Towards Probing Conformational States of Y2 Receptor Using Hyperpolarized 129Xe NMR
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of the Y2R-A202C-CrA Conjugate
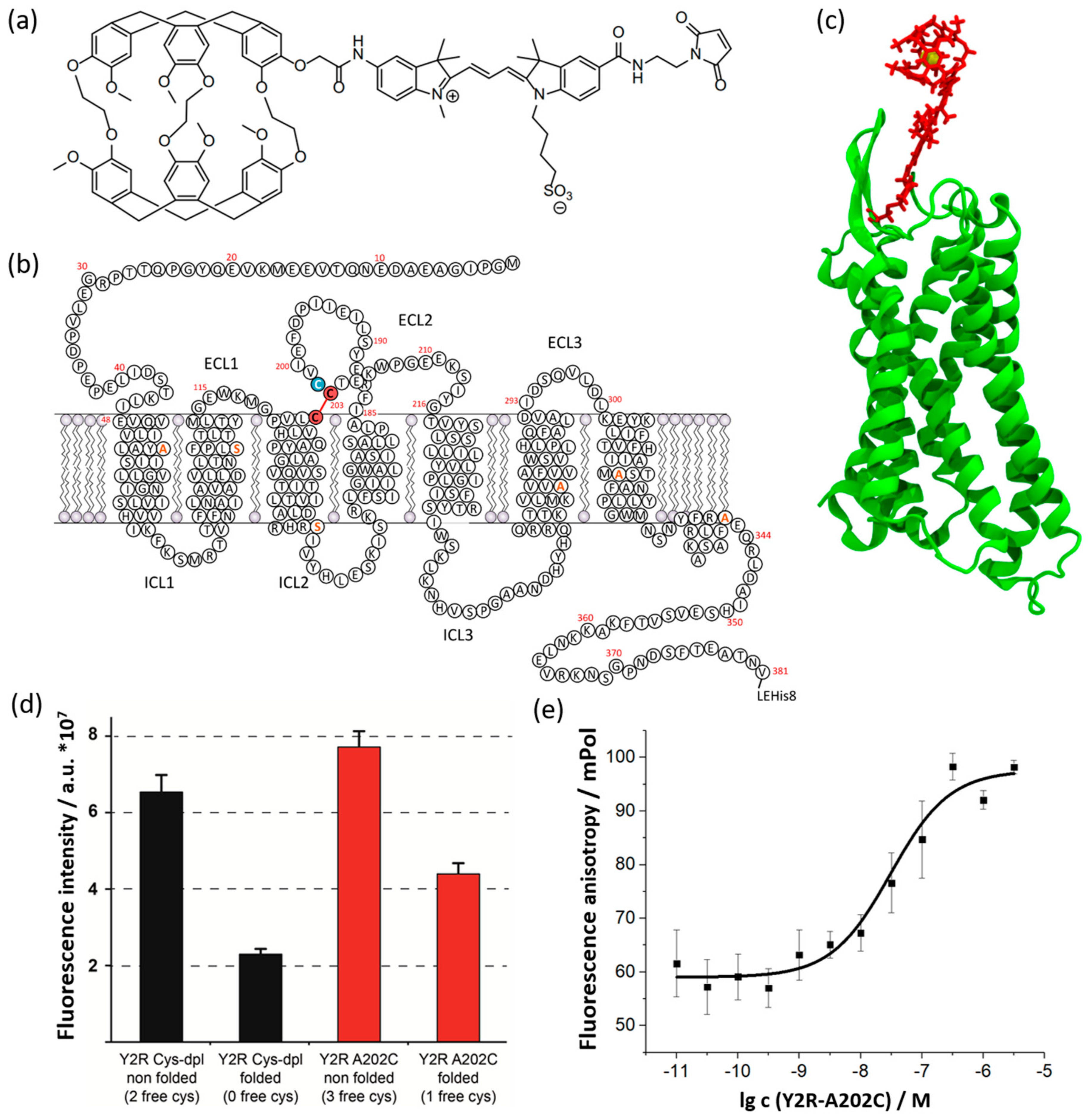
2.2. Xenon NMR
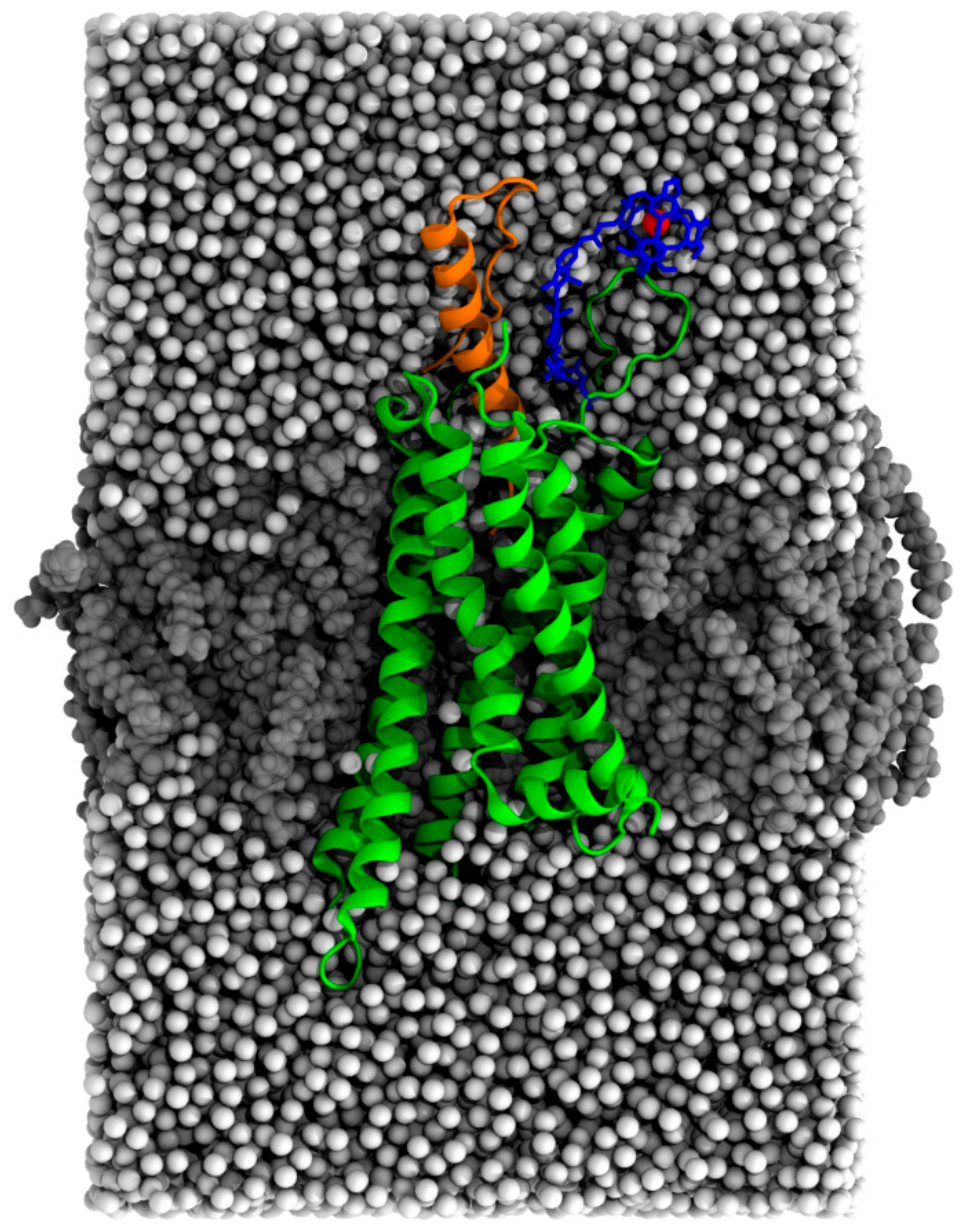
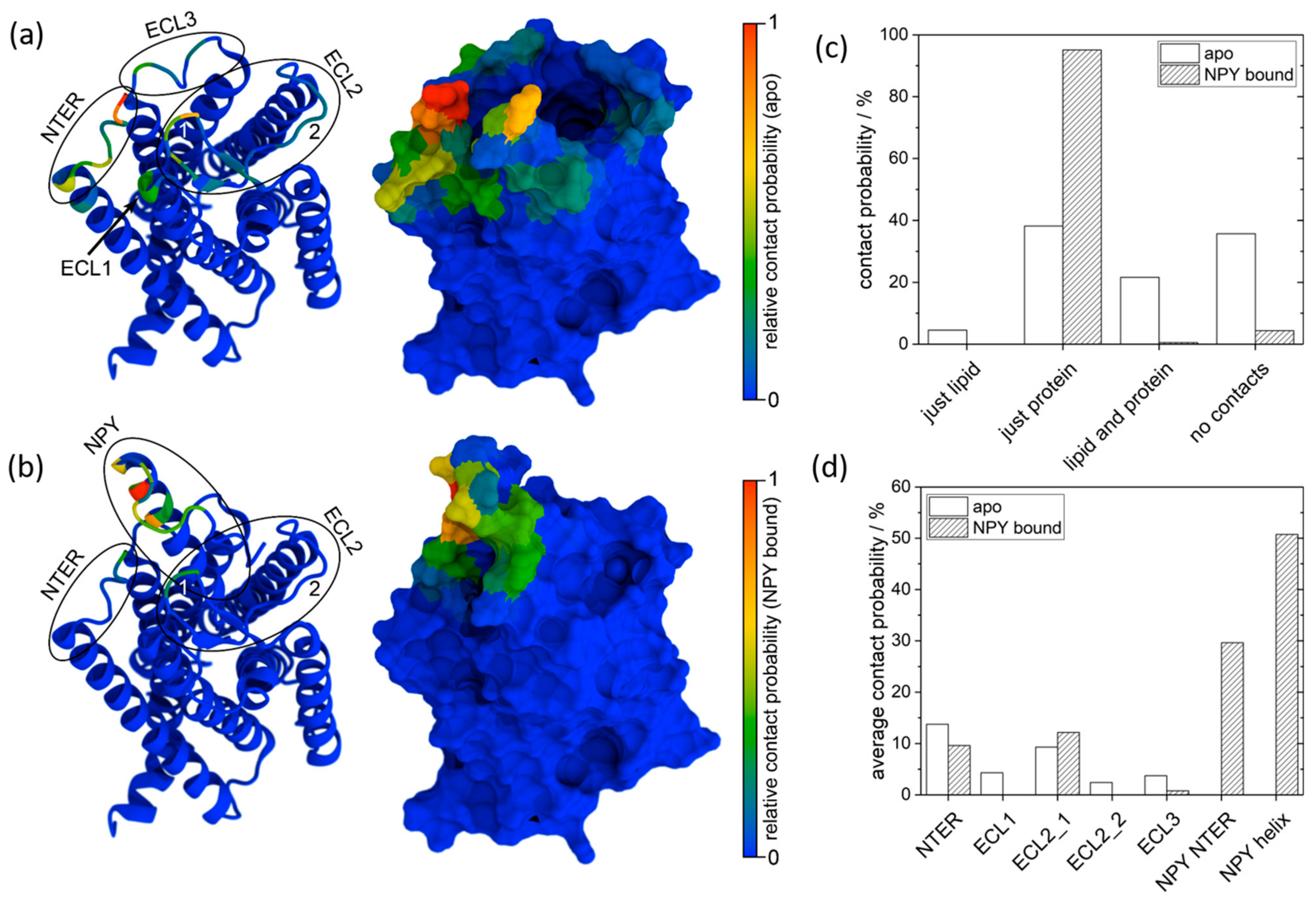
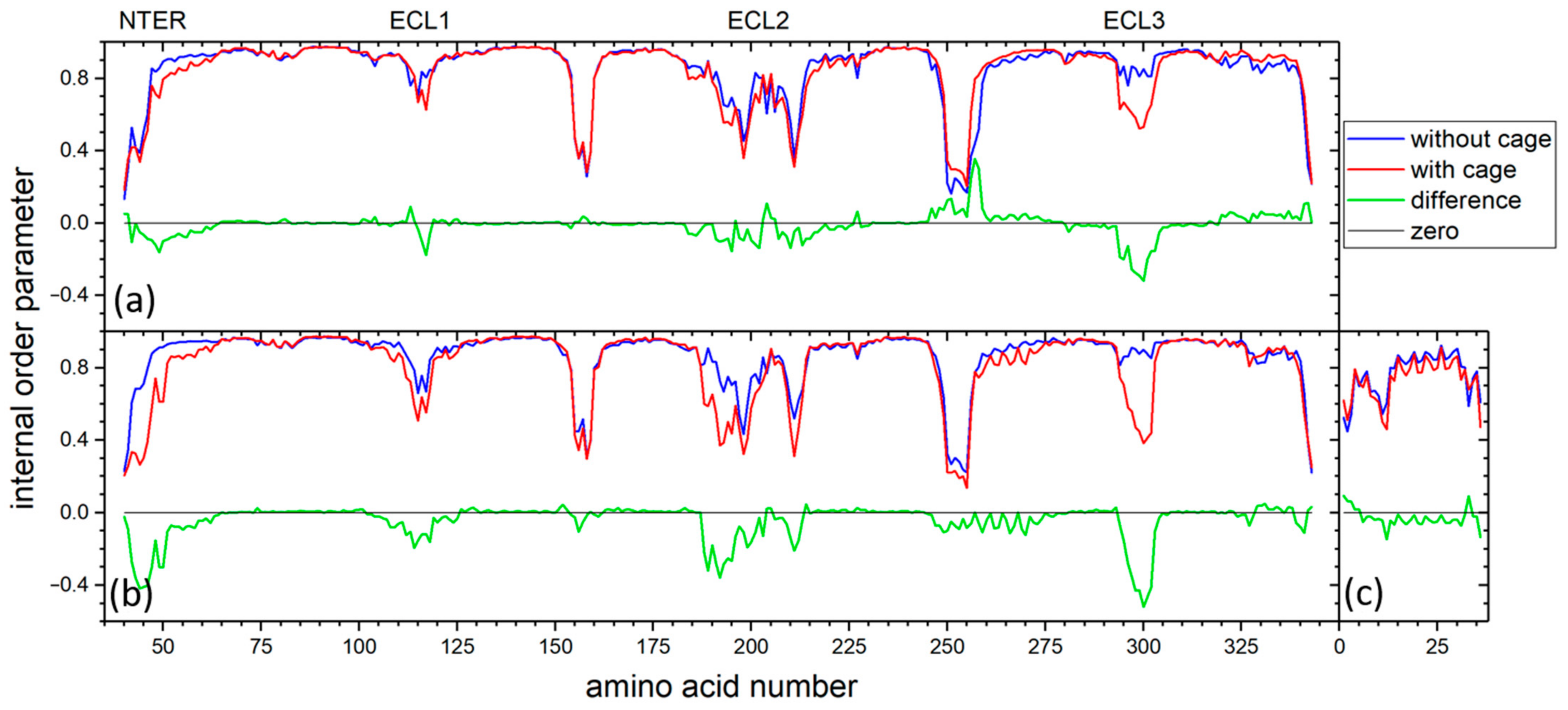
2.3. MD Simulation
2.4. Signal Assignment
3. Conclusions
4. Materials and Methods
4.1. Sample Preparation
4.2. CrA Functionalization
4.3. NMR Experiments
4.4. MD Simulations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Manglik, A.; Kim, T.H.; Masureel, M.; Altenbach, C.; Yang, Z.; Hilger, D.; Lerch, M.T.; Kobilka, T.S.; Thian, F.S.; Hubbell, W.L.; et al. Structural Insights into the Dynamic Process of β2-Adrenergic Receptor Signaling. Cell 2015, 161, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.K.; Pandey, A.; Tran, D.P.; Villanueva, N.L.; Kitao, A.; Sunahara, R.K.; Sljoka, A.; Prosser, R.S. Delineating the conformational landscape of the adenosine A2A receptor during G protein coupling. Cell 2021, 184, 1884–1894.e14. [Google Scholar] [CrossRef] [PubMed]
- Bostock, M.J.; Solt, A.S.; Nietlispach, D. The role of NMR spectroscopy in mapping the conformational landscape of GPCRs. Curr. Opin. Struct. Biol. 2019, 57, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Krug, U.; Gloge, A.; Schmidt, P.; Becker-Baldus, J.; Bernhard, F.; Kaiser, A.; Montag, C.; Gauglitz, M.; Vishnivetskiy, S.A.; Gurevich, V.V.; et al. The Conformational Equilibrium of the Neuropeptide Y2 Receptor in Bilayer Membranes. Angew. Chem. Int. Ed. Engl. 2020, 59, 23854–23861. [Google Scholar] [CrossRef]
- Park, S.H.; Das, B.B.; Casagrande, F.; Tian, Y.; Nothnagel, H.J.; Chu, M.; Kiefer, H.; Maier, K.; de Angelis, A.A.; Marassi, F.M.; et al. Structure of the chemokine receptor CXCR1 in phospholipid bilayers. Nature 2012, 491, 779–783. [Google Scholar] [CrossRef]
- Catoire, L.J.; Damian, M.; Giusti, F.; Martin, A.; van Heijenoort, C.; Popot, J.-L.; Guittet, E.; Banères, J.-L. Structure of a GPCR ligand in its receptor-bound state: Leukotriene B4 adopts a highly constrained conformation when associated to human BLT2. J. Am. Chem. Soc. 2010, 132, 9049–9057. [Google Scholar] [CrossRef]
- Schrottke, S.; Kaiser, A.; Vortmeier, G.; Els-Heindl, S.; Worm, D.; Bosse, M.; Schmidt, P.; Scheidt, H.A.; Beck-Sickinger, A.G.; Huster, D. Expression, Functional Characterization, and Solid-State NMR Investigation of the G Protein-Coupled GHS Receptor in Bilayer Membranes. Sci. Rep. 2017, 7, 46128. [Google Scholar] [CrossRef]
- Schmidt, P.; Thomas, L.; Müller, P.; Scheidt, H.A.; Huster, D. The G-protein-coupled neuropeptide Y receptor type 2 is highly dynamic in lipid membranes as revealed by solid-state NMR spectroscopy. Chemistry 2014, 20, 4986–4992. [Google Scholar] [CrossRef]
- Joedicke, L.; Mao, J.; Kuenze, G.; Reinhart, C.; Kalavacherla, T.; Jonker, H.R.A.; Richter, C.; Schwalbe, H.; Meiler, J.; Preu, J.; et al. The molecular basis of subtype selectivity of human kinin G-protein-coupled receptors. Nat. Chem. Biol. 2018, 14, 284–290. [Google Scholar] [CrossRef]
- Grahl, A.; Abiko, L.A.; Isogai, S.; Sharpe, T.; Grzesiek, S. A high-resolution description of β1-adrenergic receptor functional dynamics and allosteric coupling from backbone NMR. Nat. Commun. 2020, 11, 2216. [Google Scholar] [CrossRef]
- Horst, R.; Liu, J.J.; Stevens, R.C.; Wüthrich, K. β2-adrenergic receptor activation by agonists studied with ¹⁹F NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 2013, 52, 10762–10765. [Google Scholar] [CrossRef] [PubMed]
- Elgeti, M.; Hubbell, W.L. DEER Analysis of GPCR Conformational Heterogeneity. Biomolecules 2021, 11, 778. [Google Scholar] [CrossRef] [PubMed]
- Goodson, B.M. Nuclear magnetic resonance of laser-polarized noble gases in molecules, materials, and organisms. J. Magn. Reson. 2002, 155, 157–216. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.G.; Happer, W. Spin-exchange optical pumping of noble-gas nuclei. Rev. Mod. Phys. 1997, 69, 629–642. [Google Scholar] [CrossRef]
- Spence, M.M.; Rubin, S.M.; Dimitrov, I.E.; Ruiz, E.J.; Wemmer, D.E.; Pines, A.; Yao, S.Q.; Tian, F.; Schultz, P.G. Functionalized xenon as a biosensor. Proc. Natl. Acad. Sci. USA 2001, 98, 10654–10657. [Google Scholar] [CrossRef]
- Berthault, P.; Huber, G.; Desvaux, H. Biosensing using laser-polarized xenon NMR/MRI. Prog. Nucl. Magn. Reson. Spectrosc. 2009, 55, 35–60. [Google Scholar] [CrossRef]
- Taratula, O.; Dmochowski, I.J. Functionalized 129Xe contrast agents for magnetic resonance imaging. Curr. Opin. Chem. Biol. 2010, 14, 97–104. [Google Scholar] [CrossRef]
- Pedragosa-Badia, X.; Stichel, J.; Beck-Sickinger, A.G. Neuropeptide Y receptors: How to get subtype selectivity. Front. Endocrinol. 2013, 4, 5. [Google Scholar] [CrossRef]
- Kaiser, A.; Müller, P.; Zellmann, T.; Scheidt, H.A.; Thomas, L.; Bosse, M.; Meier, R.; Meiler, J.; Huster, D.; Beck-Sickinger, A.G.; et al. Unwinding of the C-Terminal Residues of Neuropeptide Y is critical for Y2 Receptor Binding and Activation. Angew. Chem. Int. Ed. Engl. 2015, 54, 7446–7449. [Google Scholar] [CrossRef]
- Vogel, A.; Bosse, M.; Gauglitz, M.; Wistuba, S.; Schmidt, P.; Kaiser, A.; Gurevich, V.V.; Beck-Sickinger, A.G.; Hildebrand, P.W.; Huster, D. The Dynamics of the Neuropeptide Y Receptor Type 1 Investigated by Solid-State NMR and Molecular Dynamics Simulation. Molecules 2020, 25, 5489. [Google Scholar] [CrossRef]
- Witte, K.; Kaiser, A.; Schmidt, P.; Splith, V.; Thomas, L.; Berndt, S.; Huster, D.; Beck-Sickinger, A.G. Oxidative in vitro folding of a cysteine deficient variant of the G protein-coupled neuropeptide Y receptor type 2 improves stability at high concentration. Biol. Chem. 2013, 394, 1045–1056. [Google Scholar] [CrossRef]
- Laugwitz, J.M.; Haeri, H.H.; Kaiser, A.; Krug, U.; Hinderberger, D.; Beck-Sickinger, A.G.; Schmidt, P. Probing the Y2 Receptor on Transmembrane, Intra- and Extra-Cellular Sites for EPR Measurements. Molecules 2020, 25, 4143. [Google Scholar] [CrossRef]
- Schmidt, P.; Berger, C.; Scheidt, H.A.; Berndt, S.; Bunge, A.; Beck-Sickinger, A.G.; Huster, D. A reconstitution protocol for the in vitro folded human G-protein coupled Y2 receptor into lipid environment. Biophys. Chem. 2010, 150, 29–36. [Google Scholar] [CrossRef]
- Schmidt, P.; Bender, B.J.; Kaiser, A.; Gulati, K.; Scheidt, H.A.; Hamm, H.E.; Meiler, J.; Beck-Sickinger, A.G.; Huster, D. Improved in Vitro Folding of the Y2 G Protein-Coupled Receptor into Bicelles. Front. Mol. Biosci. 2017, 4, 100. [Google Scholar] [CrossRef]
- Alexandrov, A.I.; Mileni, M.; Chien, E.Y.T.; Hanson, M.A.; Stevens, R.C. Microscale fluorescent thermal stability assay for membrane proteins. Structure 2008, 16, 351–359. [Google Scholar] [CrossRef]
- Tang, T.; Hartig, C.; Chen, Q.; Zhao, W.; Kaiser, A.; Zhang, X.; Zhang, H.; Qu, H.; Yi, C.; Ma, L.; et al. Structural basis for ligand recognition of the neuropeptide Y Y2 receptor. Nat. Commun. 2021, 12, 737. [Google Scholar] [CrossRef]
- Taratula, O.; Hill, P.A.; Khan, N.S.; Carroll, P.J.; Dmochowski, I.J. Crystallographic observation of ‘induced fit’ in a cryptophane host-guest model system. Nat. Commun. 2010, 1, 148. [Google Scholar] [CrossRef]
- Korchak, S.; Kilian, W.; Schröder, L.; Mitschang, L. Design and comparison of exchange spectroscopy approaches to cryptophane-xenon host-guest kinetics. J. Magn. Reson. 2016, 265, 139–145. [Google Scholar] [CrossRef]
- Schröder, L.; Lowery, T.J.; Hilty, C.; Wemmer, D.E.; Pines, A. Molecular imaging using a targeted magnetic resonance hyperpolarized biosensor. Science 2006, 314, 446–449. [Google Scholar] [CrossRef]
- Schröder, L.; Meldrum, T.; Smith, M.; Lowery, T.J.; Wemmer, D.E.; Pines, A. Temperature response of 129Xe depolarization transfer and its application for ultrasensitive NMR detection. Phys. Rev. Lett. 2008, 100, 257603. [Google Scholar] [CrossRef]
- Korchak, S.E.; Kilian, W.; Mitschang, L. Configuration and Performance of a Mobile (129)Xe Polarizer. Appl. Magn. Reson. 2013, 44, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, T.; Schröder, L.; Denger, P.; Wemmer, D.E.; Pines, A. Xenon-based molecular sensors in lipid suspensions. J. Magn. Reson. 2010, 205, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Sloniec, J.; Schnurr, M.; Witte, C.; Resch-Genger, U.; Schröder, L.; Hennig, A. Biomembrane interactions of functionalized cryptophane-A: Combined fluorescence and 129Xe NMR studies of a bimodal contrast agent. Chemistry 2013, 19, 3110–3118. [Google Scholar] [CrossRef] [PubMed]
- Korchak, S.; Riemer, T.; Kilian, W.; Mitschang, L. Quantitative Assessment of Xenon Exchange Kinetics with Cucurbit6uril in Physiological Saline. Chemphyschem 2018, 19, 1859–1865. [Google Scholar] [CrossRef]
- Kunth, M.; Witte, C.; Hennig, A.; Schröder, L. Identification, classification, and signal amplification capabilities of high-turnover gas binding hosts in ultra-sensitive NMR. Chem. Sci. 2015, 6, 6069–6075. [Google Scholar] [CrossRef]
- Zaiss, M.; Schnurr, M.; Bachert, P. Analytical solution for the depolarization of hyperpolarized nuclei by chemical exchange saturation transfer between free and encapsulated xenon (HyperCEST). J. Chem. Phys. 2012, 136, 144106. [Google Scholar] [CrossRef]
- Korchak, S.; Riemer, T.; Kilian, W.; Mitschang, L. Quantitative biosensor detection by chemically exchanging hyperpolarized 129Xe. Phys. Chem. Chem. Phys. 2018, 20, 1800–1808. [Google Scholar] [CrossRef]
- Lowery, T.J.; Garcia, S.; Chavez, L.; Ruiz, E.J.; Wu, T.; Brotin, T.; Dutasta, J.-P.; King, D.S.; Schultz, P.G.; Pines, A.; et al. Optimization of xenon biosensors for detection of protein interactions. Chembiochem 2006, 7, 65–73. [Google Scholar] [CrossRef]
- Sears, D.N.; Jameson, C.J. Theoretical calculations of the Xe chemical shifts in cryptophane cages. J. Chem. Phys. 2003, 119, 12231–12244. [Google Scholar] [CrossRef]
- Tang, T.; Tan, Q.; Han, S.; Diemar, A.; Löbner, K.; Wang, H.; Schüß, C.; Behr, V.; Mörl, K.; Wang, M.; et al. Receptor-specific recognition of NPY peptides revealed by structures of NPY receptors. Sci. Adv. 2022, 8, eabm1232. [Google Scholar] [CrossRef]
- Abiko, L.A.; Dias Teixeira, R.; Engilberge, S.; Grahl, A.; Mühlethaler, T.; Sharpe, T.; Grzesiek, S. Filling of a water-free void explains the allosteric regulation of the β1-adrenergic receptor by cholesterol. Nat. Chem. 2022, 14, 1133–1141. [Google Scholar] [CrossRef]
- Mitschang, L.; Korchak, S.; Kilian, W.; Riemer, T. Comprehensive Quantitative and Calibration-Free Evaluation of Hyperpolarized Xenon-Host Interaction by Multiparametric NMR. Anal. Chem. 2022, 94, 2561–2568. [Google Scholar] [CrossRef]
- Zhang, L.; Hermans, J. Hydrophilicity of cavities in proteins. Proteins 1996, 24, 433–438. [Google Scholar] [CrossRef]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D.; Pastor, R.W. Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef]
- Kestin, J.; Khalifa, H.E.; Ro, S.T.; Wakeham, W.A. The viscosity and diffusion coefficients of eighteen binary gaseous systems. Phys. A Stat. Mech. Its Appl. 1977, 88, 242–260. [Google Scholar] [CrossRef]
- Horton, G.K.; Leech, J.W. On the Statistical Mechanics of the Ideal Inert Gas Solids. Proc. Phys. Soc. 1963, 82, 816–854. [Google Scholar] [CrossRef]
- Rutkai, G.; Thol, M.; Span, R.; Vrabec, J. How well does the Lennard-Jones potential represent the thermodynamic properties of noble gases? Mol. Phys. 2017, 115, 1104–1121. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Dávila-Contreras, E.M.; Qi, Y.; Lee, J.; Monje-Galvan, V.; Venable, R.M.; et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef]
- Jo, S.; Lim, J.B.; Klauda, J.B.; Im, W. CHARMM-GUI Membrane Builder for mixed bilayers and its application to yeast membranes. Biophys. J. 2009, 97, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Brooks, C.L.; Mackerell, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Im, W. Automated builder and database of protein/membrane complexes for molecular dynamics simulations. PLoS ONE 2007, 2, e880. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Vogel, A.; Reuther, G.; Roark, M.B.; Tan, K.T.; Waldmann, H.; Feller, S.E.; Huster, D. Backbone Conformational Flexibility of the Lipid Modified Membrane Anchor of the Human N-Ras Protein Investigated by Solid-State NMR and Molecular Dynamics Simulation. Biochim. Biophys. Acta 2010, 1798, 275–285. [Google Scholar] [CrossRef]
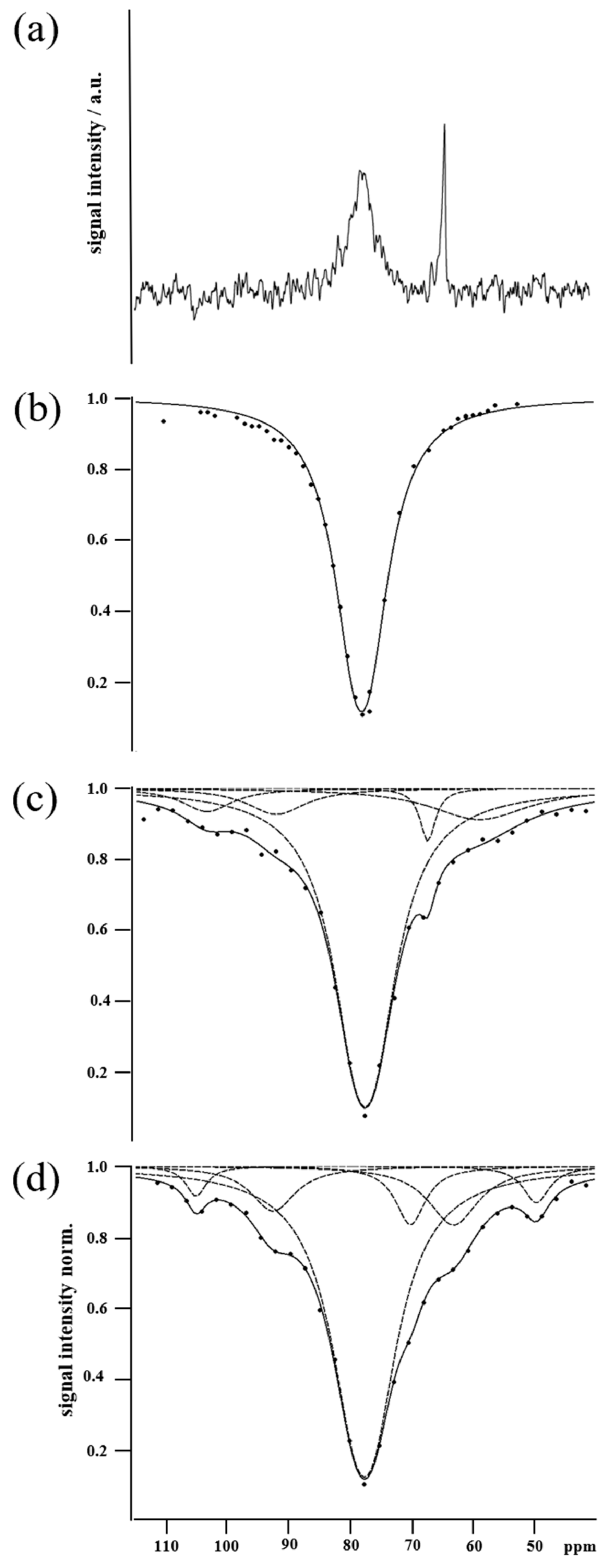

| Sample | Count | Position b/ppm | Amplitude B | Width a/π/Hz |
|---|---|---|---|---|
| conjugate | 1 | 103.3 ± 2.0 | 0.069 ± 0.047 | 820 ± 916 |
| conjugate, NPY inc. | 1 | 104.9 ± 0.4 | 0.089 ± 0.027 | 322 ± 210 |
| conjugate | 2 | 91.9 ± 2.1 | 0.077 ± 0.031 | 947 ± 797 |
| conjugate, NPY inc. | 2 | 92.4 ± 0.5 | 0.136 ± 0.018 | 704 ± 159 |
| conjugate | 3 | 77.5 ± 0.1 | 2.300 ± 0.140 | 573 ± 42 |
| conjugate, NPY inc. | 3 | 77.5 ± 0.1 | 2.120 ± 0.075 | 618 ± 33 |
| conjugate | 4 | 67.2 ± 0.8 | 0.163 ± 0.131 | 242 ± 362 |
| conjugate, NPY inc. | 4 | 70.0 ± 0.8 | 0.181 ± 0.050 | 466 ± 267 |
| conjugate | 5 | 58.8 ± 3.4 | 0.092 ± 0.054 | 1570 ± 1306 |
| conjugate, NPY inc. | 5 | 63.0 ± 0.7 | 0.182 ± 0.027 | 785 ± 179 |
| conjugate, NPY inc. | 6 | 49.5 ± 0.4 | 0.109 ± 0.018 | 417 ± 156 |
| bicelles, CrA-Cy3-mal inc. | 77.9 ± 0.1 | 2.206 ± 0.100 | 497 ± 21 | |
| Y2R-bicelles, CrA-COOH inc. | 77.3 ± 0.5 | - | 320 ± 20 | |
| Y2R-bicelles, CrA-COOH inc. | 63.7 ± 0.5 | - | 24 ± 2 |
| Contact Site | Apo Y2R-A202C-CrA | Y2R-A202C-CrA/NPY |
|---|---|---|
| NTER | L40, I41, K45 | L40, I41, S43 |
| ECL1 | L112, M113, G114 | |
| ECL2_1 | I195, P196, F198 | I195, P196, F198 |
| ECL2_2 | W207, P208, G209 | |
| ECL3 | Q296, D299, L300 | L300, K301, E302 |
| NPY NTER | G9, E10, A12 | |
| NPY helix | A14, M17, Y21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, P.; Vogel, A.; Schwarze, B.; Seufert, F.; Licha, K.; Wycisk, V.; Kilian, W.; Hildebrand, P.W.; Mitschang, L. Towards Probing Conformational States of Y2 Receptor Using Hyperpolarized 129Xe NMR. Molecules 2023, 28, 1424. https://doi.org/10.3390/molecules28031424
Schmidt P, Vogel A, Schwarze B, Seufert F, Licha K, Wycisk V, Kilian W, Hildebrand PW, Mitschang L. Towards Probing Conformational States of Y2 Receptor Using Hyperpolarized 129Xe NMR. Molecules. 2023; 28(3):1424. https://doi.org/10.3390/molecules28031424
Chicago/Turabian StyleSchmidt, Peter, Alexander Vogel, Benedikt Schwarze, Florian Seufert, Kai Licha, Virginia Wycisk, Wolfgang Kilian, Peter W. Hildebrand, and Lorenz Mitschang. 2023. "Towards Probing Conformational States of Y2 Receptor Using Hyperpolarized 129Xe NMR" Molecules 28, no. 3: 1424. https://doi.org/10.3390/molecules28031424
APA StyleSchmidt, P., Vogel, A., Schwarze, B., Seufert, F., Licha, K., Wycisk, V., Kilian, W., Hildebrand, P. W., & Mitschang, L. (2023). Towards Probing Conformational States of Y2 Receptor Using Hyperpolarized 129Xe NMR. Molecules, 28(3), 1424. https://doi.org/10.3390/molecules28031424





