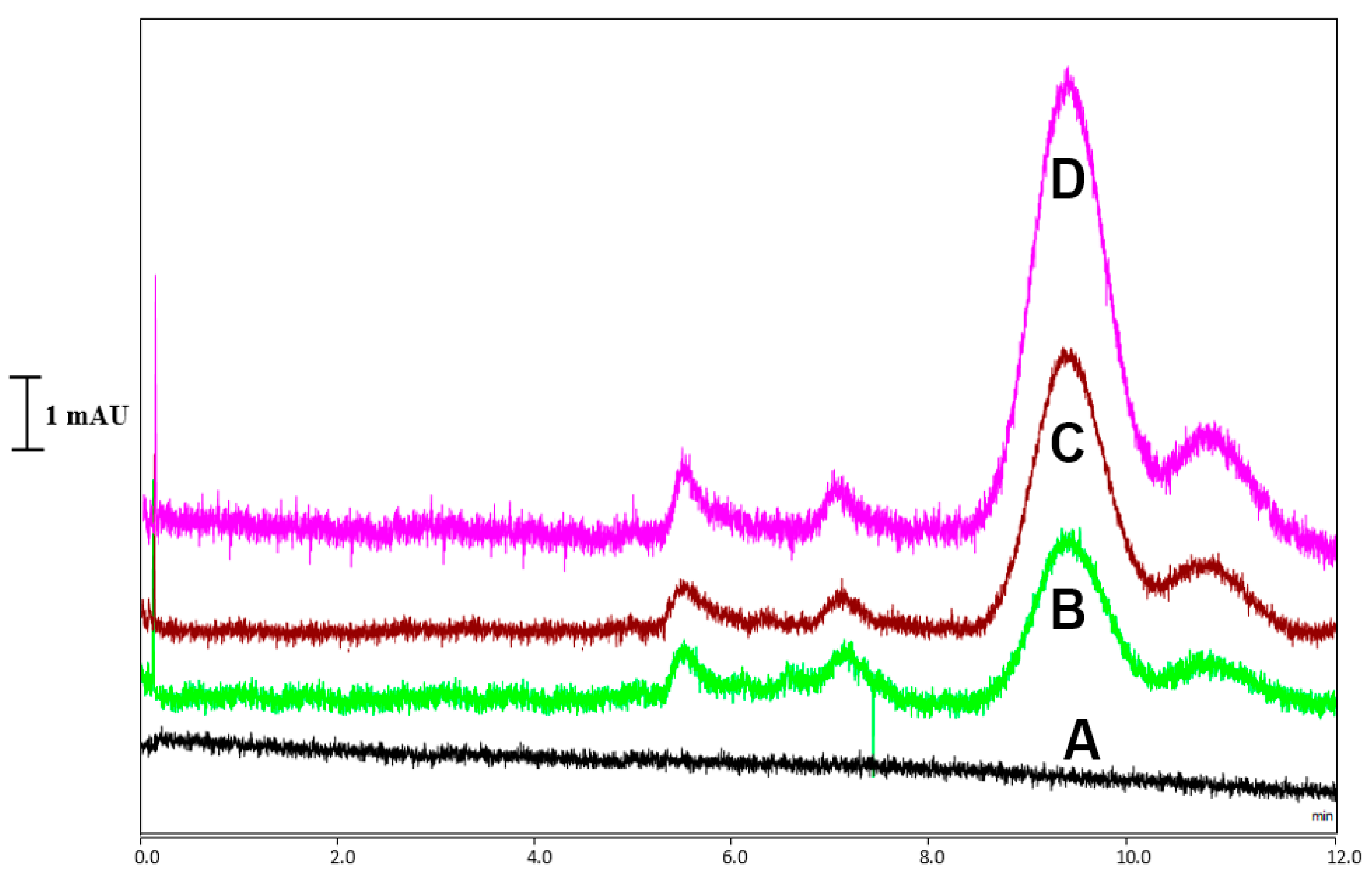
2.6. CoQ10 Analysis in Pistachio Samples
3. Materials and Methods
3.1. Chemicals
3.2. Instruments
3.3. The Preparation of Monolithic Column
3.4. Chromatographic Conditions
3.5. Extraction of Coenzyme Q10 from Pistachio Samples
3.6. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kaur:, S.; Das, M. Functional Foods: An Overview. Food Sci. Biotechnol. 2011, 20, 861. [Google Scholar] [CrossRef]
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. Coenzyme Q10 Supplementation: Efficacy, Safety, and Formulation Challenges. Compr. Rev. Food Sci. Food Saf. 2020, 19, 574–594. [Google Scholar] [CrossRef] [PubMed]
- Pravst, I.; Žmitek, K.; Žmitek, J. Coenzyme Q10 Contents in Foods and Fortification Strategies. Crit. Rev. Food Sci. Nutr. 2010, 50, 269–280. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Szczepski, K.; Poulson, B.G.; Chandra, K.; McKay, R.T.; Dhahri, M.; Alahmari, F.; Jaremko, L.; Lachowicz, J.I.; Jaremko, M. NMR as a “Gold Standard” Method in Drug Design and Discovery. Molecules 2020, 25, 4597. [Google Scholar] [CrossRef] [PubMed]
- Chandra, K.; Al-Harthi, S.; Almulhim, F.; Emwas, A.-H.; Jaremko, Ł.; Jaremko, M. The Robust NMR Toolbox for Metabolomics. Mol. Omics 2021, 17, 719–724. [Google Scholar] [CrossRef]
- Aydoğan, C.; Rigano, F.; Krčmová, L.K.; Chung, D.S.; Macka, M.; Mondello, L. Miniaturized LC in Molecular Omics. Anal. Chem. 2020, 92, 11485–11497. [Google Scholar] [CrossRef]
- Aydoğan, C.; Beltekin, B.; Alharthi, S.; Ağca, C.A.; Erdoğan, İ.Y. Nano-Liquid Chromatography with Monolithic Stationary Phase Based on Naphthyl Monomer for Proteomics Analysis. J. Chromatogr. A 2023, 1690, 463804. [Google Scholar] [CrossRef]
- Aydoğan, C. Nanoscale Separations Based on LC and CE for Food Analysis: A Review. TrAC Trends Anal. Chem. 2019, 121, 115693. [Google Scholar] [CrossRef]
- Ercan, P.; El, S.N. Changes in Content of Coenzyme Q10 in Beef Muscle, Beef Liver and Beef Heart with Cooking and in Vitro Digestion. J. Food Compos. Anal. 2011, 24, 1136–1140. [Google Scholar] [CrossRef]
- Xue, X.; Zhao, J.; Chen, L.; Zhou, J.; Yue, B.; Li, Y.; Wu, L.; Liu, F. Analysis of Coenzyme Q10 in Bee Pollen Using Online Cleanup by Accelerated Solvent Extraction and High Performance Liquid Chromatography. Food Chem. 2012, 133, 573–578. [Google Scholar] [CrossRef]
- Vasconcelos Soares Maciel, E.; de Toffoli, A.L.; Sobieski, E.; Domingues Nazário, C.E.; Lanças, F.M. Miniaturized Liquid Chromatography Focusing on Analytical Columns and Mass Spectrometry: A Review. Anal. Chim. Acta 2020, 1103, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Demir, N.; Aydoğan, C. ProFlow Nano-liquid Chromatography with a Graphene Oxide-functionalized Monolithic Nano-column for the Simultaneous Determination of Chloramphenicol and Chloramphenicol Glucuronide in Foods. J. Food Sci. 2022, 87, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Şeker, S.; Alharthi, S.; Aydoğan, C. Open Tubular Nano-Liquid Chromatography with a New Polylysine Grafted on Graphene Oxide Stationary Phase for the Separation and Determination of Casein Protein Variants in Milk. J. Chromatogr. A 2022, 1667, 462885. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Olsen, C.; Lundanes, E. Nano Liquid Chromatography Columns. Analyst 2019, 144, 7090–7104. [Google Scholar] [CrossRef]
- Shan, L.; Jones, B.R. Nano-LC: An Updated Review. Biomed. Chromatogr. 2022, 36, e5317. [Google Scholar] [CrossRef]
- Aydoğan, C.; Yılmaz, F.; Denizli, A. Cation Exchange/Hydrophobic Interaction Monolithic Chromatography of Small Molecules and Proteins by Nano Liquid Chromatography†. J. Sep. Sci. 2013, 36, 1685–1692. [Google Scholar] [CrossRef]
- Aydoğan, C.; Gökaltun, A.; Denizli, A.; El-Rassi, Z. Organic Polymer-Based Monolithic Capillary Columns and Their Applications in Food Analysis. J. Sep. Sci. 2019, 42, 962–979. [Google Scholar] [CrossRef]
- Aydoğan, C. Boronic Acid-Fumed Silica Nanoparticles Incorporated Large Surface Area Monoliths for Protein Separation by Nano-Liquid Chromatography. Anal. Bioanal. Chem. 2016, 408, 8457–8466. [Google Scholar] [CrossRef]
- Aydoğan, C.; Erdoğan, İ.Y.; El-Rassi, Z. Hydrophobic AEROSIL®R972 Fumed Silica Nanoparticles Incorporated Monolithic Nano-Columns for Small Molecule and Protein Separation by Nano-Liquid Chromatography. Molecules 2022, 27, 2306. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Dolan, J.W. Introduction to Modern Liquid Chromatography; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 978-0-470-50818-3. [Google Scholar]
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, Tissue Uptake, Metabolism and Pharmacokinetics. Free. Radic. Res. 2006, 40, 445–453. [Google Scholar] [CrossRef]
- Mattila Pirjo, Kumpulainen Jorma Coenzymes Q9 and Q10: Contents in Foods and Dietary Intake. J. Food Compos. Anal. 2001, 14, 409–417. [CrossRef]
- Aslan, H.; Günyel, Z.; Sarıkaya, T.; Golgiyaz, S.; Aydoğan, C. Determination of the Geographic Origin of 52 Honey Samples Based on the Assessment of Anionic Content Profiling with a New Algorithm Using Monolithic Column-based Micellar Nano-liquid Chromatography. J. Food Sci. 2022, 87, 4636–4648. [Google Scholar] [CrossRef] [PubMed]
- Barshop, B.A.; Gangoiti, J.A. Analysis of Coenzyme Q in Human Blood and Tissues. Mitochondrion 2007, 7, S89–S93. [Google Scholar] [CrossRef] [PubMed]
- Román-Pizarro, V.; Fernández-Romero, J.M.; Gómez-Hens, A. Automatic Determination of Coenzyme Q10 in Food Using Cresyl Violet Encapsulated into Magnetoliposomes. Food Chem. 2017, 221, 864–870. [Google Scholar] [CrossRef] [PubMed]



| Analyte | LOD µg/kg | LOQ µg/kg | R2 | Precision (RSD%) a |
|---|---|---|---|---|
| Inter day Intra day | ||||
| CoQ10 | 0.05 | 0.48 | 0.9992 | 1.5 1.9 |
| Samples | Barak Yıldızı | Tekin | Halebi | Uzun | Siirt | Kırmızı |
|---|---|---|---|---|---|---|
| CoQ10 (µg/g) | 30.5 ± 0.4 | 42.16 ± 0.6 | 17.21 ± 0.6 | 10.21 ± 0.4 | 28.21 ± 0.5 | 30.2 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydoğan, C.; Beltekin, B.; Demir, N.; Yurt, B.; El Rassi, Z. Nano-Liquid Chromatography with a New Monolithic Column for the Analysis of Coenzyme Q10 in Pistachio Samples. Molecules 2023, 28, 1423. https://doi.org/10.3390/molecules28031423
Aydoğan C, Beltekin B, Demir N, Yurt B, El Rassi Z. Nano-Liquid Chromatography with a New Monolithic Column for the Analysis of Coenzyme Q10 in Pistachio Samples. Molecules. 2023; 28(3):1423. https://doi.org/10.3390/molecules28031423
Chicago/Turabian StyleAydoğan, Cemil, Büşra Beltekin, Nurullah Demir, Bayram Yurt, and Ziad El Rassi. 2023. "Nano-Liquid Chromatography with a New Monolithic Column for the Analysis of Coenzyme Q10 in Pistachio Samples" Molecules 28, no. 3: 1423. https://doi.org/10.3390/molecules28031423
APA StyleAydoğan, C., Beltekin, B., Demir, N., Yurt, B., & El Rassi, Z. (2023). Nano-Liquid Chromatography with a New Monolithic Column for the Analysis of Coenzyme Q10 in Pistachio Samples. Molecules, 28(3), 1423. https://doi.org/10.3390/molecules28031423








